Abstract
Background. Sulodexide is a glycosaminoglycan with anticoagulant and antithrombotic activities. Although sulodexide reduced albuminuria in patients with type 1 and type 2 diabetes, long-term effects on chronic renal injury are not established. We investigated sulodexide effects and mechanisms in a rat radiation nephropathy model and in the db/db mouse model of diabetic kidney disease.
Methods. Sprague–Dawley rats received kidney radiation and were treated as follows: 15 mg/kg/day sulodexide s.c., 6 day/week (SUL) or no treatment (CONT). Subsets of animals were sacrificed after 8 weeks and 12 weeks. Blood pressure, serum creatinine, creatinine clearance (CrCl) and urinary protein excretion were measured every 4 weeks. Sclerosis and plasminogen activator inhibitor-1 (PAI-1) expression were assessed at 8 and 12 weeks, and collagen I, total collagen content and phospho-smad-2 expressions were determined at 12 weeks. Twelve-week-old db/db mice received sulodexide as above or vehicle. Albuminuria and CrCl were assessed at intervals till sacrifice at week 9 with assessment of urinary transforming growth factor-β (TGF-β) and glomerular lesions.
Results. Blood pressure, serum creatinine and CrCl were not different in radiation rat CONT vs SUL at any time. Proteinuria was significantly lower in SUL compared to CONT at 4 and 8 weeks but not at 12 weeks. Sclerosis and PAI-1 expression trended lower in SUL vs CONT at 8 weeks. There was no difference between the groups in sclerosis, collagen I mRNA, total collagen content or PAI-1 expression at 12 weeks. Phospho-smad 2 expression was significantly decreased in SUL compared to CONT at 12 weeks. Db/db mice with or without SUL showed no difference in urinary albumin/creatinine ratio, urine TGF-β or mesangial matrix expansion.
Conclusions. Our data show that sulodexide can reduce the early, but not late, proteinuria in radiation nephropathy in rats. In addition, sulodexide did not affect urine TGF-β established albuminuria or mesangial matrix expansion in a chronic model of diabetic kidney disease in mice. Although sulodexide may affect TGF-β activation in radiation nephropathy, this effect appeared insufficient in this model to inhibit the expressions of PAI-1 and collagen and reduce accumulation of extracellular matrix. These results may explain in part its lack of efficacy in recent clinical trials of chronic kidney disease.
Keywords: glomerulosclerosis, PAI-1, proteinuria, sulodexide, TGF-beta
Introduction
Sulodexide is a highly purified glycosaminoglycan (GAG) composed of a fast mobility heparin fraction (80%) as well as dermatan sulphate (20%) obtained from the porcine intestinal mucosa by a patented process [1]. Sulodexide differs from other GAGs, like heparin, by having a longer half-life and a reduced effect on systemic clotting and bleeding [1,2].
An increasing body of research has demonstrated the safety and efficacy of sulodexide in a wide range of disease settings of vascular injury. Sulodexide reduced infarct size and inflammation during reperfusion in animals with myocardial ischaemia [3]. This effect could be related to the sulodexide property of modulating complement activation following tissue injury [2]. Clinical trials have demonstrated the beneficial effects of sulodexide in the treatment of deep vein thrombosis [4] and in the treatment of venous leg ulcers [5]. GAGs exert their antithrombotic action by accelerating the inhibition of activated serine proteases such as thrombin in the coagulation cascade by interacting with serine proteases inhibitors like antithrombin III and cofactor II [6–9]. Sulodexide can also promote fibrinolysis by increasing tissue plasminogen activator (tPA) activity and decreasing plasminogen activator inhibitor-1 (PAI-1) [10,11]. Sulodexide can also exert antilipemic effects promoting the release of lipoprotein lipase [12].
In chronic kidney disease (CKD), sulodexide has been studied in diabetic nephropathy, both in animal models and in human subjects. GAGs reduced extracellular matrix deposition and transforming growth factor-β (TGF-β) overexpression in a rat model of streptozocin-induced diabetic nephropathy, a model most resembling type 1 diabetes, and inhibited TGF-β overexpression and matrix synthesis induced by high concentration of glucose in mesangial cells [13,14]. Furthermore, GAGs restored anionic charges lost from the endothelial surface [15] and reduced endothelial injury in experimental models [16]. In humans, sulodexide reduced albuminuria in subjects with type I or type II diabetes [17,18]. However, recent preliminary presentations of results from an ongoing clinical trial in diabetic kidney disease [19], the SUN-Micro-Trial, have not shown efficacy of sulodexide on microalbuminuria, and the planned phase 4 trial, so-called SUN-Macro-Trial, has been canceled [20].
Beneficial effects of sulodexide in other models of progressive kidney disease have been variable. Studies in a mild mouse adriamycin model showed decrease in early proteinuria and 0.3 vs 7.8% sclerosis with sulodexide [21], whereas there was very limited effect on renal function or histology in the rat 5/6 nephrectomy remnant model (H. Lan, personal communication). The aim of the present study was to investigate whether sulodexide treatment is effective in modifying kidney disease in a mild nonhypertensive rat model of CKD resulting from endothelial injury, namely radiation nephropathy [22], or in a model of type 2 diabetes mellitus, the db/db mouse [23], lacking the hypothalamic leptin receptor. We also investigated possible underlying mechanisms to determine possible renoprotective effects in these two models of CKD.
Materials and methods
Experimental design
Radiation nephropathy rats.
Thirty-two 250–300 g male Sprague–Dawley rats (Charles River, TN, USA) were studied. Rats were housed under normal conditions with a 12-h light/dark cycle, 70°F, with 40% humidity and 12 air exchanges/h. Rats received normal rat chow and water ad libitum (‘5001’ diet, Purina Laboratory Rodent diet, 23.4% protein, 4.5% fat, 6.0% fibre and 0.40% sodium). A dose of 12 Gy radiation was delivered to anaesthetized rats (i.p. nembutal) with a 60 γ cobalt irradiator to a band across the abdomen that included both kidneys, as we have previously reported, to induce radiation nephropathy [22]. Rats were then treated as follows: 15 mg/kg/day s.c., 6 day/week of sulodexide (SUL, dissolved in saline, n = 16) (gift from Keryx Biopharmaceuticals, Inc.) or no treatment (CONT, n = 16). Dosing of sulodexide was based on previous reports and recommendation from the manufacturer [21,24]. Sulodexide is a proprietary formulation of 80% fast-moving heparin sulfate and 20% dermatan sulphate.
Blood pressure, serum creatinine, creatinine clearance and urinary protein excretion were measured at baseline and at 4, 8, weeks and 12 weeks following radiation. Animals were sacrificed at 8 weeks (n = 6 CONT and n = 6 SUL) and at 12 weeks (n = 10 CONT and n = 10 SUL) after radiation.
Diabetic mice.
Twenty-two 12-week-old db/db male mice on C57BLKS background were studied [23]. These mice exhibit hyperglycaemia, hyperinsulinaemia and hyperleptinaemia associated with hyperphagia and obesity manifesting around 4–7 weeks after birth. We therefore started intervention after this time point (12 weeks of age) as 100% of db/db mice become frankly hyperglycaemic after 8 weeks and albuminuric at 8–12 weeks. After baseline studies of glycaemia and urinary albumin/creatinine ratio (ACR), mice were assigned to vehicle (n = 10) or sulodexide dosed as above (n = 12). Mice were housed under standard light/dark conditions with ad lib water and matched food weights ±5% daily. Body weight, plasma creatinine and urinary ACR were assessed at 6 weeks and at sacrifice at 9 weeks. Urinary TGF-β was assessed at sacrifice, and tissue was obtained for analysis of mesangial matrix expansion, as previously described [25,26].
Blood pressure and renal function measurements
Unanaesthetized rats were prewarmed for 15 min before they were placed in the blood pressure chamber. Systolic blood pressure (SBP) was measured using tail-cuff plethysmography (IITC; Life Science Inc. Woodland Hills, CA, USA) at an ambient temperature of 29°C. The tail was passed through a miniaturized cuff connected to an amplifier. The amplified pulse was recorded during automatic inflation and deflation of the cuff. Tail-cuff SBP was defined as the inflation pressure at which the waveform became indistinguishable from baseline noise. Final SBP readings were obtained averaging three successful readings.
Animals were placed in metabolic cages for 24 h for urine collection. Urinary protein concentration in rats was measured by the benzethonium chloride reaction. Serum creatinine in rats was measured by the kinetic Jaffe picric acid-based test. Both of these analytical tests were performed using a Roche/Hitachi Modular P instrument (Roche Diagnostics, Indianapolis, IN, USA). In mice, 24-h urine collections in metabolic cages were obtained for ACR, with creatinine determined by HPLC as previously described [27]. Urinary TGF-β was measured by Quantikine ELISA (R and D) as previously described and standardized per urine creatinine [28].
Sclerosis and mesangial expansion
Tissue was immersion-fixed in 4% paraformaldehyde phosphate-buffered saline (PBS) solution and routinely processed, and 4 μ paraffin sections were prepared and stained with periodic acid-Schiff. Sclerosis was defined as collapse and/or obliteration of the glomerular capillary tuft and increase of matrix. Glomerular sclerosis in rats was assessed by scoring severity of sclerosis on all glomeruli on a single section of kidney. The severity of sclerosis for each glomerulus was graded from 0 to 4+ as follows: 0 for no sclerosis, 1 for <25% of the glomerular tuft involved with sclerosis, 2 for 25 to 50%, 3 for 50 to <75% and 4 for 75 to 100% sclerosis [22]. A whole kidney average sclerosis index (SI) for radiation nephropathy rats was obtained by averaging scores from all glomeruli on one section. In db/db mice, 4% buffered formalin immersion-fixed kidneys were sectioned, stained by periodic acid-Schiff and digital images evaluated for mesangial expansion, as previously described. Briefly, the degree of mesangial matrix was semiquantitatively scored in 50 glomeruli from each mouse. The increase in mesangial matrix was defined by PAS-positive and nuclei-free area in the mesangium. The glomerular area was calculated along the borders of capillary loop [29]. The severity of matrix formation was scored from 0 to 4 where 0 = (normal glomerulus), 1 = (local, small lesions up to 25% of glomerular volume), 2 = (diffuse expansion 25–49%), 3 = (50–75%) and 4 = (>75%). An average score was calculated for each mouse. All sections were examined without knowledge of treatment.
Immunohistochemistry (IHC)
Sections were treated by 3% hydrogen peroxidase for 10 min, Powerblock (BioGenex Laboratories, San Ramon, CA, USA) for 45 min and then incubated with rabbit anti-rat PAI-1 antibody 1:50 overnight (American Diagnostica Inc., Greenwich, CT, USA) [22]. After rinsing twice with PBS, supersensitive rabbit link for mouse/rat tissue biotinylated goat anti-rabbit Ig (BioGenex) was added, incubated for 45 min, followed by rinsing two times and incubation with supersensitive label peroxidase conjugated streptavidin (BioGenex) for 45 min. After rinsing three times with PBS, diaminobenzidine (DAB) was added as a chromagen. Slides were counterstained with haematoxylin. Negative control without primary antibody showed no staining. PAI-1 protein was assessed by scoring staining intensity on all glomeruli, or up to 50 consecutive glomeruli, on a single section of the kidney. Staining intensity for each glomerulus was graded from 0 to 4: 0 no staining, 1+ trace staining, 2+ staining in <10% of the glomerular tuft, 3+ staining in 10 to 25% of the glomerulus and 4+ for >25% of the glomerulus staining. In each kidney, the average immunostaining score was then calculated and compared with average SI for those same glomeruli. In addition, average PAI-1 immunostaining for each grade of sclerosis was calculated for all glomeruli from all rats.
To further assess matrix alterations in db/db mice, fibronectin and collagen IV immunostaining was performed. Four micron sections were microwaved in 0.01 mol/L sodium citrate (pH 6.0) 4 × 5 min and stained with Ready-to-Use rabbit anti-fibronectin (Innovex Biosciences, Richmond, CA) for 1 h at RT, followed by immunoperoxidase staining with Rabbit on Rodent HRP Polymer (Biocare Medical, Concord, CA) for 30 min at RT. For collagen IV, sections were microwaved as above 3 × 5 min, stained with rabbit anti-mouse collagen IV (1:1000, Chemicon, Billerica, MA) and incubated overnight at 4°C, followed by immunoperoxidase staining with Vectastain ABC kit (Vector Laboratories, Burlingame, CA). DAB was used as a chromogen for both stains. The results of collagen IV were quantified by ImageJ software, assessing area of positive staining in 20 consecutive glomeruli from each mouse, deriving a collagen IV staining percent for each mouse and then averaging these to derive an average score for each group.
All sections were examined without knowledge of the treatment protocol.
Northern blot
The cDNA probe fragments for mouse PAI-1 mRNA were prepared by reverse transcription-polymerase chain reaction and cloned using the TA cloning kit (Invitrogen, Carlsbad, CA, USA) and harvested and purified from Escherichia coli as previously described [22]. The cDNA product was confirmed by sequence analysis. A commercial human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) cDNA probe was used as a housekeeping gene control (Promega, Madison, WI, USA). cDNA probes were labeled with [32P] deoxycytidimine triphosphate (dCTP; New England Nuclear, Boston, MA, USA) by random primer method. The collagen I cDNA probe was derived from human collagen I (ATCC, Manassas, VA, USA) as previously described [30].
Total RNA was extracted from the left kidney by the RNAzol™B method (Cinna Biotecx, Houston, TX, USA). RNA was resuspended in diethyl pyrocarbonate-treated water, and concentration was determined by absorbance at 260 nm. RNA (15 μg) was size fractionated on 1.0% formaldehyde agarose gels. Equal loading of RNA was confirmed by examination of ribosomal RNA using ethidium bromide staining. RNA was transferred to nylon membrane (Hybond N; Amersham, Picataway, NJ, USA) and cross-linked by ultraviolet irradiation. The membranes were incubated in prehybridization buffer for 2 h and hybridized with cDNA probes labeled with [32P] dCTP (New England Nuclear) for 18 to 24 h at 65°C in hybridization buffer [4× single strand conformational polymorphism, 1× Denhardt's, 1× sodium dodecyl sulphate (SDS), 100 μg/mL denatured salmon sperm DNA and 10% dextran sulphate]. Membranes were washed twice in 2× standard saline citrate (SSC), 0.1% SDS for 10 min at room temperature, once in 0.1% SDS for 20 min at 65°C and once in 0.1 SSC, 0.1% SDS for 20 min at 65°C. Membranes were air dried and exposed to XAR film (Kodak Co., Rochester, NY, USA) in intensifying screens at −70°C for 3 to 5 days. Autoradiographs were scanned by image scanner JX-330 (Sharp, Osaka, Japan), and intensity of signals was measured by NIH Image (National Institutes of Health, Bethesda, MD, USA). The ratio of specific message to the housekeeping gene GAPDH was used to quantitate expression.
In situ hybridization
35S-labeled sense and antisense riboprobes for PAI-1 were prepared by transcription of the pCR™II plasmid with insertion of the cDNA fragment by SP6 or T7 RNA polymerase (Promega) [30]. Sections were dewaxed in xylene and hydrated in graded ethanols and then 4% paraformaldehyde. After treatment by proteinase K and triethanolamine/acetic anhydride, sections were dehydrated in ethanol and air dried. Hybridization was done in buffer [50% formamide, 10% dextran sulfate, 8 mmol/L dithiothreitol, 0.2 mg/mL tRNA, 300 mmol/L NaCl, 10 mmol/L Tris–HCl, 5 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.02% polyvinylpyrolidone, 0.02% Ficoll and 0.02% bovine serum albumin] overnight at 50°C. Sections were washed in 5× SSC, 20 mmol/L β-mercaptoethanol at 50°C for 15 min, in 2× SSC, 200 mmol/L β-mercaptoethanol, 50% formamide at 68°C for 20 min, in TEN twice at 37°C for 10 min, treated with RNase at 37°C for 30 min, washed in TEN at 37°C for 10 min, in 2× SSC and 0.1× SSC each twice at 68°C for 15 min. Sections were then dehydrated in ethanol and air dried, dipped in photographic emulsion and exposed at 4°C for 14 days. The sections were developed with D-19 developer (Kodak) and counterstained with toluidine blue.
Western blotting
Tissue samples of renal cortex were lysed and prepared by RIPA plus buffer (150 mM NaCl, 50 mM Tris–HCl, 5 mM EDTA, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 100 μg/mL PMSF) with phosphate inhibitor cocktail (Sigma, St. Louis, MO, USA) and proteinase inhibitor cocktail (Roche Diagnostics). Protein concentration was measured by BCA™ protein assay kit (Pierce Biotech Inc., Rockford, IL, USA). Eighty micrograms of protein samples was electrophoresed on SDS-10% polyacrylamide gel and transferred to nitrocellulose membranes. Immunoblotting was performed with rabbit anti-phospho-Smad 2 antibody (Upstate Biotechnology Inc., Lake Placid, NY, USA). Blots were washed and incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibody (Amersham Biosciences, Piscataway, NJ, USA). Immunoreactive proteins were visualized using ECL western blotting detection reagents (Amersham Biosciences). Membranes were re-probed with mouse anti-β-actin (Sigma) and mouse anti-total Smad 2 (Cell Signaling Technology, Beverly, MA, USA) as loading control and for relative phospho-Smad 2, respectively. The expression of the target molecules was evaluated by using SCION Image Beta 4.03 (Scion Corporation, Frederick, MD, USA) as densitometric ratios relative to loading controls.
Statistical analysis
Results are expressed as mean ± standard deviation (SD). Statistical difference was assessed by t-test or Mann–Whitney U for nonparametric data as appropriate. A P-value <0.05 was considered to be significant.
Results
Renal function and blood pressure
Renal function, body weight, SBP and proteinuria were not different between the two radiation nephropathy rat groups at baseline (Table 1). No hematoma or other adverse reactions at the injection site were observed. After 4 and 8 weeks, serum creatinine, body weight and SBP were not significantly different between the two radiation nephropathy rat groups, although serum creatinine trended lower in sulodexide-treated rats (CONT vs SUL, P = 0.06). Proteinuria increased over time vs baseline. However, proteinuria was significantly reduced in sulodexide-treated animals compared to controls at these early stages (P = 0.005). At 12 weeks, there was no significant difference between the two radiation nephropathy groups in serum creatinine, body weight, SBP and proteinuria. Db/db mice randomized to control or sulodexide had no difference in body weight, glycaemia or urinary ACR at baseline. Both control and sulodexide mice gained weight similarly over the treatment period. There was no difference in ACR over time, and serum creatinine was not different at sacrifice at 9 weeks.
Table 1.
Renal function, body weight and SBP
| CONT | SUL | CONT | SUL | CONT | SUL | CONT | SUL | |
|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 8 weeks | 12 weeks | |||||
| Radiation nephropathy rats | ||||||||
| Scr (mg/dl) | 0.32 ± 0.10 | 0.28 ± 0.08 | 0.41 ± 0.10 | 0.37 ± 0.05 | 0.45 ± 0.10 | 0.39 ± 0.05 | 0.58 ± 0.15 | 0.54 ± 0.07 |
| Upr (mg/day) | 12.5 ± 5.0 | 15.4 ± 6.0 | 40.2 ± 19.0** | 41.4 ± 21.0* | 69.7 ± 37.0** | 53.6 ± 24.5** | 83.6 ± 67.8** | 65.2 ± 30.5** |
| BW (g) | 290.2 ± 23.9 | 287.6 ± 16.8 | 346.5 ± 25.9 | 338.2 ± 26.8 | 382.5 ± 25.0 | 371.9 ± 31.7 | 417.3 ± 21.9 | 425.4 ± 17.4 |
| SBP (mm Hg) | 110.1 ± 11.6 | 110.9 ± 12.2 | 115.0 ± 12.8 | 118.1 ± 30.9 | 127.5 ± 21.2 | 111.9 ± 34.1 | 127.4 ± 13.5 | 121.4 ± 12.4 |
| CONT | SUL | CONT | SUL | CONT | SUL | CONT | SUL | |
| Baseline | 2 weeks | 6 weeks | 9 weeks | |||||
| Db/db mice | ||||||||
| Glucose (mg/dl) | 423.8 ± 75.7 | 412.8 ± 87.2 | n/d | n/d | n/d | n/d | n/d | n/d |
| Scr (mg/dl) | 0.094 ± 0.048 | 0.100 ± 0.017 | ||||||
| ACR (μg/mg) | 715.7 ± 273.9 | 595.1 ± 282.1 | n/d | n/d | 179.0 ± 73.2 | 241.3 ± 180.3 | 209.7 ± 116.9 | 276.4 ± 175.1 |
| BW (g) | 42.5 ± 1.7 | 42.7 ± 2.8 | 45.5 ± 2.4 | 46.4 ± 2.6 | 50.4 ± 3.9 | 53.0 ± 3.9 | ||
Mean ± SD; CONT, control; SUL, sulodexide; Scr, serum creatinine; Upr, proteinuria; BW, body weight; SBP, systolic blood pressure; ACR, urinary albumin/creatinine ratio; n/d, not done.
P < 0.05 vs baseline.
P < 0.01 vs baseline.
Sclerosis, mesangial expansion and PAI-1 expression
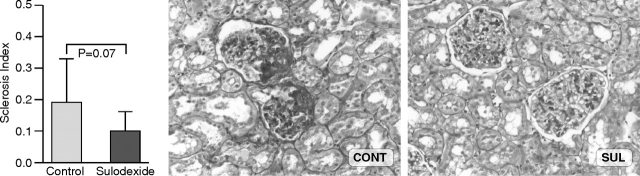
Glomerulosclerosis in sulodexide-treated rats trended to be less compared to controls (SI: 0.10 ± 0.06 vs 0.19 ± 0.10; SUL vs CONT, P = 0.07) (Figure 1). Mesangial expansion in CONT db/db mice was generally mild at sacrifice at 9 weeks, with an average of 87 ± 22% glomeruli with grade 2 mesangial expansion (range 78–94), vs 12 ± 6% with grade 3 lesions (range 24–22), similar to SUL db/db (grade 2, 84 ± 16%, range 48–96, grade 3, 16 ± 16%, range 4–52%, P = NS). Arteriolar hyaline was present in 12% of CONT and 16% of SUL.
Fig. 1.
Rats treated with sulodexide showed a trend for less glomerulosclerosis at 8 weeks compared to control animals (periodic acid-Schiff, ×200).
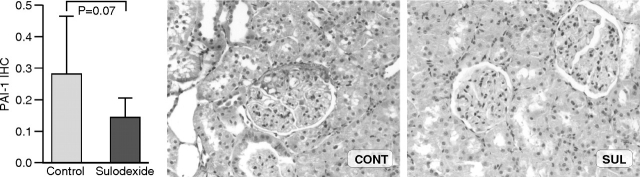
PAI-1 protein expression in radiation nephropathy by immunohistochemistry was localized to the sclerotic areas of glomeruli and also trended lower in treated animals compared to controls (0.14 ± 0.06 vs 0.28 ± 0.18; SUL vs CONT, P = 0.07) (Figure 2). PAI-1/GAPDH mRNA ratio was also reduced only numerically in sulodexide-treated animals compared to controls (0.20 ± 0.10 vs 0.15 ± 0.04; CONT vs SUL, P = 0.26) (Figure 3).
Fig. 2.
PAI-1 protein expression by immunohistochemistry was reduced numerically in sulodexide-treated animals compared to controls at 8 weeks, although not significantly (anti-PAI-1 antibody immunohistochemistry, ×200).
Fig. 3.
PAI-1/GAPDH mRNA ratio from whole kidney cortex was also reduced numerically in sulodexide-treated animals compared to controls at 8 weeks but not significantly.
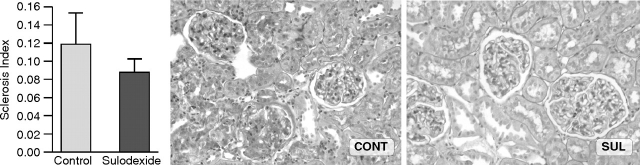
At 12 weeks glomerulosclerosis, PAI-1 protein and mRNA Northern expressions were not different between groups (SI: 0.08 ± 0.02 vs 0.12 ± 0.03; PAI-1 IHC: 0.03 ± 0.01 vs 0.05 ± 0.01; PAI-1/GAPDH: 0.26 ± 0.03 vs 0.22 ± 0.02, all SUL vs CONT, P = NS) (Figures 4 and 5). Qualitative assessment of PAI-1 by in situ hybridization (ISH) revealed occasional expression in podocytes, mesangium and parietal epithelial cells without sulodexide significantly changing its expression pattern (Figure 5A).
Fig. 4.
Glomerulosclerosis at 12 weeks was not different in control vs sulodexide-treated rats (periodic acid-Schiff, ×200).
Fig. 5.
PAI-1 mRNA by (A) or northern blot (C) and protein expression by immunohistochemistry (B) at 12 weeks were not changed by sulodexide treatment (A, B, ×200).
TGF-β activation and collagen content
TGF-β signaling was inhibited after 12 weeks of sulodexide treatment in radiation nephropathy as demonstrated by reduced phospho-Smad2 expression in sulodexide-treated animals compared to controls (phospho-Smad2/total Smad2 0.19 ± 0.01 vs 0.36 ± 0.05, SUL vs CONT, P < 0.01) (Figure 6). In contrast, urinary TGF-β was not altered in db/db mice by sulodexide (urine 24 h TGF-β 8.9 ± 12.6 pg CONT vs 19.3 ± 27.2 in SUL, n = 5 each group; urine TGF-β normalized for creatinine 0.26 ± 0.34 vs 0.48 ± 0.76, n = 5 each group, P = NS).
Fig. 6.
Phospho-Smad 2 expression was significantly decreased in sulodexide-treated compared control at 12 weeks.
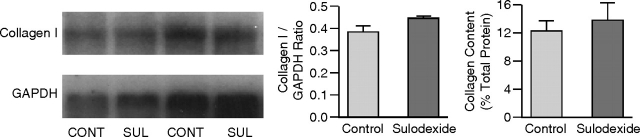
The expression of collagen I mRNA and total collagen content were not different between the two radiation nephropathy groups at 12 weeks (collagen I/GAPDH 0.43 ± 0.03 vs 0.39 ± 0.03, SUL vs CONT, P = NS; collagen content 13.86 ± 2.43 vs 12.33 ± 1.40, SUL vs CONT, P = NS) (Figure 7). To further assess possible effects of sulodexide on glomerular matrix expansion, we assessed glomerular fibronectin and collagen IV in db/db mice by immunohistochemistry. There was only minimal glomerular staining for fibronectin in diabetic mice with or without sulodexide (below levels reliably assessed by imaging software). There was modestly increased glomerular staining for collagen IV in db/db mice with further minimal increase in db/db mice treated with sulodexide (23.9 ± 0.8% in db/db vs 26.8 ± 0.7 in db/db + sulodexide).
Fig. 7.
The expression of collagen I mRNA and the total collagen content were not different between the sulodexide-treated and control groups at 12 weeks.
Discussion
Sulodexide is an old drug with a renewed interest due to several observations of its beneficial effects both in experimental models of type 1 diabetic nephropathy [13] and in pilot studies on albuminuria in human subjects with type I and II diabetes [17,18]. Two multicentre, double-masked, randomized placebo controlled trials were therefore recently designed to study the renoprotective potential of sulodexide given for 6 months to patients with type 2 diabetes, hypertension and microalbuminuria or to type 2 diabetic patients with hypertension and overt proteinuria [19]. Primary end points are conversion to normoalbuminuria and at least a 25% decrease in the urinary ACR or at least a 50% reduction in this ratio for the microalbuminuric patients and time to a composite end point of doubling of serum creatinine or ESRD in those with existing overt proteinuria. The first data presented disappointingly did not show effects on microalbuminuria, and the planned phase 4 trial has thus been canceled [20].
Based on this renewed interest in sulodexide, we aimed to study its effects on mild renal injury in a nondiabetic nonhypertensive model and in a model of renal injury due to type 2 diabetes. We chose the radiation nephropathy model because of the theoretic effects of sulodexide on PAI-1 and TGF-β, both upregulated early in this model [22], and the db/db mouse model, as it displays many metabolic features of type 2 diabetes, with associated albuminuria and mesangial expansion. It appears that impaired vascular injury, rather than direct radiation injury to parenchymal cells, underlies the parenchymal cell loss, a characteristic of late radiation injury [22,31]. Thus, endothelial cell injury and thrombosis in capillaries precede interstitial fibrosis and glomerulosclerosis in radiation nephropathy. Endothelial injury and PAI-1 are highly relevant to diabetic injury and are also hypothetically mechanisms particularly anticipated to be targeted by sulodexide.
We found that sulodexide treatment could reduce the early manifestations of radiation nephropathy as shown by a significant reduction of proteinuria at 4 and 8 weeks and by a trend in reduction of serum creatinine at 8 weeks after radiation in treated animals compared to controls. There was a corresponding trend, albeit not statistically significant, for less glomerulosclerosis in animals receiving sulodexide compared to controls at 8 weeks. Neither albuminuria nor structural lesions in db/db mice were affected by sulodexide.
However, contrasting these beneficial effects at early stages of injury, sulodexide did not prevent the manifestations associated with the late radiation injury. Indeed, our data show that at 12 weeks after radiation, there were no differences between the two groups in terms of renal function, protein excretion and severity of histologic lesions. The lack of sustained effects of sulodexide on proteinuria in this model and the lack of efficacy in a mouse model of type 2 diabetic injury parallel the recent preliminary data from the current clinical trials. Clearly, varying mechanisms of injury are active in these two models. Furthermore, even in a given model, it is highly likely that injury mechanisms are not static over time but rather are dynamically altered at different stages of evolution towards the chronically scarred kidney. For example, whereas sclerosis and loss of capillaries are hallmarks of late diabetic glomerulosclerosis, in the early stage, there is dominant angiogenesis and capillary growth [32,33]. Thus, the lack of effects of sulodexide on albuminuria, matrix and TGF-b in the db/db mouse, which only develops mild mesangial expansion as a consequence of diabetes, may not mirror effects on later stages of injury that develop in other models or in humans. A further caveat is the lack of defined relationship between proteinuria and glomerular structural lesions. Although microalbuminuria in diabetic patients is a hallmark of endothelial dysfunction, proteinuria may occur without sclerosing injury due to altered permselectivity and/or be related to hemodynamic changes [34]. As is evident from the early trials of sulodexide in diabetic patients, where microalbuminuria was decreased, and our current animal data, change in microalbuminuria does not unequivocally translate to sustained benefit on renal function or structure [17–19]. Sulodexide has antithrombotic and fibrinolytic properties and increases tPA activity and reduces PAI-1 levels in some settings [10,11]. In our study, we found that PAI-1 expression was increased after radiation injury in podocytes, mesangium and parietal epithelial cells at sites of injury, strictly associated with sclerotic areas. Although our data show that sulodexide may decrease PAI-1 expression in the early phases of injury, PAI-1 expression either at protein or mRNA levels in the late phases of injury of radiation nephropathy was not affected by sulodexide, although TGF-β signaling was decreased. Our previous studies in radiation nephropathy showed that angiotensin-converting enzyme inhibitor could prevent injury, and this was linked to decreased PAI-1, with no effect on TGF-β at the mRNA level [22]. Furthermore, we have shown that although mice deficient in β6 integrin and thus lacking αvβ6 integrin, a key activator of TGF-β, were protected from fibrosis induced by ureteral obstruction, added angiotensin or aldosterone induced PAI-1 and restored fibrosis in these mice without activating TGF-β [30]. These data point to complex interactions of the renin angiotensin aldosterone system, PAI-1 and TGF-β in effecting renal fibrosis.
GAGs reduced extracellular matrix (ECM) deposition and TGF-β overexpression in a rat model of streptozocin-induced diabetic nephropathy and inhibited TGF-β overexpression and matrix synthesis induced by high concentration of glucose in mesangial cells [13,14]. Our data showed that sulodexide significantly reduced TGF-β activation in radiation nephropathy animals compared to controls without a reduction in PAI-1 expression but did not affect urinary TGF-β or matrix accumulation in db/db mice. Furthermore, this decrease in TGF-β activation in radiation nephropathy did not change ECM accumulation. These data indicate, as also suggested by our previous studies in this model, that TGF-β is not a major mediator of sclerosis in radiation nephropathy [22].
In summary, our data suggest that sulodexide is effective in reducing the early, but not late, manifestations of radiation nephropathy in rats and has no effect on renal injury or function in db/db mice at the time point assessed. Although sulodexide significantly reduced TGF-β activation in radiation nephropathy, this effect might be insufficient in this model to inhibit the expression of both PAI-1 and collagen. Whether higher doses of the drug, or combination with other interventions, could achieve sustained results remains to be determined. These data also indicate that interpretation and extrapolation of results from animal models to humans should consider that mechanisms of fibrosis and efficacy of interventions vary significantly with differing models of CKD.
Acknowledgments
These studies were supported in part by a gift from Keryx and NIH grant NIDDK 44757. The results presented in this paper have not been published previously in whole or part, except in abstract format.
Conflict of interest statement. None declared.
References
- 1.Harenberg J. Review of pharmacodynamics, pharmacokinetics, and therapeutic properties of sulodexide. Med Res Rev. 1998;18:1–20. doi: 10.1002/(sici)1098-1128(199801)18:1<1::aid-med1>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Lauver DA, Lucchesi BR. Sulodexide: a renewed interest in this glycosaminoglycan. Cardiovasc Drug Rev. 2006;24:214–226. doi: 10.1111/j.1527-3466.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 3.Lauver DA, Booth EA, White AJ, et al. Sulodexide attenuates myocardial ischemia-reperfusion injury and the deposition of C-reactive protein in areas of infarction without affecting hemostasis. J Pharmacol Exp Ther. 2005;312:794–800. doi: 10.1124/jpet.104.075283. [DOI] [PubMed] [Google Scholar]
- 4.Errichi BM, Cesarone MR, Belcaro G, et al. Prevention of recurrent deep venous thrombosis with sulodexide: the SanVal registry. Angiology. 2004;55:243–249. doi: 10.1177/000331970405500302. [DOI] [PubMed] [Google Scholar]
- 5.Coccheri S, Scondotto G, Agnelli G, et al. Randomised, double blind, multicentre, placebo controlled study of sulodexide in the treatment of venous leg ulcers. Thromb Haemost. 2002;87:947–952. [PubMed] [Google Scholar]
- 6.Baglin TP, Carrell RW, Church FC, et al. Crystal structures of native and thrombin-complexed heparin cofactor II reveal a multistep allosteric mechanism. Proc Natl Acad Sci USA. 2002;99:11079–11084. doi: 10.1073/pnas.162232399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosmi B, Cini M, Legnani C, et al. Additive thrombin inhibition by fast moving heparin and dermatan sulphate explains the anticoagulant effect of sulodexide, a natural mixture of glycosaminoglycans. Thromb Res. 2003;109:333–339. doi: 10.1016/s0049-3848(03)00246-9. [DOI] [PubMed] [Google Scholar]
- 8.Harper PL, Daly M, Price J, et al. Screening for heparin binding variants of antithrombin. J Clin Pathol. 1991;44:477–479. doi: 10.1136/jcp.44.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverman GA, Bird PI, Carrell RW, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Quatraro A, Marchi E, et al. Impaired fibrinolytic response to increased thrombin activation in type 1 diabetes mellitus: effects of the glycosaminoglycan sulodexide. Diabete Metab. 1993;19:225–229. [PubMed] [Google Scholar]
- 11.Messa GL, La Placa G, Puccetti L, et al. Pharmacodynamic effects of sulodexide on profibrinolytic and haemorrheological patterns. Clin Drug Invest. 1995;10:165–171. doi: 10.2165/00044011-199510030-00005. [DOI] [PubMed] [Google Scholar]
- 12.Radhakrishnamurthy B, Sharma C, Bhandaru RR, et al. Studies of chemical and biologic properties of a fraction of sulodexide, a heparin-like glycosaminoglycan. Atherosclerosis. 1986;60:141–149. doi: 10.1016/0021-9150(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 13.Ceol M, Gambaro G, Sauer U, et al. Glycosaminoglycan therapy prevents TGF-β1 overexpression and pathologic changes in renal tissue of long-term diabetic rats. J Am Soc Nephrol. 2000;11:2324–2336. doi: 10.1681/ASN.V11122324. [DOI] [PubMed] [Google Scholar]
- 14.Weigert C, Brodbeck K, Haring HU, et al. Low-molecular-weight heparin prevents high glucose- and phorbol ester-induced TGF-β1 gene activation. Kidney Int. 2001;60:935–943. doi: 10.1046/j.1523-1755.2001.060003935.x. [DOI] [PubMed] [Google Scholar]
- 15.Hiebert LM, Jaques LB. The observation of heparin on endothelium after injection. Thromb Res. 1986;8:195–204. doi: 10.1016/0049-3848(76)90262-0. [DOI] [PubMed] [Google Scholar]
- 16.Kristova V, Kriska M, Babal P, et al. Evaluation of endothelium-protective effects of drugs in experimental models of endothelial damage. Physiol Res. 2000;49:123–128. [PubMed] [Google Scholar]
- 17.Gambaro G, Kinalska I, Oksa A, et al. Oral sulodexide reduces albuminuria in microalbuminuric and macroalbuminuric type 1 and type 2 diabetic patients: the Di.N.A.S. Randomized Trial. J Am Soc Nephrol. 2002;13:1615–1625. doi: 10.1097/01.asn.0000014254.87188.e5. [DOI] [PubMed] [Google Scholar]
- 18.Dedov I, Shestakova M, Vorontzov A, et al. A randomized, controlled study of sulodexide therapy for the treatment of diabetic nephropathy. Nephrol Dial Transplant. 1997;12:2295–2300. doi: 10.1093/ndt/12.11.2295. [DOI] [PubMed] [Google Scholar]
- 19.Lambers Heerspink HJ, Fowler MJ, et al. for the Collaborative Study Group Rationale for and study design of the sulodexide trials in Type 2 diabetic, hypertensive patients with microalbuminuria or overt nephropathy. Diabet Med. 2007;24:1290–1295. doi: 10.1111/j.1464-5491.2007.02249.x. [DOI] [PubMed] [Google Scholar]
- 20.Burney BO, Kalaitzidis RG, Bakris GL. Novel therapies of diabetic nephropathy. Curr Opin Nephrol Hypertens. 2009;18:107–111. doi: 10.1097/MNH.0b013e3283249c51. [DOI] [PubMed] [Google Scholar]
- 21.Kajiyama H, Lu H, Eltaraboulsi W, et al. Sulodexide reduces proteiuria and glomerular sieving in experimental focal segmental glomerulosclerosis. J Am Soc Nephrol. 2004;15:699A. (abstract) [Google Scholar]
- 22.Oikawa T, Freeman M, Lo W, et al. Modulation of plasminogen activator inhibitor-1 in vivo: a new mechanism for the anti-fibrotic effect of rennin-angiotensin inhibition. Kidney Int. 1997;51:164–172. doi: 10.1038/ki.1997.20. [DOI] [PubMed] [Google Scholar]
- 23.Sharma K, McCue P, Dunn SR. Diabetic kidney disease in the db/db mouse. Am J Physiol Renal Physiol. 2003;284:F1138–F1144. doi: 10.1152/ajprenal.00315.2002. [DOI] [PubMed] [Google Scholar]
- 24.Kristova V, Liskova S, Sotnikova R, et al. Sulodexide improves endothelial dysfunction in streptozotocin-induced diabetes in rats. Physiol Res. 2008;57:491–494. doi: 10.33549/physiolres.931506. [DOI] [PubMed] [Google Scholar]
- 25.Williams KJ, Qiu G, Usui HK, et al. Decorin deficiency enhances progressive nephropathy in diabetic mice. Am J Pathol. 2007;171:1441–1450. doi: 10.2353/ajpath.2007.070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Susztak K, Bottinger E, Novetsky A, et al. Molecular profiling of diabetic mouse kidney reveals novel genes linked to glomerular disease. Diabetes. 2004;53:784–794. doi: 10.2337/diabetes.53.3.784. [DOI] [PubMed] [Google Scholar]
- 27.Dunn S, Qi Z, Bottinger E, et al. Utility of endogenous creatinine clearance as a measure of renal function in mice. Kidney Int. 2004;65:1959–1967. doi: 10.1111/j.1523-1755.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 28.McGowan TA, Dunn SR, Falkner B, et al. Stimulation of urinary TGFβ and isoprostanes in response to hyperglycemia in humans. Clin J Am Soc Nephrol. 2006;1:263–268. doi: 10.2215/CJN.00990905. [DOI] [PubMed] [Google Scholar]
- 29.Ghosh S, Khazaei M, Moien-Afshari F, et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. Am J Physiol Renal Physiol. 2009;296:F700–F708. doi: 10.1152/ajprenal.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma LJ, Yang H, Gaspert A, et al. Transforming growth factor-beta-dependent and -independent pathways of induction of tubulointerstitial fibrosis in beta 6(-/-) mice. Am J Pathol. 2003;163:1261–1273. doi: 10.1016/s0002-9440(10)63486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law MP. Vascular permeability and late radiation fibrosis in mouse lung. Radiat Res. 1985;103:60–67. [PubMed] [Google Scholar]
- 32.Zent R, Pozzi A. Antiangiogenic therapy in diabetic nephropathy. J Am Soc Nephrol. 2006;17:325–327. doi: 10.1681/ASN.2005121290. [DOI] [PubMed] [Google Scholar]
- 33.Zhang SX, Wang JJ, Lu K, et al. Therapeutic potential of angiostatin in diabetic nephropathy. J Am Soc Nephrol. 2006;17:475–486. doi: 10.1681/ASN.2005020217. [DOI] [PubMed] [Google Scholar]
- 34.Fogo A. Nephrotic syndrome: molecular and genetic basis. Nephron. 2000;85:8–13. doi: 10.1159/000045623. [DOI] [PubMed] [Google Scholar]