Abstract
Background. Patients with end-stage renal disease (ESRD) requiring chronic haemodialysis who undergo coronary artery bypass graft surgery (CABG) are at significant risk for perioperative mortality. However, the impact of changes in ESRD patient volume and characteristics over time on operative outcomes is unclear.
Methods. Using the Nationwide Inpatient Sample database (1988–03), we evaluated rates of CABG surgery with and without concurrent valve surgery among ESRD patients and outcomes including in-hospital mortality, and length of hospital stay. Multivariate regression models were used to account for patient characteristics and potential cofounders.
Results. From 1988 to 2003, annual rates of CABG among ESRD patients doubled from 2.5 to 5 per 1000 patient-years. Concomitantly, patient case-mix changed to include patients with greater co-morbidities such as diabetes, hypertension and obesity (all P < 0.001). Nonetheless, among ESRD patients, in-hospital mortality rates declined nearly 6-fold from over 31% to 5.4% (versus 4.7% to 1.8% among non-ESRD), and the median length of in-hospital stay dropped in half from 25 to 13 days (versus 14 to 10 days among non-ESRD).
Conclusions. Since 1988, an increasing number of patients with ESRD have been receiving CABG in the USA. Despite increasing co-morbidities, operative mortality rates and length of in-hospital stay have declined substantially. Nonetheless, mortality rates remain almost 3-fold higher compared to non-ESRD patients indicating a need for ongoing improvement.
Keywords: coronary artery bypass graft, end-stage renal disease, in-hospital mortality, perioperative outcomes
Introduction
End-stage renal disease (ESRD) is a major public health problem with more than one million patients requiring renal replacement therapy worldwide [1]. In the USA, the prevalence of ESRD is expected to increase to over 650 000 by 2010 [2,3]. Cardiovascular disease remains the leading cause of death in patients with ESRD; in fact, mortality due to cardiovascular disease is 10 to 30 times higher among dialysis-dependent patients than in the general population [4]. A higher propensity for cardiac death in patients with ESRD may be explained by the increased presence of hypertension, hyperlipidaemia and abnormal calcium–phosphorus metabolism, leading to accelerated atherosclerosis [5–7].
Treatment options for coronary artery disease include percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass graft; however, PTCA has been associated with acute complications and poor long-term prognosis in patients with ESRD [6,8,9]. Coronary artery bypass graft surgery (CABG) may be a possible treatment option for dialysis-dependent patients with coronary artery disease refractory to medical therapy because of improved overall and symptom-free survival compared to PTCA [10,11]. Although some studies have concluded that ESRD patients benefit from improved survival and quality of life following CABG, other investigations suggest the opposite [12–15].
In addition to a lack of clarity regarding optimal treatment, concerns of high perioperative mortality rates following CABG have helped to maintain doubts regarding the benefits of CABG in the ESRD population. However, most of these observations include single-centre studies with small numbers of patients [10,11,13,14,16]. Furthermore, the impact of changes in ESRD patient volume and characteristics on operative outcomes over time remains unclear. Therefore, using a national database, we sought to assess rates of CABG among ESRD patients over time and associated outcomes including perioperative mortality and hospital length of stay.
Subjects and methods
We used the Nationwide Inpatient Sample (NIS), a database that was developed in 1988 by the Agency for Healthcare Research and Quality to analyse national trends in health-care utilization, quality and outcomes during inpatient hospitalizations. The NIS database contains discharge level information on all inpatients from a 20% stratified sample of all community hospitals across the USA, representing ~90% of all hospitals in the country. Data from 1988 to 2003 was used for this analysis. Because the database is publicly available and does not contain any patient identifiers in the dataset, it was approved as an exempt study by the Duke University Institutional Review Board. Each member of the research team with access to the NIS dataset has a Data Use Agreement on file with the Agency for Healthcare Research and Quality.
Study design and selection criteria
Core inpatient files were obtained for each year from 1988 to 2003. Data from NIS core files were extracted and merged with hospital-level core files for each year to obtain the total discharges with hospital data (Figure 1, box A, n = 107 500 645). Cases with a discharge procedure code pertaining to CABG surgery in any procedure data field were identified using the codes shown in Table 1. The Clinical Classification Software procedure code for CABG surgery (code 44) and valve surgery with CABG (code 43) were used to produce another analytic dataset of CABG patients from 1988 to 2003 (Figure 1, box B, n =1 424 611). Thus, subsequent references to CABG patients in this study refer to a population that includes CABG patients with and without valve surgery. In order to avoid coding errors, all discharges from hospitals reporting less than five CABG procedures annually, discharges with missing gender information and those less than 18 years of age were excluded, leaving the CABG study population of interest (Figure 1, box C, n = 1 421 891). Patients with ESRD were identified by the International Classification of Disease Ninth Revision Clinical Modification (ICD9-CM) procedure codes for peritoneal dialysis (54.98) or haemodialysis (39.95) who lacked diagnosis codes for acute renal failure (584.0, 584.5, 584.6, 584.7, 584.8 and 584.9). Use of ICD9-CM codes has been previously demonstrated to have >90% sensitivity and negative predictive value for exclusion of acute renal failure [17]. The final subset of interest (Figure 1, box D, unweighted n = 3485) included the CABG and ESRD population that were subject to additional analyses.
Fig. 1.
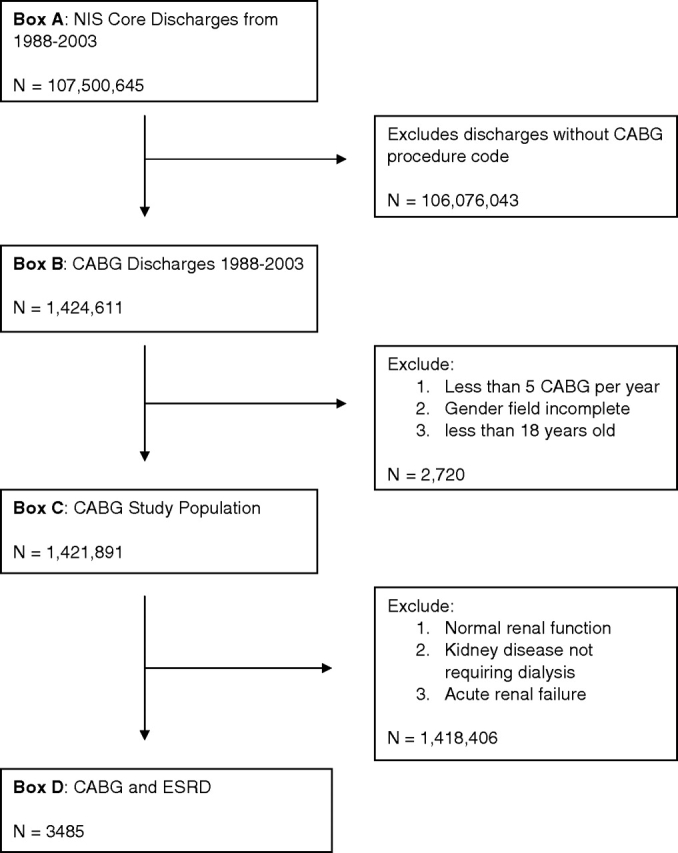
Flow diagram describing the selection criteria for discharges from core NIS data files from 1988 to 2003. Boxes A through D indicate the level of data after application of selection criteria at each step.
Table 1.
Details of the clinical classification software and ICD9-CM codes used to identify study sample
| CCS code | ICD9-CM code | Description | |
|---|---|---|---|
| Diagnosis | |||
| Acute renal failure | 157 | 584 | Acute renal failure |
| Procedures | |||
| CABG | 44 | 36.1 | Aortocoronary bypass |
| 36.2 | Revascularization by arterial implant | ||
| 36.3 | Other heart revascularization (e.g. transmyocardial laser) | ||
| Valve surgery | 43 | 35.1 | Open heart valvuloplasty without replacement |
| 35.2 | Replacement of heart valve | ||
| Dialysis | 58 | 39.95 | Haemodialysis, haemofiltration, haemodiafiltration, renal dialysis |
| 54.98 | Peritoneal dialysis | ||
Subsections of ICD-9 CM codes are available at the NIS website. CABG = coronary artery bypass graft surgery; CCS = clinical classification software; ICD9-CM = International Classification of Disease Ninth Revision Clinical Modification.
Demographic and procedural variables
Demographic and admission covariables were available in each core inpatient file. For purposes of risk adjustment, co-morbidities were identified using ICD9-CM diagnosis codes [18]. Race was not included due to incomplete availability. Principal outcome variables were in-hospital mortality, median length of hospital stay and discharge disposition. Each of the principle outcome variables are described in further detail in the statistical analysis section.
Statistical analysis
All subsequent analyses were performed on the subset of CABG patients with ESRD (Figure 1, box D). In addition, all analyses were conducted with application of discharge weights provided by the NIS, to obtain nationally representative patient-level data (weighted patients with ESRD n = 17 500). Using estimates of prevalent ESRD patients from the United States Renal Data System (USRDS), the estimated annual proportion of ESRD patients undergoing CABG surgery from 1988 to 2003 was also determined [19,20]. Trends in the three outcomes of interest were then examined. Firstly, unadjusted annual mortality rates were calculated. Secondly, annual median length of hospital stay (LOS) was determined for ESRD patients following CABG who survived until hospital discharge. Finally, because LOS may be influenced by variable discharge criteria, discharge disposition was assessed for the group of survivors. For statistical purposes, discharge disposition was categorized as ‘routine’ or ‘non-routine’. Routine discharges were to home while non-routine discharges were to a skilled nursing facility, intermediate care facility, short-term hospital, home healthcare or ‘against medical advice’.
We used multivariable regression models to determine the independent association of year with incidence of CABG patients with ESRD, mortality and mean length of hospital stay among CABG patients with ESRD, and discharge disposition among ESRD survivors. Each model included covariables described above. Significance was assessed at an alpha level <0.05. All statistical analyses were conducted using the SAS software programme, version 9.1 (SAS Institute, Cary, NC).
Results
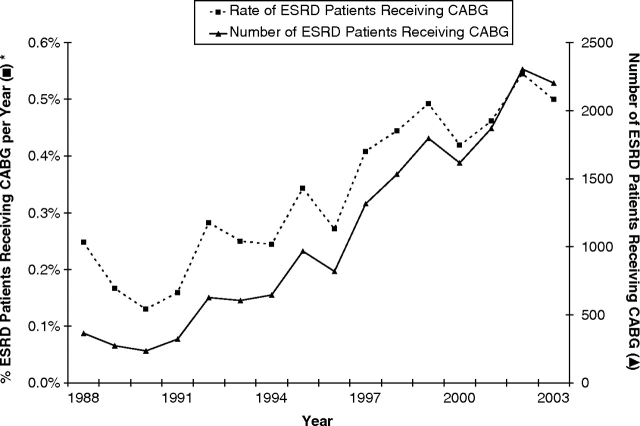
During the study period from 1988 to 2003, the proportion of patients with ESRD who underwent CABG increased (Figure 2). Expressed as a proportion of all ESRD patients, 0.24% of ESRD patients received CABG in 1988, while the proportion of CABG patients with ESRD more than doubled to 0.5% in 2003.
Fig. 2.
Percentage and number of patients with ESRD receiving CABG from 1988 to 2003.
Several characteristics of the ESRD population requiring CABG changed during the observation period (Table 2). The proportion with diabetes mellitus, hypertension, anaemia, obesity, atrial fibrillation and prior myocardial infarction increased, while the gender distribution did not change significantly. Similarly, the proportion with congestive heart failure and chronic obstructive pulmonary disease remained relatively constant from 1988 to 2003. However, the median age of patients with ESRD who underwent CABG decreased significantly over the 16-year interval.
Table 2.
Clinical and demographic characteristics of US end-stage renal disease patients undergoing coronary artery bypass surgery, by time intervals
| Variable | 1988–1991 | 1992–1995 | 1996–1999 | 2000–2003 | P-value* |
|---|---|---|---|---|---|
| Age | 65 (57–71) | 66 (56–72) | 64 (54–71) | 63 (55–70) | <0.0001 |
| Female gender | 32.74 | 35.25 | 34.51 | 37.46 | 0.0977 |
| Diabetes | 34.51 | 46.22 | 52.33 | 59.66 | <0.0001 |
| Congestive heart failure | 31.42 | 35.07 | 34.61 | 35.01 | 0.4871 |
| Hypertension | 38.94 | 53.78 | 68 | 79.4 | <0.0001 |
| Obesity | 1.33 | 0.9 | 2.71 | 3.49 | 0.001 |
| Anaemia requiring transfusion | 11.5 | 16.19 | 21.36 | 33.66 | <0.0001 |
| Atrial fibrillation | 19.47 | 25.18 | 31.44 | 31.27 | <0.0001 |
| Acute myocardial infarction | 19.47 | 17.99 | 22.48 | 22.81 | 0.0306 |
| Chronic obstructive pulmonary disease | 9.73 | 10.79 | 12.59 | 12.14 | 0.2625 |
| Length of hospital stay, days | 15 (11–27) | 14 (9–22) | 11 (7–16) | 10 (7–16) | <0.0001 |
P-values (determined by analysis of variance models comparing 4-year periods) indicate significance for trend across the 16-year period. All values are presented as percent or mean (with 95% confidence intervals).
Interquartile differences for variables were not analysed separately; instead, the P-values indicate significance over the cumulative 16-year period.
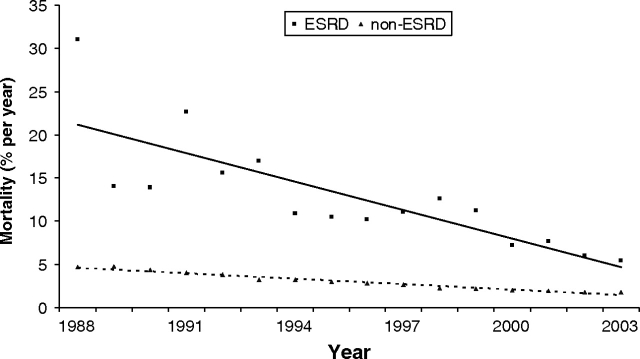
In-hospital mortality decreased over the 16-year observation period for patients with and without ESRD who underwent CABG (Figure 3). In 1988, the unadjusted mortality rate for chronic dialysis patients was higher (31%) compared to patients without ESRD (4.7%). By 2003, the mortality rates for both groups of patients had improved; in-hospital death among CABG patients with ESRD decreased significantly (5.4%). Despite improved perioperative survival, mortality rates remained over three times greater for dialysis-dependent CABG patients compared to patients without ESRD (1.8%).
Fig. 3.
Adjusted in-hospital mortality rates among patients with and without ESRD following CABG. Regression lines are shown for ESRD (straight line) and non-ESRD (dashed line) patients.
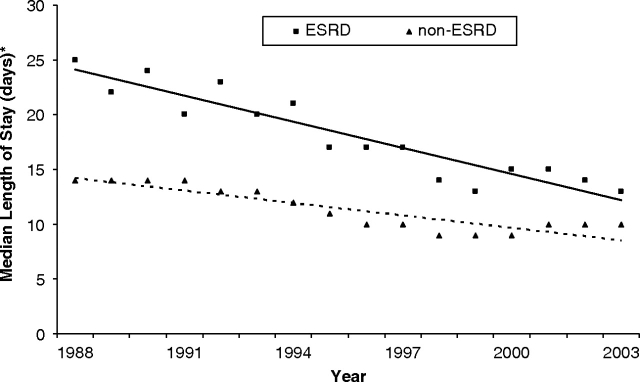
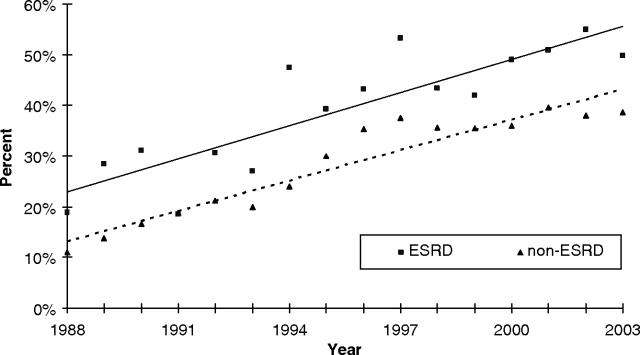
However, inpatient hospital duration among ESRD patients who survived CABG to hospital discharge declined from an average median length of stay of 25 days [95% confidence interval (CI): 20 to 30] in 1988 to 13 days (95% CI: 12 to 14) in 2003 (Figure 4). Among ESRD survivors, analysis of discharge disposition showed an increase in non-routine discharges. In 2003, 49.8% of discharges among post-CABG patients with ESRD required continuing health-care assistance compared to less than half of that amount (18.9%) in 1988 (Figure 5).
Fig. 4.
Median length of hospital stay in days among survivors with and without ESRD following CABG with vertical bars indicating the confidence intervals. Regression lines are shown for ESRD (straight line) and non-ESRD (dashed line) patients.
Fig. 5.
Percentage of post-CABG survivors with and without ESRD with non-routine discharge disposition from 1988 to 2003. Regression lines are shown for ESRD (straight line) and non-ESRD (dashed line) patients.
Discussion
Using a large national database of inpatient discharges, we found that the proportion of patients with ESRD undergoing CABG has increased significantly from 1988 to 2003. Despite an increase in CABG procedures among ESRD patients, a simultaneous decline in the annual in-hospital mortality rate was observed. Although length of stay among survivors also decreased, health-care utilization may have shifted to other health-care settings, as a 2-fold increase was observed in the proportion of non-routine discharges to a skilled nursing or intermediate care facility, short-term hospital or home healthcare.
Although dialysis improves the quality and prolongs the quantity of life for patients with ESRD, mortality rates are high with most deaths attributable to cardiovascular complications [21,22]. The higher incidence of coronary artery disease in this patient population can be attributed to the presence of co-morbid conditions that include lipid abnormalities, fluid overload, abnormal carbohydrate metabolism, platelet dysfunction and calcifications due to hyperparathyroidism [6,22]. With >30% of the cardiac deaths directly attributed to myocardial infarction [5,6], requirements for myocardial revascularization will continue to increase [1,18,19,23]. Our findings appear to support these projections by demonstrating an increasing trend of surgical revascularizations among patients with ESRD between 1988 and 2003.
While revascularization with both PTCA and CABG are technically feasible in patients with ESRD, higher rates of recurrent angina and restenosis limit the long-term benefits of PTCA. Kahn et al. first described a high incidence of recurrent angina pectoris (82%) with angiography showing restenosis in 69–100% of patients within 6 months following PTCA in 17 dialysis patients [8]. Additionally, the long-term survival benefits for CABG appear to be more favourable compared to PTCA [24]. For example, Rinehart et al. demonstrated a better clinical outcome with comparable mortality at 24 months, despite more severe cardiovascular disease in patients undergoing surgical revascularization [25]. Koyanagi et al. observed fewer myocardial infarctions and sudden cardiac deaths following CABG compared to PTCA; they also described a significantly better 5-year event-free rate following CABG (70%) compared to PTCA (18%) [10]. Furthermore, among patients with ESRD and severe cardiovascular disease, CABG was associated with lower rates of all-cause mortality, sudden cardiac death, acute myocardial infarction alone and combined acute myocardial infarction [26].
Although long-term mortality may improve with surgical revascularization in dialysis patients with coronary artery disease, perioperative mortality continues to remain higher among ESRD patients requiring CABG. One study from 1995 to 1997 reported perioperative mortality was significantly higher in the CABG group (14.5%) compared to the PTCA group (8.7%); other single centres have reported similar perioperative mortality results in ESRD patients requiring cardiac revascularization [27–29]. In a large national study using USRDS data (1978 to 1995), in-hospital mortality was also significantly higher in the CABG group (12.5%) compared to the PTCA group (5.4%) [26]. However, considerable variability exists in perioperative mortality reported among CABG patients with ESRD [30].
Despite the growing number and proportion of surgical coronary revascularization among ESRD patients, we observed a simultaneous decline in the annual morality rates despite an increasing burden of co-morbid conditions. These results suggest that a larger proportion of ESRD patients are surviving CABG over time. Other studies have also reported similar findings [26,28,31,32]. For instance, Bechtel et al. found declining 30-day mortality trends in ESRD patients undergoing CABG from 1989 to 2003 despite an increasing proportion of patients with diabetes mellitus, anaemia and myocardial infarction [32]. Similarly, Kan et al. determined no significant differences between CABG patients with and without ESRD in post-operative intensive care unit stay or in-hospital complications despite an increased prevalence of diabetes, hypertension, left main coronary artery disease and anaemia in dialysis-dependent patients [24]. These observed improvements in in-hospital mortality may be explained by a variety of advancements including: the availability and feasibility of minimally invasive and off-pump surgical techniques, specialized devices designed to reduce atheromatous embolic load and advanced bypass temperature management strategies. Moreover, improved referral of patients with ESRD for CABG may also contribute to this trend by selecting patients predicted to have better perioperative tolerance and post-operative performance status. Our data may support this explanation, as we found a trend of decreasing age among ESRD patients undergoing CABG over time. Advances in renal replacement therapy might have also improved survival by limiting fluid shifts and cardiovascular burden. Additionally, the increasing number of annual estimated CABG cases implies greater surgeon familiarity and experience with the ESRD population, potentially resulting in improved patient management.
Although our observations reveal favourable mortality trends over a 16-year period, ESRD patients continue to experience higher death rates than patients without renal failure; a finding that is similar to several other studies. For example, between 1992 and 1996, Liu et al. observed a risk of death that was 4.4 times higher (12.2% versus 3.0%) among dialysis patients versus the non-dialysis patients even after adjustment for co-morbid factors (odds ratio 3.1) [33]. Similarly, mortality rates among CABG patients with ESRD ranged from 10% to 17% in our study from 1992 to 1996; the risk of death for dialysis-dependent CABG patients was 3.3 to 5.7 times greater compared to non-dialysis patients. Furthermore, Cooper et al. identified 7152 ESRD patients requiring CABG with a 9% perioperative mortality rate between 2000 and 2003 [34]. During the same time period, our study recognized a comparable number of ESRD patients requiring CABG (7993) with perioperative mortality rates ranging from 6% to 8%. Indeed, a number of studies have previously identified impaired renal function as an independent risk factor for mortality and morbidity with CABG [6,16,21,35,36]. Possible explanations for higher death rates among ESRD patients include the previously mentioned accelerated atherosclerosis and prevalence of coronary artery disease [7,37]. Additionally, ESRD may potentiate the effects of hypertension and anaemia on cardiomyopathy [38,39]. Most patients with renal insufficiency also show left ventricular hypertrophy [39,40]. Hyperparathyroidism secondary to renal insufficiency may be associated with cardiac calcification, including heart valves and conduction tissue [40]. Furthermore, despite the presence of substantial coronary artery disease, some reports suggest minimal or the absence of anginal pain secondary to diabetic or uraemic polyneuropathy in dialysis-dependent patients [6,37,40,41]. Because of the administrative nature of the dataset used in this analysis, we were unable to examine the changes in prevalence or potential impact of these proposed mediators of accelerated cardiovascular disease.
Such pathophysiological changes in patients with ESRD may also explain higher mortality rates following CABG. During the study period, the proportion of dialysis patients with diabetes, anaemia, hypertension, obesity, atrial fibrillation and myocardial infarction increased; however, median age of CABG candidates declined. Together, a higher level of co-morbidities may have predisposed a greater proportion of patients with ESRD to accelerated development of coronary artery disease requiring CABG. More severe coronary disease among younger patients with ESRD may have accounted for the declining median age of CABG recipients with ESRD. The increasing prevalence of co-morbidities could also have increased perioperative mortality; however, we observed a decline in mortality rates among patients with ESRD who underwent CABG. Improvements in practice patterns, selection criteria, diagnostic evaluations and treatment techniques may have accounted for this observation.
Favourable in-hospital mortality rates despite increased surgical revascularization volume and reduced length of stay potentially suggest a decrease in health-care resource utilization. However, a significant increase in non-routine discharges among patients with ESRD who survived CABG suggests that decreased length of stay may be offset by increased outpatient health-care services. Cowper et al. also observed a decrease in length of hospitalization among CABG patients from 1992 to 1998 in the state of New York. They attributed the decline in length of stay to a transfer of care to non-acute health-care settings, which included home and skilled nursing facilities [42]. Thus, the net impact on health-care utilization may actually have increased despite decreased length of inpatient stay. Survivors with ESRD continue to display elevated non-traditional discharge rates compared to patients without renal failure [43].
In comparison to other studies, we provide the largest and most comprehensive observation of in-patient mortality among dialysis patients following CABG to date; however, our analysis has several limitations. Firstly, indication bias may confound the results of any observational study in that the indication for treatment may affect the likelihood of the outcome. This bias may be minimized through multivariable modelling to control for the presence of co-morbidities; however, the severity of these co-morbidities may not be adequately considered or accurately recorded. Secondly, the NIS database is based on administrative data and lacks important clinical details, including descriptions of patient anatomy, off-pump surgical technique, socioeconomic status of patients, patient diet and activity, type of congestive heart failure and type of bypass graft. In addition, the NIS may be susceptible to hospital-based practice variations leading to possible discrepancy of data coding practices. To minimize the impact of such coding errors, we excluded discharges from hospitals reporting <5 CABG procedures annually. Furthermore, the NIS does not collect information on individual surgeons who perform CABGs, which precludes considering the impact of procedural volume on outcomes. Individual surgeon and hospital CABG volume have been associated with successful outcomes [44–46]. Additionally, the NIS database may involve the possible inclusion of multiple patient admissions, which may violate statistical assumptions of independence; however, these occurrences are likely to be rare and do not bias our main findings. Furthermore, race was not included due to incomplete availability in our dataset; previous studies have identified black race as a significant independent predictor of operative mortality after CABG [47]. Finally, because of the observational nature of this study, we are limited in our ability to account for all potential cofounders that may have affected the outcomes evaluated in this study, and these findings may not reflect trends in countries other than the USA.
In summary, using nationally representative samples of US inpatients, we found that the proportion of ESRD patients receiving CABG increased from 1988 to 2003. Despite increasing co-morbidities, perioperative morality and length of in-hospital stay declined. However, mortality rates among patients with ESRD remain significantly higher than those among compared to non-ESRD patients indicating a need for ongoing improvement.
Acknowledgments
We acknowledge Carissa Baker-Smith, MD, MPH and Lawrence H. Muhlbaier, PhD for their assistance with data analytic strategies. This work was supported by grants from the National Institutes of Health [K23DK075929 (U.D.P) and KL2RR024123 (J.K.I)].
Conflict of interest statement. None declared.
References
- 1.Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol. 2002;13:S37–S40. [PubMed] [Google Scholar]
- 2.Excerpts from the United States Renal Data System 2008 Annual Data Report Atlas of Chronic Kidney Disease & End-Stage Renal Disease in the United States. Am J Kidney Dis. 2009;53:S116–S117. [Google Scholar]
- 3.Szczech LA, Lazar IL. Projecting the United States ESRD population: issues regarding treatment of patients with ESRD. Kidney Int Suppl. 2004:S3–S7. doi: 10.1111/j.1523-1755.2004.09002.x. [DOI] [PubMed] [Google Scholar]
- 4.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 5.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 6.Raggi P, Boulay A, Chasan-Taber S, et al. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 8.Kahn JK, Rutherford BD, McConahay DR, et al. Short- and long-term outcome of percutaneous transluminal coronary angioplasty in chronic dialysis patients. Am Heart J. 1990;119:484–489. doi: 10.1016/s0002-8703(05)80268-6. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed WH, Shubrooks SJ, Gibson CM, et al. Complications and long-term outcome after percutaneous coronary angioplasty in chronic hemodialysis patients. Am Heart J. 1994;128:252–255. doi: 10.1016/0002-8703(94)90476-6. [DOI] [PubMed] [Google Scholar]
- 10.Koyanagi T, Nishida H, Kitamura M, et al. Comparison of clinical outcomes of coronary artery bypass grafting and percutaneous transluminal coronary angioplasty in renal dialysis patients. Ann Thorac Surg. 1996;61:1793–1796. doi: 10.1016/0003-4975(96)00170-1. [DOI] [PubMed] [Google Scholar]
- 11.Hirose H, Amano A, Takahashi A, et al. Coronary artery bypass grafting for hemodialysis-dependent patients. Artif Organs. 2001;25:239–247. [PubMed] [Google Scholar]
- 12.Batiuk TD, Kurtz SB, Oh JK, et al. Coronary artery bypass operation in dialysis patients. Mayo Clin Proc. 1991;66:45–53. doi: 10.1016/s0025-6196(12)61174-4. [DOI] [PubMed] [Google Scholar]
- 13.Deutsch E, Bernstein RC, Addonizio P, et al. Coronary artery bypass surgery in patients on chronic hemodialysis. A case-control study. Ann Intern Med. 1989;110:369–372. doi: 10.7326/0003-4819-110-5-369. [DOI] [PubMed] [Google Scholar]
- 14.Jahangiri M, Wright J, Edmondson S, et al. Coronary artery bypass graft surgery in dialysis patients. Heart. 1997;78:343–345. doi: 10.1136/hrt.78.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaitan L, Sutter FP, Goldman SM. Coronary artery bypass grafting in patients who require long-term dialysis. Ann Thorac Surg. 2000;69:1135–1139. doi: 10.1016/s0003-4975(99)01429-0. [DOI] [PubMed] [Google Scholar]
- 16.Salem MM, Mujais S. Coronary revascularization in dialysis patients: the need for vigilance. Int J Artif Organs. 1991;14:7–9. [PubMed] [Google Scholar]
- 17.Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes for Acute Renal Failure. J Am Soc Nephrol. 2006;17:1688–1694. doi: 10.1681/ASN.2006010073. [DOI] [PubMed] [Google Scholar]
- 18.Healthcare Cost and Utilization Project (HCUP), 2000–2006. Version. Rockville, MD; 2008. computer program. [Google Scholar]
- 19.Herzog CA. Kidney disease in cardiology. Nephrol Dial Transplant. 2007;22:43–46. doi: 10.1093/ndt/gfl739. [DOI] [PubMed] [Google Scholar]
- 20.United States Renal Data System 2005 Annual Data Report. Am J Kidney Dis. 2006;47:S173–S184. [Google Scholar]
- 21.Soucie JM, McClellan WM. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996;7:2169–2175. doi: 10.1681/ASN.V7102169. [DOI] [PubMed] [Google Scholar]
- 22.Frenken M, Krian A. Cardiovascular operations in patients with dialysis-dependent renal failure. Ann Thorac Surg. 1999;68:887–893. doi: 10.1016/s0003-4975(99)00554-8. [DOI] [PubMed] [Google Scholar]
- 23.Gilbertson DT, Liu J, Xue JL, et al. Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol. 2005;16:3736–3741. doi: 10.1681/ASN.2005010112. [DOI] [PubMed] [Google Scholar]
- 24.Kan CD, Yang YJ. Coronary artery bypass grafting in patients with dialysis-dependent renal failure. Tex Heart Inst J. 2004;31:224–230. [PMC free article] [PubMed] [Google Scholar]
- 25.Rinehart AL, Herzog CA, Collins AJ, et al. A comparison of coronary angioplasty and coronary artery bypass grafting outcomes in chronic dialysis patients. Am J Kidney Dis. 1995;25:281–290. doi: 10.1016/0272-6386(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 26.Herzog CA, Ma JZ, Collins AJ. Comparative survival of dialysis patients in the United States after coronary angioplasty, coronary artery stenting, and coronary artery bypass surgery and impact of diabetes. Circulation. 22 2002;106:2207–2211. doi: 10.1161/01.cir.0000035248.71165.eb. [DOI] [PubMed] [Google Scholar]
- 27.Agirbasli M, Weintraub WS, Chang GL, et al. Outcome of coronary revascularization in patients on renal dialysis. Am J Cardiol. 2000;86:395–399. doi: 10.1016/s0002-9149(00)00953-x. [DOI] [PubMed] [Google Scholar]
- 28.Labrousse L, de Vincentiis C, Madonna F, et al. Early and long term results of coronary artery bypass grafts in patients with dialysis dependent renal failure. Eur J Cardiothorac Surg. 1999;15:691–696. doi: 10.1016/s1010-7940(99)00097-4. [DOI] [PubMed] [Google Scholar]
- 29.Ivens K, Gradaus F, Heering P, et al. Myocardial revascularization in patients with end-stage renal disease: comparison of percutaneous transluminal coronary angioplasty and coronary artery bypass grafting. Int Urol Nephrol. 2001;32:717–723. doi: 10.1023/a:1015067611958. [DOI] [PubMed] [Google Scholar]
- 30.Horst M, Mehlhorn U, Hoerstrup SP, et al. Cardiac surgery in patients with end-stage renal disease: 10-year experience. Ann Thorac Surg. 2000;69:96–101. doi: 10.1016/s0003-4975(99)01133-9. [DOI] [PubMed] [Google Scholar]
- 31.Herzog CA, Ma JZ, Collins AJ. Long-term outcome of dialysis patients in the United States with coronary revascularization procedures. Kidney Int. 1999;56:324–332. doi: 10.1046/j.1523-1755.1999.00540.x. [DOI] [PubMed] [Google Scholar]
- 32.Bechtel JF, Detter C, Fischlein T, et al. Cardiac surgery in patients on dialysis: decreased 30-day mortality, unchanged overall survival. Ann Thorac Surg. 2008;85:147–153. doi: 10.1016/j.athoracsur.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 33.Liu JY, Birkmeyer NJ, Sanders JH, et al. Risks of morbidity and mortality in dialysis patients undergoing coronary artery bypass surgery. Northern New England Cardiovascular Disease Study Group. Circulation. 2000;102:2973–2977. doi: 10.1161/01.cir.102.24.2973. [DOI] [PubMed] [Google Scholar]
- 34.Cooper WA, O’Brien SM, Thourani VH, et al. Impact of renal dysfunction on outcomes of coronary artery bypass surgery. Circulation. 2005;113:1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 35.Reddan DN, Szczech LA, Tuttle RH, et al. Chronic kidney disease, mortality, and treatment strategies among patients with clinically significant coronary artery disease. J Am Soc Nephrol. 2003;14:2373–2380. doi: 10.1097/01.asn.0000083900.92829.f5. [DOI] [PubMed] [Google Scholar]
- 36.Anderson RJ, O'Brien M, MaWhinney S, et al. Renal failure predisposes patients to adverse outcome after coronary artery bypass surgery. VA Cooperative Study #5. Kidney Int. 1999;55:1057–1062. doi: 10.1046/j.1523-1755.1999.0550031057.x. [DOI] [PubMed] [Google Scholar]
- 37.Holzmann MJ, Hammar N, Ahnve S, et al. Renal insufficiency and long-term mortality and incidence of myocardial infarction in patients undergoing coronary artery bypass grafting. Eur Heart J. 2007;28:865–871. doi: 10.1093/eurheartj/ehl508. [DOI] [PubMed] [Google Scholar]
- 38.Bennett WM, Kloster F, Rosch J, et al. Natural history of asymptomatic coronary arteriographic lesions in diabetic patients with end-stage renal disease. Am J Med. 1978;65:779–784. doi: 10.1016/0002-9343(78)90796-9. [DOI] [PubMed] [Google Scholar]
- 39.Herzog CA, Littrell K, Arko C, et al. Clinical characteristics of dialysis patients with acute myocardial infarction in the United States: a collaborative project of the United States Renal Data System and the National Registry of Myocardial Infarction. Circulation. 2007;116:1465–1472. doi: 10.1161/CIRCULATIONAHA.107.696765. [DOI] [PubMed] [Google Scholar]
- 40.Weiner DE, Tighiouart H, Amin MG, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15:1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 41.State-specific trends in chronic kidney failure—United States, 1990–2001. MMWR Morb Mortal Wkly Rep. 2004;53:918–920. [PubMed] [Google Scholar]
- 42.Cowper PA, DeLong ER, Hannan EL, et al. Trends in postoperative length of stay after bypass surgery. Am Heart J. 2006;152:1194–1200. doi: 10.1016/j.ahj.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Chen JC, Kaul P, Levy JH, et al. Myocardial infarction following coronary artery bypass graft surgery increases healthcare resource utilization. Crit Care Med. 2007;35:1296–1301. doi: 10.1097/01.CCM.0000262403.08546.A2. [DOI] [PubMed] [Google Scholar]
- 44.Hannan EL, Wu C, Ryan TJ, et al. Do hospitals and surgeons with higher coronary artery bypass graft surgery volumes still have lower risk-adjusted mortality rates? Circulation. 2003;108:795–801. doi: 10.1161/01.CIR.0000084551.52010.3B. [DOI] [PubMed] [Google Scholar]
- 45.Marcin JP, Li Z, Kravitz RL, et al. The CABG surgery volume-outcome relationship: temporal trends and selection effects in California, 1998–2004. Health Serv Res. 2008;43:174–192. doi: 10.1111/j.1475-6773.2007.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rathore SS, Epstein AJ, Volpp KG, et al. Hospital coronary artery bypass graft surgery volume and patient mortality, 1998–2000. Ann Surg. 2004;239:110–117. doi: 10.1097/01.sla.0000103066.22732.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bridges CR, Edwards FH, Peterson ED, et al. The effect of race on coronary bypass operative mortality. J Am Coll Cardiol. 2000;36:1870–1876. doi: 10.1016/s0735-1097(00)00956-6. [DOI] [PubMed] [Google Scholar]