Abstract
Several lines of evidence implicate the amygdala in face– emotion processing, particularly for fearful facial expressions. Related findings suggest that face–emotion processing engages the amygdala within an interconnected circuitry that can be studied using a functional-connectivity approach. Past work also underscores important functional changes in the amygdala during development. Taken together, prior research on amygdala function and development reveals a need for more work examining developmental changes in the amygdala’s response to fearful faces and in amygdala functional connectivity during face processing. The present study used event-related functional magnetic resonance imaging to compare 31 adolescents (9–17 years old) and 30 adults (21–40 years old) on activation to fearful faces in the amygdala and other regions implicated in face processing. Moreover, these data were used to compare patterns of amygdala functional connectivity in adolescents and adults. During passive viewing, adolescents demonstrated greater amygdala and fusiform activation to fearful faces than did adults. Functional connectivity analysis revealed stronger connectivity between the amygdala and the hippocampus in adults than in adolescents. Within each group, variability in age did not correlate with amygdala response, and sex-related developmental differences in amygdala response were not found. Eye movement data collected outside of the magnetic resonance imaging scanner using the same task suggested that developmental differences in amygdala activation were not attributable to differences in eye-gaze patterns. Amygdala hyperactivation in response to fearful faces may explain increased vulnerability to affective disorders in adolescence; stronger amygdala–hippocampus connectivity in adults than adolescents may reflect maturation in learning or habituation to facial expressions.
INTRODUCTION
During adolescence, changes in social perception, social cognition, and social emotion occur in tandem with brain maturation (Ernst, Pine, & Hardin, 2006; Nelson, Leibenluft, McClure, & Pine, 2005; Casey, Giedd, & Thomas, 2000; Spear, 2000). To explain how these cognitive and emotional processes emerge and mature over time, research needs to focus on the development of relevant brain regions, including those, such as the amygdala, that mediate aspects of social–emotional functioning. The amygdala participates in processing information about salient emotional stimuli (LeDoux, 1994), including those positive or negative in valence (Yang et al., 2002; Breiter et al., 1996; Morris et al., 1996), and appears to be particularly responsive to fearful facial expressions (Whalen et al., 1998; Adolphs, Tranel, Damasio, & Damasio, 1995). For example, adults with amygdala lesions show significant deficits in fearful face recognition, despite intact ability to identify other emotions (Adolphs et al., 1995, 1999, 2005). Functional magnetic resonance imaging (fMRI) studies also indicate that fearful facial expressions, more than neutral or other emotional expressions, engage the amygdala (Whalen et al., 2001; Breiter et al., 1996; Morris et al., 1996).
Although the ability to detect facial expressions is present quite early in life (Nelson, 1987), major questions remain about factors that lead to changes in this ability during development. In particular, little is known about the development of normative neural responses to fearful faces in the amygdala and related structures engaged during face processing. Changes during the transition from adolescence to adulthood are of particular interest, given both that affective disorders typically begin during adolescence (Kessler et al., 2005; Pine, Cohen, Gurley, Brook, & Ma, 1998) and that evidence implicates the amygdala and related structures in the onset and persistence of such conditions (Pine, 2007). As such, charting normative neurodevelopmental pathways might clarify substrates of deviant neurodevelopment and their relationship to psychopathology.
Studies in nonhuman primates suggest that amygdala lesions produce different effects on social–emotional outcomes as a function of ontogeny (Amaral, 2002, 2003; Prather et al., 2001). For example, monkeys receiving amygdala lesions in adulthood exhibit a reduction in social fears compared to controls, whereas monkeys receiving such lesions in childhood demonstrate increased social fears (Prather et al., 2001). Thus, in studies of nonhuman species, the developmental timing of amygdala damage influences a behavioral analog of human anxiety.
fMRI studies of face–emotion processing in adults and adolescents with affective disorders document dysfunctional neural circuitry involving amygdala regions, the ventral prefrontal cortex, and the anterior cingulate cortex (ACC) relative to psychiatrically healthy individuals (McClure et al., 2007; Roberson-Nay et al., 2006; Straube, Kolassa, Glauer, Mentzel, & Miltner, 2004; Stein, Goldin, Sareen, Zorrilla, & Brown, 2002; Thomas, Drevets, Dahl, et al., 2001). Because research on atypical and typical development provides mutually reinforcing insights, research comparing patterns of neural function in healthy pediatric and adult samples might provide vital data for understanding events during adolescence that lead to persistent affective disorders.
Only a few studies have examined amygdala involvement in processing fearful facial expressions among healthy adolescents. Three fMRI studies have included adolescent-only samples. First, in line with adult studies (Whalen et al., 2001; Breiter et al., 1996; Morris et al., 1996), Baird et al. (1999) reported greater amygdala activation to fearful faces than to fixation trials or nonsense stimuli in 12 adolescents (12–17 years old). A second study found that amygdala response to fearful faces varied by age and sex in 19 adolescents (9–17 years old), such that amygdala activation correlated inversely with age, particularly among women (n = 10) (Killgore, Oki, & Yurgelun-Todd, 2001). Finally, in a third study of 16 adolescents (8–15 years old), no linear relationship was found between age and amygdala activation to fearful faces, regardless of sex (Yurgelun-Todd & Killgore, 2006).
An additional three developmental fMRI studies have directly compared healthy adolescents and adults on amygdala response to fearful facial expressions. One study found sex-related developmental differences in patterns of amygdala lateralization during passive viewing of fearful faces (Killgore & Yurgelun-Todd, 2004). The other two studies yielded conflicting results (Monk et al., 2003; Thomas, Drevets, Whalen, et al., 2001). Thomas, Drevets, Whalen, et al. (2001) compared amygdala response to fearful facial expressions between six male adolescents (aged 9–13 years) and six male adults (aged 18–30 years). Within each group, amygdala activation was greater when passively viewing fearful faces versus fixations. In direct group comparisons, however, adults showed significantly greater amygdala activation to fearful relative to neutral faces than adolescents, and adolescents showed greater amygdala response to neutral versus fearful faces than adults. Monk et al. (2003) compared amygdala response in 17 adolescents (9–17 years old; 9 boys) and 17 adults (25–36 years old; 9 men) during presentations of emotionally engaging faces. In a passive-viewing condition, but not in attention-constraining conditions, adolescents showed greater amygdala activation than adults to fearful versus neutral faces. Thus, Thomas, Drevets, Whalen, et al. (2001) found that amygdala response to fearful faces was greater in adults than in adolescents, whereas Monk et al. found the opposite.
In summary, the few available studies examining amygdala functional development in humans generated contradictory findings. Thus, the current study aimed to collect data on a large sample of adolescents and adults to provide sufficient statistical power to examine the effects of age and sex on amygdala function. Because past work on the neural correlates of fearful face processing in youth primarily focused on the amygdala (Killgore & Yurgelun-Todd, 2004; Killgore et al., 2001; Thomas, Drevets, Whalen, et al., 2001; Baird et al., 1999), we also sought to expand this literature by examining developmental differences in activation of additional regions that are important for face processing: fusiform gyrus, hippocampus, ACC, and orbito-frontal cortex (OFC). Furthermore, although documenting activation in specific brain regions is important for understanding neural mediators of emotion and cognition, recent functional neuroimaging studies have begun to integrate the understanding that brain regions operate as part of highly interconnected neural circuits (Yurgelun-Todd, 2007; Nelson et al., 2005). Nevertheless, virtually no neuroimaging work considers the manner in which amygdala-based circuits show meaningful differences in functional connectivity between adolescence and adulthood. Accordingly, we examined the degree to which functional connections between the amygdala and other brain regions exhibit meaningful age-related variations during facial emotion processing.
We used the same event-related, face–emotion fMRI paradigm as Monk et al. (2003) and hypothesized that, consistent with Monk et al., adolescents would show greater amygdala activation than adults when passively viewing fearful relative to neutral faces. We also examined age as a continuous variable, given the large sample size and wide age range within each developmental group, to provide a more powerful test of continuous relationships than was possible in previous smaller studies. Based on the findings from Yurgelun-Todd and Killgore (2006) reviewed above, we hypothesized that amygdala response to fearful faces would not vary linearly by age in adolescents or adults, even in our larger sample. Other research suggests nonlinear relationships between age (ranging from 4 to 22 years old) and brain structure size (Lenroot & Giedd, 2006); thus, we explored possible nonlinear relationships between age and amygdala activation.
With regard to sex effects, prior research has yielded mixed results regarding sex-related developmental differences in amygdala activation to faces (Killgore & Yurgelun-Todd, 2004; McClure et al., 2004; Killgore et al., 2001); however, these studies, the largest of which had 34 participants, used samples that may have been underpowered to detect sex-related developmental differences reliably. Thus, a large sample would allow us to examine sex as a moderating factor of developmental differences in amygdala response during passive viewing of fearful faces.
The fusiform gyrus, the hippocampus, the ACC, and the OFC have been implicated in face processing in general, and in mood and anxiety disorders more specifically; each region has been reported to show changes in activation as a function of age and face-viewing context. For example, studies of adults suggest that the fusiform gyrus, specifically the anterior region, is involved in the perceptual identification of faces (Haxby, Hoffman, & Gobbini, 2000; Kanwisher, McDermott, & Chun, 1997) and is particularly engaged in coding fearful faces (Pessoa, McKenna, Gutierrez, & Ungerleider, 2002; Vuilleumier, Armony, Driver, & Dolan, 2001; Breiter et al., 1996). Developmental studies of fusiform gyrus response to faces indicate that children have a more distributed pattern of activation than adults when matching neutral facial expressions, but not stimulus locations (Passarotti et al., 2003), and that older versus younger children have greater fusiform activation to neutral faces than to houses (Aylward et al., 2005). However, such studies have not examined age-group differences in fusiform response to fearful versus neutral faces. Similarly, the hippocampus has been implicated in facial encoding (Haxby et al., 1996) but has been associated more strongly with memory than affect (Alkire, Haier, Fallon, & Cahill, 1998). Greater hippocampus activation has been found in adults compared to adolescents when viewing subsequently remembered neutral faces (Nelson et al., 2003), however, little is known about developmental changes in hippocampus activation during face processing generally or fearful face viewing specifically.
Turning to prefrontal regions, among adults, the OFC and ACC circuitry modulates behavior by influencing attention to emotional stimuli, including fearful or other negative facial expressions (Pessoa et al., 2002; Blair, Morris, Frith, Perrett, & Dolan, 1999; Lane, Fink, Chau, & Dolan, 1997). Across development, the OFC and ACC show functional, anatomical, and physiological changes in adolescence and by early adulthood (Eshel, Nelson, Blair, Pine, & Ernst, 2007; Gogtay et al., 2004; Adleman et al., 2002; Casey et al., 2000; Sowell, Thompson, Holmes, Jernigan, & Toga, 1999; Casey et al., 1997), and these regions have been implicated in the maturation of attentional and emotional processes, such as goal-directed attention to emotionally evocative stimuli (Bush, Luu, & Posner, 2000). Monk et al. (2003) found greater OFC and ACC activation in adolescents than in adults when passively viewing fearful versus neutral faces, as well as greater OFC activation in adults than in adolescents when focusing on emotional versus nonemotional aspects of fearful faces. As a whole, the literature indicates that, like the amygdala, the fusiform gyrus, the hippocampus, the ACC, and the OFC are involved in facial emotion processing and the limited developmental studies of these regions suggest possible age-related changes in fearful-face processing.
Recent work indicates that between-group differences in amygdala response to facial emotions are associated with between-group differences in functional connectivity among specific brain regions in adolescent anxiety patients as well as healthy adults (McClure et al., 2007; Pezawas et al., 2005). However, no previous developmental study has examined amygdala functional connectivity during facial emotion processing. A recurrent theme in theories of brain development is that adolescence is a time of neural refinement via synaptic pruning, myelination, and regulatory processes that may strengthen interconnections among brain circuits (Ernst & Spear, in press; Nelson et al., 2005; Spear, 2000; Nelson & Bloom, 1997). In theory, behavior is the net result of functional interactions among a highly integrated network of subcortical–subcortical and subcortical–cortical regions associated with the human response to emotional stimuli. Because this is the first study of developmental differences in functional connectivity during face processing, we viewed these analyses as exploratory. However, we were particularly interested in amygdala connectivity with cortical and subcortical temporal regions, such as the fusiform gyrus and the hippocampus, given their associations with face processing (Haxby et al., 2000).
Finally, to help interpret the potential roles of eye-gaze patterns and the amount of time that individuals spend looking at faces, we acquired eye movement data outside of the MRI scanner using the same face task to test whether developmental differences in neural function could relate to between-subject variability in eye gaze during passive viewing. Eye movements are an important way of indexing participants’ focus of attention while they passively view stimuli. Although we monitor participants’ eyes during fMRI scanning to ensure that they are looking at task stimuli, we conducted an additional study using an eye movement tracker outside of the scanner that permitted more precise quantification of eye movement patterns while participants completed the same task as used in the scanner. This allowed us to determine with greater accuracy whether neural activation differences between adolescents and adults might reflect differences in the location of eye gaze.
METHODS
Participants
All participants were deemed physically healthy by medical history and physical examination. Absence of psychiatric illness was confirmed with a standardized, structured psychiatric interview: the Kiddie Schedule for Affective Disorders and Schizophrenia—Present and Lifetime version (K-SADS-PL) (Kaufman et al., 1997) for adolescents and the Structured Clinical Interview for DSM-IV (SCID) (Spitzer, Williams, Gibbon, & First, 1992) for adults. Additionally, all participants had average to above-average IQ scores (≥70) on the Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 1999). Exclusion criteria included history of psychiatric illness, neurological disorders, head injury, or exposure to traumatic life events.
fMRI Sample
Thirty adults (17 men) aged 21 to 40 years (M = 31.06 ± 4.71) and 31 adolescents (16 boys) aged 9 to 17 years (M = 14.22 ± 2.44) participated in the fMRI study. WASI scores did not differ significantly between groups [adults: M = 116.63 ± 11.57; adolescents: M = 116.94 ± 11.89; t(59) = 0.10, p = .92] nor did sex distribution [χ2(n = 61) = 0.13, p = .72]. Although the main contrast in this study was the passive-viewing condition, all participants were required to have behavioral data for at least 80% of task trials from three attention-constrained conditions and to exhibit less than 2.0 mm movement during fMRI scanning. Of note, these criteria are stricter than those used in our prior work, and we applied them here to eliminate potential methodological factors that might contribute to cross-study sources of variability (e.g., movement, inadequate behavioral data). Thus, only 15 of 17 adults and 10 of 17 adolescents originally included in Monk et al. (2003) are included here. An additional 36 participants (15 adults, 21 adolescents) were studied. Data from these latter participants have not been reported previously. We combined these two samples to determine whether our past result was reproducible in a larger, more strongly powered sample by using methods identical to our past study; this combination yielded a considerably larger sample than used in any previous developmental fMRI study of face processing.
Eye Movement Sample
Sixteen adults (7 men) aged 23 to 36 years (M = 29.79 ± 4.42) and 18 adolescents (9 boys) aged 10 to 17 years (M = 14.4 ± 2.12) participated in the eye movement study outside of the scanner. The groups did not differ on the WASI [t(30) = 0.35, p = .73] or by sex [χ2(n = 34) = 0.01, p = .91]. Ten adults and 11 adolescents were included in both the fMRI and eye movement studies. Participants from the fMRI sample were invited back to perform the task with an eye tracker following a minimum period of 3 months to reduce the effects of prior exposure to the faces. The goal of measuring eye movements during the face task was to consider the degree to which different patterns of eye-gaze location (e.g., difference in length of time looking at eyes vs. mouth) might possibly account for the observed age differences in brain engagement.
Procedure
Participants were recruited through flyers and local newspaper advertisements. All adults and parents/legal guardians of adolescents gave written informed consent to participate in the study. All adolescents provided written informed assent. Study participation was compensated financially according to guidelines delineated by the National Institute of Mental Health (NIMH). The NIMH Institutional Review Board approved all study procedures.
Task and Stimuli
Participants were instructed to passively view a series of adult faces. This viewing condition was interleaved with three other conditions, during which participants were instructed to attend to specific aspects of the faces. During the latter three conditions, participants used a five-key response box developed by MRI Devices (Waukesha, WI) to rate each face from 1 (not very) to 5 (extremely) for the subjective fear it elicited (“How afraid are you?”), the hostility it conveyed (“How hostile is the face?”), and the size of a nonemotional physical feature (“How wide is the nose?”). In the passive-viewing condition, participants’ attention was unconstrained, such that they simply viewed the faces without making any ratings. Only the passive-viewing condition is discussed in this study to allow for comparison with prior developmental fMRI studies (Killgore & Yurgelun-Todd, 2004; Monk et al., 2003; Thomas, Drevets, Whalen, et al., 2001). Data on healthy adolescents and adults from the three other task conditions are presented in McClure et al. (2004) and Monk et al. (2003).
The task used a rapid, event-related design and was presented as a 160-trial, 14.2-min single run. As depicted in Figure 1, each of the four task conditions began with an instruction screen presented for 3000 msec. Following the instruction screen, 10 randomly ordered stimulus event trials (8 faces, 2 fixation crosses) were each presented for 4000 msec. The two fixation crosses were included to avoid potential collinearity between stimuli. By including one randomly occurring “null event” for every four randomly occurring face-viewing events, this method introduces random jitter between the face-viewing events of interest. Including such randomly interposed “null-event” fixation trials has been shown to mitigate collinearity in event-related analyses (Burock, Buckner, Woldorff, Rosen, & Dale, 1998). Following each event, an interstimulus interval displayed a blank screen that varied from 750 to 1250 msec (averaging 1000 msec within a 10-trial block). Unlike the random jitter introduced by the 4000-msec “null events,” this jitter of considerably shorter duration was not designed to influence interpretations of the hemodynamic response, but rather to reduce the degree to which subjects could predict onset of each face-viewing event. A single iteration of the four conditions was completed in 212 sec (each condition averaged 53 sec). Each of the four task conditions was repeated four times. Presentation order of task condition and of facial expressions was randomized across participants.
Figure 1.
Task stimuli and design. Eight facial expressions (two of happy, fearful, angry, and neutral expressions) and two fixation crosses were presented for 4000 msec individually within a given viewing condition block. For each condition, participants rated from 1 to 5 either nose width of the face, their subjective fear of the face, or perceived hostility in the face, or viewed the stimuli passively. Condition instructions were displayed for 3000 msec. Condition order was random across participants. Facial expressions displayed by the actors were also varied randomly across participants. Intertrial interval showing a blank screen varied from 750 to 1250 msec. Each of the four viewing conditions was repeated four times, yielding a single 160-trial run.
Task stimuli included faces of 56 actors selected from three standardized sets of gray-scale photographs depicting different facial expressions (Gur, 2000; Tottenham & Nelson, 2000; Ekman & Friesen, 1976). Each participant viewed 32 different actors, with each actor randomly assigned to display one of four facial expressions (happy, angry, fearful, and neutral). For example, a given actor might be randomly selected to portray “anger” across the four task conditions for one participant; this same actor might be randomly chosen to portray “fear” for another subject and “happiness” for yet another participant. This design allowed us to control for variability in nonemotional features of the actors (e.g., ethnicity, hair color). Female actors were used in half of the photographs to control for stimulus gender. A total of 32 “null-event” fixation crosses were included to facilitate data analysis. Participants viewed stimuli through the Avotec Silent Vision visual system (Stuart, FL). As a result, task compliance could be monitored continuously by visually inspecting participants’ gaze direction.
Because the current study focused on neural response to fear faces, the contrasts of interest compared activation during the passive-viewing condition for fearful faces (8 trials) versus neutral faces (8 trials) or fixation crosses (32 trials). However, the study design also included happy and angry faces to reduce habituation to a particular emotion and thereby to increase the probability of detecting a response to fearful faces (Fischer et al., 2003). The same stimuli and task were used both in and out of the scanner.
fMRI Data Acquisition
Participants were trained in an MRI simulator prior to entering the scanner. Participants were also administered a practice version of the task to ensure understanding. The practice version contained pictures of neutral facial expressions that were not shown in the MRI scanner.
Scanning occurred in a General Electric Signa 3-Tesla magnet (Waukesha, WI). Head movement was constrained by the use of foam padding. Following sagittal localization and a manual shim procedure, whole-brain blood oxygenation level-dependent (BOLD) functional T2*-weighted images were acquired. An echo-planar, single-shot, gradient-echo pulse sequence was used with the following parameters: matrix size of 64 × 64 mm, repetition time (TR) of 2000 msec, echo time (TE) of 40 msec, field of view (FOV) of 240 mm, and voxel size of 3.75 × 3.75 × 5 mm. Echo-planar images (EPI) were acquired in 23 contiguous axial slices per brain volume positioned parallel to the anterior commissure and posterior commissure (AC–PC) plane. Following EPI data collection, a high-resolution T1-weighted anatomical image was acquired using a standardized magnetization-prepared gradient-echo sequence to facilitate spatial normalization. Parameters for anatomical image acquisition were as follows: one hundred eighty 1-mm sagittal slices, matrix size of 256 × 256, TR = 11.4 msec, TE = 4.4 msec, FOV = 256, number of excitations = 1, time to inversion = 300 msec, bandwidth = 130 Hz/pixel, 33 kHz/256 pixels.
Eye Movement Data Acquisition
Following task training and initial calibration of eye position, eye movements during completion of the facial emotion task were measured by high-resolution infrared oculography using an eye tracker with remote pan and tilt optics, autofocusing lens, and magnetic head tracker (Applied Science Laboratories [ASL] Model 504; Bedford, MA). The eye tracker was used to illuminate and detect pupil and corneal reflection (CR). Gaze location was identified based on relative positions of pupil and CR and displayed as a set of cross hairs superimposed on the image from a video monitor showing the participant’s field of view. The range within which valid data can be obtained is 50° (± 258) horizontally and 35° (+ 25° to −10°) vertically, with a 0.25° visual angle of spatial accuracy. The eye tracker uses a 60-Hz sampling rate. The distance between the center of the task monitor and the participant’s eye was approximately 27 inches. A magnetic head tracking device, autofocusing lens, and chinrest were used to minimize head movement artifacts. Recordings were obtained in a room lit by standard overhead fluorescent lights.
Data Analysis
Behavioral Data
Group differences in behavioral ratings and reaction times recorded during the scan were analyzed with separate repeated measures analysis of variance (ANOVA). The ANOVA models included a between-group factor (age group) and two within-group factors (attention state, face type). Multivariate three-way interaction effects were assessed using Wilks’ Lambda F statistic; univariate effects were interpreted given a significant three-way interaction effect. Because attention and passive-viewing conditions were interspersed, analyzing behavioral data collected during the attention conditions provided a check that participants were prepared to attend to the ratings after passive viewing.
Imaging Data
Preprocessing procedures and fMRI data analyses were performed using the Statistical Parametric Mapping software (SPM99, Wellcome Department of Cognitive Neurology, London, England, 1999) and supplemental routines written in Matlab 5.3 (The Mathworks, Natick, MA, 1999). Imaging data were included for participants who moved less than 2.0 mm in any plane as assessed with MedX software (Medical Numerics, Sterling, VA). Preprocessing procedures included corrections for slice timing and motion, coregistration to the anatomical data, and spatial normalization to a Montreal Neurologic Institute (MNI) T1-weighted template image supplied with SPM99.
Individual subject-level, event-related response amplitudes were estimated using a general linear model for each event type. Event types were defined based on each face type crossed by each viewing condition; we focused only on fearful and neutral faces during the passive-viewing condition in order to interpret our results within the available literature. Fixation trials served as an implicit baseline. The waveform used to model event-related responses was a rectangular pulse (4 sec duration) convolved with the hemodynamic response function specified in SPM99. Contrast images were created for each subject using pairwise comparisons of the different event-related BOLD response amplitudes across conditions. Before performing group-level analyses, each contrast image was divided by the subject-specific voxel time-series mean, generating values proportional to percentage fMRI signal change (Zarahn, Aguirre, & D’Esposito, 1997). These normalized contrast images were then smoothed with an isotropic Gaussian kernel (full-width half-maximum = 11.4) to reduce nonstationarity in the spatial autocorrelation structure produced by the previous step (Friston et al., 2000).
For all group-level analyses, the contrast images produced for each participant were fit to a second-level random effects model. Using the full sample (n = 61), we modeled the effect of age group (adult, adolescent) and sample group (new, old) on amygdala activation during passive viewing of fearful faces, yielding df = 57. If our past amygdala results were consistent in these groups, we would then remove the sample-group factor to increase statistical power, which would result in df = 59. Because we included a subset of participants from our past study, it was important to ensure that results in the combined sample were not driven by different patterns within either of the smaller independent samples. Therefore, we examined amygdala activation during passive viewing of fearful faces in the previously studied, newly acquired, and combined samples. Based on our a priori hypotheses, the primary statistical analyses were region of interest (ROI)-based. A Gaussian random field threshold was used to determine significance of statistical comparisons. Activation had to survive the small volume correction Gaussian random field threshold (α = .05) within prespecified ROIs (Worsley et al., 1996). The primary analyses focused on the right and left amygdala and the secondary analyses focused on the ACC, the OFC, the hippocampus, and the fusiform gyrus. ROIs were defined using standard, previously validated, anatomical criteria. They were hand-traced on the single MNI template, to which fMRI data were normalized, and then applied to all normalized brains at the group level (Szeszko et al., 1999). MNI x, y, z coordinates are reported for significant results.
Functional connectivity was measured for each subject as the correlated activity between the mean BOLD signal of each amygdala ROI, as the seed region, and each voxel across the whole brain (Pezawas et al., 2005; Greicius, Krasnow, Reiss, & Menon, 2003). The mean EPI time series was extracted, mean-centered, and then normalized (root-mean-square) over each amygdala ROI throughout the entire face processing task (i.e., across passive viewing, attention-directed ratings, and all facial expressions). The resulting time series was entered into a subject-level general linear model as the sole regressor of interest against activation at each brain voxel. The model included smoothed, spatially normalized whole-brain EPI data. Both high- and low-pass filtering were used (applying a 128 sec cutoff and the SPM-provided canonical hemodynamic response function, respectively). Based on past functional connectivity studies in adults (Pezawas et al., 2005; Greicius et al., 2003) and an adolescent study using the same face-viewing task as in the present study (McClure et al., 2007), we applied a low-pass filter to mitigate the effect of high-frequency noise and to place our estimates within the typical fMRI context of measuring neural activity indirectly via the BOLD response. Regression coefficients, corresponding to the voxelwise regressor of interest, were entered into a group-level random effects model. t tests were used to examine group differences in functional connectivity for each amygdala ROI. Positive connectivity reflected increased activity in both the amygdala and voxels in a given region, whereas negative connectivity reflected increased activity in the amygdala and decreased activity in a given region or vice versa.
Eye Movement Data
Eye movement data were analyzed off-line with software (EYENAL) provided by ASL. EYENAL calculates fixations (stationary gazes) based on an algorithm that takes into account the distance of the eye to the screen for each subject. A fixation was defined as occurring when at least six consecutive data samples (corresponding to about 100 msec duration) occur within a radius of less than 0.5° visual angle. Total time (msec) looking at the eye, nose, and mouth regions of the face stimuli (i.e., total fixation duration), respectively, were the main dependent measures. Total fixation duration was analyzed with a Group (adults, adolescents) × Face region (eyes, nose, mouth) × Facial expression (fearful, neutral) repeated measures ANOVA. Effects of interest were between-group effects and interactions of group by face region and by facial expression. Multivariate effects were assessed using Wilks’ Lambda F statistic; significant multivariate effects were then interpreted by examining within- and between-group effects.
RESULTS
Between-group Differences in Behavioral Data
Mean ratings and reaction times for each face type under each attention condition are presented by group in Table 1. No significant differences were found between adolescents and adults in their task ratings [F(6, 54) = 0.69, p = .66] or reaction times [F(6, 54) = 1.85, p = .11].
Table 1.
Mean (SD) Ratings and Reaction Times for Each Face Type under Each Attention Condition for Adolescents and Adults
| Rating(1–5)a |
Reaction Time (msec)b |
|||
|---|---|---|---|---|
| Adolescents (n = 31) | Adults (n = 30) | Adolescents (n = 31) | Adults (n = 30) | |
| How Hostile Is the Face? | ||||
| Happy | 1.08 (0.13) | 1.05 (0.11) | 1605.08 (380.69) | 1292.58 (302.80) |
| Neutral | 1.73 (0.59) | 1.73 (0.65) | 1930.06 (412.27) | 1781.52 (496.06) |
| Fearful | 2.03 (0.81) | 2.14 (0.84) | 2054.56 (471.69) | 2042.79 (465.02) |
| Angry | 3.41 (1.00) | 3.61 (0.68) | 2015.25 (432.29) | 1982.83 (388.41) |
| How Afraid Are You? | ||||
| Happy | 1.10 (0.17) | 1.07 (0.14) | 1520.04 (398.27) | 1278.02 (260.16) |
| Neutral | 1.51 (0.61) | 1.62 (0.68) | 1772.99 (377.25) | 1650.57 (447.91) |
| Fearful | 2.08 (1.00) | 2.35 (0.86) | 1834.04 (419.22) | 1976.03 (365.84) |
| Angry | 2.62 (1.20) | 2.98 (0.92) | 2011.01 (470.06) | 1981.84 (326.02) |
| How Wide Is the Nose? | ||||
| Happy | 2.64 (0.46) | 2.47 (0.54) | 2051.81 (451.85) | 1895.34 (345.53) |
| Neutral | 2.21 (0.59) | 2.29 (0.50) | 1929.78 (359.07) | 1861.94 (395.75) |
| Fearful | 2.20 (0.51) | 2.23 (0.52) | 1963.72 (355.44) | 1952.11 (350.53) |
| Angry | 2.68 (0.50) | 2.57 (0.49) | 2107.99 (462.82) | 1998.82 (357.83) |
Results from repeated measures ANOVA (Group × Face type × Attention state) for ratings: F(6, 54) = 0.69, p = .66 and reaction time: F(6, 54) = 1.85, p = .11.
Each face type was rated from 1 to 5, 1 = low level, 5 = high level of hostility, fear, or nose width.
Higher reaction time = more time taken to rate the face.
Between-group Differences in Amygdala Activation
In our planned contrast of new adolescent participants versus new adult participants, when attention was unconstrained during passive viewing, displays of fearful versus neutral expressions were associated with greater bilateral amygdala engagement in adolescents than in adults [left amygdala: x = −20, y = −8, z = −6; right amygdala: x = 32, y = −8, z = −10; t(57) = 1.71, p = .046]. Next, we examined the contrast of past adolescent participants versus past adult participants from Monk et al. (2003), who met the strict criteria for task performance and movement (see fMRI Sample section). In this analysis, we similarly found increased amygdala activation in adolescents versus adults [left amygdala: x = − 16, y = −8, z = −8, t(57) = 1.79, p = .039; right amygdala: x = 34, y = −6, z = −12; t(57) = 1.67, p = .049]. Table 2 and Figure 2A and B present results from age-group analyses combining new and past participants (n = 61), which produced results similar to those obtained in each independent sample.
Table 2.
Regions of Interest with Significant Activation during Passive Viewing of Fearful Contrasted with Neutral Faces in Adolescents versus Adults
| Region | Number of Voxels (kE ) | x | y | z | t(59) | p Correcteda |
|---|---|---|---|---|---|---|
| Right amygdala | 146 | 16 | −4 | −16 | 3.34 | .007 |
| Left amygdala | 62 | −20 | −8 | −6 | 2.76 | .027 |
| Right fusiform face area/Brodmann’s area 37 | 346 | 38 | −42 | −14 | 3.53 | .024 |
Coordinates are reported in MNI space (Collins et al., 1998).
All voxelwise t values are significant at α = .05 based on a small volume correction for multiple comparisons within each region.
Figure 2.
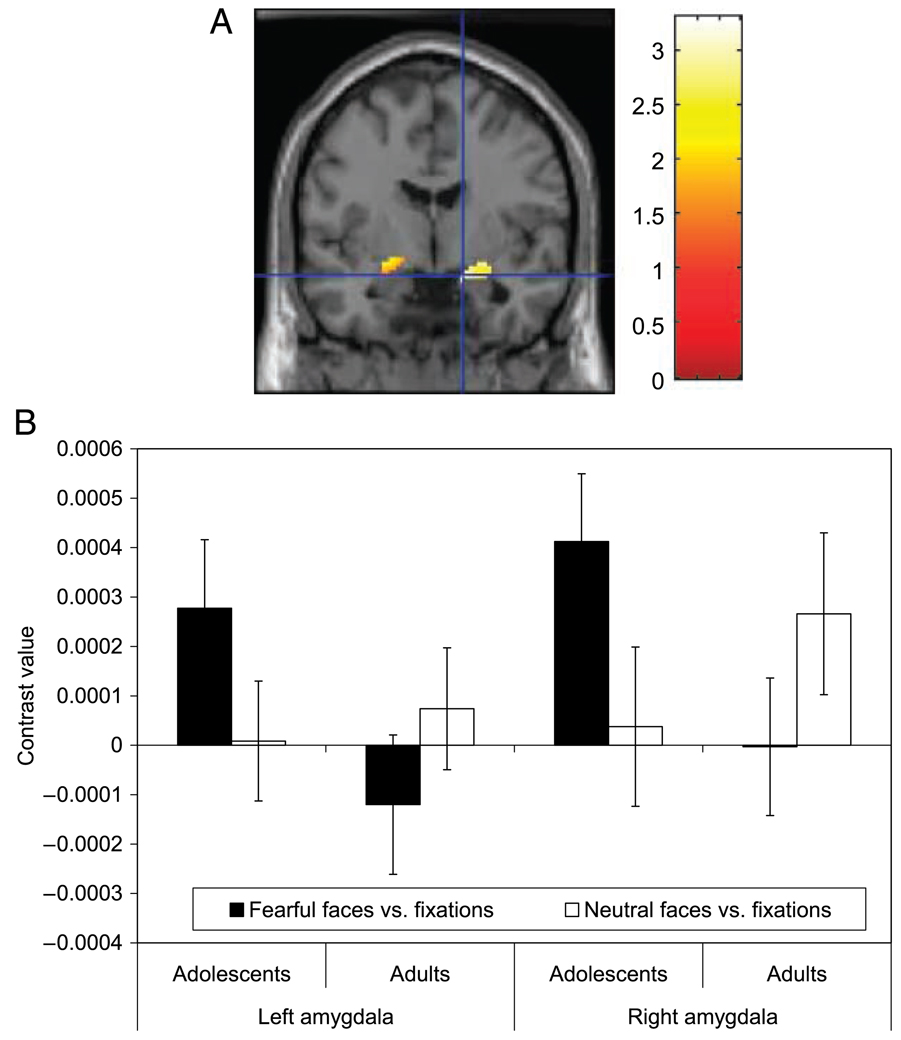
(A) Greater amygdala activation in adolescents was evident relative to adults when passively viewing fearful contrasted with neutral faces. A high-resolution anatomical overlay image in MNI space was used as provided by SPM. The figure displays a coronal slice of the left and right amygdala. Cross hairs are positioned at MNI coordinates x = 16 mm, y = −4 mm, z = −16 mm where the adolescent–adult between-group difference emerges. For visual presentation purposes, the threshold is set at p < .05 and a bilateral amygdala mask was applied. (B) Display of left (MNI coordinates: x = 30 mm, y = 53 mm, z = 23 mm) and right (MNI coordinates: x = 48 mm, y = 55 mm, z = 18 mm) amygdala voxel activation reported as a mean contrast value ± standard error. Each contrast value is derived from the percent signal change during passive viewing of fearful faces versus fixation and of neutral faces versus fixation within each age group.
In each age group separately, post hoc analyses examined amygdala activation for fearful versus neutral faces, fearful faces versus fixations, and neutral faces versus fixations. Adolescents showed increased amygdala activation in response to fearful versus neutral faces. Adults showed no differential response to fearful versus neutral faces in either amygdala ROI. Analyses comparing each facial expression during passive viewing, relative to fixations, showed significantly greater activation in response to fearful faces versus fixations in the left [x = −22, y = −6, z = −12; t(59) = 2.71, p = .026] and right amygdala [x = 18, y = −2, z = −18; t(59) = 3.54, p = .003] in adolescents, but not in adults (Figure 2B). Amygdala activation to neutral faces versus fixations was not significant in either adolescents or adults.
Because we did not detect amygdala activation to fearful versus neutral faces in adults, additional contrasts were examined post hoc to ensure that amygdala activation was measurable in adults. We found that adults had robust bilateral amygdala activation to all faces versus fixations [left amygdala: x = −18, y = −6, z = − 14, t(59) = 3.80, p = .002; right amygdala: x = 24, y = −4, z = −10, t(59) = 2.92, p = .02] as well as left amygdala activation to angry faces versus fixations [x = −20, y = −4, z = −16, t(79) = 3.54, p = .004]. However, these analyses rely on more event replicates than analyses restricted to passive viewing of fearful faces. As a result, we conducted further analyses to determine the degree to which our task was sensitive to adult amygdala engagement within a specific attention condition for specific face–emotion types. These analyses did document successful amygdala engagement in adults. For example, while rating nose width, adults had significant amygdala activation to fearful faces versus fixations [x = −20, y = −6, z = −14, t(59) = 2.73, p = .025]. Thus, the amygdala activation detected within adults varied based on the emotion and attention condition in which it was assessed.
Several post hoc analyses were also conducted to rule out the possibility that greater amygdala activation in the adolescents reflects greater general activation during face or emotion processing rather than a specific response to fearful faces, viewed passively. First, the comparison of amygdala activation to all faces versus baseline fixations between adolescents and adults was not significant. Next, comparisons of amygdala activation to happy and to angry facial expressions each contrasted against baseline fixations between adolescents and adults were also not significant.
Within-group Associations with Age
Regression analyses using curve estimation for linear, quadratic, and cubic relationships were conducted to examine associations of age (as a continuous variable) with percent signal change in activation in the left and right amygdala during passive viewing of fearful relative to neutral faces. These analyses were conducted within the adult and adolescent groups separately. Nonsignificant linear and curvilinear relationships between age and left or right amygdala engagement were found in both adolescents and adults.
Between-group Associations with Sex
Examination of sex-related developmental differences focused on the main effect of sex and the interaction between sex and group. These effects were not significant, indicating that males and females had a similar amygdala response to passively viewed fearful faces and that neither adolescent males and females nor adult males and females differed in amygdala engagement to fearful versus neutral faces.
Between-group Differences in Additional ROIs
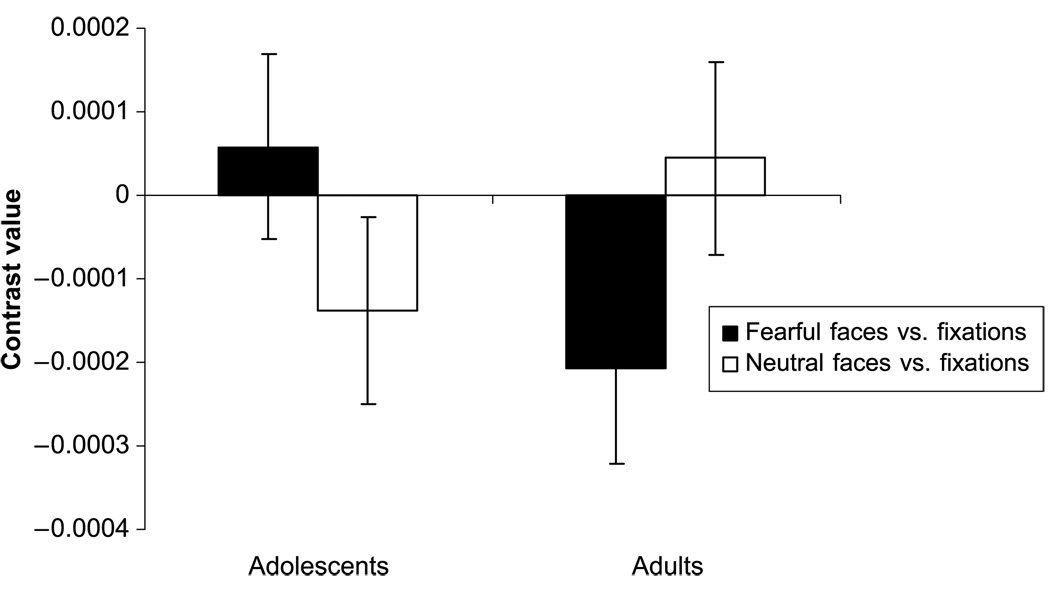
Analyses examined group differences in activation of the ACC, OFC, hippocampus, and fusiform gyrus. Significantly greater activation in the fusiform face area was found in adolescents versus adults while passively viewing fearful versus neutral faces (Table 2, Figure 3). No significant group differences were found in the other ROIs for fearful versus neutral faces.
Figure 3.
Display of fusiform voxel activation (MNI coordinates: x = 59 mm, y = 36 mm, z = 19 mm) reported as a mean contrast value ± standard error. Each contrast value is derived from the percent signal change during passive viewing of fearful vs. neutral faces, fearful faces versus fixation and of neutral faces versus fixation within each age group.
Between-group Differences in Functional Connectivity
Patterns of functional connectivity in the combined sample showed strong positive correlations between activity in the left amygdala and bilateral medial OFC, hippocampus, and fusiform gyrus, and a negative correlation between activation in the left amygdala and the right dorsal ACC (Table 3). Strong positive correlations between activity in the right amygdala and caudal ACC, hippocampus, and left fusiform gyrus were found, as well as a negative correlation with activity in the left inferior prefrontal cortex. These findings indicate that task performance is associated with positive functional connectivity between the amygdala and a distributed network of neural regions involved in face–emotion processing.
Table 3.
Voxels (MNI Coordinates) with Significant Connectivity Associations with the Amygdala
| Contrast | Region | Brodmann’s Area | Number of Voxels (kE) |
x | y | z | t(59) | p Correcteda |
|---|---|---|---|---|---|---|---|---|
| Connectivity in All Participants, Independent o f Group Status | ||||||||
| Positive connectivity with left amygdala |
L medial OFC | 47 | 231 | −26 | 18 | −6 | 6.11 | .000 |
| R medial OFC | 47 | 61 | 30 | 20 | −10 | 4.06 | .01 | |
| L hippocampus | NA | 215 | −22 | −34 | −2 | 8.21 | .000 | |
| R hippocampus | NA | 188 | 16 | −30 | −8 | 7.35 | .000 | |
| L fusiform | 36 | 138 | −22 | −34 | −14 | 5.75 | .000 | |
| R fusiform | 36 | 122 | 24 | −36 | −14 | 5.80 | .000 | |
| Negative connectivity with left amygdala |
R dorsal ACC | 32 | 336 | 14 | 36 | 28 | 4.87 | .001 |
| Positive connectivity with right amygdale |
L caudal ACC | 32/6b | 68 | 2 | −4 | 50 | 4.92 | .001 |
| R caudal ACC | 32/6b | 47 | 4 | −2 | 48 | 4.33 | .005 | |
| L hippocampus | NA | 194 | −28 | −8 | −16 | 9.44 | .000 | |
| R hippocampus | NA | 164 | 30 | −6 | −16 | 11.26 | .000 | |
| L fusiform | 37 | 138 | −34 | −54 | −16 | 5.36 | .000 | |
| Negative connectivity with right amygdala |
L inferior PFC | 45 | 40 | −24 | 34 | 8 | 3.94 | .01 |
| Connectivity in Adults vs. Adolescents | ||||||||
| Greater positive connectivity with left amygdale |
L hippocampus | NA | 2 | −26 | −40 | 0 | 3.49 | .026 |
| R hippocampus | NA | 19 | 30 | −34 | 2 | 4.09 | .005 | |
L = left; R = right; OFC = orbito-frontal cortex; ACC = anterior cingulate cortex; PFC = prefrontal cortex; NA = Brodmann’s area not defined for this subcortical region.
All voxelwise t values are significant at α = .05 based on a small volume correction for multiple comparisons within each region.
Region extends to the Supplementary Motor Area (BA 6).
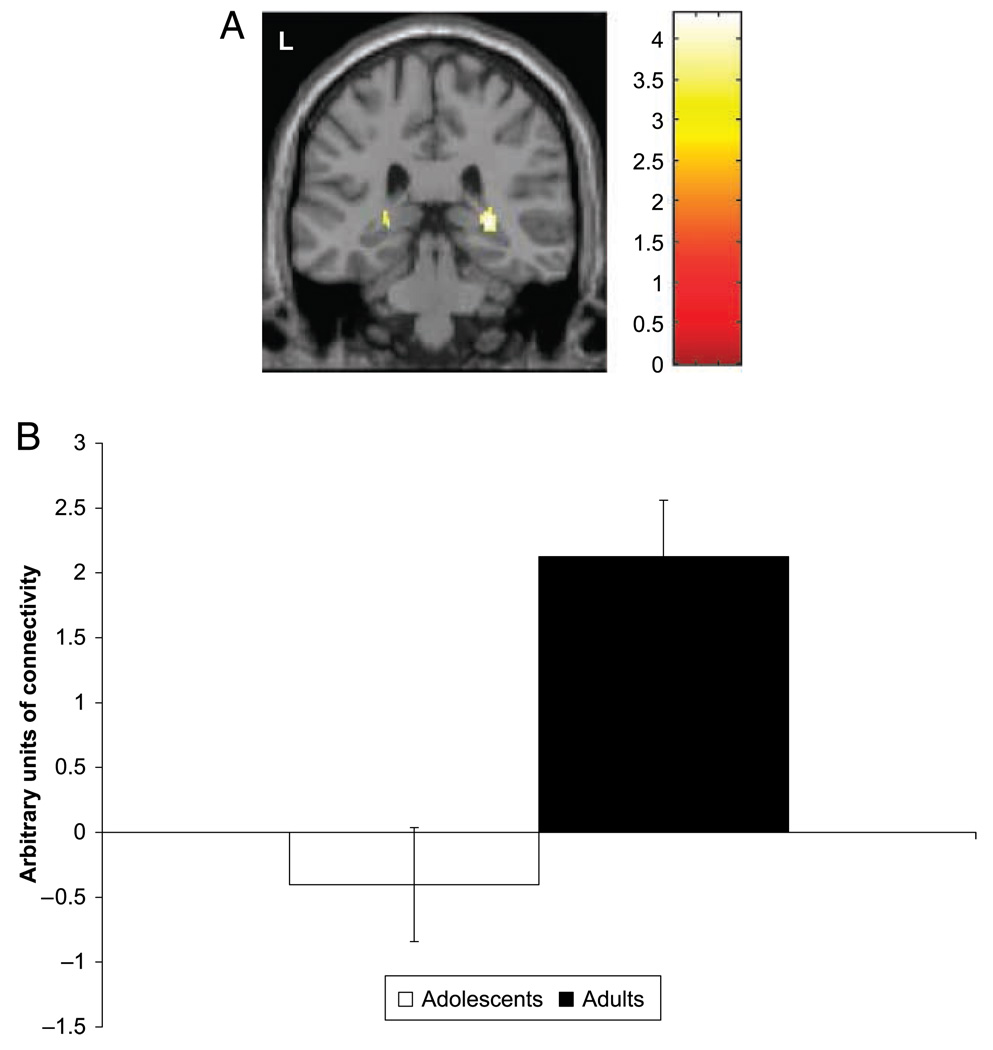
A between-group difference was found in functional connectivity between the left amygdala and bilateral hippocampus, with stronger positive connectivity in adults than in adolescents (Table 3, Figure 4A and B). Activity in both the left (x = –26, y = –40, z = 0) and right (x = 30, y = –34, z = 2) hippocampus showed significantly greater coupling with left amygdala activation in adults as compared to adolescents. No other between-group differences were evident with α = .05.
Figure 4.
Connectivity between the amygdala and the hippocampus was evident. (A and B) Adults showed significantly greater connectivity than adolescents between activation in the bilateral hippocampus (MNI coordinates left: x = −26 mm, y = −40 mm, z = 0 mm; right: x = 30 mm, y = −34 mm, z = 2 mm) and the left amygdala ROI across all trials of the face-viewing task. Highlighted areas in (A) indicate regions where the differences in activation between groups were significant. Bar graphs display mean contrast value ± standard error.
Between-group Differences in Eye Movements
There were no significant group differences or interactions between group and facial expression or face region in total fixation duration. A main effect of face region was found [F(2, 64) = 24.50, p < .001], wherein all participants spent more time looking at the eyes (M = 1.47, SE = 0.10) compared to the nose (M = 0.93, SE = 0.08, p < .01) and mouth (M = 0.57, SE = 0.07, p < .01), and at the nose compared to the mouth (p < .01). After repeating the fMRI analysis in the subgroup for which we had eye movement data, the developmental difference in amygdala activation for the passive viewing of fearful versus neutral faces contrast remained significant. Thus, the developmental difference in brain activation was found in the subsample who exhibited no developmental differences in time spent looking at different facial regions.
DISCUSSION
The present study compared adolescents (9 to 17 years old) and adults (21 to 40 years old) on amygdala response to the presentation of fearful faces, focusing on the effects of age and sex. Our current findings suggest that maturation is associated with decreased amygdala and fusiform engagement specifically when passively viewing fearful faces and with increased amygdala–hippocampus connectivity during face processing. This is not only the largest developmentally focused fMRI study on any aspect of face processing, but also the first study to document developmental differences in functional connectivity between the amygdala and activation across the whole brain, as well as developmental differences in fusiform activation to fearful faces.
Our use of a previously studied task within a larger sample adds to the growing literature on the development of the amygdala response to fearful faces (Killgore & Yurgelun-Todd, 2004; Monk et al., 2003; Killgore et al., 2001; Thomas, Drevets, Whalen, et al., 2001; Baird et al., 1999) by demonstrating the reproducible nature of developmental differences in amygdala function, an undertaking that is particularly important in order to generate and test new hypotheses based on established findings. We found that passively viewing fearful faces was associated with increased amygdala activation in adolescents relative to adults, suggesting that amygdala involvement in fearful-face processing varies between these two periods of development. This finding is consistent with specific results from Monk et al. (2003), using the same rapid event-related design, in our newly acquired sample, in the strictly defined sample culled from Monk et al., and in the large, combined sample including the new group of participants and a subset of participants from Monk et al. In particular, this developmental difference was driven by greater amygdala engagement to fearful, but not neutral, faces when compared to fixations, in adolescents. Adults, on the other hand, showed no differential amygdala response as a function of passively viewing fearful versus neutral faces or versus fixations.
One possible explanation of the present results is that greater amygdala activation in adolescents reflects greater general activation during face processing rather than a specific response to fearful faces. Post hoc fMRI analyses conducted to address this possibility showed no developmental differences in amygdala activation to faces in general or to other facial expressions. We also did not find developmental differences in the amount of time subjects spent looking at different facial regions while passively viewing fearful and neutral faces, indicating that adolescents and adults focus their eyes similarly on distinct aspects of facial stimuli presented during passive viewing and do not focus their gaze differently as a function of development. Thus, the developmental difference in amygdala response was specific to fearful facial expressions viewed passively. Amygdala activation to fearful faces also did not correlate with age within either the adult or the adolescent group, nor did it vary by sex.
Overall, the current study clarifies that, at least with an event-related task design, the previously observed developmental difference in amygdala activation to fearful versus neutral faces is reproducible, thus demonstrating the reliability of our task paradigm. Within the larger sample, we also sought to document the reliability of our past findings in amygdala activation specifically manifest during passive viewing of fearful versus neutral faces, given that this comparison previously yielded the only evidence of developmental differences in amygdala response, albeit in different directions in two studies (Monk et al., 2003; Thomas, Drevets, Whalen, et al., 2001). The consistency of the developmental amygdala finding may provide a benchmark from which new hypotheses and theories could be generated about the role of the amygdala in face processing in both typical and atypical development. Of note, however, despite observation of a consistent developmental difference across two independent samples, this study also revealed many instances where adults and adolescents exhibited similarly robust amygdala engagement. As such, adolescent immaturity of amygdala function appears to be relatively subtle: It occurs in an isolated set of attention- and emotion-specific viewing conditions.
Two novel developmental findings also emerged from the current study. First, adults had stronger amygdala– hippocampus functional connectivity than did adolescents. Second, adolescents had greater fusiform engagement to fearful versus neutral faces than did adults. With regard to functional connectivity, we found stronger coupling between the amygdala and the hippocampus in adults than in adolescents during the facial emotion viewing task. Functional connectivity, as assessed here, measures the temporal correlation of activity between different brain regions. These new findings suggest that developmental changes in the functional coupling between the amygdala and the hippocampus are likely to occur in the transition from adolescence to adulthood. Signals from the amygdala facilitate identification and detection of emotionally salient stimuli such as facial expressions. In turn, memory storage and retrieval for emotionally salient stimuli may be strengthened between these stages of development through connections to hippocampal regions (Dolcos, LaBar, & Cabeza, 2005; Richardson, Strange, & Dolan, 2004; Kilpatrick & Cahill, 2003). Indeed, evidence suggests that the relationship between cognitive and affective systems continues to develop during adolescence (Ernst et al., 2006; Nelson et al., 2005; Casey, Tottenham, & Fossella, 2002; Casey et al., 2000), and the degree to which emotion influences face–memory formation may be related to pubertal changes (Nelson et al., 2003; McGivern, Andersen, Byrd, Mutter, & Reilly, 2002). Thus, one interpretation of the present connectivity finding is that the greater amygdala– hippocampus coupling in adults is due to maturational changes in the degree to which the amygdala and hippocampus interact in forming memories of emotional faces. Among adults, emotional faces may elicit greater amygdala–hippocampus engagement due to experience and stronger memory formation of such stimuli. Although both the amygdala and the hippocampus have been found to participate in aversive memory, the mnemonic role of the amygdala appears to be confined to simpler functions such as stimulus emotion associations, whereas the hippocampus is involved in more subtle and complex processes such as context conditioning, spatial array relations, and timing-related memory. Overall, the interdependence and breadth of cognitive processes, such as memory and attention, and affective processes, such as emotion perception, may be stronger in the adult brain.
The present study is also the first to document a developmentally based difference in fusiform gyrus response to passively viewed fearful faces. Specifically, activation in the fusiform gyrus was influenced by passively viewing fearful versus neutral faces more in adolescents than in adults. These findings suggest that the neural underpinnings of facial expression perception instantiated in the fusiform face area may continue to develop through adolescence. Our regionally specific developmental findings indicated that adolescents engage the amygdala and the fusiform gyrus when attention is unconstrained during passive viewing of fearful faces more so than adults. Evidence suggests that the amygdala has strong bidirectional connections with the ventral visual processing stream (Amaral & Price, 1984) and that increased fusiform activation is related to greater input from the amygdala (Vuilleumier, Richardson, Armony, Driver, & Dolan, 2004; Morris et al., 1998). Additionally, structural changes in ventral posterior brain regions have been documented across development (Giedd et al., 1999) and the fusiform gyrus has been implicated in learning-based changes in face processing ability (Gauthier, Tarr, Anderson, Skudlarski, & Gore, 1999). Evidence of such structural and experience-based changes suggests the presence of strong plasticity in systems that process certain facial emotions (Gauthier et al., 1999), a possibility consistent with the developmental difference we documented here in fusiform function.
Adolescence is associated with prominent changes in social behavior and emotion regulation that are likely to be related partly to hormonal shifts, and structural and functional maturation in specific neural regions, such as the amygdala, and their interconnections (Nelson et al., 2005; Spear, 2000). As such, one explanation for the greater amygdala and fusiform activation in adolescents as compared to adults is that the emotional content of stimuli engages neural systems to a greater degree earlier in development. By adulthood, when control over attention has increased and other higher-level cognitive processes have matured, emotional as compared to neutral stimuli may compete less for emotion processing resources. That is, emotional stimuli may interfere less with the ability of other neural regions to engage in higher-level cognitive tasks later in development. In adolescence, greater priority may be given to resources that process fearful faces. Although the current study focused on the passive-viewing condition to isolate the influence of emotion rather than attention manipulations on neural engagement, other work suggests that changing the context of attention engages neural systems differently across development and that emotional stimuli can interfere with ongoing functions (McClure et al., 2007; Perez-Edgar et al., 2007; Monk et al., 2003; Nelson et al., 2003).
Although our primary developmental differences involved amygdala and fusiform response to fearful faces, we did not find age-related differences in amygdala–fusiform coupling during the face processing task. However, positive connectivity between the left amygdala and the fusiform face area [uncorrected p = .009; corrected p = .56, t(59) = 2.44] was found within the whole sample. This latter result is consistent with research suggesting that modulatory interactions occur between the amygdala and the fusiform gyrus (Amaral et al., 2003; Catani, Jones, Donato, & Ffytche, 2003; Vuilleumier et al., 2001), particularly during face–emotion processing. We also did not find any developmental differences in amygdala–prefrontal coupling despite strong connectivity in the entire sample between the amygdala and the ACC or the amygdala and the OFC. Both groups demonstrated similar levels of coupling between the amygdala and these prefrontal regions involved in attention allocation to emotionally salient stimuli (Adolphs, 2001; Vuilleumier et al., 2001; Critchley, Elliott, Mathias, & Dolan, 2000). Thus, across all attention–emotion conditions of the face task, we did not find age-based differences in the functional connectivity between these regions. Overall, our most robust developmental finding emerges for the right amygdala, given that Monk et al. (2003) found an age-group effect on the right amygdala only and that we did not find similar age-group differences in ACC and OFC activation to passively viewed fearful faces in either the new sample studied here or in the combined, larger sample. Thus, some effects initially noted in Monk et al. occur in only a subset of the participants studied here. As such, they represent less consistent findings than the repeatedly documented between-group differences in amygdala activation.
Of note, the developmental amygdala differences reported here contrast with those of Thomas, Drevets, Whalen, et al. (2001), who found greater amygdala response to passively viewed neutral versus fearful faces in children relative to adults. Clearly, several factors may explain these opposing findings. Nevertheless, our primary hypothesis is that the use of different fMRI task paradigms across studies remains the most influential factor, given growing evidence documenting the strong effects of task parameters on amygdala engagement. Specifically, Thomas, Drevets, Whalen, et al. used a block design, whereas Monk et al. (2003) used an event-related design. In a block design, each experimental condition is presented continuously for an extended time period and the different conditions are alternated over time (e.g., a block of fear faces, followed by a block of neutral faces). This approach yields a measure of sustained neural activation within the block. In an event-related design, stimuli are presented in multiple, independent-event types (e.g., event trials of different face emotion stimuli randomly distributed throughout the task). The event-related design measures phasic neural activation in response to stimuli for specific events. Past fMRI block-design studies in adults demonstrated increased amygdala activation to the presentation of fearful faces (Whalen et al., 2001; Breiter et al., 1996; Morris et al., 1996), but also found that the amygdala response can habituate to repeated exposure to fearful faces (Whalen et al., 1998; Breiter et al., 1996). Moreover, the stimulus duration also differs dramatically across studies. Whereas the current study presented faces for 4000 msec, requiring subjects to respond to each face in the attention conditions, Thomas, Drevets, Whalen, et al., Whalen et al. (1998), and Breiter et al. (1996) all presented faces in rapid succession (each for 500 msec), providing limited time for participants to process individual faces. The repeated, rapid contiguous presentation of fearful faces as in and Thomas, Drevets, Whalen, et al. may have influenced amygdala habituation differently than did the alternated, slow presentation of fearful faces as in Monk et al., which may account for the contradictory findings. Thus, although the findings from these two studies are opposite, they are based on different approaches to measuring functional neural activity, and this difference is likely to be the most influential factor on outcome. To better understand how amygdala activation differs based on task design, future work is needed that directly compares amygdala response to fearful faces using a block design and an event-related design.
The present study has some methodological limitations. We selected the passive-viewing context to be consistent with past studies of amygdala response to fearful faces. However, the passive-viewing condition does not allow for experimental control over subjects’ attention, possibly leading to individually generated cognitive activity during face processing. Indeed, past fMRI work suggests that cognitive processes can attenuate amygdala response (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003; Hariri, Bookheimer, & Mazziotta, 2000). The eye movement data we collected out of the scanner do not support this possibility because they indicate no differences between adolescents and adults in the amounts of time spent looking at different facial regions (i.e., eyes, nose, mouth) or facial expressions (i.e., fearful vs. neutral). Despite inherent limitations of passive viewing, using conditions implemented previously in multiple studies in adults is advantageous given the relative scarcity of data on adolescents. Further, because passive-viewing tasks have consistently been effective at evoking amygdala responses to fearful faces, they offer an established method for examining the questions of interest in the present study.
A second limitation concerns the relatively indirect implications that our between-group ROI-focused findings carry for our findings from the functional connectivity analysis. Although the former analysis focused on a subset of data, generated in two face–emotions passively viewed, the latter analysis focuses on activity across the entire task. As noted above, we focused on specific events for our ROI analysis, based on prior findings in Monk et al. (2003). For our functional connectivity analysis, we relied on methods that have been most consistently implemented in previous work (McClure et al., 2007; Pezawas et al., 2005). However, recent advances in functional connectivity analysis approaches also delineate methods for examining changes in connectivity during one or another event class.
Future studies, using alternative task designs and statistical approaches, may allow for tighter integration between ROI-based and functional connectivity-based analytic approaches, with each approach focusing on the same specific event classes. Certainly, the current task has proved useful in generating insights into the manner in which face–emotions and attention states interact. Nevertheless, as discussed below, when considering novel functional connectivity approaches, efforts to extend current insights on amygdala development might best be devoted to future studies using novel tasks that address limitations in the current task. For example, because the current study demonstrates the importance of passive viewing, future functional connectivity studies might implement designs that only collect data in this one attention state while focusing on changes in connectivity during specific face–emotion events. Exclusive focus on this one attention state would allow a novel task of similar length to the task used here to yield far more data in the most relevant classes of events than was possible in the current study.
A third limitation of the present study relates to the age range of our sample. There was a gap in study participants between the ages of 17 and 21 years, making it difficult to interpret potential changes in amygdala function that may occur across the very early ages of adulthood. In particular, the present findings do not allow for a clear demarcation of a specific age at which changes in amygdala activation to fearful faces may initiate. It is possible that, with increased age, and thus, experience, the hippocampus and associated memory functions may play a modulatory role over the amygdala; some support for this may stem from our finding of greater amygdala–hippocampus connectivity later in development. Another age-related limitation involves classifying the adolescent group solely based on age in years. It may be fruitful in future research to also define human adolescence based on pubertal status. The wide range of pubertal stages requires a study to examine a large number of adolescents who fall within each stage; unfortunately, the current study did not have enough data available regarding pubertal status to make meaningful comparisons across puberty stages. We also found that within-group variability in age did not relate to amygdala activation to fearful faces linearly or curvili-nearly. Although our result is consistent with work examining age effects on amygdala function in adolescence (Yurgelun-Todd & Killgore, 2006), other research has documented nonlinear relationships between age and the size of certain brain structures, such as the prefrontal cortex, that undergo protracted development from ages 4 through 22 years (Lenroot & Giedd, 2006). Samples with a sufficiently large number of individuals to be divided into bins of narrowly defined age groups, across specified periods of development, are needed to understand more fully how age is associated with changes in amygdala function across development.
Finally, the present study failed to detect amygdala activation in adults while they passively viewed fearful versus neutral faces. This result runs counter to the observation from adult fMRI block-design studies that consistently demonstrated amygdala activation to fearful faces (Breiter et al., 1996; Morris et al., 1996), although not all previous block-design studies of facial fear perception in adults have found amygdala activation (Sprengelmeyer, Rausch, Eysel, & Przuntek, 1998). Of note, amygdala engagement in passive-viewing, event-related fearful-face presentation paradigms, such as the one used in the current study, occurs less consistently than in block-design paradigms, as exemplified by a recent event-related fMRI study in adults that failed to detect amygdala activation to fearful faces (Deeley et al., 2007). It is possible that a failure to detect amygdala activation to fearful versus neutral faces in adults may reflect the small number of event-replicates in each attention condition, each of which included only eight trials of fearful faces. As noted above in the discussion of functional connectivity approaches, future studies should consider the advantages and disadvantages of implementing studies with novel task parameters. Specifically, the inclusion of a greater number of fearful face trials might engage the adult amygdala response on this condition; likewise, more trials could alter the adolescent response. Although alternative designs or contrasts may be more sensitive to adult amygdala response to fearful faces, one must also consider the possibility that these different approaches could potentially yield reduced sensitivity to developmental differences in amygdala activation through distinct, task-related effects on temporal processes such as fatigue, learning, or habituation.
The number of task trials cannot entirely account for the lack of adult amygdala engagement to fear-faces viewed passively. Indeed, in analyses of the nose-rating attention state, we did detect amygdala activation in adults in a similarly constructed contrast using eight fearful faces. Regardless, as noted above, future studies would benefit from a paradigm with more event replicates across fewer attention states. We used a relatively low number of task trials in the current study in order to employ the identical task paradigm from our prior study. This paradigm was designed originally with relatively few within-condition stimulus replications, in part, so that it would be tolerable to younger participants and would generate data across four attention conditions. This approach has been effective in prior research for eliciting amygdala activation in adolescents recruited from different sources and who are either healthy or at risk for or currently affected with a mental disorder (Monk et al., 2003, 2008; McClure et al., 2007; Perez-Edgar et al., 2007; Roberson-Nay et al., 2006; Nelson et al., 2003) and in two past developmental studies (Monk et al., 2003; Nelson et al., 2003). Although we have achieved within-lab reliability with this face-viewing task design, it remains important for other research groups to use the same task design to increase the comparability and generalizability of findings across studies and laboratories.
Despite these limitations, the present findings inform our understanding of fundamental changes in neuro-physiological function across development. Overall, the main results suggest that there are different balances of neural input at different periods across human development. In adolescence, there may be a greater tendency toward using neural resources for facial identification and perception, whereas in adulthood, there may be a greater bias toward using resources for memory of emotional information. For example, the greater amygdala and fusiform activation in adolescents than in adults suggests that these regions utilize neurophysiological input to a greater degree during the perception of fearful faces earlier in development. The amygdala and the fusiform gyrus are important for quick detection and recognition of emotionally salient stimuli (LeDoux, 2000; Whalen et al., 1998), and the amygdala additionally is implicated in learning and generating responses to such stimuli (LeDoux, 2000; Bechara, Damasio, Damasio, & Lee, 1999) and in modulating their consolidation into memory (Hamann, Ely, Grafton, & Kilts, 1999; Cahill & McGaugh, 1998). Activations of these regions may reflect a perception of fearful faces as more novel in adolescence than in adulthood, possibly leading to greater input via these neural regions when processing fearful faces than during adulthood. Perhaps through enhanced cognitive control and memory function that comes with age, adults by comparison use less input from the amygdala and the fusiform gyrus to perceive fearful faces.
The connectivity findings indicate that the neural circuitry underlying memory of facial emotion changes between adolescence and adulthood, suggesting that maturation of adult-level memory of emotional faces involves the functional integration of both the amygdala and the hippocampus, and perhaps a greater tightening of the communication between these two regions by adulthood. As this is the first developmental study to measure the functional connectivity of neural responses to emotional faces, these findings provide a foundation from which other studies of connectivity may build and underscore the need for more research on patterns of functional connectivity during face processing across development to increase our understanding of the brain–behavior intersection.
There are several possible extensions of the present study. First, the influence of task design remains an important methodological question, and more work is needed to tease apart how event-related and block task designs may influence amygdala response to fearful faces in different ways. Although it is beyond the scope of the present study, future studies could compare the time course of amygdala response between event-related and block task designs to understand possible task-related differences in the amygdala habituation process found to occur while viewing fearful faces. Second, further developmental investigations will be needed to monitor location of gaze during performance of this task in the scanner. Simultaneously tracking eye movements and measuring functional brain changes during face viewing may clarify neural mechanisms underlying developmental differences in processing facial emotion. A recent study indicates that a deficit in fear recognition in a patient with bilateral amygdala damage stems from an inability to use information from the eye region in faces (Adolphs et al., 2005) and other work has found amygdala response to direct eye contact to be an important social signal (Kawashima et al., 1999). Third, one direction for the functional connectivity work presented here would be to use other analytical approaches to infer directionality of influence between functionally connected regions during facial expression processing (Ramnani, Behrens, Penny, & Matthews, 2004). Finally, it will be important to use findings from typical samples such as those presented here to understand adolescence as a vulnerable time for emerging psychopathology based on developmental trajectories of emotion processing and associated changes in neural function that place adolescents at risk for affective disorders.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Mental Health, National Institutes of Health.
REFERENCES
- Adleman NE, Menon V, Blasey CM, White CD, Warsofsky IS, Glover GH, et al. A developmental fMRI study of the Stroop color-word task. Neuroimage. 2002;16:61–75. doi: 10.1006/nimg.2001.1046. [DOI] [PubMed] [Google Scholar]
- Adolphs R. The neurobiology of social cognition. Current Opinion in Neurobiology. 2001;11:231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. Journal of Neuroscience. 1995;15:5879–5891. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, Young AW, Calder AJ, Phelps EA, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37:1111–1117. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proceedings of the National Academy of Sciences, U.S.A. 1998;95:14506–14510. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: Implications for understanding social anxiety. Biological Psychiatry. 2002;51:11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The amygdala, social behavior, and danger detection. Annals of the New York Academy of Sciences. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, Lavenex P, Mason WA, Mauldin-Jourdain ML, et al. The amygdala: Is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–522. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, Parsons AC, Richards TL, Cramer SC, et al. Brain activation during face perception: Evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17:308–319. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Baird AA, Gruber SA, Fein DA, Maas LC, Steingard RJ, Renshaw PF, et al. Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:195–199. doi: 10.1097/00004583-199902000-00019. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. Journal of Neuroscience. 1999;19:5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Burock MA, Buckner RL, Woldorff MG, Rosen BR, Dale AM. Randomized event-related experimental designs allow for extremely rapid presentation rates using functional MRI. NeuroReport. 1998;9:3735–3739. doi: 10.1097/00001756-199811160-00030. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical, imaging, lesion, and genetic approaches toward a model of cognitive control. Developmental Psychobiology. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, et al. The role of the anterior cingulate in automatic and controlled processes: A developmental neuroanatomical study. Developmental Psychobiology. 1997;30:61–69. [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126:2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: A functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DL, Zijdenbos AP, Kollokian V, Sled JG, Kabani NJ, Holmes CJ, et al. Design and construction of a realistic digital brain phantom. IEEE Transactions on Medical Imaging. 1998;17:463–468. doi: 10.1109/42.712135. [DOI] [PubMed] [Google Scholar]
- Deeley Q, Daly EM, Surguladze S, Page L, Toal F, Robertson D, et al. An event related functional magnetic resonance imaging study of facial emotion processing in Asperger syndrome. Biological Psychiatry. 2007;62:207–217. doi: 10.1016/j.biopsych.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: Role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P, Friesen WV. Pictures of facial affect. Palo Alto, CA: Consulting Psychologists Press; 1976. [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Spear LP. Reward systems. In: de Hoan M, Gunnar M, editors. Handbook of developmental social neuroscience. Guilford Press; (in press). [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Wright CI, Whalen PJ, McInerney SC, Shin LM, Rauch SL. Brain habituation during repeated exposure to fearful and neutral faces: A functional MRI study. Brain Research Bulletin. 2003;59:387–392. doi: 10.1016/s0361-9230(02)00940-1. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline J. To smooth or not to smooth? Bias and efficiency in fMRI time-series analysis. Neuroimage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform ‘‘face area’’ increases with expertise in recognizing novel objects. Nature Neuroscience. 1999;2:568–573. doi: 10.1038/9224. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur R. Request for NP tasks or emotions/faces. University of Pennsylvania, Department of Psychiatry, Neuropsychiatry Section; 2000. [Accessed March 13, 2008]. Retrieved from www.med.upenn.edu/bbl/download/2Dfaces. [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: Effects of a neocortical network on the limbic system. NeuroReport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. Trends in Cognitive Sciences. 2000;4:223–233. doi: 10.1016/s1364-6613(00)01482-0. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL. Face encoding and recognition in the human brain. Proceedings of the National Academy of Sciences, U.S.A. 1996;93:922–927. doi: 10.1073/pnas.93.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, Nakamura A, Hatano K, Ito K, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122:779–783. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Oki M, Yurgelun-Todd DA. Sex-specific developmental changes in amygdala responses to affective faces. NeuroReport. 2001;12:427–433. doi: 10.1097/00001756-200102120-00047. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Sex-related developmental differences in the lateralized activation of the prefrontal cortex and amygdala during perception of facial affect. Perceptual and Motor Skills. 2004;99:371–391. doi: 10.2466/pms.99.2.371-391. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Lane RD, Fink GR, Chau PM, Dolan RJ. Neural activation during selective attention to subjective emotional responses. NeuroReport. 1997;8:3969–3972. doi: 10.1097/00001756-199712220-00024. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion, memory and the brain. Scientific American. 1994;270:50–57. doi: 10.1038/scientificamerican0694-50. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Parrish JM, Adler A, Blair RJ, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Archives of General Psychiatry. 2007;64:97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- McClure EB, Monk CS, Nelson EE, Zarahn E, Leibenluft E, Bilder RM, et al. A developmental examination of gender differences in brain engagement during evaluation of threat. Biological Psychiatry. 2004;55:1047–1055. doi: 10.1016/j.biopsych.2004.02.013. [DOI] [PubMed] [Google Scholar]
- McGivern RF, Andersen J, Byrd D, Mutter KL, Reilly J. Cognitive efficiency on a match to sample task decreases at the onset of puberty in children. Brain and Cognition. 2002;50:73–89. doi: 10.1016/s0278-2626(02)00012-x. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JLI, et al. Amygdala and nucleus accumbens activation to emotional facial expressions in adolescents at risk for major depression. American Journal of Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, et al. Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage. 2003;20:420–428. doi: 10.1016/s1053-8119(03)00355-0. [DOI] [PubMed] [Google Scholar]
- Morris JS, Friston KJ, Buchel C, Frith CD, Young AW, Calder AJ, et al. A neuromodulatory role for the human amygdala in processing emotional facial expressions. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Nelson CA. The recognition of facial expressions in the first two years of life: Mechanisms of development. Child Development. 1987;58:889–909. [PubMed] [Google Scholar]
- Nelson CA, Bloom FE. Child development and neuroscience. Child Development. 1997;68:970–987. doi: 10.1111/j.1467-8624.1997.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, et al. Developmental differences in neuronal engagement during implicit encoding of emotional faces: An event-related fMRI study. Journal of Child Psychology and Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: An fMRI study. Developmental Science. 2003;6:100–117. [Google Scholar]
- Perez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, et al. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, McKenna M, Gutierrez E, Ungerleider LG. Neural processing of emotional faces requires attention. Proceedings of the National Academy of Sciences, U.S.A. 2002;99:11458–11463. doi: 10.1073/pnas.172403899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: A genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Pine DS. Research review: A neuroscience framework for pediatric anxiety disorders. Journal of Child Psychology and Psychiatry. 2007;48:631–648. doi: 10.1111/j.1469-7610.2007.01751.x. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Penny W, Matthews PM. New approaches for exploring anatomical and functional connectivity in the human brain. Biological Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neuroscience. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, McClure EB, Monk CS, Nelson EE, Guyer AE, Fromm SJ, et al. Increased amygdala activity during successful memory encoding in adolescent major depressive disorder: An fMRI study. Biological Psychiatry. 2006;60:966–973. doi: 10.1016/j.biopsych.2006.02.018. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The Structured Clinical Interview for DSM-III-R (SCID): I. History, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sprengelmeyer R, Rausch M, Eysel UT, Przuntek H. Neural structures associated with recognition of facial expressions of basic emotions. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1998;265:1927–1931. doi: 10.1098/rspb.1998.0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M, et al. Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Archives of General Psychiatry. 1999;56:913–919. doi: 10.1001/archpsyc.56.10.913. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, et al. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, et al. Amygdala response to facial expressions in children and adults. Biological Psychiatry. 2001;49:309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Nelson CA. Research network on early experience and brain development: Face stimuli–emotion expression. 2000 Retrieved from www.macbrain.org/resources.htm. March 13, 2008.
- Vuilleumier P, Armony JL, Driver J, Dolan RJ. Effects of attention and emotion on face processing in the human brain: An event-related fMRI study. Neuron. 2001;30:829–841. doi: 10.1016/s0896-6273(01)00328-2. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Wechsler D, editor. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. Journal of Neuroscience. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Shin LM, McInerney SC, Fischer H, Wright CI, Rauch SL. A functional MRI study of human amygdala responses to facial expressions of fear versus anger. Emotion. 2001;1:70–83. doi: 10.1037/1528-3542.1.1.70. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yang TT, Menon V, Eliez S, Blasey C, White CD, Reid AJ, et al. Amygdalar activation associated with positive and negative facial expressions. NeuroReport. 2002;13:1737–1741. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Killgore WD. Fear-related activity in the prefrontal cortex increases with age during adolescence: A preliminary fMRI study. Neuroscience Letters. 2006;406:194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre GK, D’Esposito M. Empirical analyses of BOLD fMRI statistics. I. Spatially unsmoothed data collected under null-hypothesis conditions. Neuroimage. 1997;5:179–197. doi: 10.1006/nimg.1997.0263. [DOI] [PubMed] [Google Scholar]