Abstract
Background. Renal artery stenosis (RAS) causes renal injury partly via microvascular (MV) endothelial dysfunction and damage. Vascular endothelial growth factor (VEGF) is crucial for preservation of microvasculature and promotes vascular proliferation and endothelial repair. We have previously shown that MV rarefaction is associated with decreased VEGF in the kidney exposed to chronic RAS, accompanied by deteriorated renal function and fibrosis. We hypothesized that preserving the renal microcirculation in the stenotic kidney will halt the progression of renal damage.
Methods. Unilateral RAS was induced in 16 pigs. In eight, VEGF (0.05 micrograms/kg) was infused intra-renally at the onset of RAS. After 6 weeks, single-kidney haemodynamics and function were assessed using in vivo multi-detector computed tomography (CT). Renal microvessels, angiogenic pathways and morphology were investigated ex vivo using micro-CT, real-time PCR and histology.
Results. Blood pressure and degree of RAS was similar in RAS and RAS + VEGF pigs. Single-kidney renal blood flow (RBF) and glomerular filtration rate (GFR) were reduced in RAS compared to Normal (221.1 ± 46.5 and 29.9 ± 3.8 vs. 522.5 ± 60.9 and 49.3 ± 3.4 mL/min, respectively, P < 0.05), accompanied by decreased cortical MV density and increased renal fibrosis. Pre-emptive administration of VEGF preserved MV architecture, attenuated fibrosis and normalized RBF and GFR (510.8 ± 50.9 and 39.9.1 ± 4.1 mL/min, P = not significant vs. Normal).
Conclusions. This study underscores the importance of the renal microcirculation in renovascular disease. Intra-renal administration of VEGF preserved renal MV architecture and function of the stenotic kidney, which in turn preserved renal haemodynamics and function and decreased renal fibrosis. These observations suggest that preventing renal MV loss may be a potential target for therapeutic approaches for patients with chronic renovascular disease.
Keywords: computerized tomography, microcirculation, renal artery stenosis, renal haemodynamics, VEGF
Introduction
Vascular nephropathies account for over a third of all cases of end-stage renal disease. With the rising incidence and prevalence in obesity, diabetes and related co-morbidities such as atherosclerosis and hypertension, which may contribute to compromise the blood flow to the kidneys by promoting peripheral vascular disease and specifically, favoring the development of obstructive lesions in the main renal artery, as occurs in atherosclerotic renovascular disease, which accounts for a significant portion of the cases of end-stage renal disease [1,2]. Furthermore, the above-mentioned etiologies may affect the kidneys by additional mechanisms, such as inflammation, oxidative stress and fibrosis, that may deteriorate the haemodynamics and function of the kidney [3].
Renal artery stenosis (RAS), the major cause of renovascular hypertension and ischemic nephropathy, induces progressive renal injury and may evolve to end-stage renal disease. One of the ways by which RAS may progressively induce renal tissue injury is by promoting microvascular (MV) endothelial dysfunction, damage and loss. Defective microcirculation is a prominent feature in chronic renal disease. Convincing evidence supports the important role that the damage of renal microvessels and their eventual loss have on the progression of renal injury [4]. This process, also called MV rarefaction, results in a reduction in the availability of microvessels as a result of a deterioration of their function (functional rarefaction) and/or promotion of their remodeling or directly, loss (structural rarefaction) [5]. The importance of renal MV rarefaction is underscored by previous studies showing that glomerular and peritubular MV loss accentuates impairment of blood flow, development of renal ischaemia and progression of scarring in progressive renal disease [6].
We have previously shown in a model of chronic renovascular disease that the stenotic kidney develops a marked reduction in cortical MV density, accompanied by a significant deterioration of the renal function and increased glomerular and tubulo-interstitial scarring [7,8]. These changes were paralleled by a significant reduction in the expression and bioavailability of vascular endothelial growth factor (VEGF) [7]. This model allows the study of the long-term changes in MV architecture and the potential molecular mechanisms involved in the process of MV generation and repair. Hence, since we have previously shown that decreased renal VEGF parallels MV rarefaction and the functional and structural deterioration of the stenotic kidney [7,8], the current study was designed to investigate whether preservation of the renal MV architecture by an intra-renal administration of VEGF (to prevent its reduction), from the onset of RAS, will preserve the haemodynamics and function and decrease the damage of the stenotic kidney.
Materials and methods
The Institutional Animal Care and Use Committee at the University of Mississippi Medical Center approved all the protocols and procedures. Twenty-three domestic pigs (50–55 kg) were studied after 6 weeks of observation. In 13 pigs, unilateral RAS was induced at baseline by placing a local-irritant coil inside the main renal artery, which induced gradual development of RAS, as previously described [9,10]. The pigs were then randomized into two groups: those that were not further treated (RAS, n = 8) or those treated with an intra-renal infusion of VEGF (0.05 μg/kg, RAS + VEGF, n = 8) at the time of the induction of the stenosis. Administration was performed through a 5F balloon catheter, beyond the point where the coil was placed to induce RAS, as a slow bolus over 10 minutes, a procedure that does not have any effect on blood pressure [11]. The dose of VEGF used in this study has been well tolerated in humans and was selected based on a previous clinical study demonstrating a sustained increase in collateral density and perfusion of the ischaemic myocardium [12,13]. The animals were followed for 6 weeks, throughout which blood pressure was continuously measured using a telemetry system (PhysioTel, Data Sciences) implanted at baseline in the left femoral artery. Mean arterial pressure (MAP) was recorded at 5-minute intervals and averaged for each 24-hour period [9,14,15]. The other seven pigs were used as controls (Normal, n = 7).
By 6 weeks after induction of RAS, all of the pigs underwent renal angiography to quantify the degree of RAS. For this, the pigs were anaesthetized with intra-muscular telazol (5 mg/kg) and xylazine (2 mg/kg), intubated and mechanically ventilated on room air. Anaesthesia was maintained with a mixture of ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min) in normal saline, administered via an ear vein cannula (0.05 mL/kg/min). Under sterile conditions and fluoroscopic guidance, an 8F arterial catheter was advanced to the renal artery, proximal to the stenosis, and renal angiography was performed, as previously described [9,10,16,17]. Extent of the stenosis was assessed as the decrease in luminal diameter of the renal artery at the most stenotic point compared to a proximal stenosis-free segment. After angiography, the catheter was positioned in the superior vena cava, and in vivo helical multi-detector computed tomography (MDCT) flow studies were performed in the stenotic and contralateral kidneys. Briefly, sequential acquisition of 160 consecutive scans were obtained after a central venous injection of iopamidol (0.5 mL/kg/2 sec), for assessment of single-kidney renal blood flow (RBF, milliliters per minute), perfusion (milliliters per minute per gram tissue) and glomerular filtration rate (GFR, milliliters per minute), as previously detailed and validated [9,18,19]. Renal vascular resistance was calculated by dividing the mean arterial pressure (at the time of the in vivo studies) and MDCT-derived RBF. Blood samples were collected from the inferior vena cava and renal vein (from the stenotic kidney) for measurement of plasma renin activity (PRA, radio-immunoassay).
Following completion of all studies, the pigs were allowed to recover for 2 days (to allow for contrast media washout) and were then euthanized with a lethal intravenous injection of sodium pentobarbital (100 mg/kg, Sleepaway®, Fort Dodge Laboratories, Inc, Fort Dodge, IA). Kidneys were removed using a retroperitoneal incision and immersed in heparinized saline (10 units/mL). A lobe of tissue was used for micro-CT reconstruction, while another lobe of tissue was removed from one end of the kidney, snap frozen in liquid nitrogen and stored at −80°C to later assess the renal expression of renal angiogenic factors and peritubular and glomerular capillary density using real-time quantitative PCR, or preserved in 10% formalin to later perform immunohistochemistry against CD31/PECAM-1 [20] and integrin β3, and assessment of renal morphology in slices stained with trichrome [9].
Renal angiography
The degree of RAS was measured by quantitative renal angiography, as previously described [9,10,16,17]. Briefly, RAS was calculated as the decrease in luminal diameter of the renal artery at the most stenotic point compared to a proximal stenosis-free segment and expressed as % of stenosis.
MDCT analysis
Manually traced regions of interest were selected in MDCT images in the aorta, renal cortex, medulla and papilla, and their densities were sampled. Time–density curves were generated and fitted with extended gamma-variate curve-fits, and the area enclosed under each segment of the curve and its first moment calculated using the curve-fitting parameters [19]. These were used to calculate single-kidney RBF (milliliters per minute), GFR (milliliters per minute) and renal perfusion (milliliters per minute per gram tissue), using previously validated methods [9,18,19]. Finally, a volume study was performed for measurement of cortical and medullary volumes, as previously described [9,18,19].
Micro-CT
The stenotic kidney was perfused under physiological perfusion pressure (Syringe Infusion Pump 22, Harvard Apparatus, Holliston, MA) with an intravascular contrast agent, (Microfil MV122, Flow Tech, Inc., Carver, MA). The kidney samples were scanned at 0.3° increments using a micro-CT scanner and reconstructed at 9-μm resolution for subsequent analysis, as previously described [21,22]. Images were analyzed with the Analyze® software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The cortex was tomographically divided into 12 levels (starting at the juxtamedullary cortex) and the medulla into 10 levels, obtained at equal intervals [22], and the spatial density and distribution of microvessels (under 80 μm) were calculated.
Renal VEGF
Renal protein concentration and expression of VEGF in the stenotic kidney was measured in whole tissue homogenates using an enzyme immunoassay (ELISA, R&D, Minneapolis, MN) and western blotting (Santa Cruz, CA, 1:200) following standard procedures, as described previously [7].
Real-time PCR
RNA isolation, cDNA synthesis and real-time quantitative PCR were performed following standard protocols, as previously described [23–25]. Briefly, total RNA was isolated from Normal, RAS and RAS + VEGF kidneys using TRIZOL (Invitrogen, CA).Then, RNA samples were purified with chloroform and isopropyl alcohol, dissolved in diethyl pyrocarbonate (DEPC)-treated water and quantified with a spectrophotometer (A260). cDNA was synthesized using the Invitrogen SuperScript first-strand synthesis kit according to the manufacturer's instructions and as previously described [25]. To investigate the expression of endothelial nitric oxide synthase (eNOS), hypoxia-inducible factor (HIF-1α), VEGF, Flk-1, angiopoietin-1 and -2, and glyceraldehyde-3-phosphate dehydrogenase (GADPH) mRNA, real-time PCR (Bio-Rad, Hercules, CA) was subsequently performed using the SYBR Green JumpStart Taq ReadyMix kit (Sigma, St Louis, MO), as previously described [25]. The relative amount of mRNA was normalized to an internal control GAPDH and calculated by 2–ΔΔCT. The gene-specific sequences used are in Table 1.
Table 1.
Gene specific sequences
| eNOS | Upper 5′-GGGCATGAACCATGAGAAGT-3″ |
| Lower 5′-GTCTTCTGGGTGGCAGTGAT-3″ | |
| HIF-1α | Upper 5′-TTACAGCAGCCAGATGATCG-3″ |
| Lower 5′-GATTGCCCCAGGAGTCTACA-3″ | |
| VEGF | Upper 5′-CCACGAAGTGGTGAAGTTCATG-3″ |
| Lower 5′-GATGTCCACCAGGGTCTCGAT-3″ | |
| Flk-1 | Upper 5′-TGATCGGAAATGACACTGGA-3″ |
| Lower 5′-CACAACTCCATGCTGGTCAC-3″ | |
| Angiopoietin-1 | Upper 5′-TCTTTTGCAAAGGCGTTCTT-3″ |
| Lower 5′-TTGTATTGCCAGCACTCTCG-3″ | |
| Angiopoietin-2 | Upper 5′-AAAGTTGCTGCAGGGAAAGA-3″ |
| Lower 5′-TCACAGCTCAGAGCGAAGAA-3″ | |
| GADPH | Upper 5′-GGGCATGAACCATGAGAAGT-3″ |
| Lower 5′-GTCTTCTGGGTGGCAGTGAT-3″ |
Immunohistochemistry
Because of their size, microvessels under 10 μm cannot be identified by the micro-CT technique. Therefore, peritubular and glomerular capillaries were studied using immunostaining against CD31 [20] (Santa Cruz, CA, 1:80) in 5-μm paraffin-embedded mid-hilar renal cross-sections. In addition, immunoexpression for integrin β3 (Santa Cruz, CA, 1:80), a receptor for VEGF to promote vascular proliferation and a marker of sprouting angiogenesis [26,27], was also determined. Positive immunoreaction was detected using the secondary antibody, IgG Envision Plus (Dako, Carpinteria, CA), was followed by staining with the Vector NovaRED substrate kit (Vector Laboratories, Burlingame, CA), following the vendor's instructions.
Histology
Mid-hilar 5-μm cross-sections of each kidney (one per animal) were examined using a computer-aided image analysis program (NIS Element 3.0, Nikon Instruments, Melville, NY). In each representative slide, trichrome and integrin β3 staining was semi-automatically quantified in 15–20 fields by the computer program, expressed as percentage of staining of total surface area, and the results from all fields averaged [9]. To quantify CD31 immunoreactivity, randomly selected 15–20 visual fields from each sample (one slide per animal) were analyzed at ×40 magnification. Capillaries were identified as microvessels constituted of a single layer of endothelial cells [28] and quantified as the number of capillaries per visual field. Glomerular score (percentage of sclerotic glomeruli) was assessed by recording the number of sclerotic glomeruli in a total of 100 randomly selected profiles of glomeruli as previously described [9]. In addition, we evaluated glomerular area in 20 randomly selected visual fields in each section.
Statistical analysis
Results are expressed as mean ± SEM. Comparisons within groups were performed using paired Student's t-test, and among groups using ANOVA, with the Bonferroni correction for multiple comparisons, followed by unpaired Student's t-test. Statistical significance was accepted for P ≤ 0.05.
Results
Renal function
Mean arterial pressure and the angiographic degree of stenosis were similarly and significantly greater in RAS and RAS + VEGF animals compared to normal untreated controls (Table 2, Figure 1). Renal volume was similarly decreased in RAS and RAS + VEGF compared to normal untreated controls (P < 0.01 vs. RAS and RAS + VEGF, Table 2). Basal RBF and GFR were diminished in RAS, while regional perfusion remained unchanged. However, all of them were significantly augmented in RAS + VEGF pigs (Table 2), suggesting improved renovascular function. Consequently, the elevated renal vascular resistance in RAS compared to normal animals (P < 0.001 vs. Normal, Table 2) was decreased in RAS + VEGF pigs (P = not significant (NS) vs. Normal and RAS, Table 2). Plasma renin activity was similar in all the groups, as typical for chronic experimental RAS [9] (Table 2).
Table 2.
Mean arterial pressure (average of last 2 weeks prior to CT studies), degree of stenosis, basal single-kidney haemodynamics and function and plasma renin activity (mean ± SEM) in normal, RAS and RAS pigs treated with intra-renal vascular endothelial growth factor (RAS + VEGF)
| Parameter | Normal | RAS | RAS + VEGF |
|---|---|---|---|
| n = 7 | n = 8 | n = 8 | |
| Mean arterial pressure (mmHg) | 90.0.9 ± 2.1 | 132.4 ± 4.1* | 124.8 ± 3.4* |
| Degree of stenosis (%) | 0.0 ± 0.0 | 73.4 ± 4.7* | 72.8 ± 5.6* |
| Renal vascular resistance (mmHg/mL/min) | 0.21 ± 0.03 | 0.50 ± 0.03* | 0.29 ± 0.05† |
| Renal volume (cm3) cortex | 126.4 ± 8.4 | 66.0 ± 7.2* | 77.3 ± 10.7* |
| Medulla | 38.7 ± 2.3 | 23.4 ± 2.1* | 26.2 ± 3.7* |
| Renal blood flow (mL/min) | 522.5 ± 60.9 | 241.3 ± 31.2* | 494.6 ± 60.8† |
| Perfusion (mL/min/cc) cortex | 3.3 ± 0.3 | 2.9 ± 0.4 | 4.8 ± 0.6*† |
| Medulla | 2.5 ± 0.4 | 2.4 ± 0.4 | 3.5 ± 0.4† |
| Glomerular filtration rate (mL/min) | 49.3 ± 3.4 | 30.9 ± 2.8* | 41.3 ± 3.6† |
| Plasma renin activity IVC (ng/mL/h) | 0.16 ± 0.04 | 0.18 ± 0.06 | 0.22 ± 0.04 |
| Stenotic kidney | – | 0.17 ± 0.02 | 0.20 ± 0.08 |
*P < 0.05 vs. Normal.
†P < 0.05 vs. RAS.
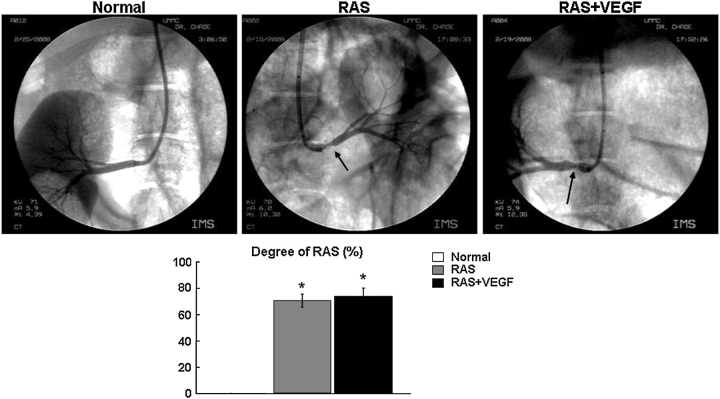
Fig. 1.
Representative renal angiography (top), quantification of the renal artery stenosis (bottom) in Normal, RAS and RAS treated with intra-renal VEGF (RAS + VEGF) animals after 6 weeks of observation. The degree of RAS and increase in blood pressure (measured chronically by telemetry) were similarly elevated in RAS and RAS + VEGF compared to normal controls, *P < 0.05 vs. Normal.
MV 3D architecture
Micro-CT reconstruction of the renal MV architecture showed that the density and distribution of microvessels under 80 μm in RAS was significantly decreased (P = 0.035 vs. Normal) across the renal cortex (inner, middle and outer cortex), but preserved in the medulla. Notably, the MV density was augmented in RAS + VEGF kidneys in both cortical and medullary compartments (despite the fact that RAS on its own did not affect MV density in the medulla) (Figure 2). This was accompanied by increased in integrin β3 immunoreactivity in RAS + VEGF kidneys (Figure 2 bottom), suggesting angiogenic effects of VEGF administration on the stenotic kidney. In addition, peritubular and glomerular capillaries (microvessels <10 um not quantified by micro-CT) followed this trend and were distinctly diminished in the RAS kidney, but augmented by VEGF administration (Figure 3).
Fig. 2.
Representative 3D tomographic images of the kidney and quantification of microvascular density of the renal cortex and medulla (top); quantification of renal expression of integrin β3 in Normal, RAS and RAS treated with intra-renal VEGF (RAS + VEGF) kidneys (bottom). Intra-renal VEGF distinctly preserved cortical and medullary MV density of those microvessels under 80 μm, partly by promoting MV proliferation, *P < 0.05 vs. Normal, †P < 0.05 vs. RAS.
Fig. 3.
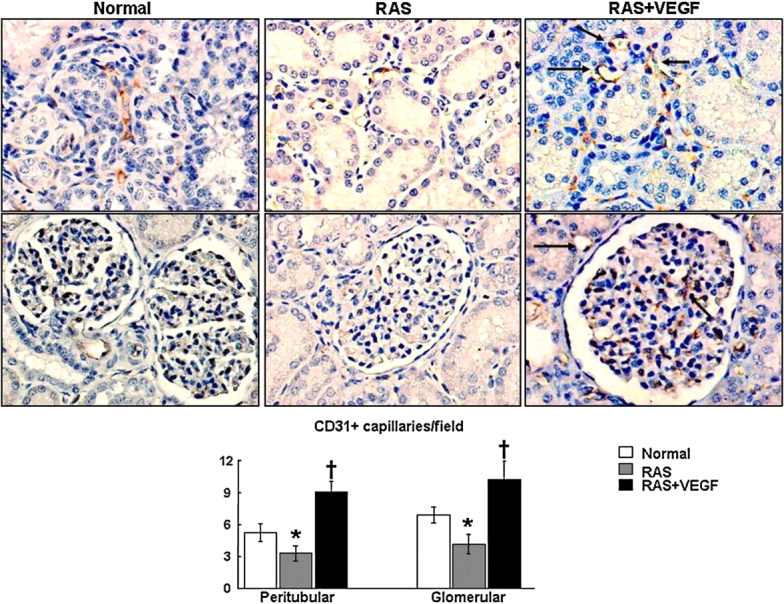
Representative CD31 immunostaining identifying peritubular (top) and glomerular (middle) capillaries (microvessels under 10 μm) and quantification (bottom) in Normal, RAS and RAS treated with intra-renal VEGF (RAS + VEGF) kidneys. Following the trend observed in the density of microvessels under 80 μm, VEGF administration in the stenotic kidney increased peritubular and glomerular immunoreactivity of CD31, suggesting augmented capillary density, †P < 0.05 vs. RAS, #P = 0.059 vs. Normal.
Angiogenic factors
Renal tissue protein content of VEGF was significantly decreased in the RAS kidney compared to normal untreated controls (0.27 ± 0.05 vs. 0.49 ± 0.06 pg/mg of protein, P = 0.01 vs. RAS) but was normalized in RAS + VEGF kidneys (0.75 ± 0.1 pg/mg of protein, P = 0.04 vs. RAS and P = NS vs. Normal). Renal expression was assessed by western blot and showed similar results (Figure 4 top). In contrast, RAS significantly increased renal mRNA expression of pro-angiogenic eNOS, HIF-1α, VEGF and its receptor Flk-1 compared to normal controls (Figure 4, bottom). However, crucial downstream mediators of VEGF such as angiopoietins-1 and -2 were downregulated in RAS. The renal mRNA levels of HIF-1α, VEGF and eNOS in RAS were normalized in RAS + VEGF, while Flk-1 remained increased. In addition, VEGF increased angiopoietin-1 and -2 (Figure 4, bottom), suggesting that VEGF administration at the onset of RAS promoted neovascularization and favoured the maturation of the new vessels in the stenotic kidney.
Fig. 4.
Representative renal protein expression of VEGF (top) (n = 7–8 per group, two representative bands shown), mRNA expression and quantification of HIF-1α, eNOS, VEGF, Flk-1 and angiopoietin-1 and -2 (bottom) in normal, RAS and RAS treated with intra-renal VEGF (RAS + VEGF) kidneys. VEGF administration in RAS-improved angiogenic signaling, *P < 0.05 vs. Normal.
Renal morphology
The RAS kidneys showed mild but significant glomerulosclerosis, accompanied by increased perivascular and tubulointerstitial fibrosis (Figure 5). These changes were attenuated by intra-renal VEGF administration (Figure 5). No apparent changes in glomerular area were evident among any of the groups (data not shown).
Fig. 5.
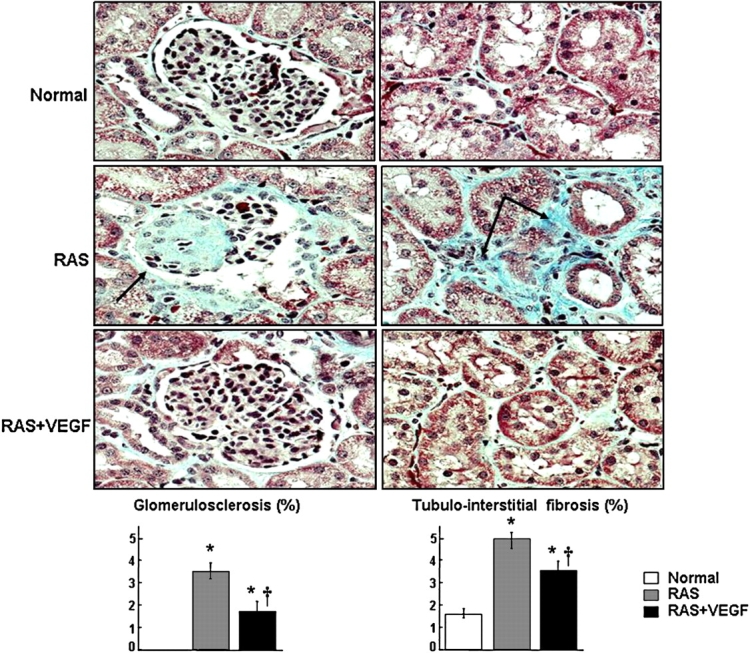
Representative renal trichrome staining showing glomerulosclerosis (left, ×40) and tubulo-interstitial fibrosis (right, ×40) and quantification in normal RAS and RAS treated with intra-renal VEGF (RAS + VEGF) kidneys. Intra-renal VEGF significantly attenuated renal fibrosis in the stenotic kidney, *P < 0.05 vs. Normal, †P < 0.05 vs. RAS.
Contralateral kidney
RBF (447.5 ± 75.9 and 574.9 ± 87.1 ml/min), GFR (70.9 ± 7.6 and 69.1 ± 10.3 ml/min) and regional perfusion (3.8 ± 0.9 and 3.3 ± 0.5 ml/min/cm3 tissue) in the contralateral kidney were similar in RAS and RAS + VEGF pigs, implying that changes due to hypertension after 6 weeks of RAS did not deteriorate the basal haemodynamics and function of the contralateral kidney compared to normal controls, and that the effects of VEGF on renal function were only in the stenotic kidney, where VEGF was infused. In addition, the contralateral kidney of RAS and RAS + VEGF showed a mild perivascular and tubulo-interstitial fibrosis (2.1 ± 0.9 and 1.8 ± 0.5%, respectively), while glomerulosclerosis was not observed (data not shown).
Discussion
This study extends our previous observations and highlights the importance of protecting the renal MV architecture and function in chronic renovascular disease. The data supports the notion that a loss in renal VEGF contributes to the reduction of the renal MV density during the evolution of RAS. Most importantly, the current study shows that preventing the decrease in renal VEGF, a key player in the angiogenic cascade, preserved the MV architecture and function, and decreased the remodeling of the stenotic kidney. In addition, this study shows feasibility of a novel therapeutic approach, suggesting potential for designing targeted interventions on the renal microcirculation to curtail progressive renal injury in chronic renovascular disease.
One of the most frequent therapeutic approaches to treat the chronically stenotic kidney is by attempting restoration of blood flow either by opening the stenotic renal artery or through bypass surgery. Notably, despite successful restoration of the blood flow to the kidneys, the renal function improves in only one-third of the cases while in the other two-thirds the renal function remains unchanged or deteriorates even further [29]. The reasons for this poor response and the determinants of the renal outcomes are unknown, and optimal therapeutic approaches are direly needed to recuperate the kidney. We hypothesize that MV rarefaction may determine the degree and extent of injury to the renal parenchyma distal to the obstruction, which may constitute a major factor impeding recovery of the stenotic kidney.
VEGF, the most important regulator of proliferation of new blood vessels, is also crucial for preservation of the microvasculature in general. By stimulating endothelial cell division, migration, survival and tube formation, VEGF plays a central role not only in the generation, but also the repair and maintenance of MV networks, including those in the kidney [6,30]. Furthermore, VEGF is also a prominent inhibitor of renal scarring. VEGF is produced constitutively in the adult kidney and impacts mainly endothelial cells [30]. The current study extends our previous studies that showed decreases in renal VEGF paralleling MV rarefaction and functional and structural deterioration of the stenotic kidney [7,8]. The reduced MV density in RAS was associated with increased renal vascular resistance, decreased RBF and GFR and significant renal scarring. Therefore, sustained levels of VEGF play a key role in preserving kidney function and structure, not only under physiological conditions, but even more importantly when facing disease as occurs in RAS.
By preventing the reduction of renal VEGF, we investigated whether and how preserving renal microvessels could protect the stenotic kidney during the evolution of RAS. We administered a single intra-renal dose of VEGF at the onset of the stenosis, based on previous clinical studies showing long-lasting effects of VEGF in augmenting the myocardial perfusion in the ischaemic heart, even after 60 days of administration [12,13]. A similar approach was also shown to increase vascular and capillary density in the hind-limb ischaemia model 40 days after induction of ischaemia [31]. We observed that exogenous VEGF in the stenotic kidney preserved cortical and medullary MV density of those vessels under 80 μm in diameter, augmented density of renal peritubular and glomerular capillaries and decreased renal vascular resistance. Preventing MV loss in RAS + VEGF kidneys also resulted in normalized RBF, GFR and significantly augmented cortical and medullary perfusion, measured 6 weeks after VEGF administration. In turn, VEGF administration led to a substantial attenuation of glomerulosclerosis and tubulo-interstitial fibrosis compared to untreated RAS. These data suggest that preventing MV rarefaction in chronic RAS resulted in a combined functional and structural renoprotective effect on the stenotic kidney. The increased MV density in RAS + VEGF was accompanied by improvement in the intra-renal angiogenic signaling compared to RAS kidneys, as suggested by normalization of HIF-1α, eNOS and angiopoietins, downstream mediators of VEGF. It is possible that the increased vascularization of the treated kidney by a pre-emptive intra-renal administration of VEGF not only resulted from proliferation of new vessels, but also from improved MV and capillary repair [32].
Our experimental approach provides a tool to elucidate the long-term changes in the vasculogenic mechanisms in the stenotic kidney that leads to defective angiogenesis and consequently, MV rarefaction. Studies have documented that acute ischaemia leads to a significant increase in VEGF [33], but evidence showing whether this response is sustained for a longer term (chronic) is scarce. We found that chronic RAS significantly increased the mRNA expression of VEGF and its upstream and downstream mediators such as HIF-1α, Flk-1 and eNOS, which likely represents a “response to injury” of the stenotic kidney to a chronic obstruction of blood flow. However, despite the upregulation of these factors, renal VEGF protein and the renal microvessels were significantly diminished in RAS, suggesting an abnormal response to the ischemic insult that failed to maintain the MV networks in the stenotic kidney. Discrepancies between VEGF mRNA and protein levels have been reported in other clinical scenarios [34], and the opposing effects of RAS on the levels of VEGF (renal mRNA vs. protein concentration) may reflect altered post-transcriptional mechanisms in the chronically stenotic kidney that may have interfered with VEGF signaling decreasing its renal bioavailability, as we have suggested [7,8], thereby explaining the significant increase in mRNA but not in the VEGF protein, nor its effects. The reasons for this disparate effect in the stenotic kidney are unclear and deserve further investigation. On the other hand, since RAS + VEGF kidneys received VEGF at the onset of the stenosis, the augmented bioavailability of this factor resulted in a sustained and more effective response to the ischaemic insult by preserving pre-existing vessels, promoting MV repair and successfully generating new ones. As a result, intra-renal microcirculation was preserved, adequate oxygen supply was provided to the RAS + VEGF kidney, and that resulted in normalization of renal VEGF, both at mRNA and protein levels.
Another potential contributory mechanism for the decrease in renal MV density in RAS could involve upregulation of endogenous VEGF inhibitors in the stenotic kidney that may have hindered VEGF vasculogenic actions [35–37]. Furthermore, the decrease in renal MV density in the stenotic kidney may have resulted from the decrease in renal angiopoietins, key downstream mediators of VEGF-induced neovascularization. A significant increase in HIF-1α signaling may upregulate VEGF and downregulate angiopoietins [38], which are necessary for stabilization and maturation of the new vessels to make them functional and thereby helpful to recover tissue perfusion [39–41]. Moreover, angiopoietins in concordance with VEGF have been shown to play a role in controlling glomerular filtration as well [40,42].
Based on our previous studies showing that MV rarefaction, renal functional deterioration, fibrosis and decreased in renal levels of VEGF progress in parallel [7,8], we first tried to elucidate the role that MV rarefaction may have in defining the progression of renal injury in the stenotic kidney, using intra-renal replenishment of VEGF as a tool to prevent those changes. In humans, the onset of renovascular disease is rarely definable, and the patients are diagnosed at different stages of the disease and with different degrees of renal compromise. Our studies were designed to first understand the role that renal microvessels may have for the progression of renal injury in a large animal model of chronic RAS mimicking clinical renovascular disease. Regional renal perfusion (blood flow/renal tissue) was preserved at this early stage of RAS as a result of a concomitant reduction in renal volume, MV density and RBF. Nevertheless, it is possible that, at a more advanced stage of the disease, this somewhat compensatory mechanism fails as renovascular disease progresses and renal injury increases [9]. In addition, deleterious changes in the contralateral kidney at a later stage may also contribute for the aggravation of hypertension and consequent renal damage. VEGF administration significantly increased regional perfusion as a result of an increased in the cortical and medullary MV density compared to untreated RAS, and despite a similar degree of stenosis and reduction in renal volume, preservation of the MV architecture was sufficient not only to augment the renal perfusion, but also to preserve the overall haemodynamics and function of the stenotic kidney. It is likely, this was the result of a combined effect of VEGF administration on both preservation of pre-existing vessels and generation of new ones, as indicated by the increased medullary MV density and augmented immunoreactivity for integrin β3, a receptor for VEGF to promote angiogenesis [26] and a marker of angiogenic vessels [27]. We are aware that the beneficial effects of VEGF administration in our model were largely preventive, and future studies investigating the potential of VEGF administration after established renal injury, at a more advanced stage of the disease, and even in the normal kidney, will be needed to further understand whether renal MV loss plays a central role for the progression and possibly, irreversibility of renal damage.
In summary, this study demonstrates a central role for MV loss in the progression of renal injury in the stenotic kidney. Using an experimental model of chronic renovascular disease, we attempted to preserve the MV architecture and function by preventing the reduction in renal VEGF. A preservation of the angiogenic cascade by VEGF was functionally consequential, as its intra-renal administration led to prevent MV rarefaction (due to both MV proliferation and likely MV repair), thereby contributing to preservation of the blood supply and renal perfusion, restoration of the haemodynamics and filtration function, and a decrease in fibrosis. These findings underscore the role of VEGF in the stenotic kidney and indicate feasibility and potential of a novel therapeutic approach to curtail renal injury, which may constitute a step forward for designing targeted interventions for management of patients with chronic renovascular disease.
Acknowledgments
Supported by grant 0830100N (Scientist Development Grant) from the American Heart Association and in part by a grant from the National Heart, Lung and Blood Institute P01 HL51971. We thank Mr. James Bailey and Mr. Fredrick Fails for their technical assistance during the in vivo MDCT studies.
Conflict of interest statement. None declared.
References
- 1.US Renal Data System: USRDS 2005 Annual Data Report; Atlas of End-Stage Renal Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes, and Digestive and Kidney Diseases; 2005. [Google Scholar]
- 2.Gomez Campdera FJ, Luno J, Garcia de Vinuesa S, et al. Renal vascular disease in the elderly. Kidney Int Suppl. 1998;68:S73–S77. doi: 10.1046/j.1523-1755.1998.06817.x. [DOI] [PubMed] [Google Scholar]
- 3.Chade AR, Lerman A, Lerman LO. Kidney in early atherosclerosis. Hypertension. 2005;45:1042–1049. doi: 10.1161/01.HYP.0000167121.14254.a0. [DOI] [PubMed] [Google Scholar]
- 4.Kang DH, Kanellis J, Hugo C, et al. Role of the microvascular endothelium in progressive renal disease. J Am Soc Nephrol. 2002;13:806–816. doi: 10.1681/ASN.V133806. [DOI] [PubMed] [Google Scholar]
- 5.Levy BI. Microvascular plasticity and experimental heart failure. Hypertension. 2006;47:827–829. doi: 10.1161/01.HYP.0000215283.53943.39. [DOI] [PubMed] [Google Scholar]
- 6.Kang DH, Johnson RJ. Vascular endothelial growth factor: a new player in the pathogenesis of renal fibrosis. Curr Opin Nephrol Hypertens. 2003;12:43–49. doi: 10.1097/00041552-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Chade AR, Zhu X, Mushin OP, et al. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 8.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 9.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 10.Lerman LO, Schwartz RS, Grande JP, et al. Noninvasive evaluation of a novel swine model of renal artery stenosis. J Am Soc Nephrol. 1999;10:1455–1465. doi: 10.1681/ASN.V1071455. [DOI] [PubMed] [Google Scholar]
- 11.Hariawala MD, Horowitz JR, Esakof D, et al. improves myocardial blood flow but produces EDRF-mediated hypotension in porcine hearts. J Surg Res. 1996;63:77–82. doi: 10.1006/jsre.1996.0226. [DOI] [PubMed] [Google Scholar]
- 12.Hendel RC, Henry TD, Rocha-Singh K, et al. Effect of intracoronary recombinant human vascular endothelial growth factor on myocardial perfusion: evidence for a dose-dependent effect. Circulation. 2000;101:118–121. doi: 10.1161/01.cir.101.2.118. [DOI] [PubMed] [Google Scholar]
- 13.Henry TD, Rocha-Singh K, Isner JM, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–880. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 14.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 15.Chade AR, Rodriguez-Porcel M, Herrmann J, et al. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 16.Chade AR, Krier JD, Rodriguez-Porcel M, et al. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004;286:F1079–F1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 17.Lerman LO, Nath KA, Rodriguez-Porcel M, et al. Increased oxidative stress in experimental renovascular hypertension. Hypertension. 2001;37:541–546. doi: 10.1161/01.hyp.37.2.541. [DOI] [PubMed] [Google Scholar]
- 18.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 19.Krier JD, Ritman EL, Bajzer Z, et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 20.Kaukinen A, Lautenschlager I, Helin H, et al. Peritubular capillaries are rarefied in congenital nephrotic syndrome of the Finnish type. Kidney Int. 2009 doi: 10.1038/ki.2009.41. [DOI] [PubMed] [Google Scholar]
- 21.Chade AR, Bentley MD, Zhu X, et al. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 22.Bentley MD, Rodriguez-Porcel M, Lerman A, et al. Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int. 2002;61:1056–1063. doi: 10.1046/j.1523-1755.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Krier JD, Textor SC, et al. Endothelin-a receptor blockade improves renal microvascular architecture and function in experimental hypercholesterolemia. J Am Soc Nephrol. 2006;17:3394–3403. doi: 10.1681/ASN.2006060635. [DOI] [PubMed] [Google Scholar]
- 24.Chade AR, Mushin OP, Zhu X, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 25.Zhu X, Rodriguez-Porcel M, Bentley M.D, et al. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 26.Mahabeleshwar GH, Feng W, Phillips DR, et al. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–2507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva R, D'Amico G, Hodivala-Dilke KM, et al. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–1713. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 28.Zhu XY, Daghini E, Chade AR, et al. Simvastatin prevents coronary microvascular remodeling in renovascular hypertensive pigs. J Am Soc Nephro. 2007;18:1209–1217. doi: 10.1681/ASN.2006090976. [DOI] [PubMed] [Google Scholar]
- 29.Textor SC. Ischemic nephropathy: where are we now? J Am Soc Nephrol. 2004;15:1974–1982. doi: 10.1097/01.ASN.0000133699.97353.24. [DOI] [PubMed] [Google Scholar]
- 30.Masuda Y, Shimizu A, Mori T, et al. Vascular endothelial growth factor enhances glomerular capillary repair and accelerates resolution of experimentally induced glomerulonephritis. Am J Pathol. 2001;159:599–608. doi: 10.1016/S0002-9440(10)61731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takeshita S, Zheng LP, Brogi E, et al. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–670. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schrijvers BF, Flyvbjerg A, De Vriese AS. The role of vascular endothelial growth factor (VEGF) in renal pathophysiology. Kidney Int. 2004;65:2003–2017. doi: 10.1111/j.1523-1755.2004.00621.x. [DOI] [PubMed] [Google Scholar]
- 33.Miraliakbari R, Francalancia NA, Lust RM, et al. Differences in myocardial and peripheral VEGF and KDR levels after acute ischemia. Ann Thorac Surg. 2000;69:1750–1753. doi: 10.1016/s0003-4975(00)01375-8. discussion 1754. [DOI] [PubMed] [Google Scholar]
- 34.Paydas S, Balal M, Tanriverdi K, et al. The relationship between the VEGF levels and VEGF mRNA expression and clinical course in different glomerulonephritis. Ren Fail. 2007;29:779–784. doi: 10.1080/08860220701540136. [DOI] [PubMed] [Google Scholar]
- 35.Favier J, Germain S, Emmerich J, et al. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol. 2005;207:358–366. doi: 10.1002/path.1833. [DOI] [PubMed] [Google Scholar]
- 36.Gupta K, Gupta P, Wild R, et al. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis. 1999;3:147–158. doi: 10.1023/a:1009018702832. [DOI] [PubMed] [Google Scholar]
- 37.Basile DP, Fredrich K, Chelladurai B, et al. Renal ischemia reperfusion inhibits VEGF expression and induces ADAMTS-1, a novel VEGF inhibitor. Am J Physiol Renal Physiol. 2008;294:F928–F936. doi: 10.1152/ajprenal.00596.2007. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Z. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- 39.Benest AV, Salmon AH, Wang W, et al. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 40.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 41.Tressel SL, Kim H, Ni CW, et al. Angiopoietin-2 stimulates blood flow recovery after femoral artery occlusion by inducing inflammation and arteriogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1989–1995. doi: 10.1161/ATVBAHA.108.175463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eremina V, Baelde HJ, Quaggin SE. Role of the VEGF-a signaling pathway in the glomerulus: evidence for crosstalk between components of the glomerular filtration barrier. Nephron Physiol. 2007;106:p32–p37. doi: 10.1159/000101798. [DOI] [PubMed] [Google Scholar]