Abstract
Background. Kidney failure is associated with muscle wasting and physical impairment. Moderate- to high-intensity strength training improves physical performance, nutritional status and quality of life in people with chronic kidney disease and in dialysis patients. However, the effect of low-intensity strength training has not been well documented, thus representing the objective of this pilot study.
Methods. Fifty participants (mean ± SD, age 69 ± 13 years) receiving long-term haemodialysis (3.7 ± 4.2 years) were randomized to intra-dialytic low-intensity strength training or stretching (attention-control) exercises twice weekly for a total of 48 exercise sessions. The primary study outcome was physical performance assessed by the Short Physical Performance Battery score (SPPB) after 36 sessions, if available, or carried forward from 24 sessions. Secondary outcomes included lower body strength, body composition and quality of life. Measurements were obtained at baseline and at completion of 24 (mid), 36 (post) and 48 (final) exercise sessions.
Results. Baseline median (IQR) SPPB score was 6.0 (5.0), with 57% of the participants having SPPB scores below 7. Exercise adherence was 89 ± 15%. The primary outcome could be computed in 44 participants. SPPB improved in the strength training group compared to the attention-control group [21.1% (43.1%) vs. 0.2% (38.4%), respectively, P = 0.03]. Similarly, strength training participants exhibited significant improvements from baseline compared to the control group in knee extensor strength, leisure-time physical activity and self-reported physical function and activities of daily living (ADL) disability; all P < 0.02. Adverse events were common but not related to study participation.
Conclusions. Intra-dialytic, low-intensity progressive strength training was safe and effective among maintenance dialysis patients. Further studies are needed to establish the generalizability of this strength training program in dialysis patients.
Keywords: haemodialysis, older adults, physical performance, Short Physical Performance Battery, strength training
Introduction
The number of patients with kidney failure treated by dialysis and transplantation in the USA has increased by 57% in the last decade, and its annual expenditure is estimated to rise to $28 billion by 2010 [1]. While long-term dialysis may extend survival, patients’ quality of life and physical function remains poor [2].
In the general population, factors associated with physical impairment and disability [3] include the loss of muscle mass and function with aging and the onset of catabolic chronic diseases [4]. Disabled individuals are unable to carry out activities of independent living (ADLs) and to maintain quality of life [4]. Disability predicts adverse health outcomes and health care needs [5]. Data from the Health, Aging and Body Composition Study [6] and the Cardiovascular Health Study [7] show that physical impairment can be detected in the early stages of chronic kidney disease. Frailty as determined by poor self-reported physical performance, exhaustion/fatigue, low physical activity and undernutrition was found to be prevalent among incident dialysis patients in the Dialysis Morbidity and Mortality Wave 2 Study [8]. Poor physical performance is associated with higher risk of death and hospitalization among dialysis patients [8,9].
Moderate- to high-intensity strength training improves physical performance, muscle mass and quality of life in chronic kidney disease (CKD) [10,11] and dialysis patients [12–16]. Strength training also improves leisure-time physical activities [17]. The reported effects of strength training on physical performance, nutritional status and physical activity suggest that this exercise modality may be promising as an adjunct to routine care of dialysis patients. However, many older dialysis patients may not be able to perform moderate- to high-intensity strength training. The objective of this pilot study was to determine the safety and efficacy of an intra-dialytic low-intensity progressive strength training program in haemodialysis patients recruited from two dialysis units in the greater Boston area.
Materials and methods
The Clinical Trials Registration for this study is NCT00363961. The study was conducted between January 2005 and August 2007.
Population
Patients undergoing haemodialysis were recruited from outpatient dialysis facilities at Tufts Medical Center (Dialysis Clinic, Inc.) and Caritas St. Elizabeth’s Medical Center (CSEMC), Boston, MA. Study procedures were approved by the Institutional Review Boards of each participating center. Informed consent was obtained from each subject prior to screening. Eligibility criteria included age ≥30 years, serum albumin <4.2 g/dl and haemodialysis thrice weekly for at least 3 months with ≥80% compliance. Exclusion criteria included unstable cardiovascular disease or any uncontrolled chronic condition; cardiac surgery, retina laser therapy, myocardial infarction, joint replacement or lower extremity fracture within the last 6 months; severe cognitive impairment; lower extremity amputation; or current strength training. Eligible participants were screened and recruited at their dialysis unit, where they had baseline testing followed by two familiarization sessions to strength and flexibility exercises. After familiarization, participants were randomized by allocation concealment to strength training or stretching exercises (attention control).
Intervention
Exercise sessions took place twice weekly during the second hour of haemodialysis for a total of 48 exercise sessions. Supervised sessions began with a 5-min warm-up and ended with a 5-min cool-down. Participants in the strength training group exercised their lower body only using ankle weights progressively in half-pound increments from 0.5 to 20 lbs (TKO, Houston, TX). Exercises included seated right/left knee extension with dorsi/plantar flexion (quadriceps muscle), seated leg curl with both legs keeping the heels pressed firmly against a chair while rolling the legs in and out (hamstrings), semirecumbent right/left inner leg raises (hip adductors), and semirecumbent dorsi/plantar flexion with straight legs (tibialis anterior, gastrocnemius and soleus muscles). Participants did a seated pelvic tilt (abdominal and lower back muscles) without using free weights. Two sets of eight repetitions were performed for each exercise with a 1.5-s concentric phase, a 0.5-s pause in the lifted position and a 3-s eccentric phase; assuring 1–2 min rest between sets. Exercise intensity was assessed by the rate of perceived exertion (RPE) modified OMNI Scale [18], with a target moderate intensity of 6 (somewhat hard) out of 10 (extremely hard), equivalent to 60% of a one-repetition maximum [19]. The first eight exercise sessions were done with none or little weight and progressed based on participants’ ability to complete two sets of eight repetitions with proper form and a RPE rating of 2–4 (easy to somewhat easy). Strength exercises were developed using information obtained from a feasibility phase conducted at Tufts Medical Center prior to the study.
Attention-control participants did stretching exercises with light resistance bands (TKO, Houston, TX), using right/left dorsi/plantar ankle flexion, right/left ankle rotation, right/left calf stretch, right/left hamstring stretch and right/left inner thigh stretch. These exercises were done in the semirecumbent position, held for 20–30 s and repeated twice. All participants were asked to continue their usual activities, including physical activity and diet, and to report any changes in health status or medications.
Safety
A Data Safety Monitoring Board (DSMB) was planned as per study protocol and assessed adverse events and their association with study participation and any proposed change in protocol. Four DSMB meetings were held.
Study outcomes
The primary study outcome was physical performance ascertained by the Short Physical Performance Battery (SPPB) score, which includes objective performance-based measures of strength (five chair stands), endurance (4-m gait speed) and balance (side-by-side, semitandem and tandem). Each component was scored from 0 to 4 and when summed yielded SPPB scores between 0 (poor) and 12 (best) performance [20]. Poor physical functioning—the hallmark of frailty—predicts disability, recurrent hospitalization, institutionalization and death in the general population [5,20] and in patients with kidney disease [8]. For example, SPPB scores <7 have been shown to be prognostic of disability, institutionalization and death [5,20], suggesting that functional status is a key aspect of quality of life and survival. Secondary outcomes included knee extensor strength measured with a Nicholas Manual Muscle Tester (Lafayette, IN), which has been shown to correlate with isokinetic knee extensor strength in the general population (r = 0.85, P < 0.01) [21]. Lean and fat mass were determined by dual X-ray absorptiometry (DXA) using a Hologic QDR2000 scanner (Waltham, MA) with coefficients of variation of 1.4% for lean and 1.8% for fat mass [22]. Self-reported measures of quality of life included physical activity and the Medical Outcomes Survey Short Form (SF-36) physical and mental component summary scores (PCS and MCS, respectively) using the eight domains of the SF-36 questionnaire [23,24]. Past, 7-day, leisure-time physical activity was assessed using the Physical Activity Scale for the Elderly, which is a 10-item questionnaire yielding scores between 0 (no activity) and 400 (greatest activity) [25]. Self-reported disability was assessed using 12 items from the Activities of Daily Living (ADL) questionnaire and summary scores calculated. Higher summary scores are indicative of disability [26].
Outcome measures were ascertained in the middle of the week prior to haemodialysis by two testers. For each participant, outcome ascertainment was performed by the same tester throughout the study to reduce inter-tester error. Measures were done at baseline (prior to randomization, ‘pretesting’) and after completion of 24 (‘mid-testing’), 36 (‘posttesting’) and 48 (‘final-testing’) exercise sessions. Blinding of testers was not feasible. However, most measurements of physical performance were taken in duplicate. At the start of the study, we had intended to use the final-testing SPPB score for the primary outcome, but based on the number of interruptions in training due to patient illnesses or hospitalizations, we decided to use the posttesting SPPB score instead and to carry forward mid-testing score data as necessary. This change in analysis plan was made 3 months into the study, prior to examination of any outcome data and with concurrence of the DSMB.
Statistical analysis
Data are shown as mean and standard deviation (SD), median and interquartile range (IQR) or numerical (and percentage). Percent changes in primary (SPPB score) and secondary outcomes (muscle strength, body composition and quality of life) were calculated as [(Post minus Pre/Pretesting) × 100%] and used for group comparisons. Comparisons between the treatment and control groups at the baseline and at the post exercise testing time points were assessed by independent sample t-test for normally distributed variables, the Mann–Whitney U-test for variables that were not normally distributed (SPPB and PASE scores) and the chi-square test or Fisher’s exact test for categorical variables. Spearman’s rank coefficient of correlation analysis was used to assess associations between primary and secondary outcomes. Analyses were performed using SPSS 16.0 for Windows (SPSS, Inc., Evanston, IL) and the SAS System for Windows 9.2 (Copyright (c) 2002–2008 by SAS Institute Inc., Cary, NC, USA). Results were considered statistically significant at a P value <0.05 (two-tailed).
Sensitivity analysis on the primary outcome was run using multilevel (mixed) model analyses of changes in SPPB over time between the treatment and control groups. For this purpose, four versions of the model were run. Version 1 was based on complete data (excluding dropouts) for pre, mid and posttesting visits with visit number (0 = pre, 1 = mid, 2 = post, 3 = final) used as the ‘time’ variable. Version 2 was run like Version 1 but included data from dropouts and from final testing. Versions 3 and 4 were similar to 1 and 2, except that calendar time (days from first initial visit) was used as the measure of time rather than the testing visit number. In Versions 3 and 4, the time points were not equally spaced for each participant in the study. Each mixed model included the randomization group as a predictor of both initial SPPB status and the rate of change in SPPB over time. These analyses were performed using Proc Mixed in SAS, Version 9.2 [27,28].
Results
Recruitment
Two hundred and fifty dialysis patients were assessed for eligibility (Figure 1), 151 at Tufts Medical Center and 99 at St. Elizabeth’s Medical Center. Of these, 59 consented to enrol in the study, of whom 50 were randomized and 44 were analyzed (35 from Tufts Medical Center and 9 from St. Elizabeth’s Medical Center). There were no differences in sociodemographic or clinical characteristics between participants enrolled (n = 59) and those refusing participation (n = 56). Similarly, there were no differences between patients assessed for eligibility by dialysis center or among those who were randomized and dropped out before the intervention (data not shown).
Fig. 1.
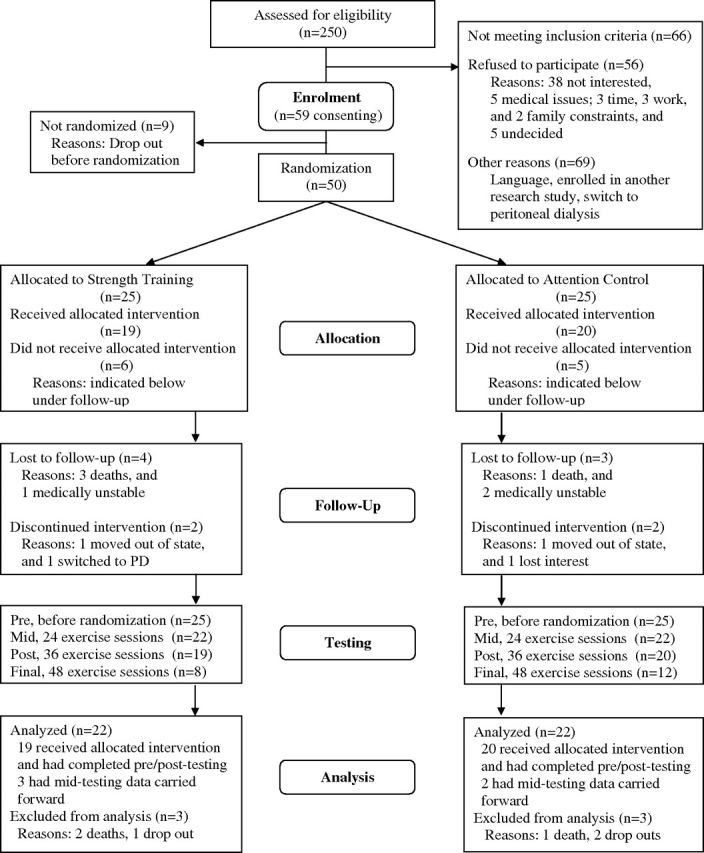
Recruitment flow chart.
Baseline characteristics
There were no differences in baseline characteristics between groups, except for a trend toward shorter duration in haemodialysis in the strength training group (Table 1). At baseline, 50% of participants had SPPB scores below 7, and 77% reported difficulty with at least one ADL. Baseline SPPB scores were inversely related with age (r = −0.46, P = 0.002).
Table 1.
Baseline subject characteristics
| Characteristic | Strength training (n = 22) | Attention control (n = 22) | P value * |
|---|---|---|---|
| Sociodemographic characteristics | |||
| Age (year) | 71.1 ± 12.6 | 66.9 ± 13.4 | 0.3 |
| Women/men, n (%) | 10 (45.5)/12 (54.4) | 11 (50.0)/11 (50.0) | 0.5 |
| College education, n (%) | 5 (22.7) | 8 (36.4) | 0.7 |
| Ethnicity | 0.9 | ||
| White, n (%) | 8 (36.4) | 7 (31.8) | |
| Asian, n (%) | 6 (27.3) | 9 (40.9) | |
| Black, n (%) | 7 (31.8) | 5 (22.7) | |
| Hispanic, n (%) | 1 (4.5) | 1 (4.5) | |
| Physiological characteristics | |||
| Estimated dry weight (kg) | 71.2 ± 17.7 | 72.9 ± 20.8 | 0.8 |
| Body mass index (kg/m2) | 25.7 ± 7.1 | 27.7 ± 7.8 | 0.4 |
| Lean body mass (kg) a | 45.8 ± 8.9 | 47.8 ± 9.0 | 0.5 |
| Fat mass (%) a | 31.3 ± 10.4 | 30.8 ± 11.2 | 0.9 |
| SPPB score, median (IQR) | 5.0 (5.2) | 6.0 (7.0) | 0.5 |
| ADL disability summary score | 6.3 ± 0.9 | 6.8 ± 1.6 | 0.2 |
| Clinical characteristics | |||
| Aetiology of kidney failure | 0.2 | ||
| Diabetes, n (%) | 5 (22.7) | 10 (45.5) | |
| Hypertension, n (%) | 9 (40.9) | 6 (27.3) | |
| Glomerular diseases, n (%) | 3 (13.6) | 0 (0) | |
| Other, n (%) | 5 (27.7) | 6 (27.3) | |
| Haemodialysis duration (year) | 2.6 ± 2.6 | 4.8 ± 5.2 | 0.08 |
| Kt/V | 1.54 ± 0.14 | 1.65 ± 0.38 | 0.2 |
| Serum creatinine (mg/dl) | 9.3 ± 2.0 | 9.5 ± 2.4 | 0.8 |
| Serum albumin (g/dl) | 3.6 ± 0.4 | 3.7 ± 0.3 | 0.7 |
| Number of chronic conditions | 7.3 ± 2.9 | 6.3 ± 2.4 | 0.2 |
| Hypertension, n (%) | 19 (86.4) | 14 (63.6) | |
| Diabetes, n (%) | 13 (59.1) | 12 (54.5) | |
| Heart disease, n (%) | 11 (50.0) | 7 (31.8) | |
| Number of medications | 9.6 ± 1.9 | 10.3 ± 3.0 | 0.3 |
| Hospitalizations during the 12 months prior to enrollment, n (%) | 17 (77.3) | 15 (68.2) | 0.4 |
Data are mean ± SD, median and interquartile range (IQR) or number (and percent) as appropriate. To convert serum creatinine in mg/dl to μmol/l, multiply by 88.4, and serum albumin in g/dl to g/L, multiply by 10. ADL disability refers to self-report difficulty with performing at least one activity of daily living.
Baseline comparisons were assessed by independent sample t-test for normally distributed continuous variables or Mann–Whitney U-test for variables not normally distributed (SPPB score) and chi-square test for categorical variables, except for ethnicity and aetiology of kidney failure for which Fisher Exact test was reported.
Dual X-ray absorptiometry (DXA) performed in 21 participants per group.
Exercise intensity
The average weight lifted and self-reported RPE of all sessions combined for each strength exercise that used free weights are shown in the online supplemental table. Exercise trainers were able to progressively increase the amount of weight lifted by the participants, while ensuring good exercise technique and safety, despite RPEs falling below 6 (moderate intensity).
Study outcomes
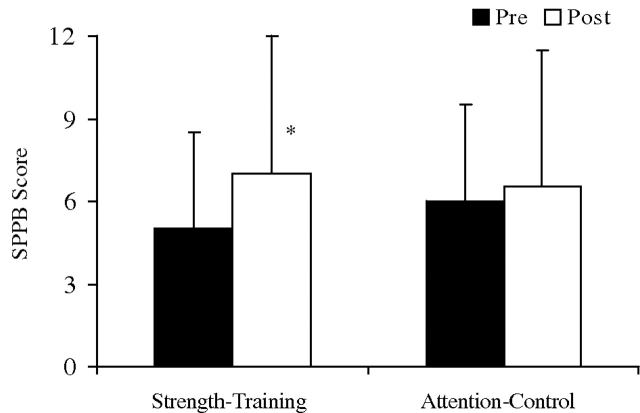
Completed posttesting measures were available in 39 participants (Figure 1). Of the 11 participants missing posttesting data, five had mid-testing data carried forward. Forty-four participants were analyzed (22 per group). SPPB scores are shown in Figure 2 and Table 2. SPPB posttesting scores significantly improved with strength training compared to the control group [21.1% (43.1%) vs. 0.2% (38.4%), respectively, P = 0.03]. The improvement was due to an improvement (reduction) in chair stand time with strength training compared to a worsening (increase) in the attention-control group (−16.4 ± 43.3% vs. +31.1 ± 34.5%, P = 0.04). Balance and gait speed did not change in either group.
Fig. 2.
Median Short Physical Performance Battery (SPPB) Scores, the primary study outcome, are shown for participants in the strength-training and attention-control groups during pre- and posttesting; Error bars represent interquartile range (IQR). (*) Baseline (pre) and post exercise comparisons between groups were assessed by Mann–Whitney U-test, P = 0.03.
Table 2.
Primary and secondary outcomes: muscle strength and body composition
| Outcome | Strength training (n = 22) | Attention control (n = 22) | P value * |
|---|---|---|---|
| SPPB score | |||
| Pre | 5.0 (5.2) | 6.0 (7.0) | 0.5 |
| Post | 7.0 (7.2) | 6.5 (4.5) | |
| % Change | 21.1 (43.1) | 0.2 (38.4) | 0.03 |
| Knee extensors strength (kg) a | |||
| Pre | 11.4 ± 5.0 | 14.8 ± 6.0 | 0.08 |
| Post | 15.8 ± 5.0 | 12.1 ± 6.1 | |
| % Change | 44.9 ± 26.3 | −18.1 ± 17.9 | 0.0001 |
| Whole-body lean mass by DXA (kg) a | |||
| Pre | 45.8 ± 8.9 | 47.8 ± 9.0 | 0.5 |
| Post | 47.9 ± 9.9 | 46.3 ± 8.7 | |
| % Change | 4.2 ± 5.6 | −3.2 ± 3.3 | 0.0001 |
| Leg lean mass by DXA (kg)a | |||
| Pre | 6.9 ± 1.7 | 7.2 ± 1.8 | 0.5 |
| Post | 7.2 ± 2.0 | 6.9 ± 1.7 | |
| % Change | 5.0 ± 7.6 | −3.2 ± 5.8 | 0.0001 |
| Whole-body fat mass by DXA (%) a | |||
| Pre | 31.3 ± 10.4 | 30.8 ± 11.2 | 0.9 |
| Post | 29.6 ± 9.8 | 33.1 ± 10.1 | |
| % Change | −2.6 ± 16.9 | 11.0 ± 20.5 | 0.02 |
Data are mean ± SD or median and interquartile range (IQR) for Short Physical Performance Battery (SPPB) Score. Leg lean mass includes both legs.
Baseline (pre) and post exercise (% changes from baseline) comparisons between the strength training and attention-control groups were assessed by independent sample t-test for normally distributed continuous variables or Mann–Whitney U-test for variables not normally distributed (SPPB score).
Knee extensors strength and dual X-ray absorptiometry (DXA) measures were available in 21 participants per group.
Sensitivity analysis showed that the changes (improvements) in SPPB over time were significantly greater in the strength training group compared to the attention-control group for Version 1 which excluded dropouts, and only data from pre, mid and posttesting visits were used. The increase in time was marginally significant (P < 0.10) when data from the final visit and dropouts were included (Version 2). In all four versions of the model, the estimated rate of change, or improvement, in SPPB over time in the treatment group was significantly greater than in the control group.
Secondary outcomes of physical performance and nutritional status are shown in Table 2. Knee extensor lower body muscle strength improved significantly with strength training compared to controls. The percentage changes seen in SPPB score were positively correlated with those seen for knee extensor strength (r = 0.33, P = 0.03). Whole-body lean mass increased significantly with strength training, while fat mass was reduced (Table 2). Self-reported physical activity, physical function and ADL disability summary scores were also improved with strength training compared to controls (Table 3).
Table 3.
Secondary outcomes: quality of life
| Outcome | Strength training (n = 22) | Attention control (n = 22) | P value * |
|---|---|---|---|
| Leisure-Time Physical Activity (PASE Score) | |||
| Pre | 47.5 (45.9) | 28.6 (36.5) | 0.2 |
| Post | 57.5 (69.3) | 22.7 (30.5) | |
| % Change | 10.3 (88.1) | −30.5 (34.3) | 0.0001 |
| SF-36 physical component summary score | |||
| Pre | 46 ± 12 | 52 ± 11 | 0.08 |
| Post | 54 ± 12 | 50 ± 11 | |
| % Change | 21 ± 36 | −2 ± 24 | 0.02 |
| SF-36 mental component summary score | |||
| Pre | 37 ± 11 | 39 ± 11 | 0.6 |
| Post | 37 ± 9 | 38 ± 9 | |
| % Change | 6 ± 27 | 1 ± 23 | 0.6 |
| ADL disability summary score | |||
| Pre | 6.3 ± 0.9 | 6.8 ± 1.6 | 0.2 |
| Post | 7.0 ± 1.4 | 6.7 ± 1.7 | |
| % Change | 10.5 ± 21.1 | −2.6 ± 8.0 | 0.02 |
Data are mean ± SD.
SF-36: Medical Outcomes Survey Short Form (SF)-36. ADL, activities of daily living.
Baseline (pre) and post exercise (% changes from baseline) comparisons between the strength training and attention-control groups were assessed by independent sample t-test for normally distributed continuous variables or Mann–Whitney U-test for variables not normally distributed (PASE score).
Study adherence
Mean adherence to exercise was 89 ± 14% in the strength training group and 90 ± 17% in the attention-control group (P = 0.8). Interruptions in exercise continuity were due to hospitalizations not associated with the study or adverse events as described below. We calculated the percentage of exercise sessions completed in 24–28 consecutive weeks with no interruptions. The percentages observed were 85 ± 15% and 89 ± 11% in the strength training and attention-control groups, respectively (P = 0.09). There were 11 participants in the strength training group and eight in the attention-control group who experienced one or more interruptions in exercise continuity and, as a result, needed more than 24–28 consecutive weeks to complete the 48 exercise sessions (P = 0.3).
Adverse events
No exercise-related injuries were reported. Occasional muscle soreness and cramping were reported during exercise that resolved spontaneously in a few days. Blood pressure and heart rate were stable during exercise. Frequency of adverse events was similar in both groups, with 13 strength training participants and 12 attention controls reporting at least one adverse event (P = 0.5). These included dialysis access issues, depression, arrhythmia, myocardial infarction (participants had to wait 6 months before being screened and re-enrolled into the study), gastrointestinal bleeding, constipation, diarrhoea, fall-related injury, hypotension due to hypovolaemia, infection, bacteraemia or death. None of these adverse events occurred during exercise. The DSMB determined that none of these events were related to study participation. However, 67% required hospitalization and medical clearance to resume exercise contributing to the low number of participants undergoing final testing.
Discussion
This randomized controlled trial shows that intra-dialytic, low-intensity progressive strength training was safe and beneficial among haemodialysis patients. Strength training resulted in significant improvements in physical performance (SPPB score and muscle strength), accompanied by significant improvements in nutritional status (increased whole-body and leg lean mass and decreased fat mass) and in self-reported quality of life.
Most participants in the present study were physically impaired, chronically ill and older. Physical impairment was evidenced by a low SPPB score, which has been shown to predict disability when scores are less than 7 [5,20]. We also found that low SPPB scores were significantly associated with older age. Objective measures of physical performance assessed with SPPB are strength, endurance and balance. These are used to discriminate functional capacity and health outcomes in populations at risk like the older adult and those with chronic conditions [20,29]. In our study, SPPB score—the primary study outcome—increased significantly with strength training. In older adults, a change in SPPB score of about 1.0 point, similar to that we observed, has been suggested as clinically meaningful [30]. It is possible that a change in SPPB of such magnitude may have the ability to discriminate and/or monitor health-related quality of life in vulnerable populations like the one we studied, but more information is needed to make such a conclusion. The change in SPPB score was directly associated with knee extensor strength, suggesting that impairment and functional capacity may be benefited in similar ways with strength training. Taken together, these findings suggest that intra-dialytic, low-intensity strength training is a promising intervention to prevent and potentially revert functional decline in dialysis patients known to be physically impaired [31,32] and disabled [8].
The beneficial effects of intra-dialytic, high-intensity strength training on physical performance, nutritional status (body composition) and physical activity have been evaluated in dialysis patients [12–16]. The study by Johansen et al. [16] was a 2 × 2 factorial trial of anabolic steroid administration and strength training for 12 weeks conducted in middle-age dialysis patients. The study by Cheema et al. compared strength training to usual care in dialysis patients who were 63 ± 14 years old. Testing measures were carried out after 12 [14] and 24 [15] weeks of training. The study by Kopple et al. [13] compared the effects of 21 weeks of strength training, endurance training or both in dialysis patients who were 43.6 ± 3.3 years old. Exercise adherence rates for these studies were similar to those we obtained.
In the Johansen et al. study [16], participants assigned to strength training had significant increases in knee extensor strength, similar to our findings. In contrast, they did not find significant increases in physical performance or changes in lean body mass by DXA, while significant (albeit small) increases in fat mass were seen. They, however, showed increases in measures of mid-thigh muscle mass, which we did not perform. Self-reported measures of physical activity and physical functioning were significantly improved with strength training in both the Johansen et al. and the present study. Possible explanations for the discrepancy in the results between these two studies may be the shorter duration of the Johansen et al. study and that the participants in the present study were more disabled and had more comorbid conditions, hence a greater potential to benefit from a strength training program of lower intensity. In the Cheema et al. studies [14,15], participants assigned to strength training had significant improvements in physical performance, muscle strength and self-reported physical function, similar to our findings. Participants in these studies were similar in age, number of chronic conditions and medications. In the Kopple et al. study [13], younger participants assigned to strength training had a significant increase in fat-free mass. These changes paralleled transcriptional changes in genes favoring skeletal muscle protein synthesis. Such molecular changes are associated with enhanced physical capacity [33]. In our study, changes in physical performance parallel those of body composition, suggesting that skeletal muscle anabolism may be possible with low-intensity progressive strength training. Such anabolic response has been reported with acute strength training incorporated to intra-dialytic oral nutritional supplementation in young (38 ± 11 year), relatively healthy, dialysis patients [12] and should be investigated further.
In the present study, physical activity outside of the study improved with strength training. This was similar to that reported in participants with poorly controlled diabetes [17]. In contrast to that study, the strength training program in this study was lower in intensity but meaningful in the changes seen in physical performance, as noted earlier, suggesting that voluntary increases in leisure-time physical activity may be the result of participants feeling better. This is relevant because patients on haemodialysis are less active than healthy sedentary controls and than those of younger age [34].
The strengths of this study include the use of an attention-control group, which reduces the confounding effect of motivation and socialization of a supervised exercise program. Exercise adherence was good considering that none of these patients had previous experience with this type of structured exercise and that 66% of the participants were of an ethnic minority background. Limitations of this study are that we did not include a long-term follow-up necessary to assess exercise effects on clinical outcomes. However, the shorter duration of the study is similar to that reported in other studies of kidney failure patients. We were unable to blind participants and testers to group allocation and behaviour change, which could influence outcome measures.
It is difficult to assess the generalizability of this pilot study. We recruited 27% of eligible patients, and less than half of these (40%) completed 48 exercise sessions and final testing. It is possible that only a small fraction of dialysis patients would be motivated and physically capable of intra-dialytic, low-intensity strength training. However, it is also possible that a higher fraction of patients would be able and willing to participate in an exercise program that was implemented as part of routine medical care rather than part of a study. This will need to be investigated further.
In conclusion, intra-dialytic low-intensity strength training was safe and effective in older adults with kidney failure. Strength training improved physical performance, nutritional status and physical activity. Our findings suggest that this exercise modality is promising as an adjunct to routine care of patients with kidney failure treated by haemodialysis. These findings need to be confirmed in larger and longer-term trials. If replicable, they may contribute to the evidence-based information necessary to safely implement strength exercise as part of routine care in dialysis units.
Supplementary Material
Acknowledgments
Grant support for this trial included the National Institute of Diabetes and Digestive and Kidney (NIDDK) R03 DK064825 and T32 DK0 07777, the USDA ARS agreement 58-1950-9-001, NIH General Clinical Research Center M01 RR000054, and the William B. Schwartz Nephrology Fund at Tufts Medical Center. Any opinions, findings, conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily represent the views of the US Department of Agriculture or any of the funding sources. There are no financial interests to disclose by any of the authors. We are indebted to the DSMB members: Klemens B. Meyer, MD, and James A. Strom, MD, for their insightful input and assistance during the study. We would like to thank Ann T. Sweeney, MD, for her assistance with DXA scan measures; Robert MacKinnon, RN, for his help with recruitment and IRB procedures; Jennifer E. Layne, PhD, for her assistance with the exercise intervention; and the dialysis unit staff of Dialysis Clinic, Inc. and St. Elizabeth’s Medical Center. The results presented in this paper have not been published previously in whole or part, except in abstract format at the 2008 XIV International Congress on Nutrition and Metabolism in Renal Disease in Marseilles, France.
Conflict of interest statement. None declared.
Supplementary data
Supplementary data are available online at http://ndt.oxfordjournals.org
References
- 1.US Renal Data System . National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases. Bethesda, MD; 2002. Annual data report: atlas on end-stage renal disease in the United States. [Google Scholar]
- 2.Kurella Tamura M, Covinsky K, Chertow G, et al. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361:1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagi SZ. An epidemiology of disability among adults in the United States. Milbank Mem Fund Q Health Soc. 1976;54:439–467. [PubMed] [Google Scholar]
- 4.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 2004;59:255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006;54:750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Stehman-Breen C, Fried LF, et al. The presence of frailty in elderly persons with chronic renal insufficiency. Am J Kidney Dis. 2004;43:861–867. doi: 10.1053/j.ajkd.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL, Chertow GM, Jin C, et al. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 9.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30:204–212. doi: 10.1016/s0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 10.Castaneda C, Gordon PL, Uhlin KL, et al. Resistance training to counteract the catabolism of a low-protein diet in patients with chronic renal insufficiency. A randomized, controlled trial. Ann Intern Med. 2001;135:965–976. doi: 10.7326/0003-4819-135-11-200112040-00008. [DOI] [PubMed] [Google Scholar]
- 11.Castaneda C, Gordon PL, Parker RC, et al. Resistance training to reduce the malnutrition-inflammation complex syndrome of chronic kidney disease. Am J Kidney Dis. 2004;43:607–616. doi: 10.1053/j.ajkd.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Majchrzak KM, Pupim LB, Flakoll PJ, et al. Resistance exercise augments the acute anabolic effects of intradialytic oral nutritional supplementation. Nephrol Dial Transplant. 2008;23:1362–1369. doi: 10.1093/ndt/gfm773. [DOI] [PubMed] [Google Scholar]
- 13.Kopple JD, Wang H, Casaburi R, et al. Exercise in maintenance hemodialysis patients induces transcriptional changes in genes favoring anabolic muscle. J Am Soc Nephrol. 2007;18:2975–2986. doi: 10.1681/ASN.2006070794. [DOI] [PubMed] [Google Scholar]
- 14.Cheema B, Abas H, Smith B, et al. Progressive exercise for anabolism in kidney disease (PEAK): a randomized, controlled trial of resistance training during hemodialysis. J Am Soc Nephrol. 2007;18:1594–1601. doi: 10.1681/ASN.2006121329. [DOI] [PubMed] [Google Scholar]
- 15.Cheema B, Abas H, Smith B, et al. Randomized controlled trial of intradialytic resistance training to target muscle wasting in ESRD: the Progressive Exercise for Anabolism in Kidney Disease (PEAK) study. Am J Kidney Dis. 2007;50:574–584. doi: 10.1053/j.ajkd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Johansen KL, Painter PL, Sakkas GK, et al. Effects of resistance exercise training and nandrolone decanoate on body composition and muscle function among patients who receive hemodialysis: a randomized, controlled trial. J Am Soc Nephrol. 2006;17:2307–2314. doi: 10.1681/ASN.2006010034. [DOI] [PubMed] [Google Scholar]
- 17.Castaneda C, Layne JE, Munoz-Orians L, et al. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341. doi: 10.2337/diacare.25.12.2335. [DOI] [PubMed] [Google Scholar]
- 18.Robertson RJ, Goss FL, Rutkowski J, et al. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med Sci Sports Exerc. 2003;35:333–341. doi: 10.1249/01.MSS.0000048831.15016.2A. [DOI] [PubMed] [Google Scholar]
- 19.Lagally KM, Robertson RJ. Construct validity of the OMNI resistance exercise scale. J Strength Cond Res. 2006;20:252–256. doi: 10.1519/R-17224.1. [DOI] [PubMed] [Google Scholar]
- 20.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 21.Roy MA, Doherty TJ. Reliability of hand-held dynamometry in assessment of knee extensor strength after hip fracture. Am J Phys Med Rehabil. 2004;83:813–818. doi: 10.1097/01.phm.0000143405.17932.78. [DOI] [PubMed] [Google Scholar]
- 22.Clasey JL, Hartman ML, Kanaley J, et al. Body composition by DEXA in older adults: accuracy and influence of scan mode. Med Sci Sports Exerc. 1997;29:560–567. doi: 10.1097/00005768-199704000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Ware J, Sherbourne C. The MOS 36-item short-form healthy survey (SF-36): conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 24.Ware J, Kosinski M, Keller S. The Health Institute. New England Medical Center, Boston; 1994. SF-36 Physical and Mental Health Summary Scales: a user’s manual. [Google Scholar]
- 25.Washburn RA, Smith KW, Jette AM, et al. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 26.Katz S, Ford AB, Moskowitz RW, et al. Studies of illness in the aged. The index of ADL: a standardized measure of biological and psychosocial function. JAMA. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 27.SAS . SAS User’s Guide: Proc Mixed. SAS Institute, Cary, NC; 2008. [Google Scholar]
- 28.Singer J, Willett J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 29.Studenski S, Perera S, Wallace D, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 30.Perera S, Mody SH, Woodman RC, et al. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Johansen KL, Shubert T, Doyle J, et al. Muscle atrophy in patients receiving hemodialysis: effects on muscle strength, muscle quality, and physical function. Kidney Int. 2003;63:291–297. doi: 10.1046/j.1523-1755.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 32.Johansen KL, Kaysen GA, Young BS, et al. Longitudinal study of nutritional status, body composition, and physical function in hemodialysis patients. Am J Clin Nutr. 2003;77:842–846. doi: 10.1093/ajcn/77.4.842. [DOI] [PubMed] [Google Scholar]
- 33.Putman CT, Xu X, Gillies E, et al. Effects of strength, endurance and combined training on myosin heavy chain content and fibre-type distribution in humans. Eur J Appl Physiol. 2004;92:376–384. doi: 10.1007/s00421-004-1104-7. [DOI] [PubMed] [Google Scholar]
- 34.Johansen KL, Chertow GM, Ng AV, et al. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.