Summary
Metabolite gradients may guide mitochondrial localization in cells, and angiogenesis in tissues [1, 2]. It is unclear if they can exist in single cells, because the length scale of most cells is small compared to expected diffusion times of metabolites. To investigate metabolic gradients, we need experimental systems in which spatial patterns of metabolism can be systematically measured and manipulated. We used concentrated cytoplasmic extracts from Xenopus eggs [3] as a model cytoplasm, and visualized metabolic gradients formed in response to spatial stimuli. Restricting oxygen supply to the edge of a drop mimicked distance to the surface of a single cell, or distance from a blood vessel in tissue. We imaged a step-like increase of NAD reduction ~600 μm distant from the oxygen source. This oxic-anoxic switch was preceded on the oxic side by a gradual rise of mitochondrial transmembrane potential (Δψ) and reactive oxygen species (ROS) production, extending over ~600 μm and ~300 μm, respectively. Addition of ATP-consuming beads mimicked local energy sinks in the cell. We imaged Δψ gradients with a decay length of ~50–300 μm around these beads, in the first visualization of an energy demand signaling gradient. Our study demonstrates that mitochondria can pattern the cytoplasm over length scales that are suited to convey morphogenetic information in large cells and tissues, and provides a versatile model system for probing the formation and function of metabolic gradients.
Results and Discussion
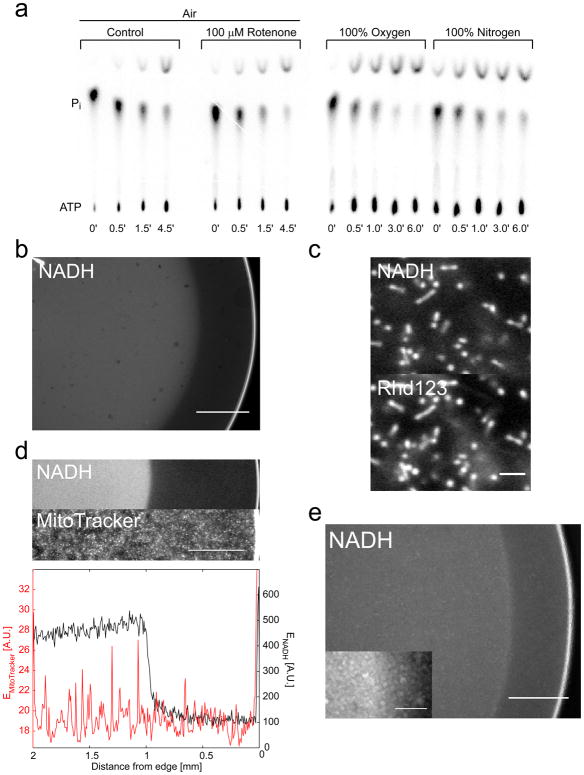
Xenopus laevis egg extracts have interesting potential for analysis of metabolic patterns. This almost undiluted model cytoplasm contains a large number of evenly distributed mitochondria and glycogen granules. These extracts recapitulate the cell cycle, form spindles that divide chromosomes, and can even undergo apoptosis. Their suitability for perturbation, fractionation and imaging has facilitated systematic investigation of cytoplasmic organizational mechanisms [3]. The metabolic activity of Xenopus egg extract was not quantified previously. We determined a concentration of ~3.4 mM for ATP and ~0.1 mM for ADP by MonoQ liquid chromatography for mitotic extract exposed to air at room temperature. Similar values were previously reported for intact eggs/oocytes [4]. Extracts made anoxic by replacing air with pure N2 exhibited ~3× increased [ADP], but no significant change in [ATP] (data not shown). Thin layer chromatography of 32P pulse-labeled extracts showed that 32P was incorporated into ATP after ~3 min (Figure 1A). From these data, we estimated an ATP production/consumption rate of ~0.02 mmol l−1 s−1, similar to that of resting muscle [5]. Within experimental error, which is limited by slow mixing times in the viscous extract, ATP turnover rates were largely oxygenation state and respiration independent.
Figure 1.
(A) Autoradiography of thin layer chromatograms of egg extracts pulsed for the indicated times with 32P potassium phosphate at the indicated drug or gas conditions. Positions of inorganic phosphate (Pi) and ATP are indicated. (B) UV autofluorescence pattern in an extract drop exposed to air at its side. (C) Higher magnification of bright zone. Co-staining with Rhd123 reveals that the UV autofluorescence signal mainly derives from mitochondria. Scale bar, 10 μm. (D) UV autofluorescence pattern in a mitochondrial suspension. Co-staining with MitoTrackerGreen. (E) UV autofluorescence pattern in a drop of HelaS3 cell suspension. Inset, magnification of border region (oxic-anoxic). Inset scale bar, 50 μm. If not otherwise indicated, scale bars correspond to 500 μm.
To spatially restrict oxygen supply, we squeezed a 4 μl drop of extract between a glass slide and a coverslip, leaving the edge of the drop exposed to air (Figure S1A). Endogenous UV-excited fluorescence served as a non-invasive measure of metabolic state. Cytoplasmic, UV-excited autofluorescence mostly derives from mitochondrial NADH, with minor contributions (~10%) from cytoplasmic NADPH [6]. A bright zone of UV-autofluorescence developed in the center of the drop over 1–4 min and quickly expanded outwards (supplementary movie). Dark zone width (DZW) reached steady state at ~300–900 μm that was stable for at least two hours (Figure 1B). As expected, the UV-excited signal derived mostly from mitochondria (Figure 1C). By calibration of the extract UV signals with pure NADH standards, we estimated concentrations of ~0.2 mM NADH in the dark- and ~0.4 mM in the bright zone (Figure S1B, C). A similar, step-like pattern of UV fluorescence was observed when mitochondria isolated from the egg extract were suspended in buffer in the presence of respiratory substrates (Figure 1D). NADH fluorescence intensity and DZW depended on mitochondrial concentration (Figure S2A). Dense suspensions of living HeLaS3 cells also exhibited a step-like transition of UV autofluorescence in the drop experiment (Figure 1E). This mimics classic observations in tissues [7], where a step-like increase in UV autofluorescence revealed tissue regions that became anoxic following experimental ischemia. DZW length scale (~300–900 μm) is approximately consistent with previously measured in vivo oxygen decay lengths in solid tumor tissue (~200–300 μm; [8]) and the distance between open capillaries in resting frog muscle (~200–500 μm; [9]).
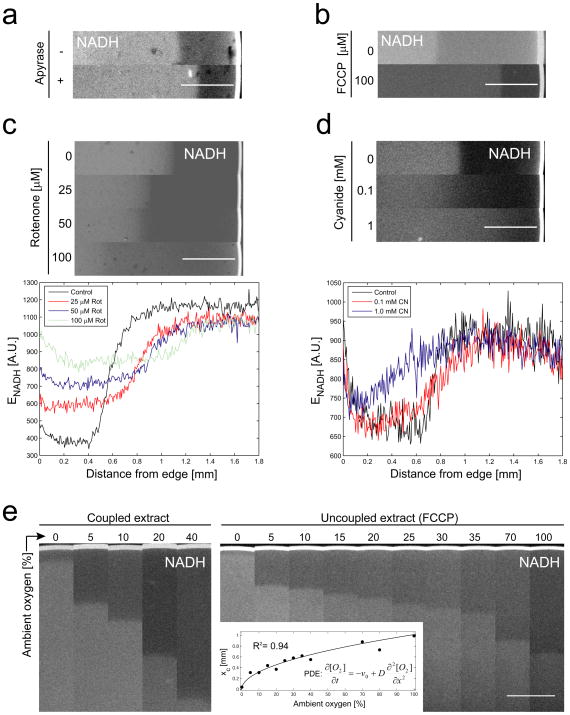
To determine the origin of the NADH patterns, we measured their response to metabolic perturbations. Increase of ATP consumption by addition of an unregulated ATPase (apyrase) to the extract resulted in a DZW decrease (Figure 2A). Mitochondrial uncoupling with FCCP had a similar effect (Figure 2B). Both treatments increase respiration by decreasing Δψ [10]. Partial inhibition of complex I by rotenone increased DZW and its baseline intensity, whereas partial inhibition of complex IV inhibition by cyanide decreased the slope of zone transition (Figure 2C, D). Both inhibitors caused loss of the dark zone at high concentrations, presumably by blocking NADH oxidation. DZW increased as a function of ambient oxygen partial pressure in the absence or presence of FCCP (Figure 2E). With FCCP, DZW could be measured over the whole range of oxygen partial pressures, providing a sufficient data set to determine the shape of the curve. A one-dimensional reaction-diffusion model assuming oxygen independence of respiration rate predicted a square root dependency of DZW (xc) on ambient oxygen partial pressure Ω0 (Experimental Procedures). This model fit our data well (Figure 2E, inset) and also qualitatively agreed with the decrease in DZW upon stimulation of respiration rate as discussed above. An alternative reaction-diffusion model assuming 1st order oxygen kinetics of respiration [11] fit significantly less well (F-Test: p = 0.009, data not shown). Our data are thus more consistent with the widely accepted notion that respiration rate in aerobic organisms is insensitive to variations in [O2] down to low concentrations [12], [13].
Figure 2.
Effect of metabolic perturbations on NADH patterns in egg extract. (A) Increasing energy demand by addition of apyrase. (B) Mitochondrial uncoupling with FCCP. (C) Complex I inhibition with rotenone. Lower panel, corresponding line profiles. (D) Complex IV inhibition with potassium cyanide. (E) Exposure to different ambient oxygen concentrations. Numbers indicate O2 percentage of N2/O2 gas mixture. Left panel, untreated extract. Right panel, FCCP treated extract. Inset, dark zone with (xc) plotted as a function of oxygen concentration and fitted with a square root function. Partial differential equation (PDE) describing the corresponding one dimensional reaction diffusion model assuming 0th order oxygen kinetics for respiration. Scale bars, 500 μm.
Our data suggested the following preliminary model for NADH patterning: Oxygen diffuses inwards from the edge into the drop and is consumed by respiring mitochondria that are homogeneously distributed throughout the extract, generating a spatial gradient of [O2] and metabolic activity. Respiration rate is constant through the dark zone (Figure 2E, inset), indicating that cytochrome c oxidase (COX) is saturated with oxygen. As [O2] approaches the oxygen-Km of COX (< 1 μM; [10]), respiration rate starts to fall, and [NADH] increases creating the bright zone. Anoxia does not reduce the complete cellular NAD(H) pool, since anaerobic ATP production, which requires cytoplasmic NAD, remains efficient (Figure 1A). The observed redox switch is probably largely restricted to the mitochondrial NAD(H) sub-pool.
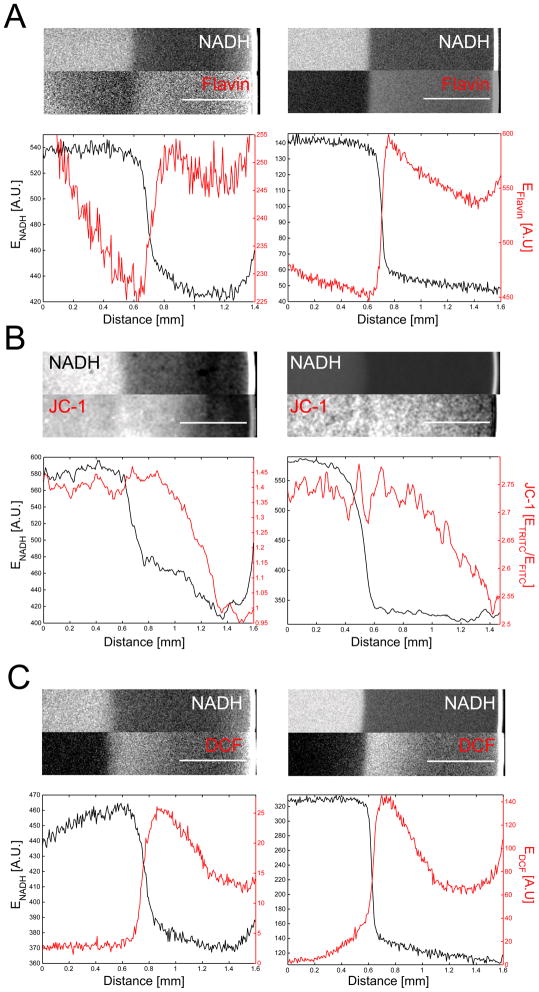
The model stated above predicted that in the dark zone, all activities coupled to the respiratory chain upstream of COX should be insensitive to changes in oxygen concentration, because COX is saturated with oxygen. Similar to NAD reduction, these activities should undergo sudden changes around the oxygen-Km of COX. In fact, we observed a sharp decrease of flavin autofluorescence spatially coinciding with the increase in NADH fluorescence (Figure 3A). The redox state of the flavin prosthetic groups in complex I (FMN) and complex II (FAD) depend on respiratory activity. Unlike NAD/NADH, flavins fluoresce in the FITC channel exclusively in their oxidized state [14, 15]. Flavin autofluorescence was completely quenched by rotenone (Figure S2B) indicating that it derived from FMN in complex I and that electron input from β-oxidation was minor. Their similar spatial responses suggested close coupling of NAD and FMN reduction state.
Figure 3.
Imaging of mitochondrial activities. (A) Simultaneous imaging of NADH- and flavin-autofluorescence in egg extracts (left panel) and mitochondrial suspensions (right panel). (B) Simultaneous imaging of NADH and mitochondrial membrane potential using JC-1. Mitochondrial potential is represented as ETRITC/EFITC emission ratio of the JC-1 sensor. High ETRITC/EFITC ratios (bright colors) indicate high mitochondrial membrane potential. Left panel, egg extract. Right panel, mitochondrial suspension. (C) Reactive oxygen species (ROS) measured as DCF emission (EDCF) in egg extracts (left panel) and mitochondrial suspensions (right panel). Bright colors indicate high concentrations of DCF/ROS. Lower panels, averaged line plots. Scale bars, 500 μm.
Using JC-1 to image mitochondrial membrane potential (Δψ), we found a gradual rise of Δψ in the dark zone over ~600 μm, that reached its maximum just before NADH started its sudden increase (Figure B). This graded change in Δψ was notably different from the step-like change in [NADH] visualized in the same extract. Constant, high Δψ was observed in the anoxic region at the center of the drop. As expected, the Δψ signal was completely quenched by FCCP (Figure S2C). Electron carrier (EC) reduction at complex I, III, and IV of the ETC is coupled to vectorial H+ transport across the inner mitochondrial membrane. At constant energy demand, Δψ provides an integrated measure of EC reduction level. As long as COX is saturated with oxygen, Δψ cannot convey information about changes in oxygen concentration to upstream ETC reactions. Hence, the observed Δψ increase disagreed with our previous assumption that the dark zone was created exclusively by oxygen saturation of COX.
ETC electron flux can be diverted to oxygen at sites upstream of COX, producing reactive oxygen species (ROS). We used 2′–7′ dichlorofluorescein diacetate (DCFDA) to image ROS and observed a gradual increase of ROS production rate in the dark zone, preceding the oxic-anoxic switch in [NADH] (Figure 3B). Unlike the Δψ gradient, the ROS gradient decayed more steeply towards the oxygen source, with a spatial scale of ~300 μm. Preferential production of ROS under hypoxic conditions ([O2] ~1–20 μM) has been observed previously in cells and tissues [16] and our data strongly confirms this view.
Similar to NADH, the Δψ and ROS patterns were not dependent on cytoplasmic reactions and could be reconstituted in pure mitochondria (Figure 3, left panels). Their more graded response conflict with our previous assumption that NADH patterning might result from oxygen saturation of COX, with all mitochondrial activities switching together as [O2] falls below the Km. These data rather suggest a model where reduction of ETC electron carriers gradually increase as [O2] decreases. As expected, increasingly reduced electron carriers cause an increase in Δψ and ROS production. But why, in this model, would NADH and flavin pools undergo switch-like responses to decreasing oxygen? One explanation might be that downstream EC substrate pools, such as ubiquinone and cytochrome c, cause kinetic buffering of upstream NAD and flavin reduction levels against changes in oxygen concentration. Only after the kinetic buffering capacity of the system is exhausted will respiration cease, resulting in the observed switch-like increase of NADH reduction. The kinetic requirements for such a buffering mechanism will be discussed in more detail elsewhere (manuscript in preparation).
[O2] gradients were observed under various physiological and pathological conditions in animal tissues [8, 17, 18]. We hypothesize they generate spatial patterns of NADH, Δψ and ROS similar to those we observe in extract. The gradual change of ETC reduction, measured as Δψ, may compensate for the effect of decreasing [O2] on COX rate, allowing aerobic ATP production rate to remain relatively independent of [O2]. The same gradual increase in ETC reduction appears to cause a gradual increase in mitochondrial ROS (mROS) production [19], up the point where [O2] drops below some critical value. mROS have been shown to stabilize HIF-1 against proteolytic degradation [2, 20], triggering transcriptional programs that drive angiogenesis and proliferation. The ~300 μm length scale and gradual nature of the mROS gradient seem well suited to convey positional information about local oxygen demand to nearby blood vessels [9]. The step-like change of [NADH] over ~30 μm, in contrast, is less suited to convey positional information, and may serve other functions. For example, the NADH patterns may spatially regulate Akt-dependent cell survival [21]. Control of survival pathways may be most important in transiently anoxic regions, to temporarily protect against cell death, in which case switch-like signaling is appropriate.
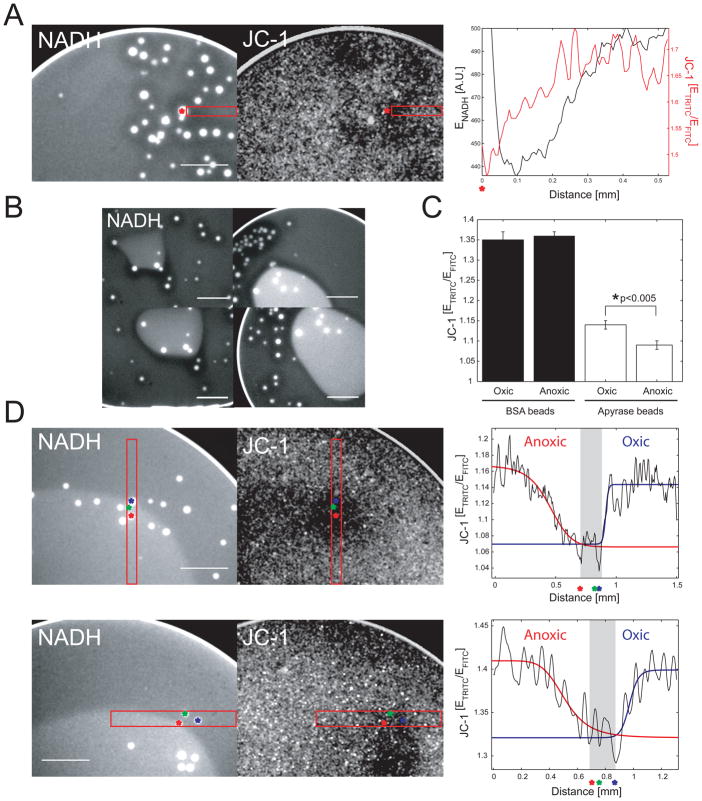
Local sites of high ATP consumption are thought to attract mitochondria in single cells [1], but such hypothetical energy demand gradients have not been visualized. To generate localized ATP consumption in our system, we covalently coupled the unregulated ATPase apyrase to small, porous beads, and added them to suspensions of purified mitochondria and egg extract. In mitochondrial suspensions provided with ATP, inorganic phosphate and pyruvate, the apyrase beads induced steady-state gradients of mitochondrial potential and [NADH]. In particular, a gradual increase of Δψ as a function of bead distance preceded a more switch-like increase in NAD reduction state (Figure 4A). Furthermore, we noticed that heterogeneous distribution of apyrase beads in mitochondrial suspensions (Figure 4B) or extracts (data not shown) gave rise to more complex geometries of the NADH pattern, indicating that localized ATP consumption actively molded the shape of the spatial oxygen field in the drop.
Figure 4.
Imaging of energy demand gradients. (A) Imaging of [NADH] and Δψ in mitochondrial suspensions containing beads coupled with apyrase. Mitochondrial potential is represented as ETRITC/EFITC emission ratio of the JC-1 sensor. Red box, averaged line scan presented in right panel. (B) [NADH] in mitochondrial suspensions with heterogeneously distributed apyrase beads. (C) Mitochondrial potential on BSA- and apyrase coated beads in egg extracts. Error bars, standard error (n > 10 beads). (D) Imaging of [NADH] and Δψ in egg extracts containing beads coupled with apyrase. Right panel, averaged line scans of image regions indicated by a red box. Red curve, anoxic energy demand gradient fitted with an EC50 function. Blue curve, oxic energy demand gradient fitted with an EC50 function. Asterisks mark bead positions. Gray shaded region marks bead cluster position. Scale bars, 500 μm.
In egg extract, the apyrase beads, but not BSA-coated beads, induced local decreases of the Δψ signal (Figure 4C). Changes in [NADH] were not observed, probably because the beads had to compete with rapid ATP hydrolysis by the cytoplasm, so the energy demand signaling gradients were less pronounced than in pure mitochondria suspensions. Alternatively, ATP demand around the beads might be satisfied by glycolysis rather than by respiration. Interestingly, the zones of reduced Δψ around apyrase beads in the extract were more pronounced in the anoxic than in the oxic zone (Figure 4C). These zones reported on the presence of energy demand signaling gradients extending ~300 μm around apyrase beads in the anoxic zone, and ~50 μm in the oxic zone (Figure 4D). Δψ is known to respond to changes in ADP concentration. With the previously measured ATP production rate in extract, a one-dimensional reaction-diffusion model predicted a decay-length of ~20–50 μm for a hypothetical oxic ADP gradient for a range of literature ADP diffusion coefficients (1–5 × 102 μm2s−1; [22, 23, 24, 25]).
To our knowledge, the Δψ gradients around ATP-consuming beads are the first direct observation of an energy demand signaling gradient in cytoplasm. Its length scale in oxic, but not in anoxic cytoplasm, is consistent with a simple ADP reaction-diffusion scheme. Our observations do not rule out that the energy demand signaling gradients are created by reaction-diffusion of ATP or AMP. Previously, it has been found that [ATP] dependent enzymatic rates vary at different subcellular localizations, and that mitochondria and glycolytic enzymes preferentially localized to subcellular regions of high energy demand [17]. Although this is consistent with the notion of intracellular [ATP] gradients, latter have not been directly observed yet.
Since most cells are oxic, the decay length of the energy demand signaling gradients we observed would allow propagation of information on local energy demand over distances of ~50 μm, a length scale relevant to positioning of mitochondria in large cells, such as axons, eggs and early blastomeres. Δψ might regulate mitochondrial position in large cells indirectly via proteolytic degradation of the mitochondrial fusion factor OPA-1 [26], or more directly, by modulating the activity of molecular motors. The observed ~50 μm length scale suggests that diffusion of ADP, relative to the time scale of metabolism, is fast enough to approximately equalize its concentration throughout the cytoplasm of smaller cells, even in the face of strong local energy demand.
In this study, we reconstituted, step-like NADH patterns previously observed in tissues in response to spatial restriction of oxygen supply [7]. We report, for the first time, length scale and shape of Δψ and ROS patterns generated by spatial restriction of oxygen supply. The gradual nature of latter gradients makes them good candidates for patterning tissue morphogenesis (Figure S3). By contrast, the step-like NADH gradient is more suited to control life-death decision making following ischemia. By adding local ATP sinks, we observed for the first time, to our knowledge, energy demand signaling gradients in cytoplasm as spatial changes in Δψ. Their length scale appears too large for conveying positional information to mitochondrial in typical cells, though they could play that role in large cells. Metabolic gradients, created by reaction-diffusion, may be an under-appreciated player in morphogenesis more generally.
Supplementary Material
Acknowledgments
PN was supported by an EMBO long term fellowship and an HFSP long term fellowship. HYK is a HHMI predoctoral fellow.
Footnotes
Experimental procedures and three figures are available online
References
- 1.Hollenbeck PJ, Saxton WM. The axonal transport of mitochondria. J Cell Sci. 2005;118:5411–5419. doi: 10.1242/jcs.02745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hickey MM, Simon MC. Regulation of angiogenesis by hypoxia and hypoxia-inducible factors. Curr Top Dev Biol. 2006;76:217–257. doi: 10.1016/S0070-2153(06)76007-0. [DOI] [PubMed] [Google Scholar]
- 3.Desai A, Murray A, Mitchison TJ, Walczak CE. The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 1999;61:385–412. doi: 10.1016/s0091-679x(08)61991-3. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin MB, Dworkin-Rastl E. Metabolic regulation during early frog development: glycogenic flux in Xenopus oocytes, eggs, and embryos. Dev Biol. 1989;132:512–523. doi: 10.1016/0012-1606(89)90246-7. [DOI] [PubMed] [Google Scholar]
- 5.Hochachka PW, McClelland GB. Cellular metabolic homeostasis during large-scale change in ATP turnover rates in muscles. J Exp Biol. 1997;200:381–386. doi: 10.1242/jeb.200.2.381. [DOI] [PubMed] [Google Scholar]
- 6.Chance B. Mitochondrial NADH redox state, monitoring discovery and deployment in tissue. Methods Enzymol. 2004;385:361–370. doi: 10.1016/S0076-6879(04)85020-1. [DOI] [PubMed] [Google Scholar]
- 7.Steenbergen C, Deleeuw G, Barlow C, Chance B, Williamson JR. Heterogeneity of the hypoxic state in perfused rat heart. Circ Res. 1977;41:606–615. doi: 10.1161/01.res.41.5.606. [DOI] [PubMed] [Google Scholar]
- 8.Helmlinger G, Yuan F, Dellian M, Jain RK. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat Med. 1997;3:177–182. doi: 10.1038/nm0297-177. [DOI] [PubMed] [Google Scholar]
- 9.Krogh A. The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919;52:409–415. doi: 10.1113/jphysiol.1919.sp001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rumsey WL, Schlosser C, Nuutinen EM, Robiolio M, Wilson DF. Cellular energetics and the oxygen dependence of respiration in cardiac myocytes isolated from adult rat. J Biol Chem. 1990;265:15392–15402. [PubMed] [Google Scholar]
- 11.Bonner JT, Segel L, Cox EC. Oxygen and differentation in Dictyostelium discoideum. J Biosci. 1998;23:177–184. [Google Scholar]
- 12.Meyerhof O. Chemische Vorgaenge im Muskel. Berlin: Springer; 1930. [Google Scholar]
- 13.Warburg O, Kubowitz F. Biochem Z. 1931:5. [Google Scholar]
- 14.Warburg O. Wasserstoffubertragende fermente. Berlin: Saenger; 1948. [Google Scholar]
- 15.Chance B. Optical method. Annu Rev Biophys Biophys Chem. 1991;20:1–28. doi: 10.1146/annurev.bb.20.060191.000245. [DOI] [PubMed] [Google Scholar]
- 16.Guzy RD, Schumacker PT. Oxygen sensing by mitochondria at complex III: the paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- 17.Jones DP. Intracellular diffusion gradients of O2 and ATP. Am J Physiol. 1986;250:C663–675. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 18.Rumsey WL, Pawlowski M, Lejavardi N, Wilson DF. Oxygen pressure distribution in the heart in vivo and evaluation of the ischemic “border zone”. Am J Physiol. 1994;266:H1676–1680. doi: 10.1152/ajpheart.1994.266.4.H1676. [DOI] [PubMed] [Google Scholar]
- 19.Misra HP, Fridovich I. The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem. 1972;247:188–192. [PubMed] [Google Scholar]
- 20.Bell EL, Klimova TA, Eisenbart J, Moraes CT, Murphy MP, Budinger GR, Chandel NS. The Qo site of the mitochondrial complex III is required for the transduction of hypoxic signaling via reactive oxygen species production. J Cell Biol. 2007;177:1029–1036. doi: 10.1083/jcb.200609074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, Hu Y, Ramdas L, Hu L, Keating MJ, Zhang W, Plunkett W, Huang P. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- 23.Jacobus WE. Theoretical support for the heart phosphocreatine energy transport shuttle based on the intracellular diffusion limited mobility of ADP. Biochem Biophys Res Commun. 1985;133:1035–1041. doi: 10.1016/0006-291x(85)91240-9. [DOI] [PubMed] [Google Scholar]
- 24.de Graaf RA, van Kranenburg A, Nicolay K. In vivo (31)P-NMR diffusion spectroscopy of ATP and phosphocreatine in rat skeletal muscle. Biophys J. 2000;78:1657–1664. doi: 10.1016/S0006-3495(00)76717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vendelin M, Kongas O, Saks V. Regulation of mitochondrial respiration in heart cells analyzed by reaction-diffusion model of energy transfer. Am J Physiol Cell Physiol. 2000;278:C747–764. doi: 10.1152/ajpcell.2000.278.4.C747. [DOI] [PubMed] [Google Scholar]
- 26.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.