Abstract
Background. Recent studies suggest that uric acid is a mediator of diabetic nephropathy. We hypothesized that elevated serum uric acid levels are a strong predictor of albuminuria in patients with type 1 diabetes.
Methods. We analyzed data from the Coronary Artery Calcification in Type 1 Diabetes study, a prospective observational study. A stepwise logistic regression model was applied to predict the development of micro- or macroalbuminuria after 6 years of follow-up in 324 participants who had no evidence of micro- or macroalbuminuria at baseline. A P-value <0.1 was used as the criteria for entry into and removal from the model.
Results. The following factors were selected in the stepwise multivariate model as predictors of micro- or macroalbuminuria at the 6-year follow-up visit: baseline serum uric acid levels, HbA1c and pre-albuminuria. For every 1-mg/dl increase in serum uric acid levels at baseline, there was an 80% increased risk of developing micro- or macroalbuminuria at 6 years (odds ratio 1.8; 95% confidence interval 1.2, 2.8; P = 0.005). Additional covariates considered in the stepwise model were sex, age, duration of diabetes, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment, waist circumference, waist/hip ratio, body mass index, systolic and diastolic blood pressure, smoking, serum creatinine, cystatin C, high-density lipoprotein cholesterol and triglycerides.
Conclusion. Elevated serum uric acid levels are a strong predictor of the development of albuminuria in patients with type 1 diabetes.
Keywords: type 1 diabetes, uric acid, albuminuria
Introduction
Diabetic nephropathy (DN) is a major cause of morbidity and mortality in patients with diabetes [1,2]. Approximately 5–10% of patients with diabetes have type 1 diabetes. While modern insulin therapy has lowered the rate of micro- and macrovascular complications in patients with type 1 diabetes, the long-term burden of diabetic complications persists in this patient population [3]. Few factors have been associated with the development of DN in patients with type 1 diabetes, including age, poor glycemic control reflected by an elevated HbA1c [4], hypertension [5] and smoking [2,6].
Over the last decade, uric acid has been re-introduced as a potential mediator of endothelial dysfunction and kidney disease [7]. Animal studies implicate uric acid as a cause of endothelial dysfunction and as a factor in the progression of kidney disease [8–10]. In patients with type 1 diabetes, high normal serum uric acid levels appear to be associated with impaired glomerular filtration rate (GFR) [11]. A recent study by Hovind et al. found that high serum uric acid levels predict the development of macroalbuminuria in patients with type 1 diabetes [12].
Microalbuminuria, in addition to being the hallmark for early detection of diabetic nephropathy, is a marker for endothelial dysfunction and a known risk factor for cardiovascular events and mortality in patients with diabetes [13]. We examined the relationship between baseline serum uric acid levels and the development of micro- or macroalbuminuria after 6 years of follow-up in participants of the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study.
Materials and methods
Study participants
The data presented in this report were collected as part of the baseline examination of the CACTI study [14]. The study enrolled 1416 individuals between 19 and 56 years of age, with no known history of coronary heart disease: 652 participants with type 1 diabetes and 764 control participants without diabetes. We limited our analysis to individuals with type 1 diabetes. Patients with type 1 diabetes were included if they had long-standing disease (mean duration 23 years, range 4–52 years), were insulin dependent within 1 year of diagnosis and were diagnosed prior to age 30 years or had positive antibodies or a clinical course consistent with type 1 diabetes. In order to assess whether baseline serum uric acid levels predict the development of micro- or macroalbuminuria at the 6-year visit, only patients with no evidence of micro- or macroalbuminuria at the baseline visit were included in the analysis (n = 455). Over 86% (n = 393) of these study participants completed a 6-year follow-up examination, and 324 of these participants had complete data on change in albuminuria status, uric acid and other covariates included in the stepwise logistic regression model, and were included in the final model.
Physical examination
Participants completed the baseline examination between March 2000 and April 2002. Anthropometric measurements were obtained, including height, weight, minimum waist circumference (measured at the smallest point between the tenth rib and the iliac crest, over bare skin, in duplicate) and hip circumference (measured at the maximum circumference of the buttocks, in duplicate). Body mass index (BMI) was calculated in kilograms per square meter. Resting systolic blood pressures and fifth phase diastolic blood pressures were measured three times while the subjects were seated, and the second and third measurements were averaged. Hypertension was defined as a blood pressure ≥140/90 mmHg or participant receiving current antihypertensive treatment. Participants completed standardized questionnaires that inquired about medical history, current medication, insulin doses, physical activity, alcohol and tobacco use, and family medical history.
Laboratory measurements
Serum uric acid levels were measured on stored baseline samples via the Clinical Analyzer utilizing a uricase-based commercial kit. These samples had been thawed twice in the past. The results were reported in milligrams per deciliter. Albumin excretion rate (AER) was used when available (n = 314) to define albuminuria status at the 6-year follow-up visit. AER was calculated from urinary albumin measured and averaged in two timed overnight urine samples. Normal AER was defined as <20 µg/min. Microalbuminuria was defined as an AER ≥20 µg/min and <200 µg/min, and macroalbuminuria was defined as an AER ≥200 µg/min [15]. Alternatively, if AER was not available at the 6-year follow-up visit (n = 10), we relied on albumin/creatinine ratio (ACR) to classify patients into the following groups according to the K/DOQI guidelines [15]: nonalbuminuria was defined as ACR <30 mg/g, microalbuminuria was defined as ACR was ≥30 mg/g and <300 mg/g, and if the ACR was ≥300 mg/g, then the subjects were considered to have macroalbuminuria. The validity of ACR in predicting diabetic kidney disease has been well documented, and it correlates well with estimates of microalbuminuria in 24-h urine collections [16,17]. Consistent with that, for the subjects who had both measurements available, AER and ACR were well correlated in our study population.
The following measurements were obtained following a 12-h fast: glucose, HbA1c, triglycerides and other lipids [total cholesterol and high-density lipoprotein (HDL) cholesterol were measured]. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald method [18]. Cystatin C was measured on stored serum samples in the clinical lab at University of Colorado Hospital in Denver, Colorado, using a commercially available particle-enhanced immunonephelometric assay (Dade-Behring) [19].
Statistical analysis
Variables were examined for normality, and non-normally distributed variables (triglycerides and urinary ACR) were log-transformed. The data are presented as arithmetic means and SDs for the continuous variables, utilizing geometric means and SD for the log-transformed variables. To evaluate serum uric acid levels as a predictor of the development of micro- or macroalbuminuria, stepwise multiple logistic regression analysis was performed with a P-value <0.1 as the criteria for entry and removal from the model. Potential covariates were included in the stepwise model based on their biological plausibility or evidence from the literature that they correlate with micro- or macroalbuminuria. The following variables were considered for entry into the model: sex, age, duration of diabetes, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment, waist circumference, waist/hip ratio, BMI, systolic and diastolic blood pressure, smoking, HbA1c, pre-albuminuria, serum creatinine, cystatin C, serum uric acid levels, HDL cholesterol and triglycerides. We included pre-albuminuria as a covariate in the analysis, as pre-albuminuria (defined as degree of proteinuria in the sub-albuminuric range with urinary ACR <30 mg/g) is reportedly associated with increased risk of cardiovascular events in the general population [20].
Informed consent
All study participants provided informed consent and the study protocol was approved by the Colorado Multiple Institutional Review Board.
Results
Baseline characteristics of the participants included in the analysis
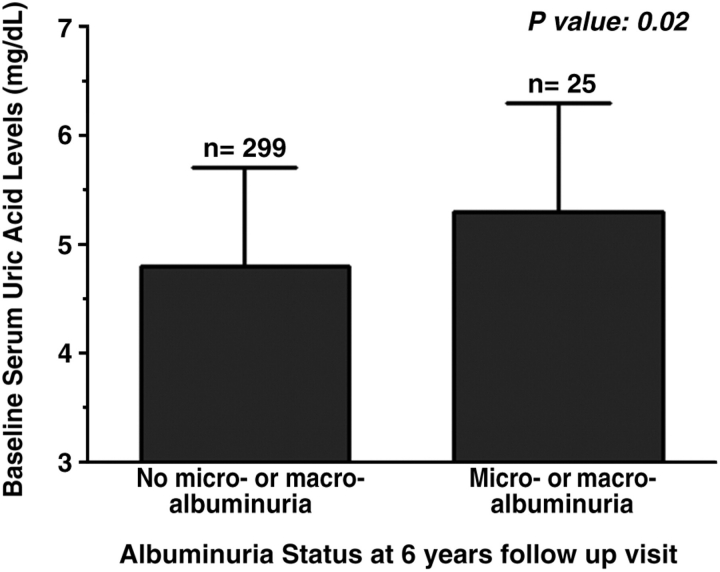
Forty-five percent of the participants were males; the average age was 37 years and the mean HbgA1C 7.7%. Of the 324 patients included in this analysis, 25 patients (8%) developed micro- or macroalbuminuria after 6 years. In Figure 1, we show the baseline serum uric acid levels for the participants with and without albuminuria at the 6-year follow-up visit. The patients with type 1 diabetes who developed micro- or macroalbuminuria had higher serum uric acid levels at baseline (5.3 ± 1.2 mg/dl) as compared to the patients who did not develop micro- or macroalbuminuria (4.8 ± 0.9 mg/dl, P-value 0.02). Table 1 illustrates the baseline characteristics for the study participants according to albuminuria status at the 6-year follow-up visit. Compared to the participants who did not develop micro- or macroalbuminuria, the patients who did develop micro- or macroalbuminuria after 6 years had higher baseline HbAIC levels, higher baseline serum triglyceride levels and higher baseline urinary ACR in the pre-albuminuric range.
Fig. 1.
Mean baseline serum uric acid levels according to albuminuria status at the 6-year follow-up visit.
Table 1.
Baseline characteristics of the participants with and without albuminuria at the 6-year follow-up visit
| Characteristic | No micro- or macroalbuminuria at 6-year follow-up visit (n = 299) | Micro- or macroalbuminuria at 6-year follow-up (n = 25) |
|---|---|---|
| Age (years) | 37 ± 9 | 37 ± 9 |
| Duration of type 1 diabetes (years) | 23 ± 9 | 22 ± 7 |
| ACE inhibitor or ARB (%) | 24 | 67 |
| Systolic blood pressure (mmHg) | 115 ± 13 | 117 ± 9 |
| Diastolic blood pressure (mmHg) | 76 ± 8 | 79 ± 7 |
| BMI (kg/m2) | 26 ± 4 | 28 ± 5 |
| Waist circumference (cm) | 84 ± 12 | 91 ± 16 |
| HbA1c (%) | 7.7 ± 1.2 | 8.5 ± 1.4* |
| Pre-albuminuria (ACR < 30 mg/g) | 5.1 ± 1.7 | 8.5 ± 2.0** |
| Serum creatinine (mg/dl) | 1.1 ± 0.2 | 1.2 ± 0.2 |
| Cystatin C (mg/l) | 0.76 ± 0.1 | 0.8 ± 0.1 |
| Total cholesterol (mg/dl) | 170 ± 32 | 173 ± 32 |
| LDL cholesterol (mg/dl) | 97 ± 28 | 98 ± 25 |
| Triglycerides (mg/dl) | 75 ± 2 | 101 ± 2 * |
| HDL cholesterol (mg/dl) | 57 ± 17 | 51 ± 16 |
ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; ACR, urinary albumin/creatinine ratio; LDL, low-density lipoprotein; HDL, high-density lipoprotein. Values above are presented as mean ± standard deviation or geometric mean (standard deviation). *P < 0.05, **P < 0.001.
Serum uric acid levels at baseline are a strong predictor of the development of albuminuria at 6 years
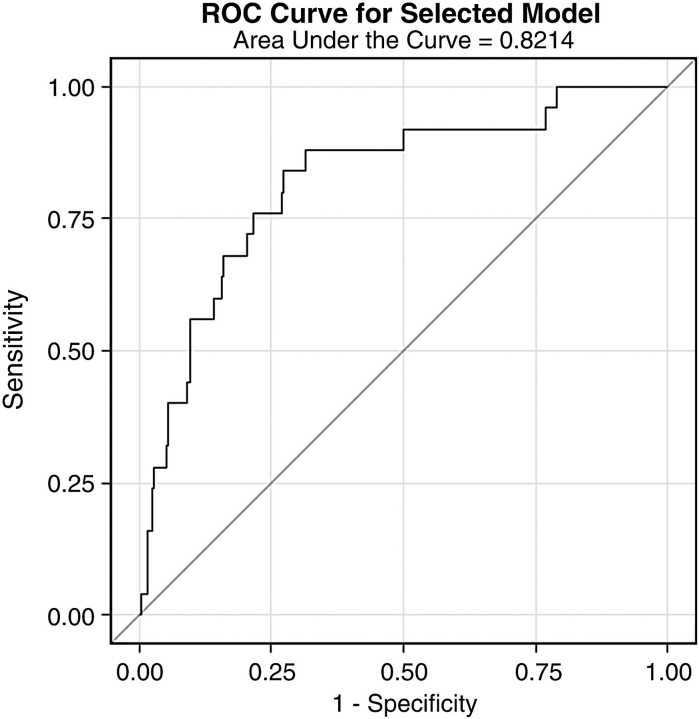
To assess if serum uric acid levels predict the development of micro- or macroalbuminuria over 6 years, we conducted a stepwise multivariate regression analysis including only the patients with no micro- or macroalbuminuria at baseline. As indicated in the Materials and methods section, factors that could potentially influence the development of albuminuria were evaluated for entry into the stepwise model, including: sex, age, duration of diabetes, angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker treatment, waist circumference, waist/hip ratio, BMI, systolic and diastolic blood pressure, smoking, in addition to HbA1c, pre-albuminuria, serum creatinine, cystatin C, serum uric acid levels, HDL cholesterol and triglycerides. Results of the stepwise multivariate model are shown in Table 2. The final model included serum uric acid levels, HbA1c and pre-albuminuria. For every 1-mg/dl increase in serum uric acid levels, there was an 80% increased risk of developing micro- or macroalbuminuria (odds ratio 1.8; 95% confidence interval 1.2, 2.8; P = 0.005). We show the receiver operating characteristic curve of the full model predicting micro- or macroalbuminuria in Figure 2. The three variables selected in the multivariate model (serum uric acid levels, HbA1C and pre-albuminuria) result in a c-statistic of 0.82, indicating that the applied model explains far more than expected by chance.
Table 2.
Comparison of the predictors of development of micro- or macroalbuminuria in patients with type 1 diabetes (n = 324)
| Characteristic | Adjusted odds ratio (95% CI) | P |
|---|---|---|
| HbA1c | 1.6a (1.1, 2.4) | 0.007 |
| Log baseline urinary ACR | 2.4a (1.6, 3.7) | <0.0001 |
| Baseline serum uric acid | 1.8a (1.2, 2.7) | 0.005 |
Factors considered for entry into the model: sex, age, duration of diabetes, ACE inhibitor or ARB treatment, waist circumference, waist/hip ratio, BMI, systolic and diastolic blood pressure, HbA1c, pre-albuminuria, serum creatinine, cystatin C, baseline serum uric acid levels, HDL cholesterol and triglycerides. Odds ratio and 95% confidence interval (CI) are per SD for log baseline urinary ACR (SD = 0.55), for baseline serum uric acid (SD = 1.0) and for baseline HbA1c (SD = 1.0).
Fig. 2.
Receiver operating characteristic (ROC) curve—the three variables selected in the multivariate model (serum uric acid levels, HbA1C and urinary ACR) result in a c-statistic = 0.82.
Discussion
In the present study, we explored the association between a potentially modifiable risk factor, serum uric acid levels and the development of either micro- or macroalbuminuria in a well-characterized cohort of patients with type 1 diabetes who had participated in the CACTI study over a 6-year follow-up period. Our results indicate that baseline serum uric acid levels are a strong predictor of the development of micro- or macroalbuminuria at 6 years, independent of HbA1c and of degree of pre-albuminuria, in patients with type 1 diabetes.
Few studies have explored the relationship between uric acid and diabetic kidney disease in patients with type 1 diabetes. A recent cross-sectional analysis by Rosolowsky et al. in patients with type 1 diabetes demonstrated that high normal serum uric acid levels were independently associated with lower GFR as estimated from the serum concentration of cystatin C [11]. In addition, a prospective observational study by Hovind et al. found baseline serum uric acid levels to be predictive macroalbuminuria over an 18-year follow-up period in 263 patients with type 1 diabetes [12]. Consistent with their results, our study demonstrates that serum uric acid levels are a powerful predictor of the future development of micro- or macroalbuminuria in patients with type 1 diabetes.
Controversy exists as to whether uric acid plays a pathological role in endothelial dysfunction and kidney disease in humans [21]. Uric acid is a potent antioxidant and when administered acutely may actually improve endothelial function [22–24]. Nevertheless, recent experimental evidence suggests that uric acid may induce oxidative stress once it enters cells, and as such it may be a mediator of disease. In animals, mild hyperuricemia induced by the administration of a uricase inhibitor results in endothelial dysfunction [8] and hypertension [9,10], both of which resolve once uric acid levels are lowered. In a recent study by Kosugi et al., allopurinol treatment of diabetic (db/db) mice significantly lowered uric acid levels, reduced albuminuria and ameliorated tubulointerstitial injury, suggesting a role for uric acid in diabetic nephropathy [25]. Mild hyperuricemia was further shown to induce renal microvascular disease independent of blood pressure, as a consequence of activation of the renin–angiotensin–aldosterone system [26], and by inhibition of intrarenal production of nitric oxide [27].
Our study has several limitations. First, albuminuria was our primary outcome and a clinically more relevant endpoint would have been end-stage renal disease or cardiovascular events and mortality; however, we are limited in sample size and duration of follow-up, and hence are not able to assess either outcome. Second, in the absence of reliable markers of early decline in GFR [28], we cannot exclude the possibility that the rise in serum uric acid is but a sensitive marker of early kidney disease. Nevertheless, this does not preclude it from being a potentially modifiable risk factor for cardiovascular and renal complications of diabetes. Finally, the higher baseline levels of serum uric acid in the patients who developed micro- or macroalbuminuria may have identified a group of individuals at greater risk of developing DN due to other manifestations of metabolic syndrome. Although examination of baseline characteristics showed no significant differences between both groups with regard to blood pressure and body weight, the CACTI study is an observational epidemiologic study and not an interventional one, so recommendations with regard to therapies aimed at actually lowering serum uric acid levels cannot be made based on these results.
In conclusion, our results indicate that serum uric acid levels are a strong predictor of micro- or macroalbuminuria in patients with type 1 diabetes. Measuring serum uric acid levels routinely may help identify a group of patients at higher risk of developing diabetic complications. Further studies are needed to confirm these findings and to examine the impact of lowering serum uric acid levels on diabetic complications in patients with type 1 diabetes.
Acknowledgments
Support for this study was provided by the National Institutes of Health grants R01 HL61753, R01 HL079611, DK-5121 and HL-68607, American Diabetes Association post-doctoral fellowship 7-09-CVD-06 (J.S.B.) and Diabetes Endocrinology Research Center Clinical Investigation Core P30 DK57516. The study was performed at the Adult General Clinical Research Center at the University of Colorado Denver Anschutz Medical Center supported by the NIH M01 RR000051, at the Barbara Davis Center for Childhood Diabetes in Denver and at the Renal Division, Department of Medicine at the University of Colorado Denver in Aurora, CO, USA.
Conflict of interest statement. R.J. is listed as an inventor on several patent applications on lowering uric acid as it relates to blood pressure and metabolic syndrome.
References
- 1. USRDS 2005 Annual Report. Atlas of End-Stage Renal Disease in the United States. National Institutes of Health, Bethesda, NIDDK, 2005.
- 2.Creager MA, Luscher TF, Cosentino F, et al. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation. 2003;108:1527–1532. doi: 10.1161/01.CIR.0000091257.27563.32. [DOI] [PubMed] [Google Scholar]
- 3.Krishnan S, Short KR. Prevalence and significance of cardiometabolic risk factors in children with type 1 diabetes. J Cardiometab Syndr. 2009;4:50–56. doi: 10.1111/j.1559-4572.2008.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkins BA, Ficociello LH, Ostrander BE, et al. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 5.Krolewski AS, Canessa M, Warram JH, et al. Predisposition to hypertension and susceptibility to renal disease in insulin-dependent diabetes mellitus. N Engl J Med. 1988;318:140–145. doi: 10.1056/NEJM198801213180303. [DOI] [PubMed] [Google Scholar]
- 6.Rossing P, Hougaard P, Parving HH. Risk factors for development of incipient and overt diabetic nephropathy in type 1 diabetic patients: a 10-year prospective observational study. Diabetes Care. 2002;25:859–864. doi: 10.2337/diacare.25.5.859. [DOI] [PubMed] [Google Scholar]
- 7.Kang DH, Nakagawa T, Feng L, et al. A role for uric acid in the progression of renal disease. J Am Soc Nephrol. 2002;13:2888–2897. doi: 10.1097/01.asn.0000034910.58454.fd. [DOI] [PubMed] [Google Scholar]
- 8.Khosla UM, Zharikov S, Finch JL, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005;67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 9.Mazzali M, Hughes J, Kim YG, et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Lozada LG, Tapia E, Soto V, et al. Treatment with the xanthine oxidase inhibitor febuxostat lowers uric acid and alleviates systemic and glomerular hypertension in experimental hyperuricaemia. Nephrol Dial Transplant. 2008;23:1179–1185. doi: 10.1093/ndt/gfm783. [DOI] [PubMed] [Google Scholar]
- 11.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovind P, Rossing P, Tarnow L, et al. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes—an inception cohort study. Diabetes. 2009;58:1668–1671. doi: 10.2337/db09-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman DJ, Mattock MB, Dawnay AB, et al. Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess. 2005;9:xiii–vi. doi: 10.3310/hta9300. xiii–163. [DOI] [PubMed] [Google Scholar]
- 14.Maahs DM, Kinney GL, Wadwa P, et al. Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diabetes Care. 2005;28:301–306. doi: 10.2337/diacare.28.2.301. [DOI] [PubMed] [Google Scholar]
- 15.KDOQI Clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 16.Zelmanovitz T, Gross JL, Oliveira JR, et al. The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy. Diabetes Care. 1997;20:516–519. doi: 10.2337/diacare.20.4.516. [DOI] [PubMed] [Google Scholar]
- 17.Nathan DM, Rosenbaum C, Protasowicki VD. Single-void urine samples can be used to estimate quantitative microalbuminuria. Diabetes Care. 1987;10:414–418. doi: 10.2337/diacare.10.4.414. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Maahs DM, Ogden LG, Kretowski A, et al. Serum cystatin C predicts progression of subclinical coronary atherosclerosis in individuals with type 1 diabetes. Diabetes. 2007;56:2774–2779. doi: 10.2337/db07-0539. [DOI] [PubMed] [Google Scholar]
- 20.Gerstein HC, Mann JF, Yi Q, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 21.Johnson RJ, Kang DH, Feig D, et al. Is there a pathogenetic role for uric acid in hypertension and cardiovascular and renal disease? Hypertension. 2003;41:1183–1190. doi: 10.1161/01.HYP.0000069700.62727.C5. [DOI] [PubMed] [Google Scholar]
- 22.Waring WS, McKnight JA, Webb DJ, et al. Uric acid restores endothelial function in patients with type 1 diabetes and regular smokers. Diabetes. 2006;55:3127–3132. doi: 10.2337/db06-0283. [DOI] [PubMed] [Google Scholar]
- 23.Scott GS, Cuzzocrea S, Genovese T, et al. Uric acid protects against secondary damage after spinal cord injury. Proc Natl Acad Sci U S A. 2005;102:3483–3488. doi: 10.1073/pnas.0500307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waring WS, Adwani SH, Breukels O, et al. Hyperuricaemia does not impair cardiovascular function in healthy adults. Heart. 2004;90:155–159. doi: 10.1136/hrt.2003.016121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kosugi T, Nakayama T, Heinig M, et al. The effect of lowering uric acid on renal disease in the type 2 diabetic db/db mice. Am J Physiol Renal Physiol. 2009;297:F481–F488. doi: 10.1152/ajprenal.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzali M, Kanellis J, Han L, et al. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 27.Mazzali M, Kim YG, Suga S, et al. Hyperuricemia exacerbates chronic cyclosporine nephropathy. Transplantation. 2001;71:900–905. doi: 10.1097/00007890-200104150-00014. [DOI] [PubMed] [Google Scholar]
- 28.Bostom AG, Kronenberg F, Ritz E. Predictive performance of renal function equations for patients with chronic kidney disease and normal serum creatinine levels. J Am Soc Nephrol. 2002;13:2140–2144. doi: 10.1097/01.asn.0000022011.35035.f3. [DOI] [PubMed] [Google Scholar]