Abstract
Background. Autosomal dominant polycystic kidney disease (ADPKD), a major cause of end-stage renal failure, results from genetic mutation of either polycystin-1 (Pkd1) or polycystin-2 (Pkd2). In order to develop novel therapies to treat the advancement of disease progression, numerous rodent models of different genetic backgrounds are available to study cyst development.
Methods. Here, a Pkd1-floxed inducible mouse model using the interferon responsive Mx1Cre-recombinase was utilized to test the effect of the small molecule triptolide. Relative to other Pkd1 inactivation models, cyst progression in this neonatal to adult transition model is attenuated. Following the characterization of inducible cyst formation in these mice, the development of kidney cysts from triptolide or vehicle-treated animals was analysed.
Results. Although Pkd1 deletion on postnatal Days P10 and P12 resulted in numerous cysts by P35, daily injections with triptolide beginning on Day P16 significantly reduced the total number of cysts per kidney, with a pronounced effect on the number of microcysts and the overall cystic burden. Additionally, renal function as assessed by blood urea nitrogen levels was also improved in triptolide-treated mice at both the P22 and P35 time points. As the Pkd1flox/flox;Mx1Cre model has not been previously used for drug development studies, the feasibility of a 6-month adult Pkd1 inactivation study was also tested. While kidney cyst formation was minimal and focal in nature, livers of these Pkd1-deficient mice were severely cystic, enlarged and pale.
Conclusions. These results suggest that the Pkd1flox/flox;Mx1Cre model of ADPKD is amenable to short-term kidney cyst formation drug studies; however, it may be problematic for long-term therapeutic research where widespread liver cysts and fibrosis could compromise drug metabolism.
Keywords: ADPKD, polycystic, triptolide
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is characterized by the progressive formation and expansion of fluid-filled kidney cysts ultimately leading to a decline in kidney function and end-stage renal disease (ESRD). A defect in the gene product of either Pkd1 (85% of all ADPKD cases) or Pkd2 (15% of cases) is thought to result in a loss of calcium signalling in kidney epithelial cells and a reversion to the proliferative phenotype [1,2]. There are numerous murine models of ADPKD available with varying genetic backgrounds, including several with Pkd1 deficiencies [3–9]. Those models utilizing inducible Pkd1 inactivation allow for the study of rapid kidney cyst formation in neonates as well as a delayed disease progression in adult mice [4,6,10], as previous findings demonstrate a difference in the timing of cystogenesis due to Pkd1 inactivation before and after murine postnatal Day 14 (P14) [4,6]. The Pkd1flox/flox;Mx1Cre inducible model has recently been described [10] where Cre-mediated recombination is dependent upon the viral response and endogenous interferon (IFN) production in response to double stranded polyinosinic–polycytidylic acid (pI:pC) injection. As target gene inactivation using the Mx1Cre mouse has been shown to be ~50% in the kidney [11,12], it was possible that this system could be used for the development of a less aggressive (i.e. fewer affected kidney epithelial cells) ADPKD disease progression model. However, while it may be possible to modulate the extent of Mx1Cre-mediated recombination of Pkd1 by pI:pC dosing, previous studies have shown that genetic inactivation of Pkd1 before P14 caused rapid cyst progression within 3 weeks, whereas adult inactivation resulted in few focal kidney cysts after several months [10].
As Pkd1 defects are the primary cause of ADPKD, the ultimate goal would be to develop a slowly progressive Pkd1-based murine model in which to test possible therapeutic agents against cystogenesis and cyst expansion. We have previously shown that the small molecule triptolide can reduce cyst formation and cystic burden in the in utero Pkd1−/− mouse model [13] and in the aggressive neonatal Pkd1flox/−;KspCre model [14]. As triptolide can induce polycystin-2 (PC2) dependent calcium release and inhibit proliferation of Pkd1−/− cells [13], we continue to test the efficacy of this potential therapeutic in murine models of ADPKD. As compared to the previous Pkd1 models tested with triptolide (Pkd1−/− and Pkd1flox/−;KspCre), the Pkd1flox/flox;Mx1Cre model has several benefits including normal kidney development until P10, a longer lifespan and a lower percentage of kidney epithelial cells with Pkd1 inactivation, thus making disease progression less aggressive than in the previous in utero or neonatal models. Since the Pkd1flox/flox;Mx1Cre model had not been tested with any drug therapy regimens and cyst formation when initiated before P14 would allow for examination of cystic burden in an otherwise healthy animal after 3 weeks, we began experiments to determine if triptolide would affect cyst formation in this less aggressive Pkd1-derived ADPKD model.
The goals of this study were to establish a baseline of reproducible cyst formation and progression following P10/P12 induction of Mx1Cre-mediated excision of Pkd1, to determine what effect triptolide would have in a more slowly advancing mouse model of cystic disease and to evaluate the feasibility of using the Pkd1flox/flox;Mx1Cre model in a long-term adult Pkd1 inactivation model for drug therapy studies.
Materials and methods
Animal studies
Mx1Cre mice were purchased from the Jackson Laboratory (Maine, USA) and Pkd1-floxed mice were generated as previously described [8]. All approved animal protocols were conducted in accordance with Yale Animal Resources Center and Institutional Animal Care and Use Committee regulations. Pkd1flox/flox mice were crossed with Pkd1flox/+;Mx1Cre mice and progeny were genotyped. For short-term studies (P35 termination), all littermates were injected intraperitoneally (i.p.) using one of the following regimens: once on postnatal Day P10, once on P12 or twice on both P10 and P12 with 250 μg pI:pC (Sigma-Aldrich, MO, USA). pI:pC was delivered in either a total volume of 12.5 or 25 μl in sterile RNase-free water. Mice were injected i.p. with triptolide or dimethyl sulfoxide (DMSO) delivered in sterile PBS beginning on Day P16 until the termination of the experiment on Day P35. Injections were daily where 0.12 mg/kg triptolide was administered from P16 to P20 and then increased to 0.25 mg/kg from P21 to P35. For long-term studies, mice were induced once at Day P30 with 250 μg pI:pC in a 50 μl volume and were euthanized 5 months after induction. Mice were anaesthetized with 30% isoflurane before submandibular puncture to collect blood for blood urea nitrogen (BUN) levels or alkaline phosphatase analyses (BioAssay Systems, CA, USA).
Tissue histology
Kidneys or livers were fixed in 4% paraformaldehyde, embedded in paraffin and stained with haematoxylin and eosin (H&E; AML Laboratories, MD, USA). Images were acquired under bright-field microscopy; cyst number and cystic burden were quantitated using Image J analysis software (NIH). Ki-67 immunohistochemistry (IHC) was performed by the Yale Pathology Core Tissue Services. Multiple fields of each kidney were photographed using a 40× or 10× objective, and cysts were counted for total cell number and the percent positive Ki-67 cells. A total of 283 cysts were counted for DMSO-treated animals and 212 cysts were counted for triptolide-treated animals. Statistical analysis was completed using KaleidaGraph software where two data sets were analysed using Student’s t-test and three or more data sets were analysed by analysis of variance (ANOVA) using Bonferroni’s post hoc analysis (α = 0.05). Significance was determined as P <0.05. All data are presented as mean ± SE.
MRI acquisition and analysis
For magnetic resonance imaging (MRI) studies, kidneys were removed and fixed in 4% paraformaldehyde before imaging. All experiments were performed on a 15-cm horizontal bore 9.4 T spectrometer (Bruker, MA, USA) with custom-made radio-frequency surface coil (15 mm diameter). Multi-slice axial images were acquired using a rapid acquisition with relaxation enhancement (RARE) spin-echo sequence. Imaging parameters were as follows: field of view = 13 mm, image matrix = 128 × 128, in-plane resolution = 100 × 100 μm, slice thickness = 500 μm, RARE factor = 8, effective echo time = 34 ms and recycle time = 5 s. All MRI images were viewed using home-written Matlab (Natick, MA) software where a threshold was used to suppress the paraformaldehyde signal and reveal the tissue water signal.
Results
Development of the Pkd1flox/flox;Mx1Cre-inducible model
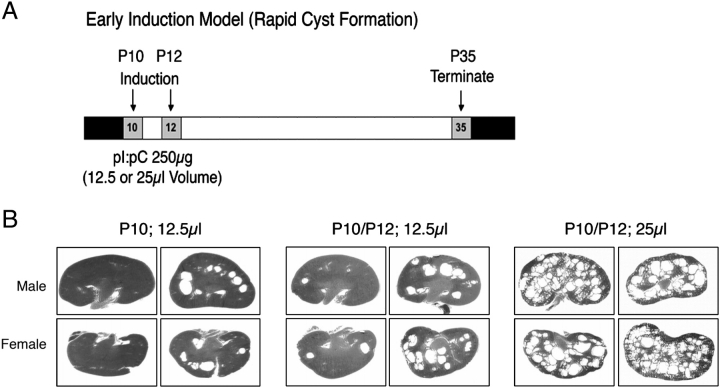
As our ultimate goal of this study was to be able to assess cystic burden and progression as a result of a therapeutic drug treatment regimen with triptolide, we first characterized cyst formation in this inducible Pkd1 deletion model. For postnatal Day P35 termination studies, we crossed Pkd1flox/flox with Pkd1flox/+;Mx1Cre mice to generate Pkd1flox/flox;Mx1Cre cystic mice. Normal development proceeded until neonatal Day P10 at which point we tested a combination of i.p. pI:pC induction strategies to achieve systemic Pkd1 inactivation. pI:pC inductions at P10, P12 or a combination of P10/P12 injections were tested and cyst progression was examined at 5 weeks of age (P35; Figure 1). In addition, given the small size of the neonate, we examined the effect of pI:pC volume on the reproducibility of Mx1Cre induction; 250 μg was delivered i.p. in either a volume of 12.5 or 25 μl.
Fig. 1.
Neonatal cyst formation is dependent on pI:pC dosing schedule. (A) Timeline for pI:pC induction experiments. (B) Figures shown are representative H&E-stained kidney sections from P35 Pkd1flox/flox;Mx1Cre male and female mice induced with 250 μg pI:pC. Variables in the induction schedule are represented as injection at Days P10, P12 or both P10/P12 and the volume of pI:pC delivered in 12.5 or 25 μl.
Results from this short-term model showed that there was high variability of cystogenesis if only one injection of pI:pC was utilized. Animals induced at P12 with a 12.5 μl volume had no reproducible kidney cyst formation by Day P35 (data not shown) and a single induction at P10 at the same volume resulted in a moderate but highly variable cystic burden that was gender independent (Figure 1B). We next tested a double pI:pC induction schedule (P10/P12) with the low 12.5 μl volume; however, results again were highly variable between animals (Figure 1B). When we increased the pI:pC volume to 25 μl, it was not only well tolerated by the neonates but also resulted in a reproducible cystic kidney phenotype present at Day P35 (Figure 1B). While we were not able to obtain consistent results to produce a mild short-term disease model of Pkd1-dependent kidney cyst formation by either single induction or low volume injection, we successfully determined the conditions necessary for robust cyst initiation in this model. It is also of note that, at Day P35, while the kidneys had many cysts of varying sizes, these mice displayed no signs of distress, decreased activity or abdominal bulging. This suggests that while there was rapid cyst formation within 3 weeks in this model, overall survival should extend past 35 days. To examine cystogenesis further in this model, we completed all subsequent experiments using dual P10/P12 induction with a 25 μl injection volume.
Cyst formation and expansion is rapid in the Pkd1flox/flox;Mx1Cre neonate
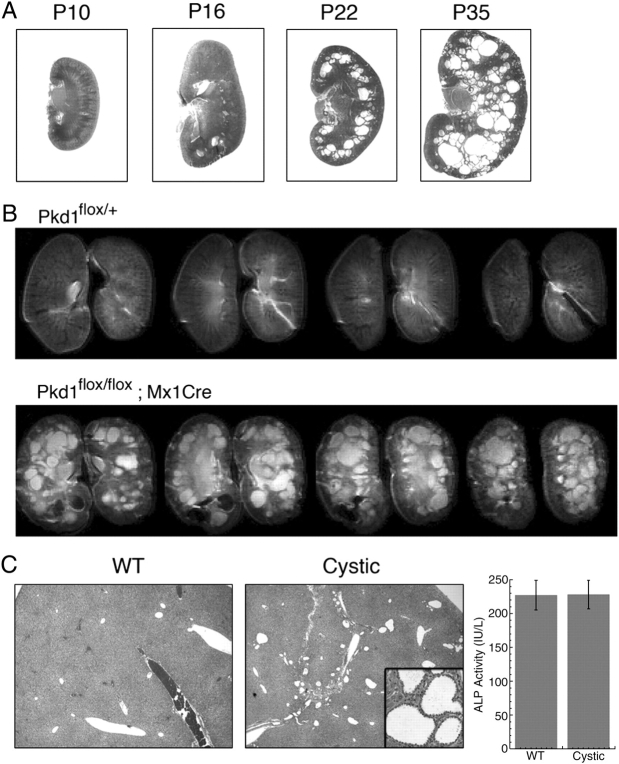
Before beginning drug studies with triptolide or DMSO vehicle control, we first wanted to establish the baseline of cystogenesis in this model. Cyst progression was therefore examined by assessing the rate of cyst growth and expansion at P16, P22 or P35. The cystic phenotype was evident by visual inspection of kidneys at both P22 and P35 as they were larger, pale and cysts were easily seen by light transmission (data not shown). While kidneys at Day P10 appeared normal, following dual P10/P12 induction, cyst formation was already evident at P16 by histological examination (Figure 2A). Additionally, microcysts were observed under higher magnification (data not shown). Cyst growth and expansion continued to proceed at a rapid rate as observed in both P22 and P35 kidneys (Figure 2A).
Fig. 2.
Kidney cystic burden increases rapidly in the Pkd1flox/flox;Mx1Cre neonatal induction model. (A) Representative H&E-stained kidney sections from Pkd1flox/flox;Mx1Cre mice showing progression of cyst formation following pI:pC induction. (B) Contiguous T2-weighted axial slices from non-cystic (Pkd1flox/+) and cystic (Pkd1flox/flox;Mx1Cre) male mice obtained using a multi-slice RARE sequence. Kidneys were harvested at P35 following pI:pC induction at P10/P12; 25 μl. Imaging parameters at 9.4 T were as follows: in-plane resolution = 100 × 100 μm, slice thickness = 500 μm, effective echo time = 34 ms and recycle time = 5 s. (C) Representative H&E-stained liver sections from P35 Pkd1flox/+ (WT) and Pkd1flox/flox;Mx1Cre (Cystic) mice (inset shows ×40 magnification of cholangiocyte-derived liver cysts). Alkaline phosphatase (ALP) activity from blood serum was also analysed as an indicator of liver function (WT n = 3, cystic n = 7; P = 0.9776).
While (H&E) staining and histological examination of each kidney provided us with evidence of the formation of numerous cysts of varying sizes, we further explored the structural changes of the entire kidney using high-resolution MRI. A kidney pair from a single cystic male mouse at Day P35 was scanned in 500 μm axial-slice sections where many cysts were evident in each section with in-plane resolution of 100 × 100 μm (Figure 2B). It appeared that there was a uniform distribution of cysts (white intense regions in lower row of Figure 2B) as they were present in both the kidney cortex and medulla. This was in contrast to a kidney pair from a non-cystic (Pkd1flox/+) male mouse where no abnormalities were evident (Figure 2B).
In addition to kidney cysts, systemic Pkd1 deletion is also known to result in liver cyst formation derived from cholangiocytes. Upon examination of H&E-stained liver sections from P35 cystic mice, many small cysts could be easily identified (Figure 2C). While the overall cystic burden of the liver was minimal, we further wanted to assess liver function by alkaline phosphatase (ALP) activity from blood serum. As compared to wild-type (WT) mice at this age, cystic mice did not show any increase in ALP activity, therefore indicating that liver function was within normal limits. Following the establishment of cyst formation and progression in the kidney and no evidence of altered liver function to affect drug metabolism, we began a series of experiments to test whether triptolide could reduce cystogenesis in this neonatal to adult transition model of Pkd1 inactivation.
Triptolide reduces cyst formation in the P35 Pkd1flox/flox;Mx1Cre mouse
Having established the dosing and timing of pI:pC-induced Pkd1 deletion in this model, we next determined the triptolide treatment regimen. As pI:pC-induced IFN production in the mouse has been established to subside after 2 days, we wanted to permit the full immune response before introducing triptolide since triptolide is a known anti-inflammatory agent [15,16]. Therefore, triptolide was not administered until P16, or 4 days after the last pI:pC injection when small cysts were already evident (Figure 2A). We began i.p. injections with 0.12 mg/kg/day triptolide as we have previously shown this concentration to be well tolerated by neonates [14]. At weaning (P21), we increased the triptolide concentration to 0.25 mg/kg/day, which we have established as safe with no adverse side effects in adult mice (unpublished data).
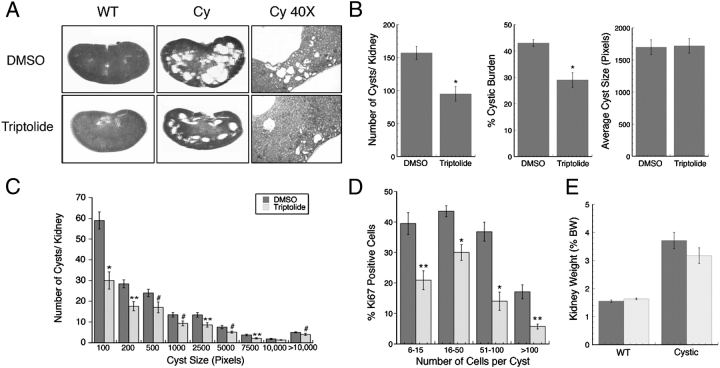
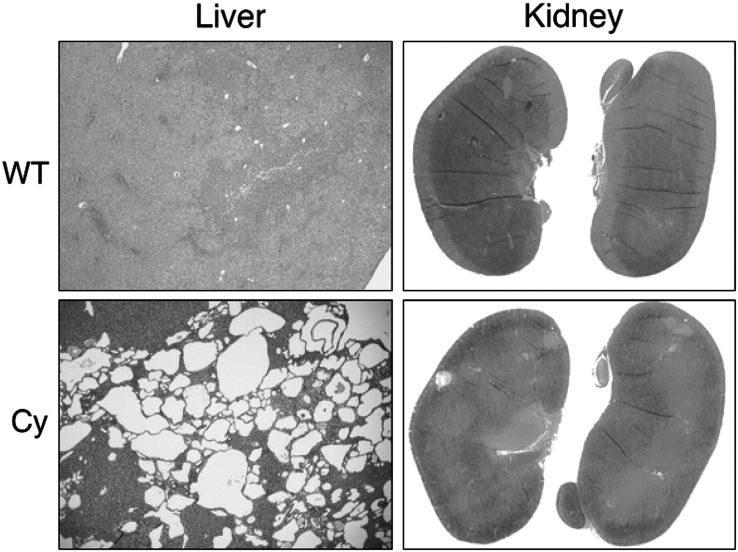
All animals during the course of the study in either treatment group had normal behaviour, and up to the P35 termination date, no gross anatomical differences such as abdominal distention could be visualized. Control WT (Pkd1flox/+ or Pkd1flox/flox) mice in both treatment groups had normal kidneys with no evidence of cyst formation (Figure 3A). Mice (Pkd1flox/flox;Mx1Cre) treated with DMSO vehicle control had large cystic kidneys at P35 as previously demonstrated (Figure 3A). In contrast, mice treated with triptolide not only had reduced overall cystic burden but it was also evident that small cysts formed at the kidney cortex periphery were frequently reduced in number (Figure 3A). Statistical analysis of kidney sections [DMSO: n = 42 (21 mice), triptolide: n = 40 (20 mice)] confirmed that triptolide reduced the total number of cysts per kidney as well as the overall cystic burden (Figure 3B). This was especially encouraging as cyst formation had already begun when triptolide treatment commenced at P16 (Figure 2A). Although there was no difference in the combined average cyst size between the two treatment groups, as we have previously observed [14] (Figure 3B), we further analysed the data by calculating the frequency of cyst sizes (i.e. indication of cyst expansion) per group as well (Figure 3C). While the most pronounced triptolide-induced decrease was detected in the smallest cyst group (up to 100 pixels) and supported our findings that triptolide acts to delay cystogenesis in the Pkd1flox/−;KspCre model [14], we also observed a significant decrease in the number of subsequent larger cysts. While it is not clear at this time if triptolide is directly affecting cyst enlargement, it is encouraging that we saw this result for the first time in a more slowly developing cyst progression model. In order to determine if triptolide could reduce proliferation of kidney epithelial cells, IHC was completed on kidney sections for the expression of the proliferation marker, Ki-67. Cysts of multiple sizes and stages of progression ranging from six cells to >200 cells were counted for both total cell number and the number of Ki-67-positive cells to determine the percent of proliferating cells per cyst. Triptolide reduced the proliferation rate independent of cyst size as determined by t-test analysis (Figure 3D). Although the largest cyst size group (>100 cells) had the lowest percentage of proliferating cells when compared to smaller cysts in DMSO-treated animals (17%), triptolide further reduced the proliferation rate to 6%.
Fig. 3.
Triptolide reduces cystic burden in the Pkd1flox/flox;Mx1Cre model of ADPKD. (A) Representative H&E-stained P35 kidney sections from wild-type Pkd1flox/+ (WT) and cystic Pkd1flox/flox;Mx1Cre (Cy) mice treated with DMSO or triptolide (×8 magnification). Far right panels are representative H&E-stained outer cortex kidney sections showing areas of small cyst formation (×40 magnification). (B) Analysis of kidney cyst number, % cystic burden and average cyst size (kidneys: n = 42 DMSO and n = 40 triptolide) from cystic P35 Pkd1flox/flox;Mx1Cre mice. (C) Frequency distribution of the number of cysts present of varying size. Cyst size is represented in pixels as determined from Image J analysis software (kidneys: n = 42 DMSO and n = 40 triptolide). (D) The percent of proliferating cells per cyst as assessed by Ki-67 immunohistochemical staining. Cells per cyst were counted from sagittal cross sections taken from DMSO- or triptolide-treated mice (The number of total cysts analysed for DMSO was 283 and for triptolide was 212). (E) Analysis of kidney weight as a percentage of body weight (%BW) from cystic (n = 21 DMSO, n = 20 triptolide) and non-cystic (WT) (n = 24 DMSO, n = 26 triptolide) P35 mice treated with either DMSO or triptolide. All mice were induced at P10/P12 using a 25 μl pI:pC volume. For all panels: *P < 0.0001, **P < 0.003 and #P < 0.05 by Student’s t-test.
No overt toxicity was detected as triptolide did not affect the total body animal weight in comparison to the respective WT or cystic DMSO control (data not shown). Additionally, although the percent kidney weight to body weight ratio tended to be lower in triptolide-treated cystic animals, this did not reach statistical significance as compared to DMSO control (Figure 3E).
Triptolide preserves renal function
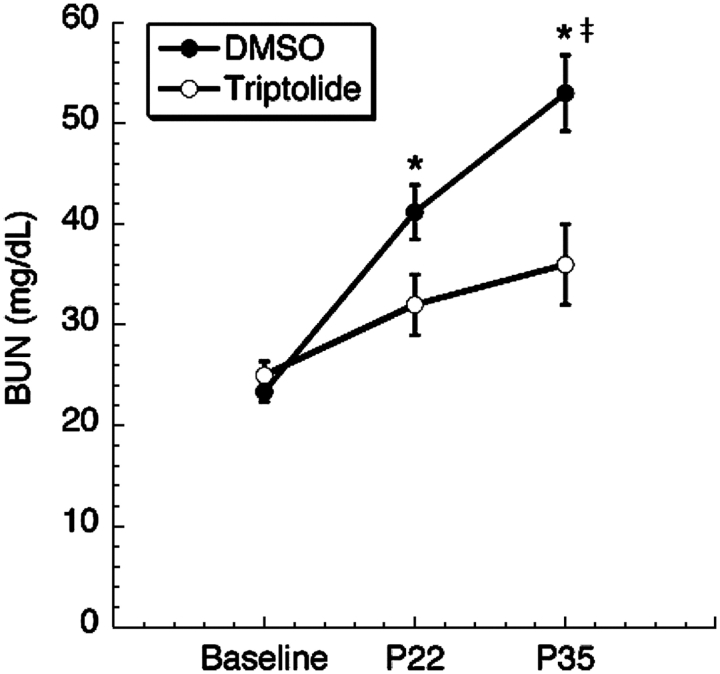
While the results from kidney histology of triptolide-treated mice were encouraging, we also wanted to determine if the reduction in cyst number translated into a benefit in renal function as assessed by BUN and, therefore, analysed BUN changes from P22 to P35. Baseline BUN values were calculated from an average of WT mice at P22 from either DMSO- or triptolide-treated animals where there was no difference between experimental groups (Figure 4). We did, however, observe a significant reduction in BUN levels from Pkd1flox/flox;Mx1Cre triptolide-treated animals as early as P22 when compared to DMSO control (DMSO n = 13: 41 ± 3, triptolide n = 11: 31 ± 3; P = 0.03202 by t-test) and this trend continued through to the P35 endpoint (DMSO n = 13: 53 ± 4, triptolide n = 11: 37 ± 3; P = 0.01047 by t-test). When the data were analysed by ANOVA to determine the relative change in BUN levels over time (i.e. baseline through P35), triptolide-treated mice showed no significant difference from baseline values (Figure 4). In contrast, there was a statistically significant increase in BUN levels over the same time course in animals treated with DMSO (P < 0.0001; Figure 4). Taken together, triptolide reduced cyst formation, cystic burden and preserved renal function in a neonatal to adult transition model where overt clinical signs of disease were not yet evident.
Fig. 4.
Triptolide preserves renal function. BUN values were measured from cystic mice over time on both P22 and P35 (n = 13 DMSO, n = 11 triptolide; asterisk indicates significance at P < 0.035 by Student’s t-test). Baseline refers to the average BUN values of non-cystic mice treated with DMSO or triptolide taken on P22. Double dagger indicates P < 0.0001 for the increase in BUN from baseline through P35 for DMSO by ANOVA using Bonferroni’s post hoc analysis (α = 0.05). There was no increase in BUN from baseline through P35 (P = 0.10322) with triptolide treatment.
Pkd1flox/flox;Mx1Cre adult induced mice have severe liver cysts and fibrosis
As we continued to test triptolide in progressively older Pkd1 animal models to more closely approximate human ADPKD disease progression, we were interested in pursuing the Pkd1flox/flox;Mx1Cre model for drug studies following adult (post-P14) Pkd1 inactivation. Therefore, we next completed an adult induction study, where cyst formation and expansion requires several months to become evident following Pkd1 deletion. We induced Pkd1flox/flox;Mx1Cre mice at P30 with a single injection of 250 μg pI:pC and examined cyst formation after 5 months. Most mice, irrespective of gender or genotype, remained active and no visible signs of disease were evident over the course of the experiment; however, one male cystic mouse at 4 months of age had to be euthanized due to severe abdominal bulging. Upon necropsy, the liver was found to be severely cystic and there was development of ascites indicative of liver damage; however, the kidneys were normal with no cystic involvement. Upon dissection of the remainder of induced Pkd1flox/flox;Mx1Cre mice at 6 months of age, fluid-filled liver cysts were visible by eye and histological examination of H&E-stained liver sections confirmed extensive cyst formation and fibrosis in all animals (Figure 5). This was in contrast to livers from WT (Pkd1flox/flox) mice where liver histology appeared normal (Figure 5).
Fig. 5.
Widespread liver cysts are present 5 months post-Pkd1 adult deletion. Representative H&E-stained liver sections (×40 magnification) from Pkd1flox/flox wild-type (WT) or induced Pkd1flox/flox;Mx1Cre cystic (Cy) mice. Normal hepatic structures such as the central vein and portal triad can be observed in the WT sections. H&E kidney sections are also represented (×8 magnification) from WT and cystic mice. All tissues were obtained at 5 months following 1× 250 μg pI:pC induction at P30.
While the livers from the long-term study mice confirmed that pI:pC induction was successful, there was little evidence of cyst formation in the kidney. A few focal cysts were apparent in the kidneys of induced animals; however, the majority of nephron structure and parenchyma were normal (Figure 5). We analysed BUN levels from Pkd1flox/flox;Mx1Cre mice and the average was 30 ± 3.2 mg/dl (n = 5), indicating that kidney function was comparable to control animals (26 ± 0.7, n = 2; P = 0.1244). While it is possible that we would need to extend our study to observe kidney cyst progression with this induction schedule, it is also possible that we might have to increase the number of pI:pC injections in the adult mouse to induce cysts in the kidney. However, as liver cyst formation is already severe with the current induction protocol, it is doubtful that the mice would survive to the point of kidney cyst formation as liver failure would likely lead to premature death.
Discussion
The Pkd1flox/flox;Mx1Cre mouse model of ADPKD has recently been described [10], although it had not yet been used to test potential therapeutic agents. In this study, we have assessed the small molecule triptolide for its ability to reduce cyst formation in this system. Although we have previously shown that triptolide is a promising candidate for ADPKD therapeutic intervention, our continued goal is to examine its efficacy in older Pkd1-derived murine models where cyst progression extends to adulthood. As the Pkd1flox/flox;Mx1Cre IFN-inducible mouse permits the specific timing of Pkd1 deletion and results in rapid (but not fatal) cyst formation within 3 weeks following neonatal (pre-P14) Pkd1 inactivation, this was an appropriate model in which to test the effect of triptolide.
Since we were testing a drug that could reduce cyst formation, we first had to confirm the reproducibility of this induction model. As the immune response to dsRNA leading to IFN production may vary between animals, we first tested the severity of kidney cystogenesis upon induction within several days of the P14 developmental window and modulation of i.p. injection volumes. Results from this short-term study demonstrate that a modest volume increase (from 12.5 to 25 μl) compounded with dual inductions decreased intrinsic experimental error and allowed us to obtain reproducible kidney cyst formation and progression without an immediate and fatal decline in kidney function. By P35, at the termination of the experiment, kidney cysts were numerous and of varying size, BUN levels had increased but had not led to renal failure or any obvious physical abnormalities and cholangiocyte-derived liver cysts were present but were not severe with no evidence of altered hepatic function.
We have previously demonstrated that triptolide reduces cystic burden in embryonic Pkd1−/− mice [13]; however, due to fatal developmental abnormalities, triptolide administration was limited to a short in utero treatment regimen. Subsequent experiments using the neonatal Pkd1flox/-;KspCre mouse model of ADPKD, in which cyst formation and enlargement is aggressive and mice commonly die by 2 weeks of age, established that triptolide reduces cystic burden by decreasing cyst formation but not cyst expansion. Although pre-P14 inactivation of Pkd1 results in an accelerated cystic disease (as compared to adult induction) in this current Pkd1flox/flox;Mx1Cre-inducible model of cystogenesis, mice remain healthy and viable at >1 month of age. Therefore, with this mouse model we can assess a longer treatment regimen of triptolide and more closely approximate any benefit resulting in the inhibition of cyst formation transitioning from the neonatal period of development to adult.
We began triptolide injections 4 days (P16) after the last pI:pC injection to allow for the full effect of IFN-induced Pkd1 inactivation, when small cysts were already evident (Figure 2A). At the termination of the experiment at Day P35, triptolide, as previously shown in more aggressive Pkd1-dependent ADPKD models, reduced the total number of cysts and overall cystic burden. Renal function, already showing signs of decline as assessed by increasing BUN levels on Day P22, was reduced in the presence of triptolide only 6 days after the start of injections and continued through to the P35 endpoint. Histological examination of H&E-stained kidney sections showed a significant reduction in the number of small, newly formed cysts, suggesting that triptolide attenuates cystogenesis or delays progression of cellular proliferation. When the distribution of cyst sizes per kidney was also analysed for any differences in the number per size group, we were surprised to observe an almost uniform decrease in cysts at all stages of expansion. These results may partially be explained by the reduction in cyst cell proliferation that we observed following Ki-67 staining. Triptolide treatment led to a decrease in the number of actively dividing cells in all cyst sizes that could then lead to the overall reduction in total cyst numbers. From these experiments, it is not yet clear what role triptolide is having on larger cysts that may have formed before drug administration; however, since this is the first longer-term and less aggressive model of Pkd1-induced cyst formation in which triptolide has been tested, it is possible that its therapeutic potential may extend beyond inhibition of the earliest stages of cyst initiation. Future studies are planned to address this as a reduction of cystic burden through attenuation of small cyst progression may directly affect pre-existing cyst expansion or further deterioration of the surrounding parenchyma. It is also possible that in a longer-term model, triptolide’s known anti-inflammatory effects may also contribute to maintaining renal integrity and function, as leukocyte infiltration into the kidney is associated with disease progression.
The use of the Pkd1flox/flox;Mx1Cre mouse model for therapeutic drug studies may be limited to pre-P14 Pkd1 deletion and a shorter time course for the evaluation of cyst progression as adult Pkd1 excision leads to aggressive liver cyst formation. Although liver cysts were reported in the study by Takakura et al. [10], they were not observed until almost 1 year of age following Pkd1 inactivation at 5 weeks. Differences in the genetic background of the mice used or differences in pI:pC concentration and frequency of injections may add to the discrepancy seen between studies. Our model and pI:pC induction schedule in adult animals results in severe cystic liver disease as Mx1Cre-mediated excision has previously been shown to be nearly 100% in the liver [11]. It is, however, interesting that Pkd1 inactivation in the neonate leads to a faster cyst expansion in the kidney while inactivation in the adult mouse leads to a more aggressive liver disease. This Pkd1flox/flox;Mx1Cre model may therefore be of use in dissecting the mechanisms of cyst formation in two relevant target organs in ADPKD as the development of hepatic cysts is a frequent extra-renal manifestation in humans [17].
The Pkd1flox/flox;Mx1Cre mouse adds another alternative to ADPKD models that are currently available and should be amenable to future therapeutic drug studies. A long-term drug study with the use of this model would have been ideal as a slowly progressive ‘adult’ model of ADPKD would more closely mimic the human condition as Pkd1 would be subject to inactivation in every organ system; however, extensive liver cyst formation makes this problematic as drug metabolism may be altered.
Triptolide, a small molecule previously known to act as an anti-inflammatory and anti-cancer compound, has shown efficacy as a possible therapeutic for the treatment of ADPKD. It causes polycystin-2-dependent calcium release, increases p21 expression and arrests Pkd1−/− renal epithelial cell proliferation. Our previous in vivo studies with triptolide in the Pkd1−/− in utero mouse and the neonatal Pkd1flox/−;KspCre mouse have shown that, even in these aggressive and rapidly fatal models, cystic burden and cyst numbers decreased. In our endeavour to study triptolide efficacy in Pkd1-dependent ADPKD, we continue to test triptolide in those models that more closely approximate the human condition such as a slow progression to ESRD. In this study, using the Pkd1flox/flox;Mx1Cre mouse, we have not only confirmed previous results that triptolide decreases cyst initiation but we have also observed a general decrease in the number of larger cysts as well. As murine Pkd1 models are developed that result in slow cyst formation and accumulation over time, we can better elucidate the full therapeutic potential of triptolide as an inhibitor of cyst initiation and what impact that may have on the expansion of mature cysts, kidney inflammation and the preservation of renal function and integrity.
Acknowledgments
This work was supported by research grants from US National Institutes of Health (AI055914 to C.M.C.; P30, NS-052519 to F.H.).
Conflict of interest statement. None declared.
References
- 1.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 2.Yamaguchi T, Hempson SJ, Reif GA, et al. Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 3.Boulter C, Mulroy S, Webb S, et al. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–12179. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, et al. Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet. 2007;16:3188–3196. doi: 10.1093/hmg/ddm299. [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Peissel B, Babakhanlou H, et al. Perinatal lethality with kidney and pancreas defects in mice with a targetted Pkd1 mutation. Nat Genet. 1997;17:179–181. doi: 10.1038/ng1097-179. [DOI] [PubMed] [Google Scholar]
- 6.Piontek K, Menezes LF, Garcia-Gonzalez MA, et al. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007;13:1490–1495. doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piontek KB, Huso DL, Grinberg A, et al. A functional floxed allele of Pkd1 that can be conditionally inactivated in vivo. J Am Soc Nephrol. 2004;15:3035–3043. doi: 10.1097/01.ASN.0000144204.01352.86. [DOI] [PubMed] [Google Scholar]
- 8.Shibazaki S, Yu Z, Nishio S, et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet. 2008;17:1505–1516. doi: 10.1093/hmg/ddn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starremans PG, Li X, Finnerty PE, et al. A mouse model for polycystic kidney disease through a somatic in-frame deletion in the 5' end of Pkd1. Kidney Int. 2008;73:1394–1405. doi: 10.1038/ki.2008.111. [DOI] [PubMed] [Google Scholar]
- 10.Takakura A, Contrino L, Beck AW, et al. Pkd1 inactivation induced in adulthood produces focal cystic disease. J Am Soc Nephrol. 2008;19:2351–2363. doi: 10.1681/ASN.2007101139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhn R, Schwenk F, Aguet M, et al. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- 12.Schneider A, Zhang Y, Guan Y, et al. Differential, inducible gene targeting in renal epithelia, vascular endothelium, and viscera of Mx1Cre mice. Am J Physiol Renal Physiol. 2003;284:F411–F417. doi: 10.1152/ajprenal.00235.2002. [DOI] [PubMed] [Google Scholar]
- 13.Leuenroth SJ, Okuhara D, Shotwell JD, et al. Triptolide is a traditional Chinese medicine-derived inhibitor of polycystic kidney disease. Proc Natl Acad Sci U S A. 2007;104:4389–4394. doi: 10.1073/pnas.0700499104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuenroth SJ, Bencivenga N, Igarashi P, et al. Triptolide reduces cystogenesis in a model of ADPKD. J Am Soc Nephrol. 2008;19:1659–1662. doi: 10.1681/ASN.2008030259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H, Hachida M, Enosawa S, et al. Immunosuppressive effect of triptolide in vitro. Transplant Proc. 1999;31:2056–2057. doi: 10.1016/s0041-1345(99)00262-6. [DOI] [PubMed] [Google Scholar]
- 16.Qiu D, Zhao G, Aoki Y, et al. Immunosuppressant PG490 (triptolide) inhibits T-cell interleukin-2 expression at the level of purine-box/nuclear factor of activated T-cells and NF-kappaB transcriptional activation. J Biol Chem. 1999;274:13443–13450. doi: 10.1074/jbc.274.19.13443. [DOI] [PubMed] [Google Scholar]
- 17.Everson GT, Taylor MR, Doctor RB. Polycystic disease of the liver. Hepatology. 2004;40:774–782. doi: 10.1002/hep.20431. [DOI] [PubMed] [Google Scholar]