Abstract
Polar auxin transport, which is required for the formation of auxin gradients and directional auxin flows that are critical for plant pattern formation, morphogenesis, and directional growth response to vectorial cues, is mediated by polarized sub-cellular distribution of PIN-FORMED Proteins (PINs, auxin efflux carriers), AUX1/AUX1-like proteins (auxin influx facilitators), and multidrug resistance P-glycoproteins (MDR/PGP). Polar localization of these proteins is controlled by both developmental and environmental cues. Recent studies have revealed cellular (endocytosis, transcytosis, and endosomal sorting and recycling) and molecular (PINOID kinase, protein phosphatase 2A) mechanisms underlying the polar distribution of these auxin transport proteins. Both TIR1-mediated auxin signaling and TIR1-independent auxin-mediated endocytosis have been shown to regulate polar PIN localization and auxin flow, implicating auxin as a self-organizing signal in directing polar transport and directional flows.
Keywords: PIN proteins, PINOID, ROP GTPases, polarity, protein traffic and secretion, signal transduction
Cell polarity is a fundamental cell property important for cell function in all the cellular organisms. Development of cell polarity is critical for all aspects of the plant life, such as the development of the single cell embryo (zygote), differentiation and morphogenesis of various cell types, short and long-range transport of signaling molecules such as auxin (Benkova et al., 2003; Campanoni et al., 2003; Mattsson et al., 2003; Reinhardt et al., 2003; DeMason and Chawla, 2004; Kim et al., 2005; Lin and Wang, 2005; Santelia et al., 2005), and responses to vectorial environmental cues (e.g. light and gravity) (Blakeslee et al., 2005; Tanaka et al., 2006; Zazimalova et al., 2007) or microorganisms (Lipka et al., 2007; Kwon et al., 2008). Consequently, cell polarity formation is an important cellular basis of plant growth and development. Understanding the mechanism underlying cell polarity formation will provide important insights into molecular linkages between cellular functions and plant development. Little is known about signals that activate polarity development and their linkages to developmental programs in plants. However, emerging evidence appears to support an important role of auxin as a cellular polarizing signal that regulates its own polar transport in response to developmental programs.
Auxin is a multi-functional phytohormone that controls a plethora of processes, concerning almost every aspect of a plant life, such as cytoskeletal organization, intracellular membrane trafficking (Paciorek et al., 2005), cell polarity and morphogenesis, cell division (Petrasek et al., 2002; Friml et al., 2003; Dhonukshe et al., 2005), cell expansion, cell differentiation (Fukuda, 2004), organ formation and growth (lateral organ and root hair production) (Weijers et al., 2006; Rahman et al., 2007), organization of plant architecture (phyllotaxis) (Newell et al., 2007), and responses to the environment (Weijers and Jurgens, 2005; Weijers et al., 2005; Jonsson et al., 2006; Weijers et al., 2006; Rahman et al., 2007). Auxin regulates these processes by activating either of the two distinct signaling pathways. One involves the TIR1 F-box family auxin receptor-mediated degradation of the IAA/AUX transcriptional repressors, releasing the expression of a plethora of auxin-induced genes (Dharmasiri et al., 2005; Laskowski, 2006; Quint and Gray, 2006). The other involves rapid activation of cytoplasmic events by the AUXIN-BINDING PROTEIN1 (ABP1) and/or unknown auxin signaling receptors, such as regulation of the plasma membrane (PM) localization of auxin transporters, PM ATPase-dependent proton pumping out of the cell, cytoskeleton reorganization, which can be independent of nuclear gene expression (Cross, 1991; Leblanc et al., 1999; Yamagami et al., 2004; Christian et al., 2006). The diverse functions of auxin are also often tightly linked to its polar transport—a property unique to auxin among various phytohormones (Paciorek et al., 2005). Plant developmental events influence polar auxin transport, which is achieved by auxin efflux and influx carriers. Polar transport of auxin is reported to occur as early as in the two-celled pro-embryo, between two asymmetric cells (Friml et al., 2003). Post-embryonic development is controlled by auxin synthesized in rapidly dividing cells in young developing leaves and roots (Aloni et al., 2003), and developmental programs influence localization of auxin transporters to generate auxin gradients or directional flows.
It is well established that polar localization of auxin carriers underpins directional or polar auxin transport. Several families of auxin carriers have been identified, including auxin efflux carriers such as PINs (PIN-FORMED) and PGP/MDR-like proteins and auxin influx carriers such as AUX1 (Geisler and Murphy, 2006; Kramer and Bennett, 2006; Teale et al., 2006). Several PIN proteins exemplify the importance of polar localization of these proteins in long-distance and regional auxin transport. For example, PIN1 is localized in the basal PM of stele cells and xylem cells in the vascular system, which is required for long-distance auxin flow from the shoot apex to the root tip (Galweiler et al., 1998; Friml et al., 2002). PIN2 is expressed in root tissues and is selectively localized onto the apical side of lateral root cap cells and epidermal cells, which fits for its role in acropetal auxin flux locally (Luschnig et al., 1998; Muller et al., 1998). Consistently, sub-cellular distribution of PIN2 is modulated and redefined by light and gravity to modulate directional growth in response to these vectorial cues (Abas et al., 2006; Laxmi et al., 2008). Interestingly, PIN7 is localized apically in two to eight-cell embryos but is shifted to the basal side in 16/32-cell embryos, indicating a developmental regulation of cell polarity switching (Friml et al., 2003). These observations clearly show that the polarity of auxin transporters is tightly controlled by both developmental and environmental cues. In recent years, there has been a great amount of interest in understanding the molecular and cellular basis determining the polarity of auxin transporters.
Evidence suggests that PIN polarity is regulated by cellular signaling events occurring at the polar PM site where PIN proteins are localized. Crucial signaling molecules such as a serine/threonine protein kinase PID (PINOID kinase) (Christensen et al., 2000; Benjamins et al., 2001; Friml et al., 2004; Lee and Cho, 2006; Zegzouti et al., 2006; Blakeslee et al., 2007; Michniewicz et al., 2007; Morita and Kyozuka, 2007) and a protein phosphatase, PP2A (Scherer, 2002; Michniewicz et al., 2007) are localized to the polar PM site. Signaling targets can be PIN proteins themselves and/or molecules involved in intracellular trafficking of these proteins in the processes such as endocytosis (PM-to-endosome), endosomal sorting/recycling, and transcytosis, which are all implicated in modulating polar localization of auxin transporters (Geldner et al., 2003; Teh and Moore, 2007; Kleine-Vehn et al., 2008). An intriguing question is, what are the developmental signals and how they are linked to signaling components and vesicular trafficking machinery to achieve the establishment of sub-cellular polarity of auxin transporters. Emerging evidence suggests that auxin itself could be a signal affecting PIN localization. This review will focus on the recent advances in the understanding of signaling mechanisms and their cellular targets underlying the polar distribution of auxin transporters.
VESICULAR TRAFFICKING PATHWAYS IN THE POLARIZATION OF PIN PROTEINS
As in the development of cell polarity in other systems, the polarization of auxin transporters is also intimately linked to vesicular trafficking, which has been demonstrated by a large body of genetic, chemical genetic, molecular, and cell biological studies (Steinmann et al., 1999; Geldner et al., 2003; Abas et al., 2006; Jaillais et al., 2006; Kleine-Vehn et al., 2006; Wisniewska et al., 2006; Jaillais et al., 2007; Teh and Moore, 2007; Kleine-Vehn et al., 2008; Robert et al., 2008). To date, endocytosis, endosomal sorting and recycling, and transcytosis have all been linked to the regulation of polar localization of auxin transporters.
GNOM Links Endosomal Sorting/Recycling to Polar PIN Localization
The first hint towards the involvement of vesicular trafficking in PIN polar localization was from gnom mutants, which mimics the effect of pin1 on early embryogenesis in Arabidoposis (Liu et al., 1993; Steinmann et al., 1999). In gnom mutants, PIN1 proteins display polarity defects responsible for the phenotypes of gnom mutants (Steinmann et al., 1999). GNOM protein is localized both in the PM and endosomes (Steinmann et al., 1999; Geldner et al., 2003) where PIN1 localizes. GNOM specifically mediates polar PIN1 localization to the PM (Bonifacino and Jackson, 2003). GNOM encodes a BFA (brefeldin A)-sensitive guanine nucleotide exchange factor (GEF) for ARF GTPases that regulate vesicle formation (Steinmann et al., 1999; Geldner et al., 2003; Robert et al., 2008). Using gnom knockout mutants, a transgenic line expressing a BFA-insensitive mutant GNOMM696L, Geldner and colleagues concluded that GNOM is the entry point for intracellular sorting and recycling of PIN1 to the PM (Geldner et al., 2003) (Figure 1).
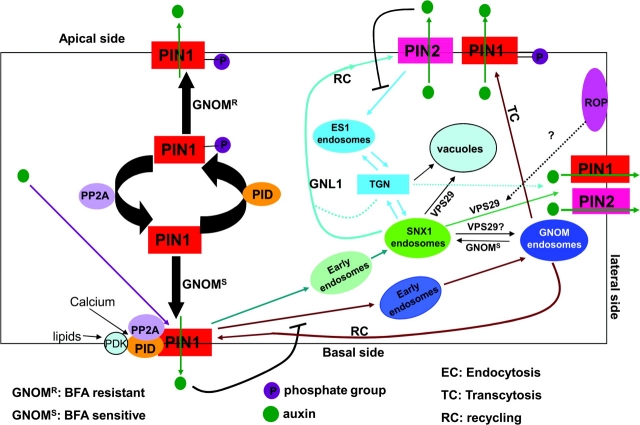
Figure 1.
Sub-Cellular and Signaling Mechanisms Underlying Polar PIN Localization.
A model of regulators and pathways contributing to polar PIN localization in roots is proposed based on results discussed in this review. Endosome-mediated trafficking of PINs operates in regulating PIN polarity. At least two distinct pathways involving early endosomes are involved, which influence PIN1 and PIN2 trafficking through ES1-independent and ES1-dependent early endosomes, respectively. The SNX1/VPS29 retromer complex and the GNOM pathway appear to reside in late endosomes that may regulate PIN sorting, degradation in vacuoles, and recycling to the PM. Switching or re-polarization of PINs seems to involve distinct trafficking pathways as well. The GNOM-dependent pathway is involved in transcytosis-mediated polarity switching between the apical and basal sides, whereas the GNOM-independent and VSP29-dependent pathway may regulate PIN re-polarization to the lateral side. The precise relationship among early endosomes, TGN, late endosomes, recycling endosomes, and lytic endosomes remains unclear and is most likely quite complex. Speculative pathways are indicated by dotted lines. Signaling mechanisms regulating PIN trafficking are emerging. PID kinase directly phosphorylates PIN1 to direct the apical localization of PIN1. PP2A phosphatases have an opposite role by directing basipetal localization of PINs via the GNOM-mediated pathway. Calcium signaling and a signaling pathway involving PDK1 kinase are suggested to act upstream of those pathways. Sterols may also play some roles in generating polarity signal or regulate the formation of membrane microdomains necessary for the polar PIN localization. ROP signaling-mediated cell polarity may also contribute to PIN polarity signaling in an unknown manner. Auxin itself is shown to inhibit both ES1-insensitive PIN1 endocytosis and ES1-sensitive PIN2 endocytosis. There is also some evidence that auxin regulates PIN localization via the TIR1-dependent signaling pathway as well as having a more indirect effect by modulating PIN protein expression.
Arabidopsis thaliana contains 12 ARF GEFs. GNL1, a GNOM-like ARF GEF, has a conserved function in ER-to-Golgi trafficking and a selective function in the endocytosis of PIN2. Interestingly, GNL1 is not involved in endosomal sorting/recycling, and there is no evidence that GNOM and GNL1 function in the trafficking of other PINs (Geldner et al., 2003; Teh and Moore, 2007). Thus, there must be other ARF GEFs or mechanisms involved in the intracellular sorting/recycling of PINs.
SNX1 and VPS29: Sorting Auxin Transporters?
The existence of signal-dependent distinct mechanisms controlling endosomal sorting/recycling of different auxin transporters is further supported by recent functional studies of the retromer by the Gaude group. The retromer is a heteropentameric complex consisting of a sorting nexin dimer (through undefined combination of SNX1, SNX2, SNX5, or SNX6) and a trimer composed of VPS26, VPS29, and VPS35 in mammalian cells (Arighi et al., 2004; Shi et al., 2006; Hierro et al., 2007; Rojas et al., 2007; Bonifacino and Hurley, 2008). The SNX dimers fulfill the recruitment of the pentameric retromer to endosomes, while the VPS22–VPS29–VPS35 triple sub-complex is proposed to bind cargos, which shuttle between endosomes and the trans-Golgi network (TGN). An Arabidopsis thaliana SORTING NEXIN 1 (AtSNX1) was also found in a novel endosomal compartment different from the GNOM-containing ‘BFA compartment’ (Jaillais et al., 2006). AtSNX1 co-localizes with endosomal markers RABF1 and RABF2b in endosomes but not in Golgi, the trans-Golgi network (TGN), and GNOM-containing endosomes (Jaillais et al., 2006). Wortmannin, a PI3K (phosphatidylinositol-3 kinase) inhibitor affecting SNX localization in mammalian cells, also induced the formation of an enlarged AtSNX1-containing compartment. Wortmannin together with cycloheximide (protein synthesis inhibitor) caused PIN2 to accumulate in Wortmannin-induced compartments. Based on these observations, it was concluded that polar PIN2 distribution is mediated by a novel AtSNX1-mediated endosomal pathway that is distinct from the GNOM-dependent PIN1-trafficking pathway, and it was proposed that two distinct populations of endosomes are involved in PIN1 and PIN2 trafficking, respectively (Figure 1). Gravity stimulation of PIN2 internalization is proposed to act through the AtSNX1-marked endosomes (Jaillais et al., 2006). Interestingly, the polarization of AUX1, which also shifts polarity in response to gravity stimulation, is AtSNX1-independent (Jaillais et al., 2006).
Another study from the Gaude group suggests that the retromer could also regulate the re-polarization of PIN1 during specific developmental programming (Jaillais et al., 2007). Phenotypes of vps29 knockout mutants are similar to those of pin and snx1 mutants with abnormal shape and number and positioning of cotyledons and reduced primary root lengths. In addition, VPS29-mediated embryonic and postembryonic developments resemble the action of PINs. VPS29 is localized to SNX1-containing endosomes and functions in the regulation of the size of SNX1-containing endosomes, suggesting that VPS29 and SNX1 form a functional complex (Jaillais et al., 2007). In vps29, PIN1 localization in the stele is normal except for additional accumulation in abnormal intracellular compartments. In lateral root initial cells, however, PIN1 was found to assemble into enlarged SNX1-labeled endosomes but not in GNOM-marked endosomes. Interestingly, PIN2 was also affected in vps29, localizing to the enlarged compartment in root cells (Jaillais et al., 2007). These results support the notion that retromer-dependent trafficking specifically regulates re-polarization of PINs in response to environmental and developmental signals (Figure 1).
Although PIN2 localization was similarly affected in snx1 and vps29 mutants, PIN1 re-polarization was only affected in vps29 but not in the snx1 mutant (Jaillais et al., 2006, 2007). AUX1 trafficking is not affected in either snx1 or vps29 mutant (Jaillais et al., 2006, 2007). The mechanism by which SNX1 and VPS29 differentially affect endosomal trafficking of these auxin transporters is not clear. However, these observations suggest that components of the retromer are able to integrate developmental and environmental signals by mediating the trafficking of auxin transporters.
An important future question is how the identical retromer differentially regulates the localization of PINs and other auxin transporters and how it is linked to environmental and developmental signals that influence their polar localization. The retromer complex is known to participate in the retrograde transport of cargo proteins from endosomes to the TGN. Based on this function, defects in the retromer are expected to cause mis-targeting of retrograde cargos to lytic lysomomes (late endosomes) or vacuoles for degradation. In Arabidopis thaliana, VPS26–VPS29–VPS35 trimer has been shown to affect the distribution of cargos to the lytic compartment (Oliviusson et al., 2006). If the retromer in Arabidopsis follows this conventional function, this would mean that certain PINs under specific developmental or environmental situations are transported through the retrograde pathway to TGN/sorting endosomes, from which they could be targeted to the PM (Figure 1). This transport mechanism would be distinct from the BFA-sensitive endosomal recycling pathway that has been shown for PIN1 polar distribution in the root stele. Further studies will be necessary to test this retromer-mediated PIN trafficking mechanism.
The Power of Chemical Genetics in Revealing Trafficking Pathways to the Polarization of Auxin Transporters
Chemical inhibitors (also known as small molecules) such as NPA (1-N-naphthylphatalymic acid), Wortmannin, and BFA have been instrumental in investigating vesicular trafficking in the regulation of auxin transporter polarization. NPA, a well known inhibitor of polar auxin transport, was shown to affect PIN localization through its effect on vesicular trafficking (Geldner et al., 2001). BFA inhibits PIN polarization to the PM by inducing PIN-containing endosome-related compartments called BFA bodies (Geldner et al., 2001). GNOM was shown to be a BFA target, and the combined use of BFA and gnom mutations provided many important insights into the mechanisms for PIN polarization (Steinmann et al., 1999; Geldner et al., 2001; Paciorek et al., 2005; Dhonukshe et al., 2007; Kleine-Vehn et al., 2008). Wortmannin, an inhibitor of phosphatidylinositol-3-OH kinase, was useful for the investigation of AtSNX1-mediated GNOM-independent PIN2 trafficking (Jaillais et al., 2006, 2007). Therefore, the discovery of new small molecules through the emerging chemical genetics approach will help to uncover new mechanisms/pathways underlying the polarization of auxin transporters.
Indeed, a recent chemical genetic study from the Raikhel group has proven that chemical genetics will be a fertile field. By screening for small molecules affecting the polar localization of a ROP GTPase-interacting protein RIP1, a chemical selectively affecting the endosomal sorting/recycling of a subset of proteins was identified and thus was named endosidin 1 (ES1) (Robert et al., 2008). ES1 induced depolarization of pollen tube growth and mis-localization of RIP1. In root cells, ES1 induced the formation of ‘ES1 bodies’ accumulating PIN2, BRI1, and AUX1, but did not affect the localization of other PM-localized proteins including PIN1 and PIN7. ES1 bodies also contain SYP61 and VHA-a1, which are known to localize to TGN/early endosomes. However, ES1 does not affect internalization of FM4-64 dye (an endocytosis marker). Interestingly, the accumulation of PIN2 in ES bodies but not the formation of these structures was blocked by auxin, which is known to inhibit endocytosis of PINs. Based on these observations, it was concluded that ES1 affects the trafficking of early endosomes and the sorting of PIN2/BRI1/AUX1 through these endosomes and that ES1 distinguishes this endosomal trafficking pathway from other pathway(s) for PIN1 and PIN7 trafficking (Robert et al., 2008) (Figure 1).
Another distinct feature of ES1 effects was that it did not alter SNX1-containing compartments where PIN2 was found to reside and which are important for PIN2 trafficking. As discussed above, AUX1 localization was shown to be independent of the retromer (Jaillais et al., 2006, 2007). One explanation for these overlapping but distinct actions of ES1 and SNX1 is that ES1 affects early endosomal trafficking of PIN2/BRI1/AUX1, whereas the retromer-mediated retrograde pathway is involved in the late endosome sorting, which determines whether endosomal cargos are recycled to the PM or targeted for degradation in vacuoles. If this is the case, existence of an intermediate sorting pathway that distinguishes PIN2 from AUX1 is expected. The pathway might be able to bypass the PIN2/SNX1 pathway. Although such pathways have yet to be confirmed by molecular and genetic studies, ES1 clearly reveals the complexity of intracellular trafficking of auxin transporters and its role in regulating their polar localization.
Shifting PIN Polarity by Transcytosis
Adding to the complexity of vesicular trafficking in regulating the polarization of auxin transporters is the participation of transcytosis in this process, as recently shown by the Friml group (Kleine-Vehn et al., 2008). Transcytosis refers to the translocation of cargos between separately polarized plasma membrane domains bypassing lysosomes. As mentioned above, GNOM is responsible for basal PIN1 localization in given cells by endocytosis and recycling pathways. A recent paper from the Friml group shows that GNOM can work on translocation of PIN1 by means of an atypical transcytosis (Kleine-Vehn et al., 2008). PINs have distinct polar PM localization on the apical, basal or lateral side of cells, based on their difference in sequences and expressed cells (Kaplinsky and Barton, 2004). In view of the trafficking pathway, Vehn et al. showed that BFA can lead to relocation of PIN1 from the basal side to the apical side of PM reversed to their normal localization in given cells if kept with sufficient time or higher concentration of BFA treatment. Interestingly, this kind of transcytosis is only effective to intrinsically basal cargoes and GNOM-dependent. Furthermore, the authors also showed that GNOM-dependent transcytosis is essential for early cell polarization. In the developmental process of provascular cells, PIN1 can successfully shift from the apical side to the basal side in a wild-type line. In contrast, the gnom mutant line loses the ability to transport apically localized PIN1 to the basal side and shows abnormalities in embryo patterning. The BFA-treated wild-type line also fails to localize PIN1 at the basal side of provascular cells. Thus, transcytosis of PIN1 is dependent on the BFA-sensitive GNOM (Figure 1) and is very essential for early events of establishing cell polarity.
In roots, PIN1 in the stele and PIN2 in the cortex are localized in the basal side of cells, whereas PIN2 is localized to the apical side of epidermal cells. Internal insertion of GFP into PIN1 (PIN1–GFP-3) leads to apicalization of the protein (Kleine-Vehn et al., 2008). It was shown that the BFA-sensitive GNOM ARF GEF is required for the basal but not apical localization of PINs. Loss-of-function gnom mutations or BFA treatment caused the basal PINs to be recruited to the apical side. The BFA-induced polarity shift of PINs was reversible in a protein synthesis-independent manner. FRAP experiments and tagging of PINs with a photoconvertible GFP show that this recruitment can directly translocate PINs between the apical and basal sides. Mutations in GNOM cause basal PIN1 to be apicalized during early embryogenesis. Based on these results, it was concluded that transcytosis through endosome-mediated trafficking plays a critical role in modulating PIN polarity (Kleine-Vehn et al., 2008) (Figure 1). An important future direction is to understand molecular pathways that are responsible for the recruitment of PIN cargos (carried by the transcytotic endosomes) to the polarized PM domains, which are most likely regulated by specific developmental signals.
The Cytoskeleton: A Role in the Trafficking of Auxin Transporters?
Endomembrane trafficking processes, such as endocytosis, exocytosis, and vesicle transport, are intimately linked to the dynamics of microfilaments and microtubules. Evidence suggests that polar distribution of auxin transporters such as AUX1 and PINs is influenced by cytoskeletal dynamics, probably through its regulation of endomembrane trafficking (Geldner et al., 2001; Kleine-Vehn et al., 2006). Application of actin disrupting drugs (Cytochalasin D or latrunculin B) reduced the recruitment of PIN1 onto the PM and changed the patterns of PIN1-labelled components (Geldner et al., 2001; Paciorek et al., 2005). Recently, it was found that several auxin transport inhibitors (ATIs) such as TIBA act to change auxin distribution by interfering with actin structures (Rahman et al., 2007; Dhonukshe et al., 2008). It is well known that plant-specific GTPase ROP regulates cytoskeleton dynamics and cell polarity (Fu et al., 2005). As discussed below, ROP2 seems to regulate root gravitropism by altering polar auxin distribution. ROP signaling may play an important role in coordinating the cytoskeletal dynamics with vesicular trafficking to regulate the polarization of auxin transporters.
Sterol Regulation of Trafficking of Auxin Transporters
Sterols are very important components of the PM and endo-membrane systems such as the trans-Golgi network. In particular, sterols play an important role in the formation of membrane microdomains such as lipid rafts in the PM, which have a significant impact on cell polarity development. Sterols are also dynamic in endocytic trafficking between the PM and endosomal compartments, which is sensitive to BFA application. When cells were treated with BFA, sterols were aggregated in BFA compartments similar to PINs (Geldner et al., 2001; Grebe et al., 2003). Interestingly, recycling of sterols is also actin-dependent (Willemsen et al., 2003). Roles of sterols in the regulation of PINs trafficking has been implicated by the genetic study of the STEROL METHYLTRANSFERASE1 (SMT) gene, which is important for the synthesis of major membrane sterols like sitosterol and campesterol (Willemsen et al., 2003). In Arabidopsis smtorc mutants, basally localized PIN1 is shifted to lateral sides in stele cells, while laterally localized PIN3 becomes uniformly distributed in the peripheral membrane in columella cells (Willemsen et al., 2003). AUX1 localization is not affected by smtorc mutations. However, AUX1 localization was altered by treatment with filipin, which change sterol composition of the PM (Kleine-Vehn et al., 2006).
How do changes in sterol composition affect the PIN localization? A recent study by Men and his colleagues provide some insights into this question (Men et al., 2008). Mutation in CPI1, which encodes the major cyclopropylsterol isomerase and is responsible for appropriate biosynthesis of sterols, leads to altered sterol composition and causes defects in PIN2 endocytosis. In the cpi1-1 mutant, PIN2 proteins are re-localized uniformly at peripheral sides of cells. FM4-64 internalization was reduced in cpi1-1 mutants, suggesting that endocytosis was compromised in the cpi1-1 mutant. Consequently, BFA application did not induce accumulation of PIN2 in the BFA bodies, in contrast to its induction of PIN2 agglomeration in wild-type cells. These results suggest that CPI1-mediated sterol biosynthesis consequently regulates endocytic trafficking of PIN2, which is critical for PIN2 polarization in the PM (Men et al., 2008).
The differential effect on the polarization of auxin transporters by different means of altering sterol composition implies that sterols may have a signaling role in the regulation of endocytosis or endomembrane trafficking, which subsequently influences PIN/AUX1 polarization. Sterols may directly participate in polarity signaling or regulate the formation of membrane microdomains, which subsequently mediates vesicular trafficking. How membrane composition such as phosphoinositides could be involved in the regulation of PIN polarity signaling will be discussed in the following section.
SIGNALING TO POLARIZE AUXIN TRANSPORTERS
Given the importance of vesicular trafficking in the polarization of auxin transporters, the next question is what is the mechanism underlying the spatial definition of vesicular trafficking to generate a localized pattern of PINs/AUX1 distribution? Intuitively, a polarity signal must exist, which must be linked to vesicular trafficking machinery. Polarity signaling could either preferentially activate an exocytic/recycling pathway, leading to preferential targeting of PIN/AUX1 proteins to the polar site, or preferential removal of these proteins from non-polar sites by an endocytic pathway. The signaling could also impact the endosomal sorting machinery to allow selective trafficking of a particular PIN/AUX protein in a given cell. The importance of signaling in determining the polarity of auxin transporters is suggested by the finding that both developmental and environmental signals could direct the changes in the polarity of PIN proteins. For example, PIN1 shifts from cell boundaries to the basal membrane in the provascular cells in early embryogenesis, while PIN2 polarity changes in response to gravity stimulation (Friml et al., 2003; Abas et al., 2006). A clear demonstration of signaling in the regulation of the polarization of auxin transporters came from a study showing that a protein kinase activity can determine the polarity of PIN1 localization.
PID (PINOID): A Kinase Activity Regulating PIN Polarity
The pid mutant phenocopies pin1 mutants, displaying pin-like inflorescence (Christensen et al., 2000), which implies PID's role in auxin transport or signaling (Vernoux et al., 2000; Benjamins et al., 2001; Broer and Brookes, 2001; Furutani et al., 2004). PID encodes a plant-specific serine/threonine protein kinase (Christensen et al., 2000; Friml et al., 2004; Lee and Cho, 2006). Overexpression of PID (35S::PID) but not of kinase-negative MPID (35S::MPID) altered hypocotyl and root gravitropism and led to root meristem collapse as well (Friml et al., 2004).
Importantly, PID overexpression caused PIN1 localization to shift from the basal to the apical end of root stele cells. In the meantime, PIN1 is re-localized from the apical to the basal end of inflorescence cells in pid knockout mutant (Friml et al., 2004). The PID protein kinase directly phosphorylates PIN1, and the status of PID-mediated PIN1 phosphorylation determines the preferential apical or basal PIN1 distribution (Figure 1). However, evidence suggests that simple phosphorylation states of PIN proteins do not fully explain differential basal and apical localization of PIN proteins. Recently, Michniewicz et al. demonstrated that PINOID can phosphorylate PIN1 in vivo, and the central hydrophilic loop, which is conserved in other PIN members, is the phosphorylated domain (Michniewicz et al., 2007). Moreover, Wisniewska and colleagues found that the polar PIN localization is controlled not only by cell-specific signals, but also by PIN-specific sequences (Wisniewska et al., 2006). Therefore, phosphorylation of the conserved hydrophilic loop together with PIN isoform-specific signals may provide a specific cargo code. An important future direction is to understand how polarity signaling specifies the code and links it to trafficking or recruitment machineries.
PP2A: A Protein Phosphatase in Determining PIN Polarity
Given the importance of PIN phosphorylation status in the regulation of PIN polar localization, PIN dephosphorylation is anticipated to modulate the polarity of PIN proteins. Indeed, a recent study shows that the PP2A phosphatase activity is required for proper polar PIN localization and auxin transport-dependent plant development (Michniewicz et al., 2007). Mutations in PP2As (in Arabidopsis, there are three closely related PP2A, including PP2A1, PP2A2, and PP2A3) cause diverse developmental aberrations consistent with defects in auxin polar transport (Rashotte et al., 2001). Genetic analysis indicated that PP2A (Ser/Thr phosphatases) and PID (Ser/Thr kinase) have opposite roles in regulating auxin-dependent embryo and root development. Cytological evidence supports a basal-to-apical shift of PIN1 localization in provascular cells in pp2aa1 pp2aa2 embryos, and of PIN2 in young cortical cells and PIN4 in proximal initials and root meristem cells during the post-embryo stage of the pp2aa1 pp2aa2 double mutant (Michniewicz et al., 2007), which confirms that PP2A is involved in auxin transport and antagonistic to PID.
What Signals Act Upstream of PID and PP2A?
An interesting question is how the kinases and phosphatases that determine the phosphorylation status of PINs are regulated and linked to the initial developmental and environmental signals that define the cellular polarity of PIN proteins.
A localized signaling pathway likely exists at the PM site where PINs are localized, because both PID and PP2A partially co-localize with PIN1 in the PM. One likely upstream component in this signaling pathway(s) is 3-phosphoinositide-dependent protein kinase 1 (PDK1), which interacts, phosphorylates, and activates PID (Zegzouti et al., 2006) (Figure 1). Calcium may also play an important role in the regulation of PID/PP2A-mediated PIN phosphorylation. PID phosphorylation activity is negatively regulated by calcium and is concentration-dependent, as a calcium level as high as 2.5 mM inhibited both the auto- and transphosphorylation obviously in the presence of 15 mM Mg2+ (Zegzouti et al., 2006). Auxin-related cell elongation and root orientation have been demonstrated to respond to changes in cytoplasmic calcium levels, and also PINOID-mediated auxin signaling involves calcium-binding protein (Benjamins et al., 2003). Cytoplamic calcium concentration can be regulated by inositol triphosphate, a metabolite of phosphatidylinositol 4,5-bisphosphate, an important membrane signaling molecule. Thus, both PDK1 and PP2A could be regulated by signaling phospholipids, which might be linked to Rho GTPases (Kost, 2008), the conserved regulator of cell polarity (see below).
Conserved Polarity Regulator: ROP/Rac GTPase
The plant-specific ROP/Rac subfamily of the highly conserved Rho-family GTPases, which controls signaling that leads to the establishment of cell pola yeast or animal cells, has been shown to regulate cell polarity formation in several cell types in plants (Fu and Yang, 2001; Gu et al., 2004; Yang, 2008). Emerging evidence supports a role for ROP signaling in the modulation of PIN polarity. GFP–ROP2 was shown to be polarly localized to the PM in a similar manner to PIN2–GFP (Li et al., 2005). Gravity stimulation was found to induce vectorial relocalization of GFP–ROP2 in a similar way to PIN2 re-localization. Furthermore, ROP2 overexpression increased PIN2–GFP polar localization and increased gravity responsiveness (Li et al., 2005). As discussed above, VPS29-mediated endosomal trafficking is required for PIN1 re-polarization to initiate lateral root formation. Interestingly, the lateral root formation defect in vps29 is epistatic to CA-rop2 induction of lateral root formation, suggesting a possible connection between ROP signaling and endosomal trafficking-mediated PIN polarization (Li et al., 2001, 2005; Jaillais et al., 2006). Consistent with the participation of the actin cytoskeleton in the endosomal pathway-mediated PIN recycling (Geldner et al., 2001; Dhonukshe et al., 2008), ROP GTPase signaling has been shown to regulate cell polarity through its effect on the dynamics of the actin cytoskeleton (Fu et al., 2002; Gu et al., 2003; Fu et al., 2005; Yang, 2008). ROP GTPase-dependent actin dynamics has been shown to regulate polarized exocytosis in pollen tubes (Lee et al., 2008). These observations raise an intriguing possibility: localized ROP GTPase signaling in the PM could provide a mechanistic linkage between polarity signaling in the PM and vesicular trafficking that modulates the polarization of PINs and other auxin transporters. In support of this possibility is the evidence that ROP GTPases participate in auxin signaling, which, by itself, appears to regulate PIN polarization (Cheung and Wu, 2004; Yang, 2008). Therefore, the investigation of the linkage between ROP signaling and polarization of auxin transporter may be another fertile field for auxin biology and cell polarity.
AUXIN: CONSTRUCTING REGULATORY LOOP FOR ITS OWN FLUX
According to the paradigm of cell polarity signaling, the formation of a specific cell polarity is initiated by a vectorial cue that specifies that polarity (Yang, 2008). The polarization of auxin transporters can be controlled by vectorial cues from the environment, such as light and gravity. However, developmental signals that determine the polarization of auxin transporters remain largely obscure. Auxin has been implicated as a developmental signal that regulates auxin transport required for organ development (Cheng et al., 2007). Zhao's group recently showed that mutations causing partial deficiency in auxin biosynthesis (yuc1 yuc4 double mutant) have a synergistic effect on leaf and flower development with pid as well as with npy1, a mutation in the homolog of NPH3, which has been shown to form a complex with PID-related protein kinase PHOT1 (Motchoulski and Liscum, 1999; Cheng et al., 2007). This synergistic effect supports a role for auxin in the regulation of the PID/NPY1 pathway to influence PIN localization (Cheng et al., 2007). Since NPH3 is involved in phototropic responses, it is likely that paralleled PID/NPY1 and PHOT1/NPH3 pathways regulate organogenesis and phototropism, respectively (Cheng et al., 2007). Importantly, two recent studies from the Friml group suggest a role for auxin in the regulation of PIN localization (Paciorek et al., 2005; Sauer et al., 2006). Thus, auxin can be the start and end point for its own flux. Evidence suggests that auxin regulates its own distribution at least through two distinct signaling pathways.
TIR1-Independent Auxin Regulation of PIN Localization
In one pathway, auxin appears to promote the polar localization of PINs in the PM by inhibiting their endocytosis from the PM (Paciorek et al., 2005). The evidence for this pathway came from the experiments showing that auxin can block the accumulation of PIN proteins, such as PIN1 and PIN2, in BFA-induced endomembrane compartments called BFA bodies. It was proposed that auxin inhibits the endocytosis of PINs from the PM (Paciorek et al., 2005). Indeed, it was shown that high concentrations of auxin (10–50 μM) could inhibit clathrin-mediated endocytosis of PINs (Dhonukshe et al., 2007). The auxin inhibition of PIN endocytosis does not require protein synthesis, implicating that this auxin action is independent of the well established TIR1-dependent auxin signaling pathway, which involves auxin-mediated gene expression (Woodward and Bartel, 2005; Quint and Gray, 2006; Teale et al., 2006). Therefore, it would be of great significance to elucidate the mechanism of TIR1-independent action mediated by auxin.
What is the developmental significance of regulation of PIN localization by the auxin-mediated endocytosis? Two possible models can explain the role of this auxin-mediated PIN localization. In the first model, auxin-mediated endocytosis of PINs could be important for the maintenance of polar PIN localization, once the initial PIN polarity is established. Polar PIN localization presumably would result in high extracelluar auxin concentrations localized at the side of PIN localization, which would locally suppress endocytosis of PIN at the side, while clathrin-mediated endocytosis would be more active at the non-PIN sides. This would generate a positive feedback mechanism to self-maintain the polarized localization of PINs. This model would be consistent with the observation that high levels of auxin are needed to inhibit endocytosis (Dhonukshe et al., 2007). This mechanism may be important for the regulation of PIN localization in cells where high concentrations of auxin are present. For example, some cells which surround quiescent center cells have higher auxin concentrations and display lower internalization of PIN proteins (Paciorek et al., 2005).
In a second model, auxin inhibition of PIN endocytosis could be involved in the initial establishment of PIN polarization. In this case, some other spatial regulation could result in a localized accumulation of a high concentration of auxin, which would then promote localized inhibition of PIN endocytosis to initiate polar distribution of PIN in the PM. Because auxin is a highly diffusible small molecule, localized accumulation of high auxin levels would require a localized auxin transport or biosynthesis system. However, it is also possible that auxin is a self-polarizing signal that can activate the polar localization of PINs to cause localized auxin accumulation. Given an environment where auxin is uniformly distributed, a stochastic localized change in auxin concentration could locally activate a self-polarizing signaling mechanism through positive feedback loops to rapidly establish PIN polarization. This type of self-polarization has been observed in animal cells, where incubation of animal cells in a solution containing a polarizing signal can induce cell polarization. Mathematical modeling shows that the stochastic self-polarization is possible (Altschuler et al., 2008). However, inhibition of PIN endocytosis by high levels of auxin is unlikely to account for this type of self-polarization, because this system would not be sensitive enough for a small stochastic change in local auxin concentration. Such localized small changes in auxin concentration most likely require a highly sensitive auxin sensing system such as the TIR1-dependent auxin sensing mechanism discussed below.
TIR1-Dependent Auxin Regulation of PIN Localization
As mentioned above, it is possible that auxin can maintain polar PIN localization and initiate re-polarization of PIN in response to stochastic changes. Auxin itself could act as a self-polarizing signal by modulating PIN localization. Recently, the Friml group demonstrated that a TIR-dependent signaling pathway can signal to re-polarize PIN proteins (Sauer et al., 2006). In given cells, auxin treatment is sufficient to shift apically localized PIN2 in cortical cells to the lateral side, and the basally localized PIN1 in pericycle and endodermis cells to lateral sides. Thus, re-polarization induced by auxin is cell type-specific. There is some evidence that re-polarization of PINs in given cells is independent of their own transcription. By utilizing promoters with no auxin-inducibility such as 35S and SCR, Paciorek et al. (2005) concluded that auxin-promoted PM PIN1 localization proceeds without de-novo PIN1 synthesis. Furthermore, auxin-induced PIN lateralization occurs in the genetic background, in which PID, GNOM, or BIG is mutated, indicating the re-polarization is independent of the PID, GNOM, and BIG pathways. Pre-treatment of roots by MG-132, a 26S proteosome inhibitor, prevented auxin-induced PIN lateralization, indicating the necessity of a TIR1-dependent signaling pathway for this process. Consistently, root hair formation is blocked when lateral auxin flow is inhibited by incorrect PIN1 lateralization caused by disruption of the TIR1 signaling pathway. Therefore, we can expect that the expression of certain genes that are dependent on the TIR1 signaling pathway is required for the lateralization of PINs.
Auxin also has a long-term effect on auxin distribution by regulating PIN protein expression. In single, double and even triple pin mutants, the remaining PIN genes appear to be ectopically expressed to compensate for the defective PINs (Blilou et al., 2005). The ectopic expression of remaining PIN genes is the consequence of redistribution of auxin in pin mutant cells. This is consistent with auxin-triggered, PLT-mediated auxin redistribution and reconstruction of auxin gradient in root tissues (Blilou et al., 2005; Galinha et al., 2007). The PLT family comprises four close members of the AP2/ERF superfamily, which act as transcriptional factors and characterize auxin-induced transcription (Aida et al., 2004; Blilou et al., 2005). In root tissues, the four members of PLT proteins are expressed in root meristem and define the fate of stem cells in a PLT dosage-dependent way (Aida et al., 2004; Galinha et al., 2007). PLT genes are thought to be secondary auxin-responsive factors or slow auxin-responding components in auxin signaling pathways and this process is inhibited by IAA14 (Aida et al., 2004). Expression of PLT genes themselves is auxin-inducible. Indeed, PLT proteins regulate the expression of PIN genes in the root cells and PIN proteins can reversely restrict expression patterns of PLT genes within particular cells (Aida et al., 2004; Blilou et al., 2005; Galinha et al., 2007).
The studies discussed above highlight several feedback loops of auxin fluxes. One is from auxin to inhibition of PIN endocytosis and to polar auxin transport, which is TIR1-independent. The second is the regulation of PIN localization by a TIR1-dependent signaling pathway to direct auxin flow. The third is from auxin to the action of PLTs, which then regulate feedback action of PIN genes and to polar auxin redistribution to maintain a maximum auxin level in the root tip. Therefore, auxin can perform to regulate its own flux and homeostasis elegantly. Many mechanistic details in these feedback loops, such as the relationship of auxin-induced gene transcription and polar localization of PIN proteins, remain to be elucidated.
CONCLUSIONS AND PERSPECTIVES
It is clear that complex vesicular trafficking pathways, which emanate from both endocytic and antegrade pathways, are integral to the polar distribution of auxin translocators in the plasma membrane. These pathways appear to be subject to the regulation by environmental and developmental cues that determine the polarity of a specific auxin translocator. A number of prominent signaling molecules such as the PID protein kinase, PP2A protein phosphatases, and ROP GTPases have been implicated in the regulation of PIN polarity. Finally, both short-term and long-term auxin signaling pathways participate in the regulation of PIN polarity, implicating auxin itself as a developmental signal influencing polar distribution of auxin translocators. Major future challenges reside in understanding what signaling mechanisms specify specific plasma membrane domains for the localization of auxin transporters, how they are linked to auxin signaling pathways, and how they orchestrate endocytosis, endosomal sorting and recycling, and transcytosis of these transporters.
FUNDING
The work in the Yang laboratory is supported by a National Institute of Health grant to Z.Y. (R01GM081451). X.G. is in part supported by a Chinese Ministry of Science and Technology 973 project (2007CB108700).
Acknowledgments
We apologize to those whose relevant work could not be cited in this review due to space limitation. No conflict of interest declared.
References
- Abas L, Benjamins R, Malenica N, Paciorek T, Wiśniewska J, Wirniewska J, Moulinier-Anzola JC, Sieberer T, Friml J, Luschnig C. Intracellular trafficking and proteolysis of the Arabidopsis auxin-efflux facilitator PIN2 are involved in root gravitropism. Nat. Cell Biol. 2006;8:249–256. doi: 10.1038/ncb1369. [DOI] [PubMed] [Google Scholar]
- Aida M, Beis D, Heidstra R, Willemsen V, Blilou I, Galinha C, Nussaume L, Noh YS, Amasino R, Scheres B. The PLETHORA genes mediate patterning of the Arabidopsis root stem cell niche. Cell. 2004;119:109–120. doi: 10.1016/j.cell.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Aloni R, Schwalm K, Langhans M, Ullrich CI. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta. 2003;216:841–853. doi: 10.1007/s00425-002-0937-8. [DOI] [PubMed] [Google Scholar]
- Altschuler SJ, Angenent SB, Wang Y, Wu LF. On the spontaneous emergence of cell polarity. Nature. 2008;454:886–889. doi: 10.1038/nature07119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS. Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J. Cell Biol. 2004;165:123–133. doi: 10.1083/jcb.200312055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Ampudia CS, Hooykaas PJ, Offringa R. PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 2003;132:1623–1630. doi: 10.1104/pp.103.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamins R, Quint A, Weijers D, Hooykaas P, Offringa R. The PINOID protein kinase regulates organ development in Arabidopsis by enhancing polar auxin transport. Development. 2001;128:4057–4067. doi: 10.1242/dev.128.20.4057. [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- Blakeslee JJ, et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell. 2007;19:131–147. doi: 10.1105/tpc.106.040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeslee JJ, Peer WA, Murphy AS. Auxin transport. Curr. Opin. Plant Biol. 2005;8:494–500. doi: 10.1016/j.pbi.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Hurley JH. Retromer. Curr. Opin. Cell Biol. 2008;20:427–436. doi: 10.1016/j.ceb.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Jackson CL. Endosome-specific localization and function of the ARF activator GNOM. Cell. 2003;112:141–142. doi: 10.1016/s0092-8674(03)00038-2. [DOI] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J. Neurochem. 2001;77:705–719. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blasius B, Nick P. Auxin transport synchronizes the pattern of cell division in a tobacco cell line. Plant Physiol. 2003;133:1251–1260. doi: 10.1104/pp.103.027953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. NPY1, a BTB-NPH3-like protein, plays a critical rolein auxin-regulated organogenesis in Arabidopsis. Proc. Natl Acad. Sci. U S A. 2007;104:18825–18829. doi: 10.1073/pnas.0708506104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AY, Wu H-M. Overexpression of an Arabidopsis formin stimulates supernumerary actin cable formation from pollen tube cell membrane. Plant Cell. 2004;16:257–269. doi: 10.1105/tpc.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- Christian M, Steffens B, Schenck D, Burmester S, Böttger M, Lüthen H. How does auxin enhance cell elongation? Roles of auxin-binding proteins and potassium channels in growth control. Plant Biol (Stuttg) 2006;8:346–352. doi: 10.1055/s-2006-923965. [DOI] [PubMed] [Google Scholar]
- Cross JW. Cycling of auxin-binding protein through the plant cell: pathways in auxin signal transduction. New Biol. 1991;3:813–819. [PubMed] [Google Scholar]
- DeMason DA, Chawla R. Roles for auxin during morphogenesis of the compound leaves of pea (Pisum sativum) Planta. 2004;218:435–448. doi: 10.1007/s00425-003-1100-x. [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M. The F-box protein TIR1 is an auxin receptor. Nature. 2005;435:441–445. doi: 10.1038/nature03543. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof YD, Friml J. Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr. Biol. 2007;17:520–527. doi: 10.1016/j.cub.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Dhonukshe P, et al. Auxin transport inhibitors impair vesicle motility and actin cytoskeleton dynamics in diverse eukaryotes. Proc. Natl Acad. Sci. U S A. 2008;105:4489–4494. doi: 10.1073/pnas.0711414105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhonukshe P, Kleine-Vehn J, Friml J. Cell polarity, auxin transport, and cytoskeleton-mediated division planes: who comes first? Protoplasma. 2005;226:67–73. doi: 10.1007/s00709-005-0104-8. [DOI] [PubMed] [Google Scholar]
- Friml J, et al. AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell. 2002;108:661–673. doi: 10.1016/s0092-8674(02)00656-6. [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. Efflux-dependent auxin gradients establish the apical–basal axis of Arabidopsis. Nature. 2003;426:147–153. doi: 10.1038/nature02085. [DOI] [PubMed] [Google Scholar]
- Friml J, et al. A PINOID-dependent binary switch in apical–basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z. Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell. 2005;120:687–700. doi: 10.1016/j.cell.2004.12.026. [DOI] [PubMed] [Google Scholar]
- Fu Y, Li H, Yang Z. The ROP2 GTPase controls the formation of cortical fine F-actin and the early phase of directional cell expansion during Arabidopsis organogenesis. Plant Cell. 2002;14:777–794. doi: 10.1105/tpc.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Yang Z. Rop GTPase: a master switch of cell polarity development in plants. Trends Plant Sci. 2001;6:545–547. doi: 10.1016/s1360-1385(01)02130-6. [DOI] [PubMed] [Google Scholar]
- Fukuda H. Signals that control plant vascular cell differentiation. Nat. Rev. Mol. Cell Biol. 2004;5:379–391. doi: 10.1038/nrm1364. [DOI] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development. 2004;131:5021–5030. doi: 10.1242/dev.01388. [DOI] [PubMed] [Google Scholar]
- Galinha C, Hofhuis H, Luijten M, Willemsen V, Blilou I, Heidstra R, Scheres B. PLETHORA proteins as dose-dependent master regulators of Arabidopsis root development. Nature. 2007;449:1053–1057. doi: 10.1038/nature06206. [DOI] [PubMed] [Google Scholar]
- Geisler M, Murphy AS. The ABC of auxin transport: the role of p-glycoproteins in plant development. FEBS Lett. 2006;580:1094–1102. doi: 10.1016/j.febslet.2005.11.054. [DOI] [PubMed] [Google Scholar]
- Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G. The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell. 2003;112:219–230. doi: 10.1016/s0092-8674(03)00003-5. [DOI] [PubMed] [Google Scholar]
- Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K. Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature. 2001;413:425–428. doi: 10.1038/35096571. [DOI] [PubMed] [Google Scholar]
- Grebe M, Xu J, Möbius W, Ueda T, Nakano A, Geuze HJ, Rook MB, Scheres B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 2003;13:1378–1387. doi: 10.1016/s0960-9822(03)00538-4. [DOI] [PubMed] [Google Scholar]
- Gu Y, Vernoud V, Fu Y, Yang Z. ROP GTPase regulation of pollen tube growth through the dynamics of tip-localized F-actin. J. Exp. Bot. 2003;54:93–101. doi: 10.1093/jxb/erg035. [DOI] [PubMed] [Google Scholar]
- Gu Y, Wang Z, Yang Z. ROP/RAC GTPase: an old new master regulator for plant signaling. Curr. Opin. Plant Biol. 2004;7:527–536. doi: 10.1016/j.pbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- Hierro A, Rojas AL, Rojas R, Murthy N, Effantin G, Kajava AV, Steven AC, Bonifacino JS, Hurley JH. Functional architecture of the retromer cargo-recognition complex. Nature. 2007;449:1063–1067. doi: 10.1038/nature06216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillais Y, Fobis-Loisy I, Miége C, Rollin C, Gaude T. AtSNX1 defines an endosome for auxin-carrier trafficking in Arabidopsis. Nature. 2006;443:106–109. doi: 10.1038/nature05046. [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Santambrogio M, Rozier F, Fobis-Loisy I, Miége C, Gaude T. The retromer protein VPS29 links cell polarity and organ initiation in plants. Cell. 2007;130:1057–1070. doi: 10.1016/j.cell.2007.08.040. [DOI] [PubMed] [Google Scholar]
- Jönsson H, Heisler MG, Shapiro BE, Meyerowitz EM, Mjolsness E. An auxin-driven polarized transport model for phyllotaxis. Proc. Natl Acad. Sci. U S A. 2006;103:1633–1638. doi: 10.1073/pnas.0509839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplinsky NJ, Barton MK. Plant biology: plant acupuncture: sticking PINs in the right places. Science. 2004;306:822–823. doi: 10.1126/science.1105534. [DOI] [PubMed] [Google Scholar]
- Kim I, Kobayashi K, Cho E, Zambryski PC. Subdomains for transport via plasmodesmata corresponding to the apical–basal axis are established during Arabidopsis embryogenesis. Proc. Natl Acad. Sci. U S A. 2005;102:11945–11950. doi: 10.1073/pnas.0505622102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Sauer M, Brewer PB, Wiśniewska J, Paciorek T, Benková E, Friml J. ARF GEF-dependent transcytosis and polar delivery of PIN auxin carriers in Arabidopsis. Curr. Biol. 2008;18:526–531. doi: 10.1016/j.cub.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Dhonukshe P, Swarup R, Bennett M, Friml J. Subcellular trafficking of the Arabidopsis auxin influx carrier AUX1 uses a novel pathway distinct from PIN1. Plant Cell. 2006;18:3171–3181. doi: 10.1105/tpc.106.042770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B. Spatial control of Rho (Rac–Rop) signaling in tip-growin plant cells. Trends Cell Biol. 2008;18:119–127. doi: 10.1016/j.tcb.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Kramer EM, Bennett MJ. Auxin transport: a field in flux. Trends Plant Sci. 2006;11:382–386. doi: 10.1016/j.tplants.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Kwon C, et al. Co-option of a default secretory pathway for plant immune responses. Nature. 2008;451:835–840. doi: 10.1038/nature06545. [DOI] [PubMed] [Google Scholar]
- Laskowski M. Teaching resources: model of the TIR1 pathway for auxin-mediated gene expression. Sci. STKE. 2006;2006:tr1. doi: 10.1126/stke.3222006tr1. [DOI] [PubMed] [Google Scholar]
- Laxmi A, et al. Light plays an essential role in intracellular distribution of auxin efflux carrier PIN2 in Arabidopsis thaliana. PLoS ONE. 2008;3:e1510. doi: 10.1371/journal.pone.0001510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblanc N, Perrot-Rechenmann C, Barbier-Brygoo H. The auxin-binding protein Nt-ERabp1 alone activates an auxin-like transduction pathway. FEBS Lett. 1999;449:57–60. doi: 10.1016/s0014-5793(99)00398-1. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cho HT. PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2006;18:1604–1616. doi: 10.1105/tpc.105.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YJ, Szumlanski A, Nielsen E, Yang Z. Rho–GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol. 2008;181:1155–1168. doi: 10.1083/jcb.200801086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shen JJ, Zheng ZL, Lin Y, Yang Z. The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol. 2001;126:670–684. doi: 10.1104/pp.126.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xu J, Xu ZH, Xue HW. Brassinosteroids stimulate plant tropisms through modulation of polar auxin transport in Brassica and Arabidopsis. Plant Cell. 2005;17:2738–2753. doi: 10.1105/tpc.105.034397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R, Wang H. Two homologous ATP-binding cassette transporter proteins, AtMDR1 and AtPGP1, regulate Arabidopsis photomorphogenesis and root development by mediating polar auxin transport. Plant Physiol. 2005;138:949–964. doi: 10.1104/pp.105.061572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipka V, Kwon C, Panstruga R. SNARE-ware: the role of SNARE-domain proteins in plant biology. Annu. Rev. Cell Dev. Biol. 2007;23:147–174. doi: 10.1146/annurev.cellbio.23.090506.123529. [DOI] [PubMed] [Google Scholar]
- Liu C, Xu Z, Chua NH. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. Plant Cell. 1993;5:621–630. doi: 10.1105/tpc.5.6.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev. 1998;12:2175–2187. doi: 10.1101/gad.12.14.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson J, Ckurshumova W, Berleth T. Auxin signaling in Arabidopsis leaf vascular development. Plant Physiol. 2003;131:1327–1339. doi: 10.1104/pp.013623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men S, Boutté Y, Ikeda Y, Li X, Palme K, Stierhof YD, Hartmann MA, Moritz T, Grebe M. Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 2008;10:237–244. doi: 10.1038/ncb1686. [DOI] [PubMed] [Google Scholar]
- Michniewicz M, et al. Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell. 2007;130:1044–1056. doi: 10.1016/j.cell.2007.07.033. [DOI] [PubMed] [Google Scholar]
- Morita Y, Kyozuka J. Characterization of OsPID, the rice ortholog of PINOID, and its possible involvement in the control of polar auxin transport. Plant Cell Physiol. 2007;48:540–549. doi: 10.1093/pcp/pcm024. [DOI] [PubMed] [Google Scholar]
- Motchoulski A, Liscum E. Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science. 1999;286:961–964. doi: 10.1126/science.286.5441.961. [DOI] [PubMed] [Google Scholar]
- Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–6911. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell AC, et al. Phyllotaxis: cooperation and competition between mechanical and biochemical processes. J. Theor. Biol. 2008;251:421–439. doi: 10.1016/j.jtbi.2007.11.036. [DOI] [PubMed] [Google Scholar]
- Oliviusson P, Heinzerling O, Hillmer S, Hinz G, Tse YC, Jiang L, Robinson DG. Plant retromer, localized to the prevacuolar compartment and microvesicles in Arabidopsis, may interact with vacuolar sorting receptors. Plant Cell. 2006;18:1239–1252. doi: 10.1105/tpc.105.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- Petrásek J, Elckner M, Morris DA, Zazímalová E. Auxin efflux carrier activity and auxin accumulation regulate cell division and polarity in tobacco cells. Planta. 2002;216:302–308. doi: 10.1007/s00425-002-0845-y. [DOI] [PubMed] [Google Scholar]
- Quint M, Gray WM. Auxin signaling. Curr. Opin. Plant Biol. 2006;9:448–453. doi: 10.1016/j.pbi.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J. 2007;50:514–528. doi: 10.1111/j.1365-313X.2007.03068.x. [DOI] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. Plant Cell. 2001;13:1683–1697. doi: 10.1105/TPC.010158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhardt D, Pesce ER, Stieger P, Mandel T, Baltensperger K, Bennett M, Traas J, Friml J, Kuhlemeier C. Regulation of phyllotaxis by polar auxin transport. Nature. 2003;426:255–260. doi: 10.1038/nature02081. [DOI] [PubMed] [Google Scholar]
- Robert S, Chary SN, Drakakaki G, Li S, Yang Z, Raikhel NV, Hicks GR. Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl Acad. Sci. U S A. 2008;105:8464–8469. doi: 10.1073/pnas.0711650105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Kametaka S, Haft CR, Bonifacino JS. Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol. Cell Biol. 2007;27:1112–1124. doi: 10.1128/MCB.00156-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Vincenzetti V, Azzarello E, Bovet L, Fukao Y, Düchtig P, Mancuso S, Martinoia E, Geisler M. MDR-like ABC transporter AtPGP4 is involved in auxin-mediated lateral root and root hair development. FEBS Lett. 2005;579:5399–5406. doi: 10.1016/j.febslet.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Sauer M, Balla J, Luschnig C, Wisniewska J, Reinöhl V, Friml J, Benková E. Canalization of auxin flow by Aux/IAA–ARF-dependent feedback regulation of PIN polarity. Genes Dev. 2006;20:2902–2911. doi: 10.1101/gad.390806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer GF. Secondary messengers and phospholipase A2 in auxin signal transduction. Plant Mol. Biol. 2002;49:357–372. [PubMed] [Google Scholar]
- Shi H, Rojas R, Bonifacino JS, Hurley JH. The retromer subunit Vps26 has an arrestin fold and binds Vps35 through its C-terminal domain. Nat. Struct. Mol. Biol. 2006;13:540–548. doi: 10.1038/nsmb1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G. Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science. 1999;286:316–318. doi: 10.1126/science.286.5438.316. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dhonukshe P, Brewer PB, Friml J. Spatiotemporal asymmetric auxin distribution: a means to coordinate plant development. Cell Mol. Life Sci. 2006;63:2738–2754. doi: 10.1007/s00018-006-6116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teale WD, Paponov IA, Palme K. Auxin in action: signalling, transport and the control of plant growth and development. Nat. Rev. Mol. Cell Biol. 2006;7:847–859. doi: 10.1038/nrm2020. [DOI] [PubMed] [Google Scholar]
- Teh OK, Moore I. An ARF–GEF acting at the Golgi and in selective endocytosis in polarized plant cells. Nature. 2007;448:493–496. doi: 10.1038/nature06023. [DOI] [PubMed] [Google Scholar]
- Vernoux T, Kronenberger J, Grandjean O, Laufs P, Traas J. PIN-FORMED 1 regulates cell fate at the periphery of the shoot apical meristem. Development. 2000;127:5157–5165. doi: 10.1242/dev.127.23.5157. [DOI] [PubMed] [Google Scholar]
- Weijers D, Sauer M, Meurette O, Friml J, Ljung K, Sandberg G, Hooykaas P, Offringa R. Auxin and embryo axis formation: the ends in sight? Curr. Opin. Plant Biol. 2005;8:32–37. doi: 10.1016/j.pbi.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Weijers D, et al. Maintenance of embryonic auxin distribution for apical–basal patterning by PIN-FORMED-dependent auxin transport in Arabidopsis. Plant Cell. 2005;17:2517–2526. doi: 10.1105/tpc.105.034637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Schlereth A, Ehrismann JS, Schwank G, Kientz M, Jürgens G. Auxin triggers transient local signaling for cell specification in Arabidopsis embryogenesis. Dev. Cell. 2006;10:265–270. doi: 10.1016/j.devcel.2005.12.001. [DOI] [PubMed] [Google Scholar]
- Willemsen V, Friml J, Grebe M, van den Toorn A, Palme K, Scheres B. Cell polarity and PIN protein positioning in Arabidopsis require STEROL METHYLTRANSFERASE1 function. Plant Cell. 2003;15:612–625. doi: 10.1105/tpc.008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW, Bartel B. Auxin: regulation, action, and interaction. Ann. Bot. (Lond.) 2005;95:707–735. doi: 10.1093/aob/mci083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagami M, Haga K, Napier RM, Iino M. Two distinct signaling pathways participate in auxin-induced swelling of pea epidermal protoplasts. Plant Physiol. 2004;134:735–747. doi: 10.1104/pp.103.031294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Cell polarity signaling in Arabidopsis. Ann. Rev. Cell Dev. Biol. 2008;24:551–745. doi: 10.1146/annurev.cellbio.23.090506.123233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazímalová E, Krecek P, Skůpa P, Hoyerová K, Petrásek J. Polar transport of the plant hormone auxin: the role of PIN-FORMED (PIN) proteins. Cell Mol. Life Sci. 2007;64:1621–1637. doi: 10.1007/s00018-007-6566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegzouti H, Anthony RG, Jahchan N, Bügre L, Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc. Natl Acad. Sci. U S A. 2006;103:6404–6409. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]