Cardiac hypertrophy is defined as an increase in the size of the heart caused by an increase in the size of the cardiomyocytes therein, which is achieved by an increase in the number of sarcomeres, a basic unit of a muscle's cross-striated myofibril [1-3]. Cardiac hypertrophy is caused by hemodynamic overload, which includes pressure overload (PO) caused by aortic stenosis and high blood pressure, and volume overload (VO) caused by valvular insufficiency, chronic myocardial infarction, arteriovenous shunt, and pregnancy [1, 2]. PO and VO induce distinct forms of cardiac hypertrophy with different morphologies, mechanical properties, and gene expression profiles, at least at early phases. PO causes “concentric” hypertrophy, characterized by increased ventricular wall thickness and little chamber dilation, where parallel addition of sarcomeres increases the size of cardiomyocytes in both minor and major axes. In contrast, VO results in “eccentric” hypertrophy, characterized by increased ventricular volume with little increase in the wall thickness, where serial addition of sarcomeres increases the size of cardiomyocytes primarily by elongation [1-3].
Molecular, biological and functional properties in the heart are differentially regulated between PO- and VO-induced hypertrophy (Table 1). For example, synthesis of contractile proteins is increased during the early phase of PO cardiac hypertrophy [4-8]. In contrast, an increase in LV mass may be at least initially caused by reduced protein degradation, with protein synthesis becoming significant only at a later phase, in VO cardiac hypertrophy [4]. Perhaps one of the most interesting differences between PO and VO hypertrophy in terms of their functional significance and the underlying signaling mechanisms is the expression of fetal sarcomere proteins. Calderone and colleagues have shown that alpha-skeletal actin (αSKA), a fetal isoform of the contractile protein, is up-regulated in myocardium subjected to PO, but not in myocardium under VO in rats [9]. Other studies also demonstrated that αSKA is a marker of concentric, rather than eccentric hypertrophy, both in rodents and humans [10-12]. Since increased expression of αSKA is associated with increased ventricular contractility [13], and its expression decreases when concentric hypertrophy develops into the eccentric phenotype with reduced cardiac function [16], it may be possible that αSKA is essential, especially at the initial phase of PO, for the heart to offset the systolic impedance and maintain cardiac output[14]. The functional significance of the fact that αSKA is not up-regulated during VO hypertrophy remains unknown.
Table 1.
Morphological and molecular characteristics of concentric and eccentric hypertrophy, and their extra- and intracellular mediators. CM= cardiomyocyte; EM=extracellular matrix, FAK=focal adhesion kinase.
| Pathological hypertrophy | |||
|---|---|---|---|
| Morphologic and molecular characteristics | Concentric | Eccentric | |
| Gravimetric, morphological and structural parameters | Ventricular mass | ↑↑ | ↑↑ |
| Ventricular volume | =/↓ | ↑↑ | |
| Relative wall thickness | ↑↑ | = | |
| Cardiomyocyte growth in width | ↑↑↑ | =/↑ | |
| Cardiomyocyte growth in length | ↑ | ↑↑↑ | |
| Sarcomere replication | Parallel | Series | |
| CM protein turnover and fetal gene up-regulation [4, 6, 7, 9, 29] | Contractile protein synthesis | ↑↑ | =/↑ |
| Contractile protein degradation | ↓↓ | ↓↓ | |
| Fetal myosin switch | ↑↑ | =/↑ | |
| Alpha-skeletal actin expression | ↑↑ | = | |
| Atrial natriuretic factor | ↑↑ | ↑↑ | |
| Tubulin amount | ↑↑ | = | |
| EM protein turnover [30] | EM accumulation | ↑↑ | ↑ |
| MMP-9 activity | ↑ | ↑↑↑ | |
| MMP-1/TIMP-1 ratio | ↓ | =/↓ | |
| Biomechanical, neurohumoral and intracellular mediators [1, 2, 10, 11, 20, 31-36] | Biomechanical sensors signals | Melusin, ILK-1, FAK (activation) | FAK, gp130 and paxillin (reduced signaling), |
| Neuro-humoral mediators | Angiotensin II, Endothelin-1, norepinephrine | CT-1, LIF, IGF-1 | |
| Receptors | GPCR | gp130, LIF receptor | |
| Signaling molecules | cAMP, Ca2+, RhoA PI3K/Akt, MEK1/2, ERK1/2, calcineurin, Elf-4E | Ca2+, Gab1 SHP2, MEK5/ERK5, calcineurin, calmodulin kinases II-IV | |
Two major triggers of intracellular signaling pathways which modulate cardiac hypertrophy include biomechanical signaling and neurohumoral factors [1, 2]. Biomechanical stress activates stretch-sensitive molecules in the heart, including ion channels, integrins, G protein-coupled receptors, and other cytoskeletal proteins, which convert mechanical signals to chemical ones and transmit them to sarcomeres, second messengers, and the nucleus [1, 2]. In addition, neurohormonal factors, such as angiotensin II, endothelin-1, CT-1 and LIF, are secreted from cardiomyocytes and non-myocytes as autocrine or paracrine factors in response to mechanical forces, thereby causing cardiac hypertrophy, fibrosis, cell death, contractile dysfunction and rhythm disturbances [1, 2, 15-17].
Interestingly, the heart appears to have an ability to sense distinct forms of mechanical loading and initiate unique signaling mechanisms to induce different forms of hypertrophy. For example, the direction of mechanical stretch is a crucial determinant of growth in cardiomyocytes [18]. A stretch in the same direction as the cardiomyocyte sarcomere axis does not stimulate synthesis and turnover of contractile proteins. On the other hand, a stretch across the short axis of cardiomyocytes, which mimics stretch of cardiomyocytes in the heart under PO, leads to increased contractile protein synthesis and decreased degradation, resulting in protein accumulation [18]. Mechanical load of cardiomyocytes during the systolic phase is associated with increased activation of MEK1/2/ERK1/2 compared to cardiomyocytes subjected to diastolic load in vitro [19]. Increased diastolic stress may promote eccentric hypertrophy through reduced tyrosine phosphorylation of focal adhesion kinase and decreased interaction between focal adhesion kinase and cytoskeletal proteins, such as p130 and paxillin [20]. Systolic loading in vivo is associated with increased JNK activity, whereas increased diastolic stress causes a rapid activation of p38 MAPK [21].
Activation of stimulus-specific signaling molecules, either directly or indirectly through secretion of specific cytokines, mediates either PO- or VO-induced cardiac hypertrophy. Importantly, however, the causative roles of the aforementioned stimulus-specific signaling molecules in mediating VO-induced eccentric hypertrophy remain to be elucidated.
In this issue of the Journal, Nakaoka and colleagues [22] describe a new insight regarding the signaling mechanism mediating eccentric cardiac hypertrophy. Previously, the authors showed that LIF-induced elongation of cardiomyocytes is mediated by activation of SHP2, an SH2 domain-containing tyrosine phosphatase, and its association with Gab1, an adaptor protein [11]. In the present study, the authors demonstrated that LIF-induced activation of SHP2 negatively regulates expression of αSKA through inhibition of RhoA. The work not only confirms the importance of SHP2 activation in mediating the phenotype of eccentric hypertrophy, but also provides a specific explanation as to why eccentric hypertrophy is not accompanied by upregulation of αSKA. Specifically, inhibition of RhoA, together with activation of ERK5, plays an important role in mediating the cardiac phenotype observed in LIF-induced cardiac hypertrophy. RhoA is activated by PO [23] and Gαq-agonists inducing concentric hypertrophy [24], whereas it is inhibited by LIF [22]. Since RhoA regulates transcription mediated through SRF [25], RhoA may play an important role in mediating distinct gene expression profiles between concentric and eccentric hypertrophy. It should be noted that, since suppression of SHP2 allows LIF to induce activation of RhoA and expression of αSKA, LIF should be able to independently activate RhoA, which is negatively regulated by SHP2. It has been shown that activation of MEK5/ERK5 not only promotes elongation of myocytes but also inhibits parallel sarcomere assembly [26]. Thus, the signaling mechanism mediating eccentric hypertrophy appears to have cross-talk with that mediating concentric hypertrophy. It is possible that suppression of SHP2 may convert LIF-induced hypertrophy from eccentric to concentric. Even if this hypothesis is incorrect, the fact that SHP2 co-regulates cell shape and expression of αSKA is intriguing.
Although it is becoming increasingly clear that cytokines activating gp130 and the downstream signaling pathway could induce a cardiac phenotype mimicking eccentric hypertrophy, surprisingly, the involvement of these mechanisms in VO-induced eccentric hypertrophy has not been clearly demonstrated in vivo. For example, whether or not the IL6 family cytokines are selectively upregulated in VO-induced hypertrophy compared to PO-induced hypertrophy, and, if so, whether they are involved in the development of eccentric hypertrophy remain to be elucidated. The involvement of gp130, SHP2 or ERK5 in VO-induced hypertrophy also remains to be shown. Perhaps the most straightforward experiment would have been to test whether the development of eccentric hypertrophy by VO or by LIF can be inhibited in the absence of these molecules. Unfortunately, interpreting these experiments may not actually be straightforward, however, because gp130, SHP2 and ERK5 have multiple cellular functions, most importantly promoting cell survival mechanisms. Hemodynamic overload in the complete absence of these molecules induces cell death, and the consequent LV dysfunction independently affects cardiac hypertrophy and the gene expression profile, which makes interpretation of experimental results difficult. For example, the homozygous deletion of gp130 [27] or SHP2 [28] is sufficient to stimulate cardiac dilation secondary to cardiac dysfunction. The use of heterozygous deletion may partially alleviate this problem.
Since we now know that the gp130/SHP2 pathway induces eccentric hypertrophy in cardiomyocytes, future investigations should clarify the nature of the biomechanical sensors that are selectively activated during VO stress, and their intracellular mediators. In addition, neurohumoral factors that are specifically released in response to VO should be investigated. Eventually, how the biomechanical sensors, cytokines and the gp130/SHP2 pathway are connected to one another should be clarified (Figure 1). Intracellular signaling cascades involved in the promotion of eccentric hypertrophy may positively or negatively regulate cell survival, which is the major determinant of transition from compensated hypertrophy to cardiac failure. Since many of the signaling mechanisms described by Nakaoka are involved in cell survival, they may be involved in the initial (adaptive) phase of eccentric hypertrophy. We speculate that the signaling mechanisms mediating dilation of the heart during transition from compensated (concentric) to uncompensated (eccentric) hypertrophy could be distinct from the gp130/SHP2 pathway described in this work since such a process should be accompanied by cell death and consequent cardiac dysfunction. Elucidating the signaling mechanism mediating the development of eccentric hypertrophy at both adaptive and maladaptive phases may allow us to identify novel strategies to modulate ventricular remodeling and gene expression and delay or even prevent the onset of heart failure in patients with cardiac disease characterized by VO.
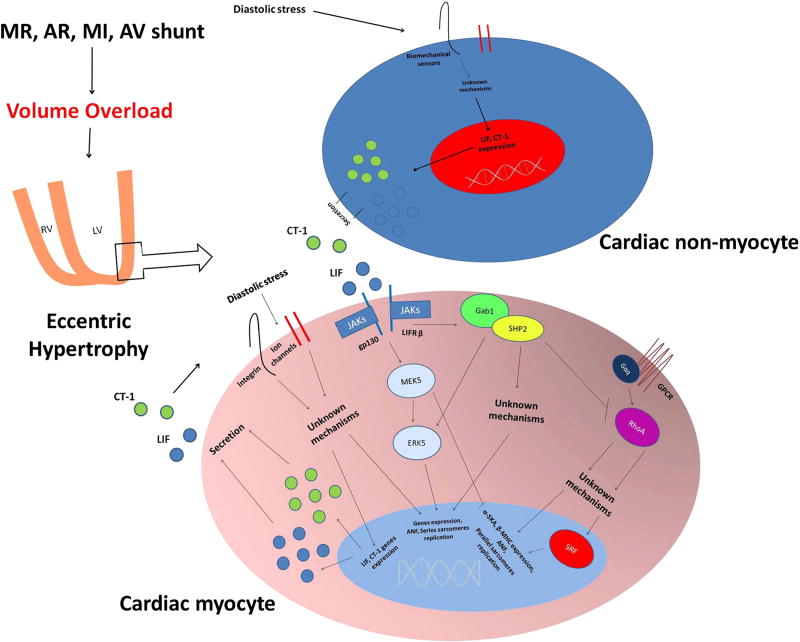
Figure 1.
Proposed mechanisms promoting the development of compensated eccentric hypertrophy following volume overload. An increase in diastolic stress might activate biomechanical sensors which activate intracellular signals that, in turn, modulate gene expression favoring eccentric hypertrophy development, and stimulate LIF and CT-1 transcription, translation and secretion. At the same time, cardiac non-myocytes may secrete LIF and CT-1 in response to VO. Thus, through autocrine and paracrine effects, secreted LIF and CT-1 activate the cardiomyocyte intracellular Gab1-SHP2 complex, by activating gp 130 and LIF receptors. SHP2 promotes cardiomyocyte elongation through ERK5 activation and perhaps also through other unknown mechanisms. At the same time SHP2 also inhibits RhoA, which regulates gene expression through SRF. MR: mitral regurgitation; AR: aortic regurgitation; MI: myocardial infarction; AV: arteriovenous.
Acknowledgments
The authors thank Daniela Zablocki for critical reading of the manuscript. This work was supported in part by U.S. Public Health Service Grants HL 59139, HL67724, HL69020, HL91469, and AG27211 and Foundation Leducq Transatlantic Network of Excellence
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006 Aug;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006 Jan 28;367(9507):356–67. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 3.Grossman W, Jones D, McLaurin LP. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975 Jul;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsuo T, Carabello BA, Nagatomo Y, Koide M, Hamawaki M, Zile MR, et al. Mechanisms of cardiac hypertrophy in canine volume overload. Am J Physiol. 1998 Jul;275(1 Pt 2):H65–74. doi: 10.1152/ajpheart.1998.275.1.h65. [DOI] [PubMed] [Google Scholar]
- 5.Nagatomo Y, Carabello BA, Hamawaki M, Nemoto S, Matsuo T, McDermott PJ. Translational mechanisms accelerate the rate of protein synthesis during canine pressure-overload hypertrophy. Am J Physiol. 1999 Dec;277(6 Pt 2):H2176–84. doi: 10.1152/ajpheart.1999.277.6.H2176. [DOI] [PubMed] [Google Scholar]
- 6.Imamura T, McDermott PJ, Kent RL, Nagatsu M, Cooper Gt, Carabello BA. Acute changes in myosin heavy chain synthesis rate in pressure versus volume overload. Circ Res. 1994 Sep;75(3):418–25. doi: 10.1161/01.res.75.3.418. [DOI] [PubMed] [Google Scholar]
- 7.Moalic JM, Bercovici J, Swynghedauw B. Protein synthesis during systolic and diastolic cardiac overloading in rats: a comparative study. Cardiovasc Res. 1981 Sep;15(9):515–21. doi: 10.1093/cvr/15.9.515. [DOI] [PubMed] [Google Scholar]
- 8.Dool JS, Mak AS, Friberg P, Wahlander H, Hawrylechko A, Adams MA. Regional myosin heavy chain expression in volume and pressure overload induced cardiac hypertrophy. Acta Physiol Scand. 1995 Dec;155(4):396–404. doi: 10.1111/j.1748-1716.1995.tb09989.x. [DOI] [PubMed] [Google Scholar]
- 9.Calderone A, Takahashi N, Izzo NJ, Jr, Thaik CM, Colucci WS. Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation. 1995 Nov 1;92(9):2385–90. doi: 10.1161/01.cir.92.9.2385. [DOI] [PubMed] [Google Scholar]
- 10.Wollert KC, Taga T, Saito M, Narazaki M, Kishimoto T, Glembotski CC, et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy. Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J Biol Chem. 1996 Apr 19;271(16):9535–45. doi: 10.1074/jbc.271.16.9535. [DOI] [PubMed] [Google Scholar]
- 11.Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, et al. Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes. Circ Res. 2003 Aug 8;93(3):221–9. doi: 10.1161/01.RES.0000085562.48906.4A. [DOI] [PubMed] [Google Scholar]
- 12.Suurmeijer AJ, Clement S, Francesconi A, Bocchi L, Angelini A, Van Veldhuisen DJ, et al. Alpha-actin isoform distribution in normal and failing human heart: a morphological, morphometric, and biochemical study. J Pathol. 2003 Mar;199(3):387–97. doi: 10.1002/path.1311. [DOI] [PubMed] [Google Scholar]
- 13.Hewett TE, Grupp IL, Grupp G, Robbins J. Alpha-skeletal actin is associated with increased contractility in the mouse heart. Circ Res. 1994 Apr;74(4):740–6. doi: 10.1161/01.res.74.4.740. [DOI] [PubMed] [Google Scholar]
- 14.Berni R, Savi M, Bocchi L, Delucchi F, Musso E, Chaponnier C, et al. Modulation of actin isoform expression before the transition from experimental compensated pressure-overload cardiac hypertrophy to decompensation. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1625–32. doi: 10.1152/ajpheart.01057.2008. [DOI] [PubMed] [Google Scholar]
- 15.Pemberton CJ, Raudsepp SD, Yandle TG, Cameron VA, Richards AM. Plasma cardiotrophin-1 is elevated in human hypertension and stimulated by ventricular stretch. Cardiovasc Res. 2005 Oct 1;68(1):109–17. doi: 10.1016/j.cardiores.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 16.Wang F, Seta Y, Baumgarten G, Engel DJ, Sivasubramanian N, Mann DL. Functional significance of hemodynamic overload-induced expression of leukemia-inhibitory factor in the adult mammalian heart. Circulation. 2001 Mar 6;103(9):1296–302. doi: 10.1161/01.cir.103.9.1296. [DOI] [PubMed] [Google Scholar]
- 17.Serneri GG, Boddi M, Cecioni I, Vanni S, Coppo M, Papa ML, et al. Cardiac angiotensin II formation in the clinical course of heart failure and its relationship with left ventricular function. Circ Res. 2001 May 11;88(9):961–8. doi: 10.1161/hh0901.089882. [DOI] [PubMed] [Google Scholar]
- 18.Simpson DG, Majeski M, Borg TK, Terracio L. Regulation of cardiac myocyte protein turnover and myofibrillar structure in vitro by specific directions of stretch. Circ Res. 1999 Nov 12;85(10):e59–69. doi: 10.1161/01.res.85.10.e59. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto K, Dang QN, Maeda Y, Huang H, Kelly RA, Lee RT. Regulation of cardiomyocyte mechanotransduction by the cardiac cycle. Circulation. 2001 Mar 13;103(10):1459–64. doi: 10.1161/01.cir.103.10.1459. [DOI] [PubMed] [Google Scholar]
- 20.Sabri A, Rafiq K, Seqqat R, Kolpakov MA, Dillon R, Dell'italia LJ. Sympathetic activation causes focal adhesion signaling alteration in early compensated volume overload attributable to isolated mitral regurgitation in the dog. Circ Res. 2008 May 9;102(9):1127–36. doi: 10.1161/CIRCRESAHA.107.163642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sopontammarak S, Aliharoob A, Ocampo C, Arcilla RA, Gupta MP, Gupta M. Mitogen- activated protein kinases (p38 and c-Jun NH2-terminal kinase) are differentially regulated during cardiac volume and pressure overload hypertrophy. Cell Biochem Biophys. 2005;43(1):61–76. doi: 10.1385/CBB:43:1:061. [DOI] [PubMed] [Google Scholar]
- 22.Nakaoka Y, Kunimoto S, Arita Y, Higuchi K, Yamamoto K, Fujio Y, Nishida K, Kuroda T, Hirota H, Yamauchi-Takihara K, Hirano T, Komuro I, Mochizuki N. SHP2 mediates gp130-dependent cardiomyocyte hypertrophy via negative regulation of skeletal alpha-actin gene. Journal of Molecular and Cellular Cardiology. 2010 doi: 10.1016/j.yjmcc.2010.03.001. in press. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi N, Horinaka S, Mita S, Nakano S, Honda T, Yoshida K, et al. Critical role of Rho-kinase pathway for cardiac performance and remodeling in failing rat hearts. Cardiovasc Res. 2002 Sep;55(4):757–67. doi: 10.1016/s0008-6363(02)00457-1. [DOI] [PubMed] [Google Scholar]
- 24.Aikawa R, Komuro I, Nagai R, Yazaki Y. Rho plays an important role in angiotensin II- induced hypertrophic responses in cardiac myocytes. Mol Cell Biochem. 2000 Sep;212(1-2):177–82. [PubMed] [Google Scholar]
- 25.Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, Balasubramanyam A, et al. RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem. 1998 Nov 13;273(46):30287–94. doi: 10.1074/jbc.273.46.30287. [DOI] [PubMed] [Google Scholar]
- 26.Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN. Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy. EMBO J. 2001 Jun 1;20(11):2757–67. doi: 10.1093/emboj/20.11.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirota H, Chen J, Betz UA, Rajewsky K, Gu Y, Ross J, Jr, et al. Loss of a gp130 cardiac muscle cell survival pathway is a critical event in the onset of heart failure during biomechanical stress. Cell. 1999 Apr 16;97(2):189–98. doi: 10.1016/s0092-8674(00)80729-1. [DOI] [PubMed] [Google Scholar]
- 28.Kontaridis MI, Yang W, Bence KK, Cullen D, Wang B, Bodyak N, et al. Deletion of Ptpn11 (Shp2) in cardiomyocytes causes dilated cardiomyopathy via effects on the extracellular signal-regulated kinase/mitogen-activated protein kinase and RhoA signaling pathways. Circulation. 2008 Mar 18;117(11):1423–35. doi: 10.1161/CIRCULATIONAHA.107.728865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsutsui H, Tagawa H, Kent RL, McCollam PL, Ishihara K, Nagatsu M, et al. Role of microtubules in contractile dysfunction of hypertrophied cardiocytes. Circulation. 1994 Jul;90(1):533–55. doi: 10.1161/01.cir.90.1.533. [DOI] [PubMed] [Google Scholar]
- 30.Nagatomo Y, Carabello BA, Coker ML, McDermott PJ, Nemoto S, Hamawaki M, et al. Differential effects of pressure or volume overload on myocardial MMP levels and inhibitory control. Am J Physiol Heart Circ Physiol. 2000 Jan;278(1):H151–61. doi: 10.1152/ajpheart.2000.278.1.H151. [DOI] [PubMed] [Google Scholar]
- 31.De Acetis M, Notte A, Accornero F, Selvetella G, Brancaccio M, Vecchione C, et al. Cardiac overexpression of melusin protects from dilated cardiomyopathy due to long-standing pressure overload. Circ Res. 2005 May 27;96(10):1087–94. doi: 10.1161/01.RES.0000168028.36081.e0. [DOI] [PubMed] [Google Scholar]
- 32.Lu H, Fedak PW, Dai X, Du C, Zhou YQ, Henkelman M, et al. Integrin-linked kinase expression is elevated in human cardiac hypertrophy and induces hypertrophy in transgenic mice. Circulation. 2006 Nov 21;114(21):2271–9. doi: 10.1161/CIRCULATIONAHA.106.642330. [DOI] [PubMed] [Google Scholar]
- 33.Takahashi N, Saito Y, Kuwahara K, Harada M, Tanimoto K, Nakagawa Y, et al. Hypertrophic responses to cardiotrophin-1 are not mediated by STAT3, but via a MEK5-ERK5 pathway in cultured cardiomyocytes. J Mol Cell Cardiol. 2005 Jan;38(1):185–92. doi: 10.1016/j.yjmcc.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Wada H, Ivester CT, Carabello BA, Cooper Gt, McDermott PJ. Translational initiation factor eIF-4E. A link between cardiac load and protein synthesis. J Biol Chem. 1996 Apr 5;271(14):8359–64. doi: 10.1074/jbc.271.14.8359. [DOI] [PubMed] [Google Scholar]
- 35.Kato T, Sano M, Miyoshi S, Sato T, Hakuno D, Ishida H, et al. Calmodulin kinases II and IV and calcineurin are involved in leukemia inhibitory factor-induced cardiac hypertrophy in rats. Circ Res. 2000 Nov 10;87(10):937–45. doi: 10.1161/01.res.87.10.937. [DOI] [PubMed] [Google Scholar]
- 36.DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, et al. Myocyte- restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006 Sep 15;99(6):636–45. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]