Abstract
Background. In Fabry nephropathy, alpha-galactosidase deficiency leads to accumulation of glycosphingolipids in all kidney cell types, proteinuria and progressive loss of kidney function.
Methods. An international working group of nephrologists from 11 Fabry centres identified adult Fabry patients, and pathologists scored histologic changes on renal biopsies. A standardized scoring system was developed with a modified Delphi technique assessing 59 Fabry nephropathy cases. Each case was scored independently of clinical information by at least three pathologists with an average final score reported.
Results. We assessed 35 males (mean age 36.4 years) and 24 females (43.9 years) who mostly had clinically mild Fabry nephropathy. The average serum creatinine was 1.3 mg/dl (114.9 μmol/l); estimated glomerular filtration rate was 81.7 ml/min/1.73 m2 and urine protein to creatinine ratio was 1.08 g/g (122.0 mg/mmol). Males had greater podocyte vacuolization on light microscopy (mean score) and glycosphingolipid inclusions on semi-thin sections than females. Males also had significantly more proximal tubule, peritubular capillary and vascular intimal inclusions. Arteriolar hyalinosis was similar, but females had significantly more arterial hyalinosis. Chronic kidney disease stage correlated with arterial and glomerular sclerosis scores. Significant changes, including segmental and global sclerosis, and interstitial fibrosis were seen even in patients with stage 1–2 chronic kidney disease with minimal proteinuria.
Conclusions. The development of a standardized scoring system of both disease-specific lesions, i.e. lipid deposition related, and general lesions of progression, i.e. fibrosis and sclerosis, showed a spectrum of histologic appearances even in early clinical stage of Fabry nephropathy. These findings support the role of kidney biopsy in the baseline evaluation of Fabry nephropathy, even with mild clinical disease. The scoring system will be useful for longitudinal assessment of prognosis and responses to therapy for Fabry nephropathy.
Keywords: chronic kidney disease, Fabry disease, pathology, sclerosis, scoring
Introduction
Fabry disease is an X-linked genetic disorder with cellular accumulation of globotriosylceramides (GL-3) due to lysosomal alpha-galactosidase enzyme activity deficiency [1,2]. Males classically develop chronic kidney disease (CKD) and progress to end-stage renal disease (ESRD) before their fifth decade [1–3]. Enzyme replacement therapy (ERT) reduces GL-3 deposits [4–6] and slows progression of Fabry nephropathy [7].
Females have variable disease severity [1]; they can be asymptomatic, but most of them develop clinically apparent disease [8,9] and risk premature death [10]. Proteinuria and/or reduced glomerular filtration rate (GFR) may be found in 40% of adult females [8,11], and some are severely affected, reaching ESRD at the same median age as males [3,11].
Renal intracellular GL-3 deposits are present, even in patients with normal GFR and minimal proteinuria [12,13]. Vacuolization of podocytes and epithelial cells is a characteristic histologic finding [12,14–16]. Mesangial expansion, segmental and global glomerulosclerosis, tubular atrophy and interstitial fibrosis are also seen, even in early stages of the disease [12–17]. The original descriptions of Fabry nephropathy were in males [16]. Females can develop the same kidney histopathology as males [12,16,18], but fewer female cases have been described [12,15,16, 18–20].
We systematically scored histologic lesions in kidney biopsies in a set of 35 adult males and 24 adult females with Fabry disease, the majority with clinically mild nephropathy. In addition, a morphometric index of chronic damage was obtained for each biopsy [21]. A validated scoring system was developed, with the long-term goal to determine whether baseline histologic information can be related to the rate of progression and/or response to ERT in Fabry nephropathy.
Subjects and methods
The International Study Group of Fabry Nephropathy (ISGFN) was formed by pathologist and nephrologist pairs, from 10 different major referral centres for Fabry disease, and by a central nephropathology site (Dr A.B. Fogo, Nashville, TN, USA). The central site coordinated the collection, distribution and circulation of available biopsies among the participating centres and data entry into a computerized database. Kidney biopsies were from white adults with the diagnosis of Fabry disease, as confirmed by mutational analysis of the alpha-galactosidase gene.
Clinical assessments
Demographic and clinical data were abstracted from the clinical records closest to the time of the biopsy. Biopsies were performed between January 1999 and September 2006. GFR was estimated (eGFR) with the Modification of Diet in Renal Disease simplified equation [22]. Urine protein values were expressed as urine protein/creatinine ratios (UPCR). When only 24-h protein measurements were available, daily creatinine excretions were estimated as the product of eGFR and serum creatinine.
Histological scoring
General approach.
Disease-specific GL-3 storage-related and nonspecific histologic features of Fabry nephropathy were included in the score sheet (see Appendix for details). The scoring matrix was developed and refined in several round-robin slide reviews and face-to-face scoring sessions, using a modified Delphi technique [23].
Biopsies were coded and the exact tissue sections to be scored were marked on the slides at the central site, and slides were distributed on a blinded basis for review. A subset of 20 representative biopsies was initially circulated to all centres for training and scoring validation. Subsequent scoring was done by three centres for each case.
Interstitial fibrosis and index of chronic damage.
In addition to the semi-quantitative histologic assessment on interstitial fibrosis, the index of chronic damage was morphometrically determined for each biopsy.
Statistical analysis
The results are presented as mean ± standard deviation (SD), with medians and ranges where indicated. Bivariate determinations are presented as percentages of the number scored or enumerated. Trend analysis was done with linear regression of the median of variables for each CKD stage [24] against the stage ordinal (stages 4 and 5 were combined).
The reliability of the scoring among the pathologists was assessed with intraclass correlation coefficient (ICC) analysis [25,26] for the semi-quantitative assessment of interstitial fibrosis and morphometric assessment of the chronic damage index, and for the podocyte vacuolization on periodic acid Schiff-stained light microscopic sections and podocyte GL-3 deposits on semi-thin sections stained with toluidine blue. Because each case was not scored by every pathologist, a generalization of the standard linear model that allows for fixed and random effects was used for ICC analysis instead of analysis of variance [27].
The interclass correlation, using Spearman's correlation coefficient, was used to compare the overall assessment of interstitial fibrosis obtained with semi-quantitative scoring by the pathologists with the scores obtained for the same cases using a published morphometric technique [21]. The semi-quantitative and morphometric scores of interstitial fibrosis also underwent Bland–Altman analysis [28]. Interclass correlations, using Spearman's correlation coefficient, were also used to compare the overall assessment of podocyte vacuoles on light microscopy with podocyte GL-3 inclusions on semi-thin sections.
Statistical comparisons were done with unpaired two-tailed t-tests and two-tailed Fisher exact tests. The F-statistic was used to evaluate the significance of trends. P-values < 0.05 were considered to be significant. Calculations were carried out with SAS, version 9.1 (SAS Institute, Inc. Cary, NC, USA).
Results
Summary of clinical assessments
All patients had defined mutations, and five male patients had the p.N215S missense mutation, previously described in the late-onset cardiac variants of Fabry disease [1]. The mean age was 39.4 ± 13.2 years (range 16–70), and the serum creatinine was 1.3 ± 1.2 mg/dl (range 0.6–7.1) [114.9 ± 106.1 μmol/l (range 53.0–627)]. UPCR was 1.08 ± 1.34 g/g (range 0.01–5.62) [122.0 ± 151.4 mg/mmol (range 1.1–635.1)] (Table 1). Forty-six patients (78.0%, 25 males) had stage 1 or 2 CKD, 8 (13.6%, 7 males) had stage 3 CKD and 5 males (8.5%) had stage 4 or 5 CKD (Table 2). Males with CKD stage 1 were significantly younger than the females (28.1 ± 10.3 versus 42.4 ± 10.6 years, P < 0.003).
Table 1.
Values are presented as mean ± SD and median, and (range; median) for UPCR
| All patients (n = 59) | Male (n = 35) | Female (n = 24) | P-value | |
|---|---|---|---|---|
| Age (years) | 39.4 ± 13.2 (40.0) | 36.4 ± 14.0 (37.0) | 43.9 ± 10.6 (43.5) | 0.030* |
| Serum creatinine (mg/dl) | 1.3 ± 1.2 (0.9) | 1.6 ± 1.4 (1.2) | 0.8 ± 0.2 (0.8) | 0.007* |
| eGFR (ml/min/1.73 m2) UPCR (g/g) | 81.7 ± 34.8 (81.9) 1.08 ± 1.34 | 79.0 ± 42.3 (80.0) 1.28 ± 1.48 | 85.8 ± 19.6 (84.0) 0.80 ± 1.09 | NS |
| (0.01–5.62; 0.42) | (0.04– 5.62; 0.51) | (0.01–3.47; 0.17) | NS | |
| Systolic BP (mmHg) | 125.7 ± 13.0 (126.0) | 126.1 ± 13.1 (125.0) | 125.3 ± 13.3 (129.0) | NS |
| Diastolic BP (mmHg) | 75.6 ± 10.5 (75.0) | 74.3 ± 11.5 (73.0) | 77.4 ± 8.9 (77.5) | NS |
Values are presented as mean ± SD and median, and (range; median) for UPCR. To convert UPCR to mg/mmol, multiply by 113.12.
*P-values; males compared to females, two-tailed t-test.
eGFR, estimated glomerular filtration rate; UPCR, urine protein to creatinine ratio; BP, blood pressure; NS, non-significant.
Table 2.
Summary of patient characteristics by CKD stage
| Stage 1 | Stage 2 | Stage 3 | Stages 4, 5 | |
|---|---|---|---|---|
| Males (n) | 15 | 8 | 7 | 5 |
| Age (years) | 28.1 ± 10.3 | 39.9 ± 14.1 | 46.4 ± 11.3 | 41.5 ± 16.6 |
| eGFR | 119.1 ± 21.9 | 73.4 ± 7.8 | 43.9 ± 8.9 | 16.3 ± 10.3 |
| UPCR | 0.73 ± 0.83 (0.04–2.39; 0.37) | 0.81 ± 0.85 (0.04–2.18; 0.55) | 0.86 ± 0.59 (0.20–1.97;0.71) | 4.23 ± 1.25 (2.3–5.6; 4.29) |
| Females (n) | 10 | 13 | 1 | 0 |
| Age (years) | 42.4 ± 10.6* | 43.5 ± 9.9 | 63.0 | NA |
| eGFR | 104 ± 12.8 | 74.8 ± 8.4 | 46.0 | NA |
| UPCR | 0.44 ± 0.48 (0.04–1.46; 0.24) | 1.00 ± 1.36 (0.01–3.47; 0.15) | 1.83 | NA |
Values are presented as mean ± SD and (range; median).
eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); UPCR, urine protein to creatinine ratio (g/g; to convert to mg/mmol, multiply by 113.12.); NA, not applicable.
*P < 0.003; males compared to females, two-tailed t-test.
Average systolic and diastolic blood pressures were 125.7 ± 13.0 (range 100–167) and 75.6 ± 10.5 mmHg (range 54–105; Table 1), respectively. Systolic blood pressure was >135 mmHg in 13 (22%) patients, and diastolic blood pressure was >85 mmHg in 9 patients (15.3%). Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers were given to 24 patients (40.7%).
Nine patients started ERT 1.5 ± 1.0 months (range 0.6–3.9, median 1.4) before biopsy at a dose of 0.2 (n = 3) or 1.0 mg/kg every 2 weeks (n = 6). Forty-six patients started ERT 8.2 ± 13.1 months (range 0.03–76.2, median 4.0) after biopsy at a dose of 0.2 (n = 13) or 1.0 mg/kg every 2 weeks (n = 33).
Biopsy findings
The histologic evaluations are summarized in Tables 3–6. On average, 16.1 ± 11.9 glomeruli were present for light microscopic assessment (range 1–67), and 2.6 ± 1.9 (range 0–8) glomeruli were examined on toluidine blue-stained semi-thin sections. Two cases were not scored on the semi-thin sections because of inadequate staining.
Glomerular sclerosis.
There was a wide range of glomerular sclerosis scores (Table 3). Mild or severe segmental sclerosis was observed in 19 of 35 males (9 stage 1 CKD, 3 stage 2 CKD, remainder with more advanced CKD), and 11 of 24 females (2 stage 1 CKD, 8 stage 2 CKD, 1 patient with more advanced CKD). The average extent of segmental sclerosis was 4.7 ± 7.8% of glomeruli, with mild and severe lesions seen in the same biopsy. Global sclerosis was present in 14.8 ± 22.7% of glomeruli. Ten of the 15 males (66.7%) with stage 1 CKD had segmental or global sclerosis in 6.2 ± 8.7% (median 2.9) of their glomeruli, and 7 of the 10 females with stage 1 CKD had segmental or global sclerosis in 5.7 ± 9.0% (median 3.0) of their glomeruli. On average, 43.5 ± 23.0% of the glomeruli had global sclerosis for the 13 patients with eGFR <60 ml/min/1.73 m2, with only 1 without global sclerosis.
Table 3.
Kidney morphologic findings in Fabry patients
| All (n = 59) | Male (n = 35) | Female (n = 24) | |
|---|---|---|---|
| Glomeruli, number in biopsy (light microscopy sections) | 16.1 ± 11.9 (13.6) | 17.3 ± 12.8 (14.3) | 14.3 ± 10.3 (13.6) |
| Segmental sclerosis; (mild + severe) (%) | 4.7 ± 7.8 (0.9) | 5.1 ± 8.5 (1.9) | 4.0 ± 6.7 (0.0) |
| Global sclerosis (%) | 14.8 ± 22.7 (2.9) | 18.3 ± 24.3 (5.9) | 9.8 ± 19.5 (0.5) |
| Glomeruli without sclerosis (%) | 69.9 ± 27.3 (75.2) | 66.3 ± 30.4 (75.2) | 75.2 ± 21.6 (77.7) |
| Interstitial fibrosis (%) | 16.2 ± 22.9 (6.0) | 20.4 ± 25.0 (10.0) | 10.1 ± 18.3 (2.5) |
| Arterial sclerosis (0–3) | 0.71 ± 0.72 (0.43) | 0.75 ± 0.77 (0.33) | 0.64 ± 0.66 (0.47) |
| Podocyte vacuoles (light microscopy; 0–3)* | 2.2 ± 0.8 (2.5) | 2.3 ± 0.7 (2.6) | 1.9 ± 0.8 (1.9) |
| Podocyte inclusions (semi-thin sections; 0–4)* | 3.0 ± 1.2 (3.5) | 3.3 ± 1.1 (3.9) | 2.4 ± 1.2 (2.8) |
Values are presented as mean ± SD and (median). Assessment from standard light microscopy sections unless otherwise indicated. Percentage represents proportion of glomeruli with lesion. Of note, glomeruli with periglomerular fibrosis, extensive corrugation, ischaemia or adhesion were not scored as ‘non-sclerotic’, and thus the sum of global or segmental sclerosis and ‘non-sclerotic’ glomeruli does not necessarily equal 100%.
*P < 0.03, two-tailed t-test, females compared to males.
Interstitial fibrosis and chronic damage index.
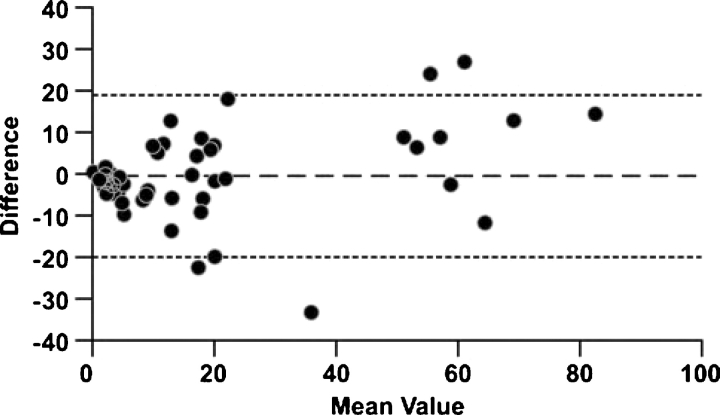
The semi-quantitative and morphometric scores for fibrosis were closely correlated (top section, Table 4; Spearman's coefficient = 0.871, P < 0.001). Bland–Altman analysis (Figure 1) [28] showed that the semi-quantitative assessment had no significant bias compared to the morphometry (mean difference of average score minus index of chronic damage = +0.79%; 95% Confidence Interval; −1.74 to +3.32%). The limits of agreement (mean difference ± 2 SD) ranged between −18.6% and 20.2%.
Table 4.
Interclass and intraclass correlation coefficients for interstitial fibrosis, podocyte vacuoles and podocyte GL-3 inclusions
| Scoring (n) | Mean (%) | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|
| Morphometric score (59) | 16.22 | 22.94 | 6.00 | 0 | 89.0 |
| Average semi-quantitative score (59) | 17.01 | 10.56 | 8.33 | 0 | 75.0 |
| Spearman's interclass correlation coefficient = 0.871, P < 0.001 | |||||
| Scoring (scale) | Mean (%) | SD | Median | Minimum | Maximum |
| Podocyte vacuoles (1–3) | 2.19 | 0.80 | 2.5 | 0 | 3.00 |
| Podocyte inclusions (1–4) | 2.95 | 1.21 | 3.4 | 0 | 4.00 |
| Spearman's interclass correlation coefficient = 0.5774, P < 0.0001 | |||||
| Scoring (n) | Variance of cases (V) (SE) | Residual variance (R) (SE) | ICC (V/V+R) | Within case variance(1-ICC)/ICC | |
| Interstitial fibrosis score (59) | 340.3 (71.8)* | 180.1 (18.9)* | 0.6539 | 52.9% | |
| Podocyte vacuoles (59) | 0.557 (0.119)* | 0.235 (0.0310)* | 0.7030 | 42.2% | |
| Podocyte inclusions (57) | 1.344 (0.278)* | 0.3443 (0.047)* | 0.7961 | 25.7% | |
*P < 0.0001.
ICC, intraclass correlation coefficient; R, residual variance; V, variance of cases.
Fig. 1.
Interstitial fibrosis (%): comparison of semi-quantitative scoring to morphometric assessment. Bland–Altman plot compares the difference (index of chronic damage – semi-quantitative score; solid horizontal line) with their mean. The limits of agreement (±2 SD) are shown as dashed horizontal lines.
The reliability of scoring among the pathologists was assessed with ICC analysis [25,26]. The variance in scoring attributable to the cases and the residual variance were used to estimate the ICC (0.6539). The variance within cases was 52.9% of the variance between cases (Table 4), indicating reasonably good agreement between the different pathologists with respect to the semi-quantitative scoring of interstitial fibrosis.
Podocytes vacuoles and inclusions.
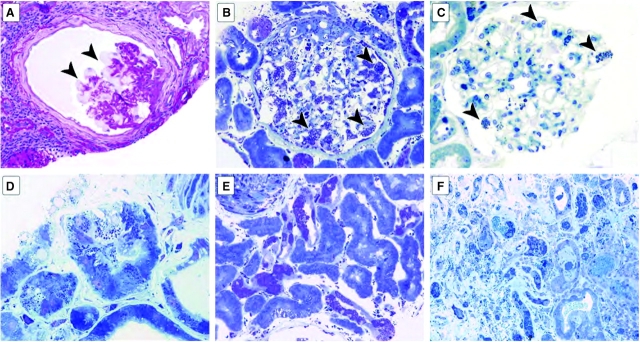
Vacuoles were frequently observed in podocytes (Figure 2A), corresponding to extracted GL-3 deposits. Vacuole average severity (0–3 scale) was 2.3 ± 0.7 (median 2.6) for males and 1.9 ± 0.8 (median 1.9) for females (P < 0.03, Table 3). GL-3 inclusions were scored for the semi-thin sections stained with toluidine blue (0–4 scale). The distribution of GL-3 inclusions was quite variable (Figure 2B and C). Women had significantly fewer deposits than men (2.4 ± 1.2 versus 3.3 ± 1.1; P < 0.03; Table 3). The most common pattern in male patients was a mixture of large expanded deposits with concurrent small deposits.
Fig. 2.
Vacuolization and glycosphingolipid deposits in Fabry nephropathy. (A) The glomerulus shows vacuolization of podocytes (arrowheads), and mild corrugation of glomerular basement membranes, with periglomerular and interstitial fibrosis (periodic acid-Schiff, x400). (B) The glomerulus shows massive expanded deposits in most podocytes (arrowheads) and also in mesangial and endothelial cells and parietal epithelial cells in this male Fabry patient (toluidine blue, ×400). (C) The glomerulus shows occasional small deposits in podocytes (arrowheads) and rare deposits in mesangial areas in this female patient (toluidine blue, ×400). (D) There are prominent deposits in some proximal tubular cells and peritubular capillary endothelium (toluidine blue, ×1000). (E) Numerous deposits in distal tubules and very rare deposits in proximal tubules. The vascular smooth muscle of a large artery (top left corner) also shows deposits, as do parietal epithelial cells lining the Bowman's capsule (bottom) (toluidine blue, ×1000). (F) Numerous deposits in distal tubules with rare deposits in proximal tubules and frequent deposits in peritubular capillaries and interstitium (toluidine blue, ×200).
Of patients with glomeruli available on semi-thin sections, one 46-year-old woman did not have any GL-3 deposits, and seven additional female patients and one male patient had small deposits (i.e. inclusion score <1.5); none had received ERT. The female patient without podocyte deposits had distal tubular and arterial medial deposits and was known to have mutation p.R363P. Parietal epithelial inclusions were present (Figure 2B), but were not reliably scored.
The podocyte vacuole scores and GL-3 inclusion scores agreed reasonably well (Table 4). The reliability of the scoring among the pathologists was assessed with ICC analysis [25,26]. The variance attributable to the cases, and the residual variance were used to estimate the ICC. The variance component within cases was 42.2% and between cases was 25.7%, respectively, for the scorings of podocyte vacuoles on light microscopy and GL-3 inclusions on semi-thin sections. Of the three ICC comparisons, the reliability of the scoring of the podocyte GL-3 inclusions appeared to be superior to the semi-quantitative scoring of interstitial fibrosis or podocyte vacuoles.
Tubular inclusions.
Distal tubule inclusions were present in 42 of the 56 cases (75.0%) (Table 5, Figure 2D–F). Proximal tubule inclusions were more prevalent in males than females (47.1% versus 19.0%, P = 0.046). Inclusions in both proximal and distal tubules were present in 14 males and 4 females, and 14 males and 9 females only had distal tubule inclusions.
Table 5.
Kidney morphologic findings in Fabry patients
| All (n = 59) | Male (n = 35) | Female (n = 24) | P-valuea | |
|---|---|---|---|---|
| Distal tubule inclusions | 42/14 (75.0%) | 28/6 (82.4%) | 14/8 (63.6%) | NS |
| Proximal tubule inclusions | 20/35 (36.4%) | 16/18 (47.1%) | 4/17 (19.0%) | 0.046 |
| Peritubular capillary inclusions | 33/23 (58.9%) | 26/8 (76.5%) | 7/15 (31.8%) | 0.002 |
| Vascular intimal inclusions | 19/19 (50.0%) | 16/9 (64.0%) | 3/10 (23.1%) | 0.038 |
| Vascular medial inclusions | 25/13 (65.8%) | 17/8 (68.0%) | 8/5 (61.5%) | NS |
| Arteriolar hyalinosis (light microscopy sections) | 34/21 (61.8%) | 22/12 (64.7%) | 12/9 (57.1%) | NS |
| Arterial hyalinosis (light microscopy sections) | 22/33 (40.0%) | 9/25 (26.5%) | 13/8 (61.9%) | 0.012 |
Data are presented as present/absent and percentage present/(present + absent);
Excluded cases are those that could not be scored due to insufficient sampling for that variable. Lesions scored on semi-thin sections except as noted.
aFisher exact two-tailed test statistic, females compared to males;
NS, non-significant.
Capillary and arterial inclusions.
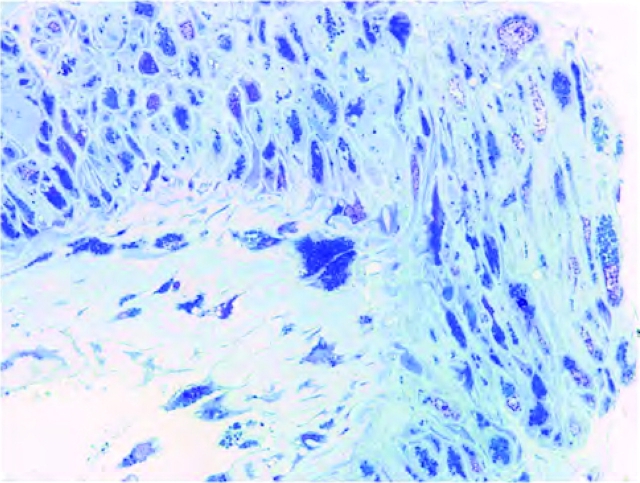
Peritubular capillary inclusions were identified in 26 males and 7 females (P = 0.002). Intimal vascular inclusions were more prevalent in males than females. Vascular medial inclusions were present in 25 out of 38 patients (Figure 3). Lesions included typical arteriosclerotic lesions and severe medial injury and necrosis with inclusions, often associated with luminal dilatation. Arterial sclerosis was mild (Table 3). Hyalinosis was present in arterioles of 34 patients and in arteries of 22 patients out of 55 evaluated (Table 5). Arteriolar hyalinosis was scored in 22 of 34 males (64.7%) and 12 of 21 females (57.1%). Arterial hyalinosis was present in 9 of 34 males (26.5%) and 13 of 21 females (61.9%; P = 0.012).
Fig. 3.
Vascular lesions in Fabry nephropathy. Numerous inclusions in endothelial cells and vascular smooth muscle cells in this large artery are seen in the toluidine blue-stained semi-thick scout section (toluidine blue, ×1000).
Trend analysis.
Arterial sclerosis was more severe with advanced CKD (P = 0.0003), but blood pressure was not correlated (not shown). Glomerular sclerosis was more severe with more advanced CKD. In stage 1 CKD, 7.8 ± 17.6% (median 0.85) of the glomeruli had segmental or global sclerosis despite minimal proteinuria.
Discussion
We have described the renal biopsies of mild Fabry nephropathy in adult patients. Histologic evidence of kidney involvement precedes clinical signs in early Fabry nephropathy, confirming previous reports [16,17,19,29]. Glomerulosclerosis and interstitial fibrosis were observed in both genders, even in patients with minimal or no proteinuria in our series. In the evaluation of early Fabry nephropathy, clinical assessments lack sensitivity, potentially delaying initiation of ERT [30], highlighting the utility of kidney biopsies as part of the baseline assessment.
A similar scoring strategy was used for lupus nephritis [31] and focal segmental glomerulosclerosis [32]: inclusion of pathologists and clinicians; multiple iterations; validation and adjudication of difficult lesions; and refinement of definitions using a modified Delphi technique [23]. We evaluated disease-specific GL-3 lesions and secondary alterations that occur in Fabry nephropathy [33], as well as any other form of CKD (e.g. arterial and glomerular sclerosis, and interstitial fibrosis). Interstitial fibrosis, global and segmental sclerosis and podocyte inclusions were robustly scored. The latter parameter showed the highest degree of inter-individual agreement. The semi-quantitative scoring of interstitial fibrosis showed similar reliability when compared to the morphometric assessment. Other lesions (e.g. parietal epithelial cell inclusions, interstitial inflammation) could not be reliably scored with the current approaches.
Glomerulosclerosis occurs early in Fabry nephropathy and is more severe with more advanced CKD. Only 37% of our patients with eGFR >60 ml/min/1.73 m2 did not have any glomerular sclerotic lesions, and ~13% had sclerosis in >20% of glomeruli. Although proportions of segmental and global sclerosis varied among cases, no significant histologic differences between genders could be identified in early CKD. This finding may have important prognostic implication; Germain et al. [6] have described a marked loss of GFR in male Fabry patients with segmental and global glomerular sclerosis in >50% of their glomeruli.
GL-3 inclusions in podocytes were larger in males than in females, despite the 7.5-year higher mean age of females. Even in early CKD, half of the males had large podocyte inclusions involving >50% of the glomerular tuft. The podocyte scores for vacuolization and inclusions were significantly correlated, with only two patients having discordant findings.
Most of our patients (78%) had mild nephropathy (stage 1–2 CKD). Nearly half of the stage 1–2 CKD patients were females. Some conspicuous gender differences were observed. Clinical disease was milder in the female cohort, with corresponding lesser degree of global sclerosis and less podocyte, peritubular, vascular and proximal tubule inclusions than in the males. However, segmental and global glomerulosclerosis, interstitial fibrosis, distal tubular inclusions, arteriolar hyalinosis and vascular medial inclusions were of similar degrees in females as in males. Arterial hyalinosis was the only lesion more prevalent in females than males, which may be related to their higher mean age. These gender differences indicate that prognostic scoring elements may be assessed differently in male and female Fabry patients. Arterial sclerosis increased with CKD stage, and there appears to be a non-linear relationship between interstitial fibrosis and CKD stage, with minimal fibrosis scores in the stage 1–2 biopsies (Table 6).
Table 6.
Summary of trend analyses by CKD stage
| Stage 1 (n = 25) | Stage 2 (n = 21) | Stage 3 (n = 8) | Stages 4, 5 (n = 5) | P-value* | |
|---|---|---|---|---|---|
| eGFR | 113.1 ± 20.0 (110.0) | 74.3 ± 8.0 (72) | 44.2 ± 8.3 (45.5) | 16.3 ± 10.3 (13.6) | 0.002 |
| Arterial sclerosis, 0–3 | 0.46 ± 0.68 (0.00) | 0.62 ± 0.57 (0.50) | 0.93 ± 0.39 (1.00) | 1.93 ± 0.72 (1.66) | 0.003 |
| No sclerosis, % glomeruli | 82.3 ± 18.7 (88.4) | 74.5 ± 20.9 (78.8) | 50.2 ± 28.9 (55.1) | 20.7 ± 14.2 (21.7) | 0.027 |
| Mild + severe sclerosis, % glomeruli | 2.8 ± 5.5 (0.00) | 4.3 ± 6.5 (1.06) | 6.9 ± 10.9 (1.35) | 11.9 ± 13.2 (5.26) | NS |
| Global sclerosis, % glomeruli | 5.0 ± 17.1 (0.00) | 8.7 ± 11.3 (5.9) | 35.9 ± 24.9 (32.7) | 55.5 ± 14.6 (50.0) | 0.024 |
| Mild + severe + global sclerosis, % glomeruli | 7.8 ± 17.6 (0.85) | 13.1 ± 15.7 (7.1) | 42.9 ± 26.9 (46.4) | 67.4 ± 11.6 (69.8) | 0.030 |
| Interstitial fibrosis, % | 8.4 ± 15.9 (2.0) | 9.4 ± 16.2 (3.0) | 27.8 ± 19.7 (21.5) | 65.6 ± 13.9 (61.0) | 0.027 |
Values are presented as mean ± SD and (median).
eGFR, estimated glomerular filtration rate (ml/min/1.73 m2); NS, non-significant.
*P-value for the regression calculated with the F-test; interstitial scores transformed as log(score) before analysis.
Attempts to develop prognostic markers and/or pathogenic insights from renal biopsies have been undertaken in other forms of CKD. Glomerulosclerosis, interstitial fibrosis, endocapillary proliferation and surprisingly, mesangial proliferation were signs of poor prognosis in IgA nephropathy [34]. In the African-American Study of Kidney Diseases, global sclerosis was minimally related to mean arterial pressure, but was predicted by systolic blood pressure, serum cholesterol and reciprocal of serum creatinine [35]. With longitudinal studies of Fabry patients, a similar strategy may support the development of a chronicity index that reflects the long-term outcome. The variables summarized in Tables 3–5 may serve as the basis for such studies. The amount of chronic renal damage at the time of biopsy is likely to be related to the rate of loss of kidney function [23]. In addition, some lesions may influence the rate of decline, as well as baseline GFR. There may also be an indication as to response to therapy for some of the scored variables.
Several limitations of our study are evident. (1) We have assigned CKD stages to the patients based on baseline serum creatinine values and calculated eGFR. The majority of patients had GFR >60 ml/min/1.73 m2, beyond the current validation range of the MDRD. UPCR was used as an index of proteinuria. KDOQI [24] defined CKD as either kidney damage or eGFR <60 ml/min/1.73 m2 for ≥3 months, with kidney damage defined as pathologic abnormality or a marker of damage. Since all of our patients had biopsy-proven Fabry nephropathy, we feel justified in using CKD staging to describe the severity of their kidney damage. Furthermore, the significant increases in the various sclerosis scores with CKD stage, with more severe scores in stage 2 than stage 1 (Table 6), lend further credence to the use of CKD staging, bearing in mind the reservations about using estimating equations for GFR. (2) Nine patients received ERT before biopsy, but the median time before biopsy was only 1.4 months, with none more than 4 months. It is not likely that short-term ERT would influence sclerotic lesions. (3) Biopsies were performed according to the local standard of care, and the criteria for submitting cases for this study were not restrictive. This retrospective, cross-sectional design raises a possible selection bias for inclusion in the study. (4) Few patients with advanced CKD were included, limiting the trend analysis as well as comparisons between genders. Nevertheless, the pathology was similar in both genders, although developing at a later age in females.
The present study focused on histologic sections for developing the scoring system. For this purpose, standard histologic sections provided sufficient glomeruli and more extensive vascular and interstitial sampling, overcoming the limited tissue sampling with semi-thin sections used in previous studies [5]. On the other hand, histological findings suggestive of Fabry nephropathy (e.g. vacuolization) may be missed on routine histologic sections; semi-thin sections are best for characterizing podocyte GL-3 inclusions. This conclusion is strengthened by the ICC analysis in Table 4.
In conclusion, our description and validation of a scoring system of histologic involvement in Fabry nephropathy are notable because of the inclusion of a large number of adult females. Chronic glomerular and interstitial damage develop early in the course of Fabry disease, and the absence of typical clinical signs of CKD does not rule out Fabry nephropathy. Overall, our results are in agreement with previous, smaller series of Fabry patients, showing significant histologic changes before renal function is decreased [12,16,19]. Access to detailed illustrations and definitions of lesions (see Appendix) should enhance the utility and application of the scoring system for future studies of Fabry nephropathy. Future development of a chronicity index based on longitudinal data may be useful for describing severity of pathologic changes and prognostic implications for progressive Fabry nephropathy. For now, we conclude that important information is provided by kidney biopsy in Fabry nephropathy that is not available from routine assessment of kidney function and proteinuria. Our results support the role of kidney biopsy in the baseline evaluation of all Fabry patients, even with mild clinical disease.
Acknowledgments
The authors would like to acknowledge the efforts of Ellen Donnert at Vanderbilt University; Department of Pathology, for coordinating the exchange of slides sets and preparation of the scoring sheets. Dr Gary Cutter of the Department of Biostatistics, University of Alabama at Birmingham, provided guidance for the statistical analyses in this paper. Dr Noël would like to acknowledge the collaborative effort of Dr B. Laurent, Dr D.P. Germain, Professor G. Choukroun, and Dr C. Cordonnier to contribute Fabry nephropathy biopsy specimens. The patients described in this report are registered in the Fabry Outcome Survey (Shire Human Genetic Therapies, Boston, MA, USA) and the Fabry Registry (Genzyme Corporation, Cambridge, MA, USA); Institutional Review Board approval was obtained at each centre as part of their registry activity. A.B.F, D.G.W, F.Br., L.B. and J.P.O are members of the Steering Committee of the ISGFN, chaired by J.P.O. A preliminary report of these findings was presented at the 2006 Annual Meeting of the American Society of Nephrology in San Diego, CA, USA [37]. This work was supported by an unrestricted educational grant from Genzyme Corporation, Cambridge, MA, USA, and in addition, Hans Ebels (Genzyme Corporation) assisted with the preparation of the manuscript for submission. The authors are fully responsible for the contents of this paper. Partial support for the analyses was provided by the UAB-UCSD O’Brien Core Center (NIH 1P30 DK 079337).
Conflict of interest statement. A.B.F. has received consultant and past grant support from Genzyme Corporation. L.B. has received travel assistance from Genzyme Corporation. E.S. has received speaker fees from Genzyme Corporation. W.J.C. has received travel assistance from Genzyme Corporation. S.M. has received travel assistance from Genzyme Corporation. F.Ba. has received speaker fees and travel assistance from Genzyme Corporation and Shire Human Genetic Therapies Inc. L.G. has nothing to disclose. M.W. has received speaker fees, consultant fees and/or research support from Genzyme Corporation, Shire Human Genetic Therapies Inc. and Amicus Therapeutics Inc. D.F. has received travel assistance from Genzyme Corporation. B.V. has received speaker fees and research grant support from Genzyme Corporation and Shire Human Genetic Therapies Inc. A.J.H. has received travel assistance from Genzyme Corporation. A.B. has received travel assistance from Genzyme Corporation. R.R. has nothing to disclose. S.W. is a member of the European Advisory Board of the Fabry Registry, sponsored by Genzyme Corporation, and is compensated for the services by Genzyme Corporation. S.W. is a paid consultant for Genzyme Corporation on matters relating to Fabry disease, has received research grants from Genzyme Corporation related to Fabry disease, and speaking fees from Genzyme Corporation and Shire Human Genetic Therapies Inc. L.H.N. has received travel assistance from Genzyme Corporation. J.P.G. has received travel assistance from Genzyme Corporation and Transkaryotic Therapies Inc. and is a consultant for Genzyme Corporation but did not receive consultancy fees. C.V. has received a non-restricted research grant from Genzyme Corporation. J.P.O. is a member of the European Advisory Board of the Fabry Registry, sponsored by Genzyme Corporation, and has received speaker fees and research support from Genzyme Corporation. J.M. has received travel assistance from Genzyme Corporation. F.Br. has received travel assistance and speaking fees from Genzyme Corporation and Shire Human Genetic Therapies Inc. D.G.W. is a paid consultant for Genzyme Corporation and has received speaker fees and research support from Genzyme Corporation.
Appendix. Histologic scoring
General approach
The initial effort involved six cases coded and blinded as to origin of slides and was intended to identify common features in the biopsies (fibrosis, sclerosis, vascular changes, GL-3 deposits, etc.) that would be included in the final scoring sheet. Round-robin slide reviews and two face-to-face scoring sessions with a modified Delphi technique [23] were used to refine the scoring system. Then, slides from 20 cases were circulated, with each group scoring each element specified in the score sheet (Figure A1). In a face-to-face meeting, cases with difficult lesions were discussed for further refinement and clarification of definitions. This sequence of scoring, Delphi consensus process and rescoring, was adopted to validate the scoring system. The final 59 cases were then circulated with each biopsy scored by three different centres. Final scores of all 59 cases represent the average in each case from all validated scorings from these centres. An average for each parameter was calculated, with a range. For lesions scored as present or absent, cases with divergent scoring were reviewed for a final score by A.B.F. Electron micrographs were not examined in the current project. Morphometry was done on all slides by one centre (see below).
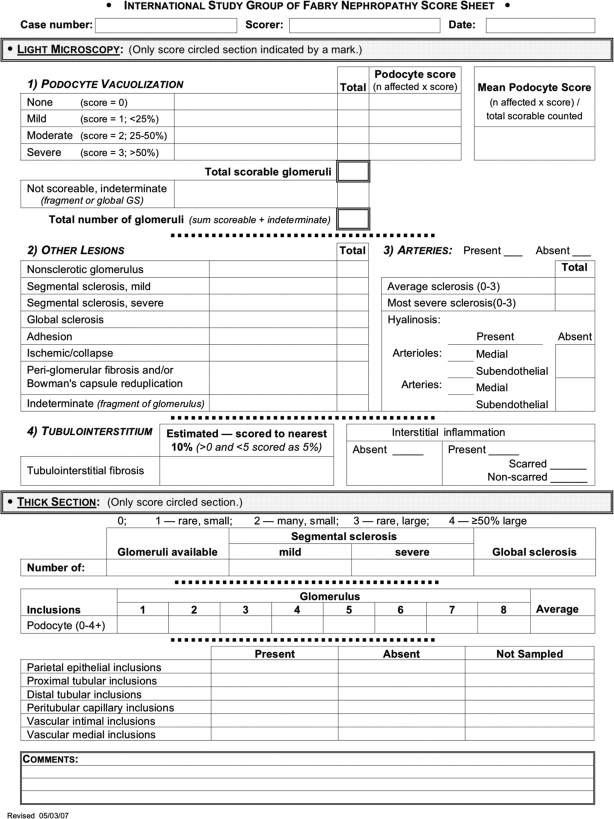
Fig. A1.
Scoring sheet for Fabry nephropathy by light microscopy.
Lesions were scored on the same sections on the circulated slide, so that all observers assessed the exact same tissue. For most cases, tissue was assessed on periodic acid-Schiff (PAS)-stained slides. In a few cases, when PAS-stained slides were not available, Jones’ stained slides were scored instead. Of note, more than one lesion could be present on light microscopy in any one glomerulus, e.g. segmental sclerosis and ischaemia/collapse could coexist in the same glomerulus, and both features were then scored as ‘present’ for that glomerulus. From our experience, optimal slides for scoring should ideally include >10 glomeruli for light microscopic assessment, and at least 3 glomeruli for semi-thin section scoring.
Light microscopic slide scoring
Glomeruli that could not be scored due to, for instance, incomplete sections or global sclerosis were indicated in the box as ‘indeterminate’ for scoring. Glomeruli with <25% of the tuft represented in the section were also marked as ‘indeterminate’ for vacuolization scoring. The total was calculated as a sum of all scored and non-scored glomeruli.
Podocyte vacuolization was scored for each individual glomerulus (‘0’ none, and ‘1’, ‘2’, or ‘3’, respectively, when <25%, 25–50%, and >50% of podocytes showed cytoplasmic vacuoles). A final average score was calculated for each biopsy.
The total number of glomeruli without any degree of sclerosis, as well as with no adhesions, evidence of ischaemia or periglomerular fibrosis, was counted and reported as nonsclerotic glomeruli. Segmental sclerosis was defined as obliteration of capillary lumen with a increased matrix involving a portion of the glomerulus (<50%, ‘mild’; or ≥50% of the tuft affected, ‘severe’). Global sclerosis was scored when the entire glomerular tuft was sclerosed. Adhesions were scored when there was continuity of connective tissue between the glomerular tuft and Bowman's capsule without concomitant well-defined sclerosis. Ischaemia/collapse was scored when >50% of the glomerular tuft showed corrugation of the glomerular basement membrane and retraction of the tuft. Periglomerular fibrosis and/or Bowman's capsule reduplication were defined as present when >25% of the circumference of Bowman's capsule was affected by these processes, in the absence of global glomerulosclerosis.
Of note, as a consequence of our definition of nonsclerotic glomeruli, the sum of the percentages of globally and segmentally sclerotic glomeruli and non-sclerotic glomeruli is not mathematically 100%.
Arterial sclerosis was scored based on lesions in vessels as an average and also as a separate score for the most severely affected as described by Remuzzi et al. [36]: ‘0’, vascular lesions absent; ‘1+’, wall thickness increased but to a degree less than the diameter of the lumen; ‘2+’, wall thickness equal or slightly greater than the diameter of the lumen; and ‘3+’, wall thickness far exceeding the diameter of the lumen with extreme luminal narrowing or occlusion. Arteriolar or arterial hyalinosis was scored as ‘present’ or ‘absent’ and specified as involving the media or subendothelial location.
Interstitial fibrosis was semi-quantitatively estimated on cortical tissue. Interstitial inflammation was noted as ‘present’ or ‘absent’. If present, its location in scarred areas or non-scarred areas or both was noted. However, this parameter was variably scored. Any area involved with either tubular atrophy and/or interstitial fibrosis was scored as ‘involved with interstitial fibrosis’. Each ×20 field was assessed, and degree of involvement with interstitial fibrosis estimated. An average of all cortical fields was then calculated, and scored as the nearest 10%, except for minimal fibrosis of ≤5%, which was scored as 5%. The results were compared with morphometric assessment on the same slides by the method published by Howie et al. [21].
Additional scoring was done on the epoxy-embedded toluidine blue-stained semi-thin section slides. The number of glomeruli available on the single section was designated. The extent of segmental and global sclerosis, defined as above, was noted. A podocyte inclusion score (see below) was given separately for each glomerulus, and an average score was thereby calculated for each biopsy. Podocyte inclusions were scored as follows: ‘0’, no deposits; ‘1+’, rare small inconspicuous deposits; ‘2+’, more frequent small deposits; ‘3+’, <50% of the tuft involved with large expanded deposits in the presence or absence of concurrent small deposits; and ‘4+’, ≥50% of the tuft involved with large expanded deposits. Inclusions in parietal epithelial, proximal and distal tubular, peritubular capillary, vascular intimal and vascular medial cells were scored as ‘present’, ‘absent’ or ‘not sampled’ in the specimen.
Morphometric assessment of chronic renal damage
Each biopsy specimen was examined to give a measure of the amount of chronic damage called ‘the index of chronic damage’ [21]. Briefly, images of the cortex were captured on a computer, and areas with chronic damage, defined as globally sclerosed glomeruli, atrophic tubules and interstitial fibrosis, were outlined using an interactive image analysis program. The index of chronic damage in a specimen was the total cross-sectional area with chronic damage expressed as a percentage of the cortical cross-sectional area.
The mean estimates of the extent of interstitial fibrosis were compared with the index of chronic damage in two ways, firstly by calculation of the Spearman's correlation coefficient, and secondly by assessment of the agreement between them using the Bland–Altman method [28]. This method gave the mean difference, or bias, with 95% confidence interval, and the limits of agreement, which were two standard deviations of the difference on each side of the mean difference. Preliminary analysis of 20 specimens showed that exclusion of the area of globally sclerosed glomeruli, which was not included in semi-quantitative estimation of the extent of interstitial fibrosis, made little difference to the index of chronic damage, because inclusion of globally sclerosed glomeruli added only 1% to the index. Subjective scoring of interstitial fibrosis was generally recorded to the nearest 10%, or to 5% for minimal fibrosis, and so 1% on the index was considered insignificant. The values of the morphometric index used were those including the area of globally sclerosed glomeruli.
References
- 1.Desnick R, Ioannou Y, Eng C. Alpha-galactosidase A deficiency: Fabry disease. In: Scriver CR, Beaudet A, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill; 2001. pp. 3733–3774. [Google Scholar]
- 2.Branton MH, Schiffmann R, Sabnis SG, et al. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore) 2002;81:122–138. doi: 10.1097/00005792-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Schiffmann R, Warnock D, Banikazemi M, et al. Fabry disease: progression of nephropathy, and prevalence of cardiac and cerebrovascular events before enzyme replacement therapy. Nephrol Dial Transplant. 2009;24:2102–2111. doi: 10.1093/ndt/gfp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A—replacement therapy in Fabry's disease. N Engl J Med. 2001;345:9–16. doi: 10.1056/NEJM200107053450102. [DOI] [PubMed] [Google Scholar]
- 5.Thurberg BL, Rennke H, Colvin RB, et al. Globotriaosylceramide accumulation in the Fabry kidney is cleared from multiple cell types after enzyme replacement therapy. Kidney Int. 2002;62:1933–1946. doi: 10.1046/j.1523-1755.2002.00675.x. [DOI] [PubMed] [Google Scholar]
- 6.Germain DP, Waldek S, Banikazemi M, et al. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol. 2007;18:1547–1557. doi: 10.1681/ASN.2006080816. [DOI] [PubMed] [Google Scholar]
- 7.Fervenza F, Torra R, Warnock D. Safety and efficacy of enzyme replacement therapy in the nephropathy of Fabry disease. Biologics: Targets Ther. 2008;2:1–22. doi: 10.2147/btt.s3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deegan PB, Baehner AF, Barba Romero MA, et al. Natural history of Fabry disease in females in the Fabry Outcome Survey. J Med Genet. 2006;43:347–352. doi: 10.1136/jmg.2005.036327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilcox WR, Oliveira JP, Hopkin RJ, et al. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112–128. doi: 10.1016/j.ymgme.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 10.MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 60 obligate carrier females. J Med Genet. 2001;38:769–775. doi: 10.1136/jmg.38.11.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortiz A, Oliveira JP, Waldek S, et al. Nephropathy in males and females with Fabry disease: cross-sectional description of patients before treatment with enzyme replacement therapy. Nephrol Dial Transplant. 2008;23:1600–1607. doi: 10.1093/ndt/gfm848. [DOI] [PubMed] [Google Scholar]
- 12.Gubler MC, Lenoir G, Grunfeld JP, et al. Early renal changes in hemizygous and heterozygous patients with Fabry's disease. Kidney Int. 1978;13:223–235. doi: 10.1038/ki.1978.32. [DOI] [PubMed] [Google Scholar]
- 13.Sessa A, Toson A, Nebuloni M, et al. Renal ultrastructural findings in Anderson-Fabry disease. J Nephrol. 2002;15:109–112. [PubMed] [Google Scholar]
- 14.Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol. 2002;13(Suppl 2):S134–S138. [PubMed] [Google Scholar]
- 15.Fischer EG, Moore MJ, Lager DJ. Fabry disease: a morphologic study of 11 cases. Mod Pathol. 2006;19:1295–1301. doi: 10.1038/modpathol.3800634. [DOI] [PubMed] [Google Scholar]
- 16.Valbuena C, Carvalho E, Bustorff M, et al. Kidney biopsy findings in heterozygous Fabry disease females with early nephropathy. Virchows Arch. 2008;453:329–338. doi: 10.1007/s00428-008-0653-2. [DOI] [PubMed] [Google Scholar]
- 17.Sessa A, Meroni M, Battini G, et al. Renal pathological changes in Fabry disease. J Inherit Metab Dis. 2001;24(Suppl 2):66–70. doi: 10.1023/a:1012423924648. [DOI] [PubMed] [Google Scholar]
- 18.Svarstad E, Bostad L, Kaarboe O, et al. Focal and segmental glomerular sclerosis (FSGS) in a man and a woman with Fabry's disease. Clin Nephrol. 2005;63:394–401. doi: 10.5414/cnp63394. [DOI] [PubMed] [Google Scholar]
- 19.Tondel C, Bostad L, Hirth A, et al. Renal biopsy findings in children and adolescents with Fabry disease and minimal albuminuria. Am J Kidney Dis. 2008;51:767–776. doi: 10.1053/j.ajkd.2007.12.032. [DOI] [PubMed] [Google Scholar]
- 20.Tosoni A, Nebuloni M, Zerbi P, et al. Ultrastructural study of renal involvement in two females with Anderson-Fabry disease. Ultrastruct Pathol. 2005;29:203–207. doi: 10.1080/01913120590951202. [DOI] [PubMed] [Google Scholar]
- 21.Howie AJ, Ferreira MA, Adu D. Prognostic value of simple measurement of chronic damage in renal biopsy specimens. Nephrol Dial Transplant. 2001;16:1163–1169. doi: 10.1093/ndt/16.6.1163. [DOI] [PubMed] [Google Scholar]
- 22.Stevens LA, Coresh J, Greene T, et al. Assessing kidney function—measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 23.Hutchings A, Raine R, Sanderson C, et al. A comparison of formal consensus methods used for developing clinical guidelines. J Health Serv Res Policy. 2006;11:218–224. doi: 10.1258/135581906778476553. [DOI] [PubMed] [Google Scholar]
- 24.KDOQI clinical practice guidelines and clinical practice recommendations for diabetes and chronic kidney disease. Am J Kidney Dis. 2007;49:S12–S154. doi: 10.1053/j.ajkd.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 25.Shrout PE, Fleiss JL. Intraclass correlations: uses in assessing rater reliability. Psycholol Bull. 1979;86:420–428. doi: 10.1037//0033-2909.86.2.420. [DOI] [PubMed] [Google Scholar]
- 26.Ounpraseuth S, Rafferty TM, McDonald-Phillips RE, et al. A method to quantify mouse coat-color proportions. PLoS ONE. 2009;4:e5414. doi: 10.1371/journal.pone.0005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24:323–355. [Google Scholar]
- 28.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 29.Grunfeld JP. How to improve the early diagnosis of Fabry disease? Kidney Int. 2003;64:1136–1137. doi: 10.1046/j.1523-1755.2003.00196.x. [DOI] [PubMed] [Google Scholar]
- 30.Aakre KM, Tondel C, Brun A, et al. The MDRD equation may mask decline of glomerular filtration rate in Fabry patients with normal or nearly normal kidney function. Clin Nephrol. 2009;71:118–124. doi: 10.5414/cnp71118. [DOI] [PubMed] [Google Scholar]
- 31.Weening JJ, D’Agati VD, Schwartz MM, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65:521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Agati V. Pathologic classification of focal segmental glomerulosclerosis. Semin Nephrol. 2003;23:117–134. doi: 10.1053/snep.2003.50012. [DOI] [PubMed] [Google Scholar]
- 33.Barbey F, Lidove O, Schwarting A. Fabry nephropathy: 5 years of enzyme replacement therapy—a short review. NDT Plus. 2008;1:11–19. doi: 10.1093/ndtplus/sfm022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feehally J, Barratt J, Coppo R, et al. International IgA nephropathy network clinico-pathological classification of IgA nephropathy. Contrib Nephrol. 2007;157:13–18. doi: 10.1159/000102283. [DOI] [PubMed] [Google Scholar]
- 35.Fogo A, Breyer JA, Smith MC, et al. AASK Pilot Study Investigators. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) Trial. Kidney Int. 1997;51:244–252. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 36.Remuzzi G, Cravedi P, Perna A, et al. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343–352. doi: 10.1056/NEJMoa052891. [DOI] [PubMed] [Google Scholar]
- 37.Fogo A, Oliveira JP, Waldek S, et al. Developing a chronicity index for kidney involvement in Fabry disease; report of the International Fabry kidney biopsy working group. J Am Soc Nephrol. 2006;17:626. abstract. [Google Scholar]