Over the last hundred years, the diagnosis of hypertension has rested upon the indirect measurement of blood pressure (BP) through the auscultation of Korotkoff sounds. Among patients on haemodialysis, BP measurement is particularly important because disparate outcomes are obtained depending on the timing, location, frequency and technique of measurement of BP [1]. This disparity of outcomes has profound implications for the management of hypertension especially among haemodialysis patients. Why home BP monitoring should become the standard of care among patients on haemodialysis is the subject of this review.
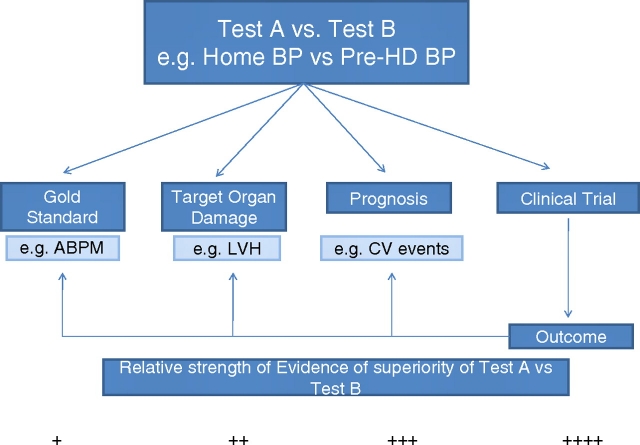
To compare tests, such as one that tests home BP to pre-dialysis BP, a diagnostic test study must be performed. A diagnostic test study can have one of the following four paradigms (Figure 1):
Test A (e.g. home BP) is compared to test B (e.g. pre-dialysis BP) using a ‘gold-standard’ or reference test. If test A performs better than test B, then test A is preferred. Whether test A should be favoured over test B depends on a variety of considerations such as its cost, practicality, invasiveness and acceptability.
The two tests may be compared not to a reference standard but to some intermediate end point. A valid intermediate end point among hypertensive patients is the presence of target organ damage such as left ventricular hypertrophy. In other words, home BP can be compared to pre- or post-dialysis BP and the results compared in their ability to predict echocardiographic left ventricular hypertrophy. If home BP measurement is more strongly related to target organ damage then, compared to paradigm 1, it provides a higher level of evidence that it is superior to pre-dialysis or post-dialysis BP.
The two tests can be compared with respect to prognosis, for example, all-cause mortality. For example, with respect to outcomes such as all-cause mortality, dialysis unit BP measurements can be compared to home BP measurements. If home BP measurement is more strongly related to all-cause mortality then, compared to paradigm 2, it provides even a higher level of evidence that it is superior to pre-dialysis or post-dialysis BP.
Finally, a randomized controlled trial can be performed to assess the value of a diagnostic test. For example, management of the patient based on home BP monitoring vs dialysis unit BP measurements can be compared in a randomized trial. If the outcomes are better with home BP monitoring, then home BP monitoring would be said to be superior. The outcomes can be one of three outcomes: the reference test, in this case ambulatory BP, regression of left ventricular hypertrophy or improvement in all-cause mortality. This paradigm would provide the highest level of evidence of the superiority of home BP recordings over dialysis unit BP recordings.
Fig. 1.
Relative strength of evidence of superiority of test A vs test B.
This review will focus on the data which support the use of out-of-office BP monitoring among patients on haemodialysis. The use of out-of-office BP monitoring among patients with chronic kidney disease who are not on haemodialysis is discussed elsewhere [2].
Comparison of home BP recordings with reference tests
Paradigm 1: Ambulatory BP monitoring
The feasibility of home BP monitoring among haemodialysis patients was reported in just 20 patients more than a decade ago [3]; substantial refinements have been made since then. Agarwal and Lewis compared pre-dialysis and post-dialysis BP measurements to ambulatory BPs [4]. The area under the curve of the receiver operating characteristic (ROC) curve, though acceptable, was not good enough for clinical decision making. There was no threshold at which there was sufficient sensitivity and specificity to diagnose ambulatory hypertension. Furthermore, they found wide agreement limits between pre- and post-BP measurement as compared to ambulatory BP. Accordingly, they concluded that pre- and post-dialysis BP measurements can be useful in a qualitative sense but are not useful quantitatively. These results were subsequently supported by a meta-analysis that demonstrated that pre- and post-dialysis BP measurements are inadequate surrogates of ambulatory BPs [5]. The pre-dialysis BP measurement, in this meta-analysis, overestimated ambulatory BP by over 16.7 mm Hg. However, the agreement limits were wide. Even for post-dialysis BP, a measurement which was less biased compared to pre-dialysis measurements, the agreement limits were wide. Accordingly, pre- and post-dialysis BP measurements were not held to be valid surrogates of ambulatory BPs at the patient level.
BP recorded in the above studies was not measured using any specified technique; these were ‘routine’ BP recordings. Rahman et al. have reported that BP measured using routine methods can be quite different compared to those obtained by using a proper technique [6]. In 55% of patients, the post-dialysis systolic BP measured in the dialysis unit was at least 10 mm Hg higher than the standard reading. Thus, routine and standardized readings could not be used interchangeably. To address these issues, in a subsequent study, Agarwal et al. measured BP using a standardized technique wherein BPs were recorded in triplicate before and after dialysis for six consecutive dialysis treatments [7]. They then compared pre- and post-dialysis BP measurements by both routine and standardized methods and home BP measurement to the reference standard of ambulatory BP monitoring. The area under the curve of the ROC curve for home BP was 0.89. Thus, if home BP monitoring was used to make clinical decisions regarding the presence or absence of hypertension in this unselected population of haemodialysis patients, the correct diagnosis would be reached 89% of the time. The threshold of 150 mm Hg systolic had the optimal sensitivity and specificity for diagnosed hypertension. Sensitivity at this threshold was 80% and specificity 84%. However, pre- and post-dialysis BP measurements regardless of routine or standardized measurement methods did not share this optimal combination of sensitivity and specificity. Thus, a threshold BP at which there would be an acceptable classification of patients into normotensive and hypertensive groups was not achieved using dialysis unit BP recordings. Accordingly, even averaged standardized BP measurement compared to home BP recordings does not share an adequate combination of sensitivity and specificity to be useful for diagnosing hypertension.
Rohrscheib et al. have recently reported that the variability of BP recorded before and after dialysis between patients is as much as the variability of BP within patients [8]. Their results from a US dialysis chain were based on a large number of recordings. Despite these large numbers of measurements, the intra-class correlation coefficients of pre-dialysis or post-dialysis BPs were not deemed clinically useful to make diagnostic decisions.
Paradigm 2: Target organ damage
Agarwal et al. reported the value of pre-dialysis, post-dialysis, home BP and ambulatory BP measurement in diagnosing left ventricular hypertrophy [9]. Left ventricular hypertrophy was taken as evidence of target organ damage among hypertensive haemodialysis patients. They found that home and ambulatory BPs were equally good in predicting left ventricular hypertrophy. Out-of-dialysis unit BP measurements such as pre- or post-dialysis BPs even when obtained using a standardized BP measurement technique were not useful in detecting the presence of left ventricular hypertrophy. Reports from Japan using weekly average BPs, using a combination of home BP measurements as well as dialysis unit BP measurements, suggest that the weekly averages have a stronger relationship with left ventricular hypertrophy and pulse wave velocity [10]. Thus, when target organ damage is used as an outcome variable, these results underscore the superiority of home BP measurements over dialysis unit BP.
Paradigm 3: Prognosis
Alborzi et al. compared pre-dialysis, post-dialysis BPs, home BPs and ambulatory BPs in predicting all-cause and cardiovascular mortality among 150 patients who were followed for a median of 2 years [11]. They found that ambulatory BPs had the best relationship between the level of systolic BP and outcomes. Home BPs were next most useful in predicting all-cause mortality. A dose–response relationship between increasing quartiles of both home BP and ambulatory BP and all-cause mortality and cardiovascular mortality was seen. However, pre- or post-dialysis BP measurements were not useful in predicting all-cause or cardiovascular mortality.
Subsequently, Moriya et al. reported that among haemodialysis patients single measurements of pre-dialysis BP recording were insufficient to predict cardiovascular events or all-cause mortality [12]. However, weekly averaged BP was noted to be an independent prognostic marker.
Agarwal, in a recent study of 326 haemodialysis patients followed for up to 7 years, reported that only home and ambulatory BPs were useful for predicting all-cause mortality [13]. A dose–response relationship between systolic BP and mortality was seen. Pre- and post-dialysis BP measurements were not useful in predicting outcomes. A complex relationship between BP and outcomes emerged. At very low BPs, <110 for ambulatory and <120 for home, there was an increase in mortality noted. Mortality was lowest when home systolic BP was between 120 and 130 mm Hg and ambulatory systolic BP was between 110 and 120 mm Hg. A limitation of this study was that the outcomes were defined by single sessions of home and ambulatory BP recordings.
Paradigm 4: Clinical trials
Kauric-Klein and Artinian randomized 17 chronic haemodialysis patients to receive home BP monitor intervention in addition to usual care, whereas an additional 17 participants were randomized to usual care without home BP monitoring [14]. Patients randomized to the home BP group had significant reductions in systolic average weekly home BP.
A recent study by da Silva et al. randomly assigned BP management based on pre-dialysis BP measurement or home BP measurement [15]. The authors reported that at 6-months BPs, as measured by ambulatory, were better when management of hypertension was guided by home BP monitoring. Although left ventricular hypertrophy regression was not seen, this may have been due to the small sample size.
At least two studies have reported that BP changes among dialysis patients participating in randomized trials can be detected using home BP recordings. For example, in the dry-weight reduction in hypertensive haemodialysis patients (DRIP) study [16], home BP could detect the fall in BP as well as ambulatory BPs [17]. Furthermore, home BP measurements were not biased unlike pre-dialysis or post-dialysis measurements. The agreement between home BPs and ambulatory BPs was the tightest. In the second study, which was a randomized, controlled trial tested the notion that the automatic feedback system of dialysis will improve hypertension control compared with standard dialysis [18]. Over the 6-month duration of the trial, the primary end point was assessed using BP measured at home. The intervention groups had a drop in systolic BP from 147.8 to 139.8 mm Hg and the control group from 141.9 to 135.2 mm Hg (P = 0.005 for change from baseline). Despite a limited number of patients, a remarkable improvement in systolic BP was evident with the use of home BP monitoring.
Are intradialytic BP recordings of any value?
Agarwal et al. reported that if median intradialytic BP was obtained during a midweek dialysis it could, in an unbiased way, detect ambulatory BP at a population level [19]. However, the agreement limits between median ambulatory BP and ambulatory BP were sufficiently wide to preclude individual decision making. In a subsequent study in the DRIP trial participants, they reported again that median intradialytic BP could detect the change in ambulatory BP. Although it appears that median intradialytic BP over the midweek dialysis is better than pre- or post-dialysis BP recordings, it is not a substitute for ambulatory (or home) BP measurements.
Are oscillometric measurements valid?
Semret et al. measured BP using oscillometric and auscultated techniques among haemodialysis patients [20]. They found that the oscillometric measurement was accurate for systolic BP but underestimated the auscultated diastolic BP. Systolic BP measured simultaneously by digitized sound and pressures agreed closely for systolic pressure (grade A, British Hypertension Society protocol) but not for diastolic pressure (grade C). More recently, Czarkowski et al., evaluating the same oscillometric monitor, reported that although the monitor met accuracy requirements for systolic BP it failed on diastolic BP [21]. Similar results were reported by Thompson et al. [22]. Furthermore, Thompson et al. reported that the magnitude of error between auscultated and oscillometric diastolic BP was related to the magnitude of arterial stiffness. Peixoto et al. have validated the use of an oscillometric ambulatory BP monitor (Spacelabs 90207) among haemodialysis patients [23]. Although the device received a passing grade C for systolic BP and grade B for diastolic BP, the authors noted that the device underestimated systolic BP in higher ranges and overestimated BP in lower ranges. Importantly, even the presence of non-functioning arteriovenous grafts and fistulas in the ipsilateral arm did not alter these results significantly.
How frequently and when should home BP measurements be recorded?
Patients on haemodialysis have a linear increase in BP over two dialysis treatments. After 48 h, this relationship between BP and time flattens. Accordingly, Agarwal and Light reported that measurement of BP three times a day would capture the linear response of BP to time [24]. In a post hoc study, they proposed that BP measurements if made after a midweek dialysis twice a day for 4 days would be sufficient to detect the presence of left ventricular hypertrophy and outcomes in these patients [25].
Beyond averages: Other applications of ambulatory BP monitoring
Frequent measurements of BP over the interdialytic interval can yield averages that are reproducible [26]. Frequent measurements of BP also yield discernable patterns of BPs that can be analysed. These patterns have been traditionally analysed using the dichotomous definition of dipping and non-dipping [27,28]. Dipping is said to be present when the systolic BP declines by >10% and is associated with more target organ damage and adverse outcomes [29,30]. However, more sophisticated, albeit more complex, recognition of patterns is possible using circular statistics. Statistically, using the trended cosinor model, these patterns can be described by an intercept, slope, amplitude and a phase [31]. The phase at the time of maximal BP is called the acrophase and the time of minimum BP is called the bathyphase. The significance of these patterns is discussed further.
The intercept BP is related to the number of medications; the greater the number of medications the higher the intercept [31]. This probably indicates confounding by indication. Those patients who have the highest BPs are exposed to the highest number of medications. The intercept is also associated with increased aortic stiffness [32]. The aortic stiffness measured by aortic pulse wave velocity was elevated in patients who had highest BPs [32]. With improvement in dry weight, the intercept BP was reduced which suggests that the intercept BP can be modulated by changes in volume state [33].
The slope of the BP is also related to the volume state [33]. In patients who had dry-weight reduction, steeper slopes were seen. Conversely, blunted slopes may indicate volume excess. Indeed, a higher number of medications are associated with blunter slopes indicating that latent volume overload may be treated with medications [31]. High interdialytic weight gain may also lead to steeper slopes [32]. Again, sodium restriction may potentially reduce the slopes and therefore improve overall BP.
The phase and amplitude of BP variation may indicate the phenomena of dipping. Dipping was not restored in the DRIP trial by proving dry weight indicating that dipping may be related to factors other than volume [28–33].
Beyond BP: Measurement of arterial mechanics
Structural and functional alterations of the conduit blood vessels are thought to contribute to the morbidity and mortality among haemodialysis patients, and various techniques have been applied to detect these alterations [34]. Although augmentation index is useful to assess pulse reflection, the aortic to femoral pulse wave velocity is held to be the reference standard for the measurement of arterial stiffness. The mechanical properties of the aorta are profoundly altered even among children on haemodialysis [35]. But despite the high prevalence of arterial stiffness, not all studies find that it is related to outcomes [36]. Perhaps the serial measurement of arterial mechanics can serve as an important prognostic tool to assess the effectiveness of anti-hypertensive therapy among hypertensive haemodialysis patients [37].
Uncertainties
There are several uncertainties that exist in the assessment of hypertension. Davenport et al. reported that there was an increased frequency of intradialytic hypotension when dialysis units in London tried to achieve the guideline recommended goals using pre- and post-dialysis BP [38]. Whether home BP guided therapies will reduce these episodes of intradialytic hypotension is unclear. No study has definitively shown whether BP reduction is causally associated with an improvement in cardiovascular or mortal outcomes among dialysis patients. Although two recent meta-analyses indicate that anti-hypertensive therapies are useful, no single trial has proven this [39,40]. Furthermore, it is unclear as to what the targets are for BP lowering. A large simple trial that explores the hypothesis whether BP should be lowered among hypertensive haemodialysis patients needs to be conducted. Such a trial would explore different levels of BP control. BP control would be guided not by pre-dialysis and post-dialysis measurements but by home BP monitoring. Without such a trial, despite the two meta-analyses, we will not have robust data to lower BP among hypertensive haemodialysis patients [41]. In the absence of randomized controlled trial data, from observational studies, it appears that home BP-guided management of anti-hypertensive drug and non-drug therapy in the 120–140 mm Hg range may be useful.
It is also not entirely clear why out-of-dialysis unit BP measurements have greater prognostic significance. Several reasons are possible why ambulatory or home BP may be superior to dialysis unit recordings which include the following: greater number of measurements, sampling over a wide variety of volume and uraemic states, sampling over periods of rest and activity and possibly a better measurement technique.
Conclusion
Despite uncertainties, the American Heart Association [42] and European Society of Hypertension [43] both recommend that in all patients with hypertension home BP monitoring should be performed. The studies discussed above suggest that home BP monitoring can be successfully used to make management decisions among haemodialysis patients. National health organizations and insurance companies should pay for the equipment, training and time required for home BP monitoring among haemodialysis patients. These measurements would allow the detection of volume overload and hypertension that has the potential to translate to better outcomes in these vulnerable patients. The time to act is now!
Conflict of interest statement. None declared.
Acknowledgments
This study is dedicated to the memory of Dr Thomas G. Pickering, a leading hypertension expert of our times, who arguably was among the most ardent supporters of home BP monitoring. This study was supported by National Institutes of Health (2RO1-062030-06).
References
- 1.Agarwal R. Hypertension and survival in chronic hemodialysis patients-past lessons and future opportunities. Kidney Int. 2005;67:1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 2.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Out-of-office blood pressure monitoring in chronic kidney disease. Blood Press Monit. 2009;14:2–11. doi: 10.1097/MBP.0b013e3283262f58. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R. Role of home blood pressure monitoring in hemodialysis patients. Am J Kidney Dis. 1999;33:682–687. doi: 10.1016/s0272-6386(99)70219-2. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Lewis RR. Prediction of hypertension in chronic hemodialysis patients. Kidney Int. 2001;60:1982–1989. doi: 10.1046/j.1523-1755.2001.00997.x. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre and post dialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 6.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT, Jr, Smith MC. A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis. 2002;39:1226–1230. doi: 10.1053/ajkd.2002.33395. [DOI] [PubMed] [Google Scholar]
- 7.Agarwal R, Andersen MJ, Bishu K, Saha C. Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int. 2006;69:900–906. doi: 10.1038/sj.ki.5000145. [DOI] [PubMed] [Google Scholar]
- 8.Rohrscheib MR, Myers OB, Servilla KS, et al. Age-related blood pressure patterns and blood pressure variability among hemodialysis patients. Clin J Am Soc Nephrol. 2008;3:1407–1414. doi: 10.2215/CJN.00110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 10.Moriya H, Ohtake T, Kobayashi S. Aortic stiffness, left ventricular hypertrophy and weekly averaged blood pressure (WAB) in patients on haemodialysis. Nephrol Dial Transplant. 2007;22:1198–1204. doi: 10.1093/ndt/gfl732. [DOI] [PubMed] [Google Scholar]
- 11.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 12.Moriya H, Oka M, Maesato K, et al. Weekly averaged blood pressure is more important than a single-point blood pressure measurement in the risk stratification of dialysis patients. Clin J Am Soc Nephrol. 2008;3:416–422. doi: 10.2215/CJN.03490807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauric-Klein Z, Artinian N. Improving blood pressure control in hypertensive hemodialysis patients. CANNT J. 2007;17:24–26. [PubMed] [Google Scholar]
- 15.da Silva GV, de Barros S, Abensur H, Ortega K, Mion D., Jr Home blood pressure monitoring in blood pressure control among haemodialysis patients: an open randomized clinical trial. Nephrol Dial Transplant. 2009;24:3805–3811. doi: 10.1093/ndt/gfp332. [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Alborzi P, Satyan S, Light RP. Dry-weight reduction in hypertensive hemodialysis patients (DRIP): a randomized, controlled trial. Hypertension. 2009;53:500–507. doi: 10.1161/HYPERTENSIONAHA.108.125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal R, Satyan S, Alborzi P, et al. Home blood pressure measurements for managing hypertension in hemodialysis patients. Am J Nephrol. 2009;30:126–134. doi: 10.1159/000206698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deziel C, Bouchard J, Zellweger M, Madore F. Impact of hemocontrol on hypertension, nursing interventions, and quality of life: a randomized, controlled trial. Clin J Am Soc Nephrol. 2007;2:661–668. doi: 10.2215/CJN.04171206. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal R, Metiku T, Tegegne GG, et al. Diagnosing hypertension by intradialytic blood pressure recordings. Clin J Am Soc Nephrol. 2008;3:1364–1372. doi: 10.2215/CJN.01510308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Semret M, Zidehsarai M, Agarwal R. Accuracy of oscillometric blood pressure monitoring with concurrent auscultatory blood pressure in hemodialysis patients. Blood Press Monit. 2005;10:249–255. doi: 10.1097/01.mbp.0000172713.28029.84. [DOI] [PubMed] [Google Scholar]
- 21.Czarkowski M, Staszkow M, Kostyra K, Shebani Z, Niemczyk S, Matuszkiewicz-Rowinska J. Determining the accuracy of blood pressure measurement by the Omron HEM-907 before and after hemodialysis. Blood Press Monit. 2009;14:232–238. doi: 10.1097/mbp.0b013e328331d5b5. [DOI] [PubMed] [Google Scholar]
- 22.Thompson AM, Eguchi K, Reznik ME, Shah SS, Pickering TG. Validation of an oscillometric home blood pressure monitor in an end-stage renal disease population and the effect of arterial stiffness on its accuracy. Blood Press Monit. 2007;12:227–232. doi: 10.1097/MBP.0b013e328108f544. [DOI] [PubMed] [Google Scholar]
- 23.Peixoto AJ, Gray TA, Crowley ST. Validation of the SpaceLabs 90207 ambulatory blood pressure device for hemodialysis patients. Blood Press Monit. 1999;4:217–221. [PubMed] [Google Scholar]
- 24.Agarwal R, Light RP. Chronobiology of arterial hypertension in hemodialysis patients: implications for home blood pressure monitoring. Am J Kidney Dis. 2009;54:693–701. doi: 10.1053/j.ajkd.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal R, Andersen MJ, Light RP. Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol. 2007;28:210–217. doi: 10.1159/000110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peixoto AJ, Santos SF, Mendes RB, et al. Reproducibility of ambulatory blood pressure monitoring in hemodialysis patients. Am J Kidney Dis. 2000;36:983–990. doi: 10.1053/ajkd.2000.19100. [DOI] [PubMed] [Google Scholar]
- 27.Covic A, Haydar AA, Goldsmith D. Ambulatory blood pressure monitoring in hemodialysis patients: a critique and literature review. Semin Dial. 2004;17:255–259. doi: 10.1111/j.0894-0959.2004.17322.x. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal R. Regulation of circadian blood pressure: from mice to astronauts. Curr Opin Nephrol Hypertens. 2010;19:51–58. doi: 10.1097/MNH.0b013e3283336ddb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covic A, Goldsmith DJ, Sambrook P, Venning MC, Ackrill P. Analysis of blood pressure variability derived from ambulatory blood pressure monitoring in 92 uraemic patients. Contrib Nephrol. 1996;119:157–160. doi: 10.1159/000425467. [DOI] [PubMed] [Google Scholar]
- 30.Covic A, Goldsmith DJ, Covic M. Reduced blood pressure diurnal variability as a risk factor for progressive left ventricular dilatation in hemodialysis patients. Am J Kidney Dis. 2000;35:617–623. doi: 10.1016/s0272-6386(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 31.Kelley K, Light RP, Agarwal R. Trended cosinor change model for analyzing hemodynamic rhythm patterns in hemodialysis patients. Hypertension. 2007;50:143–150. doi: 10.1161/HYPERTENSIONAHA.107.091579. [DOI] [PubMed] [Google Scholar]
- 32.Agarwal R, Light RP. Arterial stiffness and interdialytic weight gain influence ambulatory blood pressure patterns in hemodialysis patients. Am J Physiol Renal Physiol. 2007;294:F303–F308. doi: 10.1152/ajprenal.00575.2007. [DOI] [PubMed] [Google Scholar]
- 33.Agarwal R. Volume-associated ambulatory blood pressure patterns in hemodialysis patients. Hypertension. 2009;54:241–247. doi: 10.1161/HYPERTENSIONAHA.109.136366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Covic A, Gusbeth-Tatomir P, Goldsmith DJ. Arterial stiffness in renal patients: an update. Am J Kidney Dis. 2005;45:965–977. doi: 10.1053/j.ajkd.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 35.Covic A, Mardare N, Gusbeth-Tatomir P, et al. Increased arterial stiffness in children on haemodialysis. Nephrol Dial Transplant. 2006;21:729–735. doi: 10.1093/ndt/gfi196. [DOI] [PubMed] [Google Scholar]
- 36.Covic A, Mardare N, Gusbeth-Tatomir P, Prisada O, Sascau R, Goldsmith DJ. Arterial wave reflections and mortality in haemodialysis patients–only relevant in elderly, cardiovascularly compromised? Nephrol Dial Transplant. 2006;21:2859–2866. doi: 10.1093/ndt/gfl307. [DOI] [PubMed] [Google Scholar]
- 37.Agarwal R. Antihypertensive agents and arterial stiffness: relevance to reducing cardiovascular risk in the chronic kidney disease patient. Curr Opin Nephrol Hypertens. 2007;16:409–415. doi: 10.1097/MNH.0b013e3282063b86. [DOI] [PubMed] [Google Scholar]
- 38.Davenport A, Cox C, Thuraisingham R. Achieving blood pressure targets during dialysis improves control but increases intradialytic hypotension. Kidney Int. 2007;73:759–764. doi: 10.1038/sj.ki.5002745. [DOI] [PubMed] [Google Scholar]
- 39.Heerspink HJ, Ninomiya T, Zoungas S, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldsmith D, Covic A. Blood pressure control in CKD stage 5D patients–are we more or less certain what to do in 2009? Nephrol Dial Transplant. 2009;24:3597–3601. doi: 10.1093/ndt/gfp562. [DOI] [PubMed] [Google Scholar]
- 42.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parati G, Stergiou GS, Asmar R, et al. European Society of Hypertension guidelines for blood pressure monitoring at home: a summary report of the Second International Consensus Conference on Home Blood Pressure Monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]