Abstract
Introduction
The study objectives were to examine smoking abstinence and reinstatement effects on subjective experience and cognitive performance among adolescent smokers.
Methods
Adolescents (aged 14–17 years, 60 daily smokers and 32 nonsmokers) participated. Participants completed baseline assessments (Session 1) and returned to the laboratory 1–3 days later to repeat assessments (Session 2); half of the smokers were randomly assigned to 15–17 hr tobacco abstinence preceding Session 2.
Results
During Session 2, abstaining smokers reported significantly greater increases in withdrawal symptoms, smoking urges, and negative affect compared with smokers who did not abstain and compared with nonsmokers. Smoking reinstatement reversed abstinence effects, returning to baseline levels for smoking urges and negative affect. Abstaining smokers showed significantly enhanced cognitive performance on two of six tasks (two-letter search compared with nonabstaining smokers; serial reaction time compared with nonsmokers); smoking reinstatement resulted in significant decrements on these two tasks relative to nonabstaining smokers.
Discussion
Effects of smoking abstinence and reinstatement on self-report measures are consistent with earlier research with adolescent as well as adult smokers and may help to elucidate the motivational underpinnings of smoking maintenance among adolescent smokers. Effects found on cognitive performance were contrary to hypotheses; further research is needed to understand better the role of cognitive performance effects in smoking maintenance among adolescents.
Introduction
Cigarette smoking among adolescents is a public health concern, with nearly 1.5 million adolescents initiating smoking and more than 400,000 becoming daily smokers in the United States each year (Substance Abuse and Mental Health Services Administration 2005). Although many adolescent smokers report wanting to quit, few remain abstinent after making a cessation attempt (Mermelstein, 2003; Stanton, McClelland, Elwood, Ferry, & Silva, 1996; Zhu, Sun, Billings, Choi, & Malarcher, 1999). Even with treatment, the large majority of adolescents relapse back to smoking (Colby & Gwaltney, 2007; Sussman, Sun, & Dent, 2006). Enhancing our understanding of the motivational determinants of smoking in adolescents may facilitate the development of more effective interventions.
Negative reinforcement models provide a framework for understanding the motivational processes that sustain smoking behavior once nicotine dependence is established (Baker, Brandon, & Chassin, 2004; Watkins, Koob, & Markou, 2000). Tobacco addiction is proposed to be maintained by negative reinforcement processes in which smoking abstinence induces undesirable withdrawal effects, which in turn generate motivation to reinstate smoking to alleviate symptoms. Accordingly, investigating the nature of tobacco abstinence and reinstatement effects may be informative. Extensive experimental investigation of these effects has been conducted in adults (see Hughes, 2007 for a review); comparable research in adolescents is relatively limited. Adolescents may differ from adults in their responses based on developmental differences, briefer smoking histories, and more variable smoking patterns.
A review of studies of the effects of smoking abstinence in adolescent smokers concluded that (a) withdrawal symptoms are commonly reported by adolescents queried about a prior quit attempt; (b) craving/desire to smoke is the most commonly reported effect of acute abstinence; and (c) prevalence of abstinence effects is related to baseline heaviness of smoking (Colby, Tiffany, Shiffman, & Niaura, 2000). Nonexperimental studies of abstinence effects in adolescents are limited by tending to rely on retrospective self-report; reports can be biased by the outcome of the quit attempt (Colby et al., 2000) or based on adolescents’ expectations about withdrawal (McNeill, West, Jarvis, Jackson, Brryant, 1986). Prospective studies have also found acute abstinence to produce increased subjective withdrawal symptoms in adolescent smokers (Dozois, Farrow, Miser, 1995; Killen et al., 2001). More recently, a study involving adolescents receiving behavioral smoking cessation therapy found significant increases in craving, restlessness, and total withdrawal score following the initiation of a quit attempt (Smith, Cavallo, McFetridge, Liss, & Krishnan-Sarin, 2008). A similar study of adolescent smokers in cessation treatment found craving to be the most problematic effect of abstinence over time; moreover, nicotine withdrawal was found to be strongly related to baseline nicotine dependence levels (Bailey et al., 2009).
Experimental studies of smoking abstinence effects in adolescents
Three controlled experimental studies of smoking abstinence effects in adolescents have been published (Jacobsen, Slotkin, Westerveld, Mencl, & Pugh, 2006; Jacobsen, Mencl, Constable, Westerveld, & Pugh, 2007; Jacobsen et al., 2005). Acute (24-hr) smoking abstinence increased craving and withdrawal symptoms in adolescent smokers in all three studies. Jacobsen et al. (2005) found that abstinence disrupted smokers’ working memory and short-term verbal memory while performance on selective, divided, and sustained attention and verbal learning tasks was not affected. Jacobsen et al. (2007) found smokers’ cognitive performance on most tasks was unaffected by abstinence; however, on a binaural two-back task, abstinence reduced accuracy. Jacobsen et al. (2006) found that effects of abstinence on visuospatial memory were moderated by prenatal smoking exposure. The pattern of findings was complex, but generally, abstinence improved recall in participants with no prenatal exposure and worsened recall among prenatally exposed participants. Two of these studies also found nonsmokers to outperform smokers on working memory tasks, unrelated to the effects of abstinence (i.e., Jacobsen et al., 2005, 2007).
Experimental studies of smoking reinstatement effects in abstinent adolescent smokers
Two experimental studies of smoking reinstatement effects in adolescents have been published. An initial study by Zack, Belsito, Scher, Eissenberg, and Corrigall (2001) found that smoking reinstatement among 16 overnight-abstinent adolescent smokers reduced general smoking urges and urges to smoke to relieve withdrawal. Among the heavier smokers (≥11 cigarettes/day), smoking reinstatement enhanced performance (i.e., decreased intrusion of smoking-related stimuli) on a modified Stroop task. Reinstatement also enhanced accuracy on a rapid information processing task in heavier smokers, but impaired performance in lighter smokers. In an expanded sample (N = 42), Corrigall, Zack, Eissenberg, Belsito, and Scher (2001) found smoking reinstatement to decrease smoking craving in nondaily as well as daily adolescent smokers. During reinstatement, daily and nondaily smokers did not differ on smoking intensity or corresponding increases in expired carbon monoxide (CO), salivary nicotine, and heart rate.
The current study
The current study examined the effects of experimentally manipulated acute smoking abstinence (15–17 hr) and the suppression of abstinence effects by smoking reinstatement among 31 adolescent smokers compared with 29 control ad-lib smokers and 32 nonsmokers. Prior studies of smoking reinstatement effects among adolescents have not directly compared abstinent and nonabstinent states within the same study, so it is unclear whether these findings are due to the suppression of abstinence symptoms or the acute effects of smoking. Directly contrasting the effects of acute smoking among abstinent and nonabstinent adolescent smokers is necessary to clarify whether abstinence symptom relief is a motivational influence underlying smoking maintenance in this population. We examine effects across a battery of subjective (nicotine withdrawal symptoms, craving, positive affect, negative affect) and cognitive performance (e.g., search and recognition, spatial memory, working memory, reaction time, paired associate learning) assessments. We hypothesized that abstinence would increase smokers’ withdrawal symptoms, urge to smoke, and negative affect; decrease positive affect; and impair cognitive performance and that smoking reinstatement would suppress these effects.
Methods
Participants
Participants were recruited from area public schools. Interested students completed a brief telephone screen to establish eligibility. Adolescents were required to be 13–19 years old with 12 or fewer years of education. “Daily smokers” reported smoking one or more cigarettes daily over the past 30 days. “Nonsmokers” reported having smoked fewer than 100 cigarettes in their lifetime and none in the past 6 months. Daily use of alcohol or illicit drugs was excluded to avoid confounding abstinence effects from substances other than tobacco; however, nondaily use was permitted to increase generalizability to the population of adolescent smokers (cf., Brown, Lewinsohn, Seeley, & Wagner, 1996). Current psychiatric treatment was exclusionary but past treatment was permitted.
Informed assent and parent consent were obtained for minors; participants 18–19 years old provided their own informed consent. Following study completion, all participants received $50 and a brief (15-min) educational session about smoking. All procedures were approved by the Brown University Institutional Review Board.
Design and test procedures
The study used a mixed between- and within-subjects experimental design. Participants were measured at three assessment points: (a) Session 1 (S1, baseline); (b) Session 2-Time 1 (S2-T1, before smoking reinstatement to evaluate abstinence effects); and (c) Session 2-Time 2 (S2-T2, after smoking reinstatement). Responses at these assessments were compared in the following groups: (a) Nonsmokers (NONSMK; n = 32); (b) Abstainers: smokers randomly assigned to abstain from smoking after midnight prior to S2 (ABST; n = 31); and (c) Ad-lib smokers: smokers randomly assigned to smoke according to their usual pattern prior to S2 (ADLIB; n = 28).
Standard pre-session instructions included no use of alcohol, illicit drugs, or nonprescription drugs for 24 hr; no caffeine for 4 hr; and no dairy products or large meals with 1 hr of each session. Smokers were instructed to bring their cigarettes to each session and to smoke normally prior to S1. Participants signed an affidavit at the beginning of each session stating that they had complied with all of the instructions or they were rescheduled. Those who did not follow the instructions at the next session were withdrawn from the study. All sessions started between 3–5 p.m. because (a) this allowed for 15–17 hr smoking abstinence, sufficient to induce withdrawal (Heishman, Taylor, & Henningfield, 1994); (b withdrawal symptoms are stronger in the afternoon than morning or evening (Perkins, Briski, Fonte, Scott, & Lerman, 2009; Schneider & Jarvik, 1984); and (c) standardized session times are important when using cognitive tasks affected by diurnal variation.
Laboratory sessions were conducted by a research assistant following a scripted protocol. Participants sat in an observation room and were monitored through a one-way mirror; instructions were read verbatim to each participant and delivered via intercom system. In S1 (approximately 2 hr), participants began by providing breath and saliva samples for expired CO and cotinine levels, completed background questionnaires, and practiced the computerized cognitive performance measures (70–90 min). Next, smokers were instructed to smoke a cigarette (5 min); nonsmokers took a 5-min break. Participants then completed the baseline assessments against which S2 would be compared: self-reported withdrawal symptoms, smoking urges, positive and negative affect, and cognitive tasks. Finally, expired CO was reassessed. ABST smokers were told to refrain from smoking cigarettes after midnight prior to S2. ADLIB smokers were instructed to smoke at their usual rate. Pre-S2 instructions were provided verbally and in writing.
S2 (approximately 1 hr) occurred 24–72 hr after S1; participants’ CO levels were tested upon arrival. ABST participants were required to exhibit CO ≤ 10 ppm; those with CO > 10 ppm were rescheduled. ABST participants who failed to meet CO criteria a second time were withdrawn from the study. Prior to beginning the assessments, ADLIB smokers were instructed to smoke a cigarette (5 min). S2-T1: To evaluate smoking abstinence effects, participants completed the same measures (withdrawal symptoms, smoking urges, affect, and cognitive tasks) as at S1. S2-T2: To evaluate smoking reinstatement effects, ABST smokers were instructed to smoke a cigarette (5 min) while ADLIB smokers took a break and had their CO tested, then affect, urge, and cognitive measures were readministered to all smokers (15–20 min); then ABST smokers had their CO reassessed. Nonsmokers did not participate in the smoking reinstatement phase of the experiment.
Physiological measures
Breath samples were analyzed to determine CO levels using a Bedfont Smokerlyzer. Saliva samples were taken at baseline and shipped in dry ice to an external laboratory where cotinine levels were determined via gas chromatography (Jacob, Wilson, & Benowitz, 1981).
Background questionnaires
Demographic variables included age, gender, race, and years of education. Tobacco Use History queried age of various tobacco-use milestones and history of quit attempts. Smoking rate was obtained using the 30-day Timeline Followback for Smoking, a calendar-assisted retrospective recall of cigarettes per day that has been validated for use with adolescents (Lewis-Esquerre et al., 2005). Nicotine dependence was assessed using the Stanford Dependence Index, a 5-item questionnaire with established reliability and validity in adolescents (Rojas, Killen, Haydel, & Robinson, 1998). The scale score is a sum ranging from 5 to 25 (α in this sample = .54).
Self-report measures of abstinence and reinstatement effects
The Minnesota Nicotine Withdrawal Scale (MNWS; Hughes & Hatsukami, 1986; Hughes, 1992) includes seven symptoms rated from 0 (not present) to 4 (severe); the scale score is a mean of these items (α in this sample = .82). “Desire to smoke” was rated but not included in the scale score to measure desire and withdrawal separately (Hughes & Hatsukami, 1998). The MNWS queried participant experience over the entire day; it was not readministered at S2-T2 because the elapsed time did not seem long enough for significant change to have occurred.
The Questionnaire on Smoking Urges (QSU) Brief Form is a 10-item abbreviated version of the 32-item QSU (Tiffany & Drobes, 1991). The QSU has a total score (α in this sample = .91) plus two empirically derived factors; Factor 1 (α = .84) reflects the intention and desire to smoke as well as desire to smoke for reward and Factor 2 (α = .83) reflects intense desire to smoke as well as desire to smoke to relieve withdrawal. Items were rated from 1 (strongly disagree) to 7 (strongly agree); total and scale scores are means and also range from 1 to 7.
The Positive Affect and Negative Affect Scale (PANAS; Watson, Clarke, & Tellegen, 1988) is a widely used 20-item measure with reliable subscales for evaluating positive and negative affect (α values in this sample = .86 and .84, respectively). The PANAS has been used to study the acute effects of nicotine in an adolescent sample (Kassel et al., 2007). Participants rated adjectives describing their affect “right now” from 1 (very slightly or not at all) to 5 (extremely); scale scores are sums with possible ranges of 10–50.
Cognitive performance tasks
The Walter Reed Performance Assessment Battery (PAB; Thorne, Genser, Sing, & Hegge, 1985) is an extensive computerized cognitive test battery including psychomotor, perceptual, and cognitive tasks. Six PAB tasks were selected based on the ability of adolescents with low levels of education or cognitive ability to master them (unreported pilot data) and demonstrated sensitivity to the effects of nicotine withdrawal in adults (Heishman et al., 1994). Respondents must first be trained to asymptotic or near-asymptotic levels of performance on each task and training time varies considerably (Snyder, Davis, & Henningfield, 1989). To standardize the training time and thus length of S1 across participants, each respondent practiced each task a total of 10 times before completing the baseline PAB. Because this took about 100 min, resulting in marked participant fatigue (pilot data not reported), we constructed three separate PABs (three to four tasks each; task order within each PAB was randomized), reducing practice time to about 60 min. Participants were randomly assigned to one of the three PABs, completing it at all three time points, so the n for each task is roughly two thirds that of the total N (except on pattern recognition which was in all three PABs). The tasks included two letter search, pattern recognition, four choice serial reaction time, running memory, code substitution, and delayed recall.
Two-letter search: This visual search and recognition task is considered a test of selective attention; two target letters appear at the top of the screen while a string of 20 letters appears in the middle of the screen; the participant determines as quickly as possible whether or not the target letters are also in the string. Pattern recognition: This is a visuospatial memory task in which a random pattern of 14 asterisks is displayed for 1.5 s followed by a 3.5-s retention interval (blank screen). A second pattern is then displayed and the participant determines as quickly as possible whether the pattern is the same as the first or different. Four-choice serial reaction time: This visual vigilance continuous performance task is considered a measure of attentional control; a box with four quadrants appears on the screen and a single light within each quadrant lights up in random order. The goal is to press the corresponding key as quickly and accurately as possible when the light appears. Running memory: This continuous reaction time task assesses working memory and sustained attention. The participant is required to decide as quickly as possible whether the current letter displayed is the same or different from the prior letter. Code substitution: In this complex attention and incidental learning task, a code key of nine letter–digit pairs is displayed on the screen; the participant responds to the presentation of a series of individual letters by pressing the digit coded to each letter. Delayed recall: In this short-term memory task, the participant repeats one block of the code substitution task without the code key appearing on the screen.
Each PAB task yields three variables: percent correct, reaction time, and throughput (i.e., accurate responses per working min). To limit the number of statistical tests conducted, throughput was selected for analysis, incorporating both speed and accuracy.
Data analysis
One-way between-subjects analyses of variance (ANOVAs) and chi-square tests were used to examine potential group differences on baseline variables. Descriptive statistics were calculated to determine the mean level of mastery (percent correct) on each task on the final practice trial. To analyze effects of smoking abstinence, one-way ANOVAs were used comparing NONSMK, ABST, and ADLIB on the change score from S1 to S2-T1, which is mathematically equivalent to a Group by Time interaction (Maxwell & Delaney, 1990). Because the ADLIB and NONSMK groups both provided separate controls for ABST smokers, their comparison to each other was not of interest. Therefore, two planned comparisons of the change from S1 to S2-T1 were conducted for each ANOVA: (a) ABST versus ADLIB and (b) ABST versus NONSMK. To analyze effects of smoking reinstatement during S2, one-way between-subjects ANOVAs were used comparing ABST and ADLIB responses on the change score from T1 (presmoking for ABST) to T2 (postsmoking for ABST). We also report Cohen’s d statistics as effect size estimates (Cohen, 1988) to interpret the robustness of each effect (small d = .20, medium d = .50, large d ≥ .80). Two-tailed alpha was set at .05. ANOVAs were tested in SAS using PROC GLM for unbalanced cell sizes (SAS Institute Inc., 2003).
Results
Participant characteristics
A total of 220 individuals completed the screening interview, 129/220 (59%) met inclusion criteria, 127/129 (98%) verbally agreed to participate, 96/127 (76%) attended S1, and 92/96 (96%) completed S2. The final sample (N = 92) is described in Table 1. Compared with the two Smoker groups, the NONSMK group was more racially diverse and reported significantly less frequent alcohol and marijuana use; the three groups did not differ in age, gender, or years of education. The ABST and ADLIB groups did not differ on any baseline smoking variable.
Table 1.
Baseline demographic and smoking characteristics, by group
| NONSMK (n = 32) | ABST (n = 31) | ADLIB (n = 29) | F/χ2 | p value | |
| Demographic characteristics | |||||
| Female, n (%) | 19 (59.4) | 18 (58.1) | 18 (62.1) | 0.10 | .95 |
| Age, M (SD) | 15.5 (1.3) | 15.6 (1.5) | 15.6 (1.3) | 0.04 | .95 |
| Race/ethnicity, n (%)a | – | – | – | 22.8 | .03 |
| Native American | 2 (6.1) | 1 (3.2) | 2 (6.9) | ||
| Asian/Pacific Islander | 0 (0.0) | 1 (3.2) | 2 (6.9) | ||
| Black/African American | 3 (9.4) | 2 (3.2) | 2 (6.9) | ||
| Cape Verdean | 4 (12.5) | 1 (3.2) | 1 (3.5) | ||
| Hispanic | 9 (28.1) | 3 (9.6) | 2 (6.9) | ||
| Non-Hispanic White | 12 (37.5) | 24 (77.4) | 22 (75.9) | ||
| Other | 2 (6.2) | 0 (0.0) | 0 (0.0) | ||
| Total years education, M (SD) | 9.8 (1.1) | 9.9 (1.2) | 9.8 (1.1) | 0.04 | .96 |
| Smoking characteristics, M (SD) | |||||
| Cigarettes/day in past 30 days | – | 10.5 (5.7) | 10.7 (5.7) | 0.03 | .86 |
| Years regular smoker | – | 2.6 (1.9) | 3.0 (1.7) | 0.14 | .70 |
| Stanford Dependence Index | – | 14.5 (3.5) | 14.7 (3.1) | 0.04 | .83 |
| Expired carbon monoxide (ppm) | – | 12.9 (7.7) | 11.8 (7.0) | 0.34 | .56 |
| Salivary cotinine (ng/ml) | – | 169.8 (86.8) | 169.2 (92.5) | <0.01 | .98 |
| Substance use, M (SD)b | |||||
| Days used alcohol (of past 30) | 0.53 (1.4) | 2.5 (2.8) | 2.3 (3.3) | 5.3 | .007 |
| Days used marijuana (of past 30) | 0.56 (2.7) | 7.2 (8.7) | 6.8 (8.6) | 8.6 | <.001 |
Note. ABST = abstinent smokers; ADLIB = nonabstinent smokers; NONSMK = nonsmokers.
Pairwise chi-square comparisons indicated that NONSMK were significantly different from ABST and ADLIB groups (p < .05); ABST versus ADLIB pairwise contrasts were nonsignificant (p = .86).
Post-hoc tests indicated that NONSMK used alcohol and marijuana significantly less often than ABST and ADLIB groups (p < .05); ABST and ADLIB did not differ (p = .76 and .83, respectively).
Preliminary analyses
PAB mastery.
On the final PAB practice trial in S1, average levels of accuracy were high (99% on two-letter search, 91% on pattern recognition, 100% on serial reaction time, 99% on running memory, 97% on code substitution, and 92% on delayed recall) and did not differ by group as confirmed by one-way ANOVAs (all ps > .20).
Abstinence manipulation check.
Changes in expired CO from S1 to S2 confirmed the effect of the abstinence manipulation, F(2, 88) = 52.0, p < .001, d = 2.17. ABST smokers showed a large decrease in CO levels, while ADLIB and NONSMK levels were similar across both sessions.
Smoking abstinence effects and reinstatement effects
The M (SD) of each dependent variable by group and assessment time point is reported in Table 2. Results of ANOVAs for specific group comparisons are reported in Table 3.
Table 2.
Summary statistics of dependent variables by group and assessment time point
| NONSMK (n = 32) |
ABST (n = 31) |
ADLIB (n = 29) |
||||||||||||||
| S1 |
S2-T1 |
S1 |
S2-T1 |
S2-T2 |
S1 |
S2-T1a |
S2-T2 |
|||||||||
| M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | M | SD | |
| Self-report measures | ||||||||||||||||
| MNWS totalb | 1.02 | 0.71 | 0.63 | 0.71 | 1.40 | 0.94 | 1.86 | 0.97 | 1.33 | 0.89 | 1.24 | 0.96 | ||||
| Angry/irritable/frustrated | 0.94 | 1.13 | 0.50 | 0.76 | 1.48 | 1.29 | 2.29 | 1.53 | 1.34 | 1.08 | 1.46 | 1.20 | ||||
| Anxious/nervous | 1.25 | 1.16 | 0.84 | 1.02 | 1.58 | 1.20 | 2.03 | 1.38 | 1.34 | 1.08 | 0.89 | 1.29 | ||||
| Difficulty concentrating | 1.13 | 0.91 | 0.69 | 1.15 | 1.61 | 1.28 | 2.16 | 1.29 | 1.17 | 1.23 | 1.36 | 1.16 | ||||
| Impatient/restless | 1.34 | 1.33 | 0.78 | 0.83 | 1.48 | 1.12 | 2.71 | 1.32 | 1.90 | 1.35 | 1.36 | 1.34 | ||||
| Appetite/hunger | 1.03 | 1.43 | 0.56 | 0.95 | 1.19 | 1.33 | 1.77 | 1.33 | 1.55 | 1.43 | 1.32 | 1.36 | ||||
| Waking at night | 0.81 | 1.18 | 0.78 | 1.29 | 1.23 | 1.41 | 0.97 | 1.40 | 1.07 | 1.25 | 1.11 | 1.34 | ||||
| Depressed | 0.63 | 1.01 | 0.22 | 0.75 | 1.19 | 1.42 | 1.06 | 1.29 | 0.97 | 1.27 | 1.21 | 1.40 | ||||
| Desire to smoke | 0.00 | 0.00 | 0.00 | 0.00 | 2.61 | 1.05 | 3.74 | 0.63 | 2.41 | 1.02 | 2.11 | 1.29 | ||||
| PANAS-Negative Affect | 14.2 | 5.60 | 12.6 | 5.49 | 15.9 | 5.61 | 18.4 | 6.57 | 14.7 | 5.69 | 17.0 | 5.76 | 14.5 | 5.31 | 14.3 | 5.04 |
| PANAS-Positive Affect | 30.0 | 8.13 | 29.8 | 7.83 | 27.0 | 7.82 | 25.7 | 9.50 | 25.5 | 9.15 | 24.5 | 7.18 | 28.4 | 7.77 | 22.3 | 7.54 |
| QSU-Total | 1.03 | 0.14 | 1.00 | 0.00 | 1.78 | 0.78 | 5.41 | 1.17 | 1.98 | 1.20 | 1.99 | 1.15 | 1.71 | 1.16 | 2.13 | 1.46 |
| QSU-Factor 1 | 1.04 | 0.21 | 1.00 | 0.00 | 1.90 | 0.95 | 6.33 | 0.97 | 2.17 | 1.40 | 2.03 | 1.27 | 1.74 | 1.16 | 2.41 | 1.66 |
| QSU-Factor 2 | 1.03 | 0.18 | 1.00 | 0.00 | 1.66 | 0.78 | 4.49 | 1.52 | 1.80 | 1.11 | 1.94 | 1.09 | 1.69 | 1.20 | 1.86 | 1.32 |
| Cognitive performance tasks (throughput; accurate responses per working minute) | ||||||||||||||||
| Code substitutionc | 28.6 | 6.14 | 34.0 | 8.09 | 30.1 | 6.61 | 36.0 | 7.88 | 35.0 | 6.23 | 32.4 | 8.57 | 36.4 | 6.85 | 37.4 | 8.41 |
| Delayed recallc | 25.1 | 14.1 | 39.1 | 19.0 | 27.4 | 15.6 | 42.0 | 16.0 | 34.5 | 17.5 | 28.2 | 17.5 | 38.0 | 16.7 | 39.7 | 18.6 |
| Two-letter searchd | 14.3 | 3.30 | 16.1 | 4.82 | 15.2 | 3.24 | 17.6 | 3.91 | 16.0 | 4.14 | 19.4 | 12.8 | 16.1 | 3.74 | 16.9 | 3.64 |
| Pattern recognition | 37.7 | 11.8 | 38.3 | 11.0 | 36.1 | 11.3 | 37.1 | 12.3 | 36.1 | 13.2 | 45.2 | 15.1 | 41.6 | 17.7 | 42.3 | 15.6 |
| Running memorye | 1745 | 66.8 | 204 | 91.4 | 182 | 66.9 | 223 | 94.9 | 223 | 74.1 | 162 | 83.2 | 176 | 86.2 | 195 | 91.1 |
| Four-choice serial RTd | 1589 | 33.7 | 165 | 34.1 | 158 | 29.1 | 175 | 26.7 | 166 | 26.9 | 153 | 27.2 | 162 | 22.6 | 169 | 23.3 |
Note. ABST = abstinent smokers; ADLIB = nonabstinent smokers; MNWS = Minnesota Nicotine Withdrawal Scale; NONSMK = nonsmokers; PANAS = Positive and Negative Affect Schedule; QSU = Questionnaire of Smoking Urges—Brief; RT = reaction time; S1 = Session 1; S2 = Session 2; T1 = Time 1 (presmoking for ABST); T2 = Time 2 (postsmoking for ABST).
n = 28 because of missing data.
MNWS not administered at S2-T2.
Task administered to a subset of participants (NONSMK, n = 20; ABST, n = 20; ADLIB, n = 19).
NONSMK (n = 22), ABST (n = 21), ADLIB (n = 21).
NONSMK (n = 22), ABST (n = 21), ADLIB (n = 18).
Table 3.
Comparisons of smoking abstinence and reinstatement effects across groups
| Abstinence effecta |
Reinstatement effectb, c |
|||||||
| Omnibus test |
ABST vs. NONSMK |
ABST vs. ADLIB |
ABST vs. ADLIB |
|||||
| F | d | F | d | F | d | F | d | |
| Self-report measures | ||||||||
| MNWS totald | 12.24† | 1.06 | 23.86† | 1.37 | 9.52** | 0.72 | ||
| Angry/irritable/frustrated | 8.37*** | 0.87 | 16.73† | 1.05 | 4.45* | 0.51 | ||
| Anxious/nervous | 4.89* | 0.67 | 7.06** | 0.64 | 7.52** | 0.75 | ||
| Difficulty concentrating | 3.81* | 0.59 | 7.52** | 0.67 | ns | – | ||
| Impatient/restless | 19.66† | 1.37 | 30.72† | 1.46 | 27.85† | 1.31 | ||
| Appetite/hunger | 4.49* | 0.64 | 7.98** | 0.78 | 5.08* | 0.53 | ||
| Insomnia | ns | – | ns | – | ns | – | ||
| Depressed | 6.59** | 0.77 | ns | – | 4.93* | −0.57 | ||
| Craving | 15.45† | 1.19 | 17.74† | 1.39 | 27.35† | 1.09 | ||
| PANAS-Negative Affect | 12.78† | 1.08 | 15.97† | 0.94 | 21.71† | 1.17 | 13.39*** | −0.97 |
| PANAS-Positive Affect | ns | – | ns | – | ns | – | ns | – |
| QSU total | 170.65† | 3.94 | 249.88† | 3.58 | 258.90† | 3.45 | 161.32† | −3.37 |
| QSU-Factor 1 | 216.66† | 4.44 | 318.95† | 4.17 | 327.06† | 3.88 | 206.62† | −3.80 |
| QSU-Factor 2 | 89.96† | 2.86 | 130.63† | 2.55 | 137.54† | 2.52 | 82.30† | −2.41 |
| Cognitive performance tasks | ||||||||
| Code substitutione | ns | – | ns | – | ns | – | ns | – |
| Delayed recalle | ns | – | ns | – | ns | – | ns | – |
| Two-letter searchf | 3.15* | 0.64 | ns | – | 8.90** | 0.60 | 5.87* | −0.75 |
| Pattern recognition | ns | – | ns | – | ns | – | ns | – |
| Running memoryg | ns | – | ns | – | ns | – | ns | – |
| Four-choice serial RTg | ns | – | 4.09* | 0.67 | ns | – | 11.46** | −1.04 |
Note. Nonsignificant findings not displayed. ABST = abstinent smokers; ADLIB = nonabstinent smokers; MNWS = Minnesota Nicotine Withdrawal Scale; NONSMK = nonsmokers; PANAS = Positive and Negative Affect Schedule; QSU = Questionnaire of Smoking Urges—Brief; RT = reaction time; S1 = Session 1; S2 = Session 2; T1 = Time 1 (presmoking for ABST); T2 = Time 2 (postsmoking for ABST).
Comparisons based on S2-T1 to S1difference score.
Comparisons based on S2-T2 to S2-T1 difference score.
n = 28 because of missing data.
MNWS not administered at S2-T2.
Task administered to a subset of participants (NONSMK, n = 20; ABST, n = 20; ADLIB, n = 19).
NONSMK (n = 22), ABST (n = 21), ADLIB (n = 21).
NONSMK (n = 22), ABST (n = 21), ADLIB (n = 18).
*p < .05; **p < .01; ****p < .00; †p < .0001.
Withdrawal symptoms.
There were significant overall group differences in the change from S1 to S2-T1 on the MNWS total score and most MNWS item scores. Planned comparisons showed that ABST smokers reported greater increases than ADLIB smokers and NONSMK on the MNWS scale and the anger/irritability, anxiety, hunger, impatience/restlessness, and desire to smoke items; effect sizes were largest for desire to smoke and impatience/restlessness. While ABST smokers reported significantly greater increases in difficulty concentrating than NONSMK (who reported reductions on this item), ABST smokers did not differ from ADLIB smokers. Unexpectedly, ABST smokers reported a slight decrease on the depressed item from S1 to S2-T1 (mean change = −0.13, SD = 0.76), which was significantly different from ADLIB smokers who reported an increase on this item (mean change = 0.23, SD = 0.82).
Smoking urge and affect.
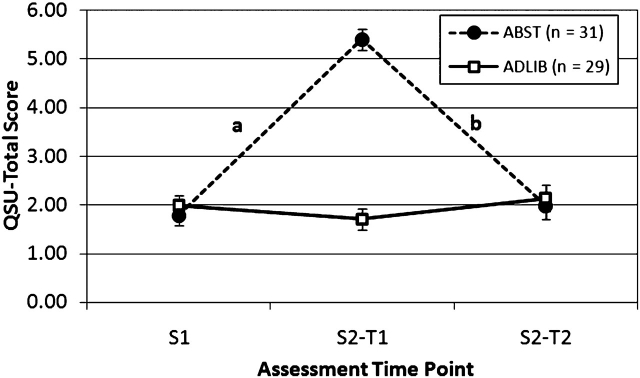
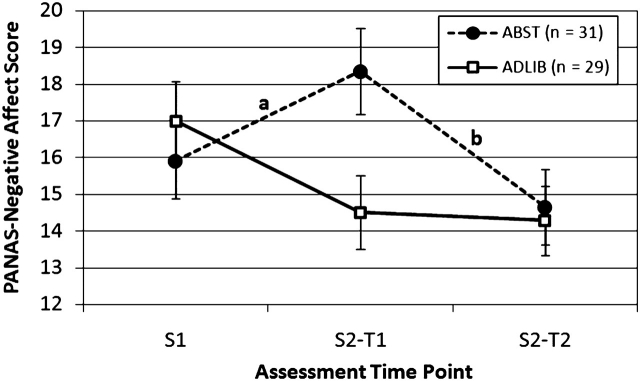
There were significant differences in PANAS-Negative Affect and QSU-Total and both QSU Factor scores between groups for the change from S1 to S2-T1 (abstinence effect) as well as the change from S2-T1 to S2-T2 (reinstatement effect). ABST smokers reported substantial increases from S1 to S2-T1 in negative affect and craving, which were significantly different from ADLIB smokers and NONSMK, who reported little change from S1 to S2-T1. Similarly, ABST smokers reported substantial reductions from S2-T1 (presmoking) to S2-T2 (postsmoking) in negative affect and craving, which were significantly different from ADLIB smokers who showed little change. The trajectory of abstinence and reinstatement effects for negative affect and craving are illustrated in Figures 1 and 2. The effects on craving were particularly robust (ds ranging from 2.41 to 4.44). Abstinence and reinstatement effects on PANAS-Positive Affect were not significant.
Figure 2.
Mean (SE) of QSU-total score by group and assessment time point. aSignificant group (ABST vs. ADLIB) effects on the change from S1 to S2-T1, F(1, 57) =169.00, p < .0001, d = 3.45. bSignificant group (ABST vs. ADLIB) effects on the change from S2-T1 to S2-T2, F(1, 57) = 161.32, p < .0001, d = −3.37. ABST = abstinent smokers; ADLIB = nonabstinent smokers; QSU = Questionnaire of Smoking Urges-Brief; T1 = Time 1 (presmoking for ABST); T2 = Time 2 (postsmoking for ABST).
Figure 1.
Mean (SE) of PANAS-Negative Affect score by group and assessment time point. aSignificant group (ABST vs. ADLIB) effects on the change from S1 to S2-T1, F(1, 57) =19.80, p < .0001, d = 1.17. bSignificant group (ABST vs. ADLIB) effects on the change from S2-T1 to S2-T2, F(1, 57) = 13.30, p = .0006, d = −0.97. ABST = abstinent smokers; ADLIB = nonabstinent smokers; PANAS = Positive and Negative Affect Schedule; QSU = Questionnaire of Smoking Urges-Brief; S1 = Session 1; S2 = Session; T1 = Time 1 (presmoking for ABST); T2 = Time 2 (postsmoking for ABST).
Cognitive performance.
There were no significant group differences in changes in performance across any of the time points for code substitution, delayed recall, pattern recognition, or running memory. Unexpectedly, ABST smokers showed improved performance on the two-letter search task from S1 to S2-T1 (mean change = 2.38, SD = 2.92), which was significantly different from ADLIB smokers who showed a performance decrement (M = −3.25, SD = 13.0). Removal of an outlier in the ADLIB smokers group who had high throughput on the two-letter search task at S1 did not change the outcome of analyses; the data point was therefore retained. ABST smokers showed a decrement in performance on the two-letter search from S2-T1 to S2-T2 (M = −1.62, SD = 3.42), which was significantly different from ADLIB smokers who showed a slight improvement in performance (M = 0.80, SD = 3.04). Similarly, on the Four-Choice Serial Reaction Time, ABST smokers’ performance improved significantly more from S1 to S2-T1 (mean change = 17.1, SD = 11.7) than NONSMK (M = 6.8, SD = 18.0). Upon smoking reinstatement, ABST smokers showed a reduction in performance on this task (M = −9.28, SD = 17.33), which was significantly different from ADLIB smokers who showed improvement (M = 7.63, SD = 14.9). The abstinence and reinstatement effects on cognitive performance ranged in size from moderate (ABST vs. ADLIB on change from S1 to S2-T1; d = 0.60) to large (ABST vs. ADLIB on change from S2-T1 to S2-T2; d = −1.04). Analysis of abstinence and reinstatements effects using accuracy and speed as the dependent variables yielded comparable results to the primary analyses based on throughput.
Discussion
This study is the first to evaluate both smoking abstinence and smoking reinstatement effects in adolescent smokers within the same experiment. Other strengths include (a) the use of two comparison groups which controlled for smoking status and the effects of repeated assessments and (b) very low attrition from Session 1 to Session 2, preventing biases associated with differential attrition among those smokers who may find it difficult to abstain from smoking. Furthermore, this study employed minimal exclusion criteria in order to enhance generalizability to the overall population of adolescent smokers. Baseline smoking characteristics in this sample are similar to data from intervention studies with adolescents (e.g., Colby et al. 1998, 2005) lending support for the clinical relevance of the findings.
Overall, our findings are supportive of withdrawal relief and negative reinforcement models of smoking maintenance and progression for adolescents. Adolescent smokers experienced increases in aversive symptoms and craving following acute smoking abstinence, which were reversed to baseline levels immediately upon smoking a cigarette. This study was the first to investigate smoking abstinence and reinstatement effects on positive as well as negative affect in adolescents. We found no effect of smoking abstinence or reinstatement on positive affect. These findings elucidate motivational factors in adolescent smoking and have direct implications for behavioral and pharmacological intervention development. Negative reinforcement models have been well supported in the empirical literature on adult smokers, but fewer studies have directly addressed the potential role of negative reinforcement in adolescent smoking.
The abstinence effects we found for negative affect, withdrawal symptoms, and smoking urges are consistent with earlier experimental studies of adolescent smokers (Jacobsen et al., 2005, 2006, 2007). Furthermore, the smoking reinstatement effects in the current study replicate and extend those of Corrigall et al. (2001) and Zack et al. (2001) who found smoking reinstatement to reduce smoking urges but did not directly demonstrate abstinence effects within the same studies. The current study demonstrated that overnight smoking abstinence increased withdrawal symptoms, smoking urges, and negative affect above baseline levels, and smoking reinstatement returned urge and affect measures to baseline levels, while nonabstinent smokers and nonsmokers showed little change over time.
The lack of effect of smoking abstinence on positive affect in adolescents is inconsistent with several studies with adults (al’Absi, Hatsukami, Davis, & Wittmers, 2004; al’Absi, Hatsukami, & Davis, 2005; Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008), though even in studies of adult smokers, effects on positive affect tend to be smaller than effects on negative affect. While the current findings require replication, the results may suggest some differences in smoking motivation between adolescents relatively early in their smoking trajectories compared with adults with more long-established smoking.
We presented the relative magnitude of smoking abstinence effects on individual withdrawal symptoms. Significant effects were found for most symptoms with impatience/restlessness and desire to smoke affected most strongly. The effects on restlessness and desire to smoke are consistent with findings published by Smith et al. (2008) that showed that these two symptoms increased in treatment-receiving adolescent smokers when they initiated a quit attempt. Among the abstaining smokers in the current study, most symptoms were rated in the “mild” to “moderate” range while desire to smoke was rated as “moderate” to “severe.” Effects on the item “waking at night” were not significant and would not be expected unless a longer abstinence period was used. It is unclear why effects on the “felt depressed” item were anomalous. Abstaining smokers reported a slight decrease from baseline while nonabstainers reported an increase. Though this item correlated with the MNWS scale at each observation, changes in the item as a function of abstinence did not correlate with changes in MNWS (data not shown).
Prior research suggests that effects of smoking abstinence and reinstatement on cognitive function may be relatively subtle and complex in adolescents. In the current trial, abstinence-induced improvement was found on selective attention and attentional control tasks (two-letter search and serial reaction time tasks respectively). In other studies, abstinence-induced decrements have been shown in a different cognitive domain using working memory and short-term verbal memory tasks (Jacobsen et al., 2005, 2007); this pattern is more consistent with research with adult smokers. On a visuospatial recall task, Jacobsen et al. (2006) found abstinence to improve performance in adolescent smokers who had no prenatal exposure to nicotine but worsened recall among prenatally exposed participants.
Less work has focused on the effects of smoking reinstatement on cognitive function. We found no effect on most tasks examined, but smoking reinstatement resulted in performance decrements in selective attention (two-letter search) and attentional control (serial reaction time). This is consistent with findings from a study by Zack et al. (2001), in which smoking reinstatement disrupted performance on a rapid information processing task among lighter-smoking adolescents. In contrast, Zack et al. found that smoking reinstatement enhanced performance on the same task among heavier smokers. Furthermore, smoking reinstatement improved performance on a selective attention measure (a modified Stroop task) in the same study, decreasing intrusion of smoking-related stimuli. The latter findings are more consistent with findings from research with adult smokers.
The pattern of findings indicates that abstinence and reinstatement effects are evident on certain cognitive performance tasks but not others, and the direction of effects can vary based on participant characteristics. Unfortunately there has been little consistency in the actual tasks examined across studies and the potential moderators evaluated. Collectively, findings suggest the possibility of a relationship between exposure to nicotine in a population (e.g., prenatally and/or based on recent smoking) and the effects of smoking abstinence and reinstatement. Research in this area could be advanced by more systematic evaluation of this possibility.
Interpretation of the current findings is limited by a design feature that resulted in reduced sample size for data on the cognitive performance tasks. Thus, outcomes on the subjective versus cognitive measures were not derived on directly comparable samples. As a result, we cannot rule out the possibility that the findings on the cognitive tasks were influenced by the smaller sample available for each analysis. Another limitation of this study is that the effects of smoking reinstatement on cognitive performance (i.e., performance decrements on two tasks) cannot be disentangled from potentially reduced motivation to perform quickly and accurately following smoking. Future research might incorporate contingent incentives for maintaining effortful performance postsmoking reinstatement.
Adolescent smokers tend to have little success quitting smoking either on their own or with treatment (Colby & Gwaltney, 2007; Sussman et al., 2006). Based on the current findings and negative-reinforcement models of smoking, treatments that directly target the reduction of aversive symptoms during abstinence (pharmacotherapies that block these effects or behavioral coping skills training that may limit their effects) or treatments that target smoking reinstatement effects may have efficacy for reducing smoking among adolescents. To date, intervention trials for adolescents have largely emphasized effects on smoking point prevalence and smoking rate at follow-up, but have focused less on effects on potentially important mediating variables more proximal to intervention delivery. The current laboratory paradigm for evaluating these effects may be a useful tool for intervention evaluation and development.
Funding
This research was supported by grant R01 CA80255 awarded to SMC from the National Cancer Institute. Additional support was provided by a research career scientist award to PMM from the Department of Veterans Affairs.
Declaration of Interests
The authors have no competing interests to declare.
Supplementary Material
References
- al'Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al'Absi M, Hatsukami D, Davis GL, Wittmers LE. Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug and Alcohol Dependence. 2004;73:267–278. doi: 10.1016/j.drugalcdep.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Bailey SR, Harrison CT, Jeffery CJ, Ammerman S, Bryson SW, Killen DT, et al. Withdrawal symptoms over time among adolescents in a smoking cessation intervention: Do symptoms vary by level of nicotine dependence? Addictive Behaviors. 34:1017–1022. doi: 10.1016/j.addbeh.2009.06.014. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Brandon TH, Chassin L. Motivational influences on cigarette smoking. Annual Review of Psychology. 2004;55:463–491. doi: 10.1146/annurev.psych.55.090902.142054. [DOI] [PubMed] [Google Scholar]
- Brown RA, Lewinsohn PM, Seeley JR, Wagner EF. Cigarette smoking, major depression and other psychiatric disorders among adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35:1602–1610. doi: 10.1097/00004583-199612000-00011. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, MI: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- Colby SM, Gwaltney CJ. Pharmacotherapy for adolescent smoking cessation. Journal of the American Medical Association. 2007;298:2182–2184. doi: 10.1001/jama.298.18.2182. [DOI] [PubMed] [Google Scholar]
- Colby SM, Monti PM, Barnett NP, Rohsenow DJ, Weissman K, Spirito A, et al. Brief motivational interviewing in a hospital setting for adolescent smoking: A preliminary study. Journal of Consulting and Clinical Psychology. 1998;66:574–578. doi: 10.1037//0022-006x.66.3.574. [DOI] [PubMed] [Google Scholar]
- Colby SM, Monti PM, O'Leary Tevyaw TA, Barnett NP, Spirito A, Rohsenow DJ, et al. Brief motivational intervention for adolescent smokers in a hospital setting. Addictive Behaviors. 2005;30:865–874. doi: 10.1016/j.addbeh.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Colby SM, Tiffany S, Shiffman S, Niaura RS. Are adolescent smokers dependent on nicotine? A review of the evidence. Journal of Drug and Alcohol Dependence. 2000;59:S83–S95. doi: 10.1016/s0376-8716(99)00166-0. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Zack M, Eissenberg T, Belsito L, Scher R. Acute subjective and physiological responses to smoking in adolescents. Addiction. 2001;96:1409–1417. doi: 10.1046/j.1360-0443.2001.961014095.x. [DOI] [PubMed] [Google Scholar]
- Dozois DN, Farrow JA, Miser A. Smoking patterns and cessation motivation during adolescence. International Journal on Addiction. 1995;30:1485–1498. doi: 10.3109/10826089509055844. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: A review of effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345–395. [Google Scholar]
- Hughes JR. Tobacco withdrawal in self-quitters. Journal of Consulting and Clinical Psychology. 1992;60:689–697. doi: 10.1037//0022-006x.60.5.689. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Measurement of the effects of abstinence from tobacco: A qualitative review. Psychology of Addictive Behaviors. 2007;21:127–137. doi: 10.1037/0893-164X.21.2.127. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. Errors in using tobacco withdrawal scale. Tobacco Control. 1998;7:92–93. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob P, Wilson M, Benowitz NL. Improved gas chromatographic method for the determination of nicotine and cotinine in biologic fluids. Journal of Chromotography A. 1981;222:61–70. doi: 10.1016/s0378-4347(00)81033-6. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57:56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl) 2007;193:557–566. doi: 10.1007/s00213-007-0797-9. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Slotkin TA, Westerveld M, Mencl WE, Pugh KR. Visuospatial memory deficits emerging during nicotine withdrawal in adolescents with prenatal exposure to active maternal smoking. Neuropsychopharmacology. 2006;31:1550–1561. doi: 10.1038/sj.npp.1300981. [DOI] [PubMed] [Google Scholar]
- Kassel JD, Evatt DP, Greenstein JE, Wardle MC, Yates MC, Veilleux JC. The acute effects of nicotine on positive and negative affect in adolescent smokers. Journal of Abnormal Psychology. 2007;116:543–553. doi: 10.1037/0021-843X.116.3.543. [DOI] [PubMed] [Google Scholar]
- Killen JD, Ammerman S, Rojas N, Varady J, Haydel F, Robinson TN. Do adolescent smokers experience withdrawal effects when deprived of nicotine? Experimental and Clinical Psychopharmacology. 2001;9:176–182. doi: 10.1037//1064-1297.9.2.176. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & Tobacco Research. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis-Esquerre JM, Colby SM, O’Leary-Tevyaw T, Eaton CA, Kahler CW, Monti P. Validation of the timeline follow-back in the assessment of adolescent smoking. Drug and Alcohol Dependence. 2005;79:33–43. doi: 10.1016/j.drugalcdep.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing experiments and analyzing data: A model comparison perspective. Mahwah, NJ: Lawrence Erlbaum; 1990. [Google Scholar]
- McNeill AD, West RJ, Jarvis M, Jackson P, Brryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology. 1986;90:533–536. doi: 10.1007/BF00174074. [DOI] [PubMed] [Google Scholar]
- Mermelstein R. Teen smoking cessation. Tobacco Control. 2003;12(Suppl. 1):i25–i34. doi: 10.1136/tc.12.suppl_1.i25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Briski J, Fonte C, Scott J, Lerman C. Severity of tobacco abstinence symptoms varies by time of day. Nicotine & Tobacco Research. 2009;11:84–91. doi: 10.1093/ntr/ntn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas NL, Killen JD, Haydel KF, Robinson TN. Nicotine dependence among adolescent smokers. Archives of Pediatric and Adolescent Medicine. 1998;152:151–156. doi: 10.1001/archpedi.152.2.151. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows. Version 9.1. Cary NC: SAS Institute Inc; 2003. [Google Scholar]
- Schneider NG, Jarvik ME. Time course of smoking withdrawal symptoms as a function of nicotine replacement. Psychopharmacology. 1984;82(1/2):143–144. doi: 10.1007/BF00426399. [DOI] [PubMed] [Google Scholar]
- Smith AE, Cavallo DA, McFetridge A, Liss T, Krishnan-Sarin S. Preliminary examination of tobacco withdrawal in adolescent smokers during smoking cessation treatment. Nicotine & Tobacco Research. 2008;10:1253–1259. doi: 10.1080/14622200802219357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder FR, Davis FC, Henningfield JE. The tobacco withdrawal syndrome: Performance decrements assessed on a computerized test battery. Drug and Alcohol Dependence. 1989;23:259–266. doi: 10.1016/0376-8716(89)90090-2. [DOI] [PubMed] [Google Scholar]
- Stanton WR, McClelland M, Elwood C, Ferry D, Silva PA. Prevalence, reliability and bias of adolescents’ reports of smoking and quitting. Addiction. 1996;91:1705–1714. [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2005 National Survey on Drug Use and Health (Vol. DHHS Publication No. SMA 05-4061.2005) Rockville, MD: Office of Applied Studies; 2005. [Google Scholar]
- Sussman S, Sun P, Dent CW. A meta-analysis of teen cigarette smoking cessation. Health Psychology. 2006;25:549–557. doi: 10.1037/0278-6133.25.5.549. [DOI] [PubMed] [Google Scholar]
- Thorne DR, Genser SG, Sing HC, Hegge FW. The Walter Reed performance assessment battery. Neurobehavioral Toxicology and Teratology. 1985;7:415–418. [PubMed] [Google Scholar]
- Tiffany ST, Drobes DJ. The development and initial validation of a questionnaire on smoking urges. British Journal of Addiction. 1991;86:1467–1476. doi: 10.1111/j.1360-0443.1991.tb01732.x. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Koob GF, Markou A. Neural mechanisms underlying nicotine addiction: Acute positive reinforcement and withdrawal. Nicotine & Tobacco Research. 2000;2:19–37. doi: 10.1080/14622200050011277. [DOI] [PubMed] [Google Scholar]
- Watson D, Clarke LA, Tellegen A. Development and validation of measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Zack M, Belsito L, Scher R, Eissenberg T, Corrigall WA. Effects of abstinence and smoking on information processing in adolescent smokers. Psychopharmacology (Berl) 2001;153:249–257. doi: 10.1007/s002130000552. [DOI] [PubMed] [Google Scholar]
- Zhu SH, Sun J, Billings SC, Choi WS, Malarcher A. Predictors of smoking cessation in U.S. adolescents. American Journal of Preventive Medicine. 1999;16:202–207. doi: 10.1016/s0749-3797(98)00157-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.