Abstract
Progress has been slow in defining molecular requirements for human B lymphopoiesis in part because of differences from experimental animals and also because of the lack of culture conditions that efficiently support the process. We recently found that human CD10+ lymphocytes were produced when CD34+ hematopoietic stem and progenitor cells were cultured in contact with human mesenchymal stem cells (hMSC). Further investigation revealed that it occurred even when progenitors were separated from hMSC by membrane filters. Experiments with neutralizing antibodies suggested that important heat labile factors produced by hMSC are unlikely to be IL-7, TSLP, CXCL12 or hemokinin-1. Further manipulation of culture conditions revealed that optimal lymphopoiesis required careful selection of fetal calf serum lots, maintenance of high cell densities, as well as recombinant cytokines (SCF, FL and G-CSF). G-CSF was particularly important when adult bone marrow rather than umbilical cord blood derived CD34+ cells were used to initiate the cultures. These improved methods should facilitate identification of molecules that can be used to speed regeneration of the humoral immune system following chemotherapy and might suggest ways to inhibit growth of B lineage malignancies.
Keywords: Human, B lymphopoiesis, G-CSF, Stromal cell-free cultures, Mesenchymal stem cell
1. Introduction
B lineage lymphocytes are produced throughout life from hematopoietic stem cells (HSC) within bone marrow (BM) (Nunez et al., 1996; Rossi et al., 2003; Stephan et al., 1998). While steps in the process have been extensively investigated in mice, progress in understanding human B lymphopoiesis has been hampered by lack of efficient ways to study progenitors in culture (Bertrand et al., 2000; Lebien, 2000). This is partially due to species differences and a growth factor that selectively drives formation of human B cells has not been identified (Pribyl and Lebien, 1996). Some success has been obtained by placing human stem and progenitor cells on monolayers of murine stromal cells (Kurosaka et al., 1999; Nishihara et al., 1998; Ohkawara et al., 1998; Rawlings et al., 1995), and we recently found that human mesenchymal stem cells (hMSC) were even more effective (Ichii et al., 2008). For example, umbilical cord blood (CB) CD34+ cells generated more CD33+ CD13+ myeloid cells and CD10+ CD19+ B lineage lymphocytes in hMSC co-cultures than when the murine MS5 stromal cell line was used. In addition to their support of hematopoiesis (Koc et al., 2000; Muguruma et al., 2006; Zhang et al., 2004), multipotential hMSC are capable of chondrocyte, osteoblast or adipocyte differentiation (Abdallah and Kassem, 2008; Chamberlain et al., 2007; Pittenger et al., 1999). Diffusible factors or membrane ligands on the adherent cells provide signals required for survival, proliferation and differentiation in the B lineage, but the nature of those molecules is poorly understood. Addition of recombinant growth and differentiation factors can be helpful, but optimal conditions have not been found.
A robust, stromal cell-free culture procedure that is based on human factors would have many advantages over existing methods. For example, drugs or biologicals could be screened for direct effects on hematopoietic cells, identifying molecules with therapeutic potential. We have now systematically manipulated culture conditions, finding that hMSC produce soluble, heat-labile factors that support human B lymphopoiesis. The same is true for undefined substances in selected lots of fetal calf serum (FCS), and the active principal was not IL-7, thymic stromal lymphopoietin (TSLP), CXCL12 or hemokinin 1. High cell densities and addition of recombinant granulocyte colony stimulating factor (G-CSF), Stem cell factor (SCF) and flt-3 ligand (FL) were all critical for efficient B lymphopoiesis. While lymphocyte production from adult BM progenitors in culture is much more difficult than umbilical CB cells, both are responsive to G-CSF. This information should now facilitate basic studies of immune system replenishment, improving differentiation schemes useful for classifying malignancies as well as the discovery of cytokines that can counter immunodeficiency resulting from mutations or chemotherapy.
2. Materials & methods
2.1. Origin and isolation of cells
CB cells were collected from healthy, full-term neonates immediately after Caesarean section or normal delivery. BM cells were collected from normal donors. All samples were collected after informed consent, using protocols approved by the Investigational Review Boards at Osaka University and O.M.R.F. Mononuclear cells were separated by Ficoll-Paque PLUS (GE Healthcare Bio-Science AB, Uppsala, Sweden) or Lymphocyte Separation Medium (Mediatech, Inc., Manassas, VA) and centrifugation. Purification of CB and BM CD34+ cells was performed using Direct human CD34 Progenitor Cell Isolation Kit (Miltenyi Biotec, Auburn, CA). Human mesenchymal stem cells were purchased from Lonza (Walkersville, MD), and maintained in Mesenchymal Stem Cell Growth Medium (MSCGM, Lonza). Flow cytometric analysis confirmed that the cultured hMSC expressed CD105, CD166, CD29, and CD44, but not CD14, CD34, or CD45.
2.2. Co-cultures for human B lymphocytes
Co-cultures of CB CD34+ cells on hMSC were performed as previously described (Ichii et al., 2008). hMSC were seeded in 12-well tissue plates 1 or 2 days before setting up the co-cultures. Isolated CD34+ cells (2×103 cells/well) were plated on sub-confluent hMSC layers in MSCGM in the presence of 10 ng/ml SCF and 5 ng/ml FL. Recombinant human SCF and FL proteins were purchased from R&D systems (Minneapolis, NY). Half of the culture medium was replaced with fresh medium containing the same cytokines twice a week. In some experiments, direct contact between hMSC and cultured cells was prevented with 0.45 μm polyethylene terephthalate membranes (FalconTM Cell Culture Inserts; Becton Dickinson Labware, Franklin Lakes, NJ).
2.3. Stromal cell-free cultures for human B lymphocytes
Isolated CD34+ cells were seeded with cytokines as indicated in the figures. The cultures were usually maintained in QBSF®60 (Quality Biological, Inc., Gaithersburg, MD) containing10 % FCS, 100 U/ml penicillin, and 100 mg/ml streptomycin). Recombinant human IL-7 and G-CSF proteins were purchased from R&D Systems. Half of the culture medium was replaced with fresh medium containing the same cytokines once a week. Supernatants of one week hMSC cultures were collected, filtered and added to lymphocyte cultures. This conditioned medium was incubated for one hour at 56°C to assess lability and also used in experiments with neutralizing antibodies to IL-7, CXCR4 or TSLP (R&D Systems). A hemokinin-1 inhibitor (L732138) was obtained from Sigma-Aldrich (St. Louis, MO).
2.4. Flow cytometry and cell sorting
Flow cytometric analysis was performed with a FACSCalibur or FACS LSRII (BD Biosciences Immunocytometry Systems, San Jose, CA) using standard multicolor immunofluorescent staining protocols. Mouse monoclonal Abs against the following human cell surface molecules were purchased: PE-CD3, PE-CD10, APC-CD10, PE-CD19, PE-CD20, FITC-CD33, PE-CD34, APC-CD34, FITC-CD45, PE-CD56, and PE-glycophorin A (GPA) from BD Biosciences/BD Pharmingen (Franklin Lakes, NJ); phycoerythrin 5-succinimidylester (PC5)-CD19 from Beckman Coulter (Marseilles, France); and FITC-IgM from Southern Biotechnology Associates (Birmingham, AL).
2.5. Transplantation of cultured cells into immunodeficient mice
Xenotransplantation of cultured cells from CB CD34+ cells were performed using a previously reported method (Hiramatsu et al., 2003). Briefly, 8- to 12-week old NOD/SCID/common γnull (NOG) mice received 240 cGy radiation divided in two fractions. Isolated CB CD34+ cells or cells generated in stromal cell-free cultures were injected via the tail vein. At intervals after transplantation, peripheral blood cells were taken and assessed for human lineage specific marker expression by flow cytometry. The same mice were sacrificed more than 2 months after transplantation, and phenotypes of BM and spleen cells were analyzed using flow cytometry.
2.6. Statistical analyses
Student’s t-test was performed to assess statistical differences. All results are shown as mean values ± SD.
3. Results
3.1. Mesenchymal stem cells produce unknown, heat-labile factors that promote human B lymphopoiesis
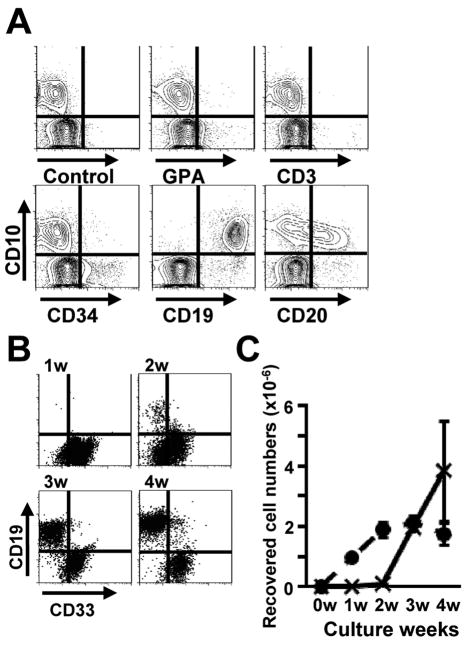
Membrane inserts have been extensively used to probe requirements for physical contact in hematopoietic cell cultures (Miller et al., 1998; Nishihara et al., 1998; Verfaillie, 1993). We used this approach to determine that hMSC or conditioned medium from them supported formation of CD10+ CD33− lymphoid cells in cultures of CD34+ CB (Figure 1A, 1B). The yield of lymphocytes was much less than when hematopoietic cells were in direct contact with hMSC, suggesting that the diffusible factors might be short-acting, and they were completely inactivated by heating for 1 hour at 56°C (Figure 1C).
Figure 1. Human mesenchymal stem cells (hMSC) produce unknown, heat-labile factors that promote human B lymphopoiesis.
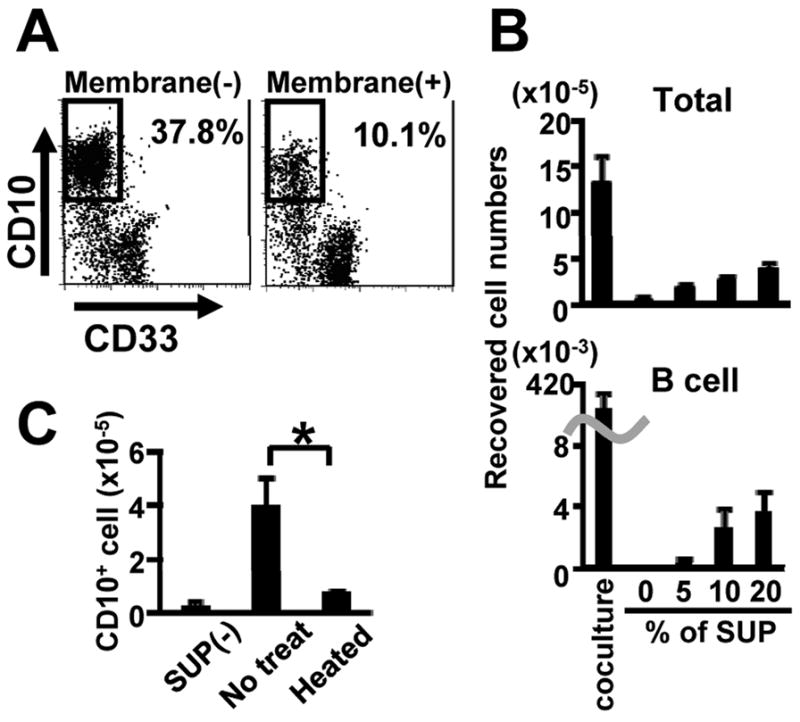
(A) CB CD34+ cells (1×103 cells/ml) were cultured on hMSC in combinations of SCF and FL with or without membrane filters for 4 weeks. The generated cells were stained with PE-CD10 and FITC-CD33, and were analyzed by flow cytometry. Similar results were obtained in three independent experiments. (B) The indicated concentrations of hMSC supernatant (SUP) were added to the cultures of CB CD34+ cells (1×103 cells/ml) in the presence of SCF and FL without stromal layers. The numbers of total and CD10+ cells after 4 weeks of cultures are shown. Similar results were obtained in three independent experiments. (C) hMSC supernatants were heated at 56 °C for 1 hour. CB CD34+ cells (5×103 cells/ml) were cultured with or without hMSC supernatant (10%) in the presence of SCF and FL without stromal layers. The numbers of CD10+ cells after 4 weeks of cultures are shown. Statistical differences from results with untreated supernatant are indicated with asterisks (p<0.01). Similar results were obtained in three independent experiments.
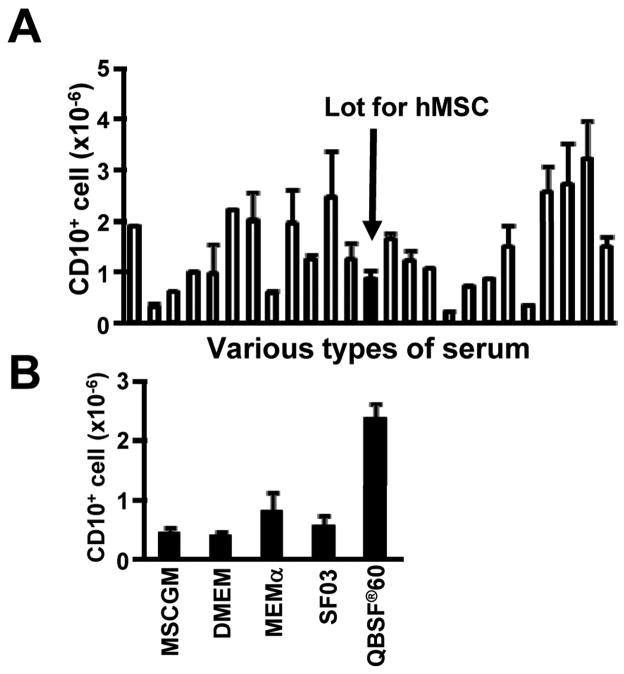
Stromal cell derived IL-7 is essential for murine adult B lymphopoiesis (Peschon et al., 1994; Von Freeden-Jeffry et al., 1995) and parts of the IL-7 receptor are used to recognize the cytokine TSLP (Levin et al., 1999; Vosshenrich et al., 2003). Chemokines recognized by the CXCR4 receptor and the hemokinin-1 peptide also influence lymphopoiesis in the mouse (Milne et al., 2004; Tokoyoda et al., 2004; Zhang et al., 2000; Zhu et al., 2007). Addition of neutralizing antibody to IL-7 had no effect on human cord blood cell cultures (Figure 2A). The same was true for antibodies to the CXCR4 receptor or TSLP, or addition of an inhibitor for hemokinin-1 (Figure 2B, 2C). The experiments described above were conducted with a batch of FCS that is optimal for growth of hMSC, and screening revealed no correlation with efficiency of lymphopoiesis (Figure 3A). All subsequent studies were done with a batch that was optimal in this respect. Medium selection was another important variable, and QBSF®60 was selected for all of our studies (Figure 3B). We conclude that while direct contact with stromal cells is not essential for human B lymphopoiesis, unknown stromal cell-derived factors do facilitate this process.
Figure 2. IL-7, CXCR4, TSLP and Hemokinin-1 are not essential for human B lymphopoiesis.
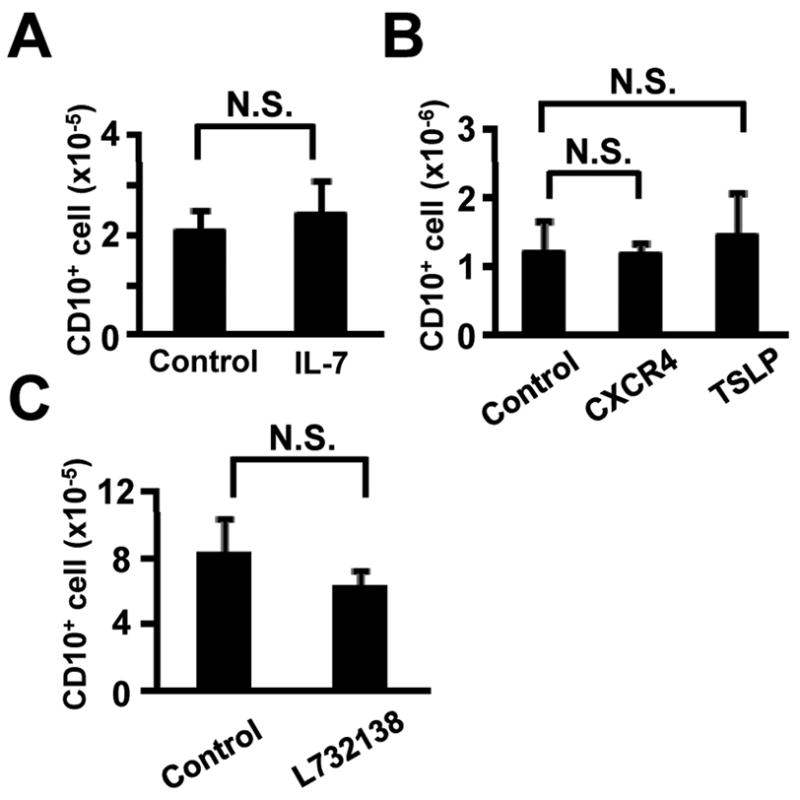
(A) CB CD34+ cells (1×104 cells/ml) were cultured with a control (10μg/ml Isotype-matched Ab) or a neutralizing antibody to IL-7(10μg/ml) in QBSF®60 medium in the presence of hMSC supernatant (10%), SCF and FL for 4 weeks. Numbers of recovered CD10+ cells determined by flow cytometry are shown. (B) CB CD34+ cells (1×104 cells/ml) were cultured with control (10μg/ml Isotype-matched Ab) or neutralizing antibodies (10μg/ml) to CXCR4 or TSLP in QBSF®60 medium in the presence of hMSC supernatant (10%), SCF, FL and IL-7 for 4 weeks. (C) CB CD34+ cells (1×104 cells/ml) were cultured with control (DMSO) or an inhibitor to Hemokinin-1 (20μM L732138) in QBSF®60 medium in the presence of hMSC supernatant (10%), SCF, FL and IL-7 for 4 weeks. Similar results were obtained in three independent experiments. N.S.; differences were not significant.
Figure 3. FCS and medium selection were important variables for stromal cell-free cultures that support human B lymphopoiesis.
(A) CB CD34+ cells (1×104 cells/ml) were cultured in QBSF®60 medium supplemented with 10% of various lots of fetal calf sera in the presence of hMSC supernatant (10%), SCF, FL and IL-7 for 4 weeks. The solid bar indicates CD10+ cell numbers in cultures with the serum batch selected for maintaining hMSC. Similar results were obtained in three independent experiments. (B) CB CD34+ cells (1×104 cells/ml) were cultured in various types of media with 10% FCS in the presence of hMSC supernatant (10%), SCF and FL for 4 weeks. Numbers of recovered CD10+ cells are shown. Similar results were obtained in three independent experiments. Data are shown as means ± SD numbers of CD10+ cells generated in triplicate samples.
3.2. Characteristics of cells generated in cultures with hMSC conditioned medium
We found that cultures initiated with 1×104 highly enriched CB CD34+ cells together with 10% hMSC conditioned medium, SCF, FL, and IL-7 generated approximately 2×106 CD10+ lymphoid cells within 4 weeks. Figure 4A shows representative flow cytometry results for cultures held one week longer. Approximately 5% of the cultured cells still expressed CD34, but neither GPA+ erythroid cells nor CD3+ T lineage cells were ever detected. Most of the generated CD10+ cells also expressed CD19, while more than half of them were CD20+. CD33+ myeloid cells peaked after 2 weeks of culture and then CD10+ CD19+ B lineage cells appeared (Figure 4B, 4C).
Figure 4. Characteristics of cells generated in stromal cell-free cultures.
(A) CB CD34+ cells (1×104 cells/ml) were cultured for 5 weeks in the presence of hMSC supernatant (10%), SCF, FL and IL-7. The generated cells were stained with FITC-CD33 and APC-CD10 as well as the indicated PE-conjugated Abs. All of the flow cytometry results shown in panel A were gated for CD33− cells. Isotype-matched Abs were used as negative controls. Similar results were obtained in three independent experiments. (B, C) CB CD34+ cells (1×104 cells/ml) were cultured with hMSC supernatant (10%), SCF, FL and IL-7 for 4 weeks. The cultured cells were collected, and surface expression of CD10, CD19 and CD33 was determined weekly by flow cytometry. Numbers of CD33+ myeloid cells (dotted line) and CD10+ CD19+ lymphoid cells (solid line) were calculated, and similar results were obtained in three independent experiments.
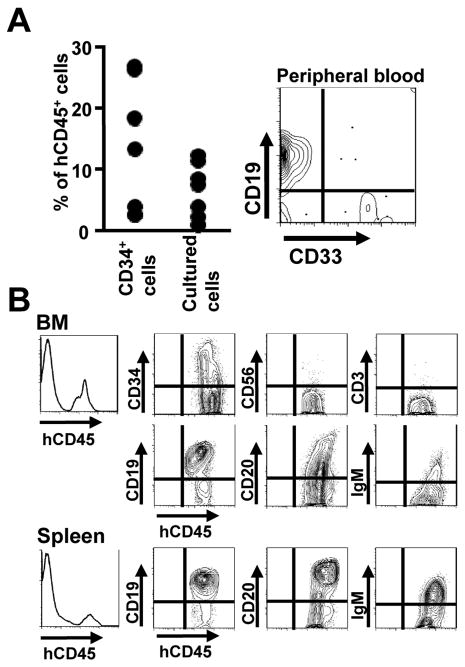
We then assessed the differentiation potential of cells expanded in three week cultures by transplanting 1×107 of them into sub-lethally irradiated, immunodeficient NOG mice. Control animals received 5×104 freshly isolated CB CD34+ cells. CD45+ human cells were detected in peripheral blood of all transplanted mice six weeks later and B lineage lymphoid cells predominated (Figure 5A). The animals were then sacrificed 3 months after transplantation and more detailed flow cytometry was performed with BM and spleen (Figure 5B). Human CD19+, CD20+ and IgM+ lymphocytes, but not NK or T cells were found in both sites. Similar results were obtained when transplanted mice were examined six months after transplantation. Thus, these culture conditions support the generation of human B lymphoid cells and cultured progenitors retain substantial potential for B lymphopoiesis in chimeric mice.
Figure 5. Differentiation of cultured human hematopoietic cells in immunodeficient mice.
(A) NOG mice (n=5 for each group) were each transplanted with 5×104 freshly isolated CB CD34+ cells or 1×107 cells recovered from 3 week stromal cell-free cultures. Six weeks later, peripheral blood samples were analyzed by flow cytometry. Percentages of human CD45+ cells are shown. Similar results were obtained in three independent experiments. Representative flow cytometry data for CD19 and CD33 expression by gated human CD45+ cells is also shown. (B) CB CD34+ cells were held 3 weeks in stromal cell-free cultures in the presence of hMSC supernatant (10%), SCF, FL and IL-7. Then, 1×107 recovered cells were transplanted into NOG mice. After 12 weeks, their BM and spleen cells were collected and subjected to flow cytometry analysis. Cells of culture origin were gated with APC-anti human CD45 and staining is shown with the indicated Abs. Results obtained in 5 independent experiments were very similar.
3.3. Lymphopoiesis can be observed in stromal cell-free, hMSC supernatant-free cultures if cell density is high
Some studies have stressed the importance of high cell density for lymphocyte viability in culture (Milne et al., 2004; Zhang et al., 2000), and we found this was also the case for human lymphoid progenitors (Figure 6A). In fact, significant numbers of lymphocytes were made even in the absence of hMSC or their products when cultures were initiated with greater than 5 × 103 CB CD34+ cells/ml. These generated cells had similar surface phenotypes to the cultured cells with 10% hMSC conditioned medium shown in Figure 4A (data not shown). A series of cultures were then set up with a starting density of 1× 104 cells/ml and subgroups were then diluted to this same density at intervals of 1, 2 or 3 weeks of culture. Total nucleated cells expanded approximately 500 fold in the control, unmanipulated group and this was the most efficient condition for generating lymphocytes (Figure 6B). Adjustment of the density at any subsequent time, and especially during the first two weeks, compromised lymphocyte recovery. Note that non-lymphoid cells represented in the total nucleated cell counts expanded well when culture cell numbers were readjusted on weeks two and three. Also, crowding of cells in round bottom rather than flat bottom 96 well trays improved cloning efficiencies for human progenitors (data not shown). Therefore, contact between maturing hematopoietic progenitors favors their survival, proliferation and/or differentiation.
Figure 6. High cell density favors lymphopoiesis in stromal cell-free cultures.
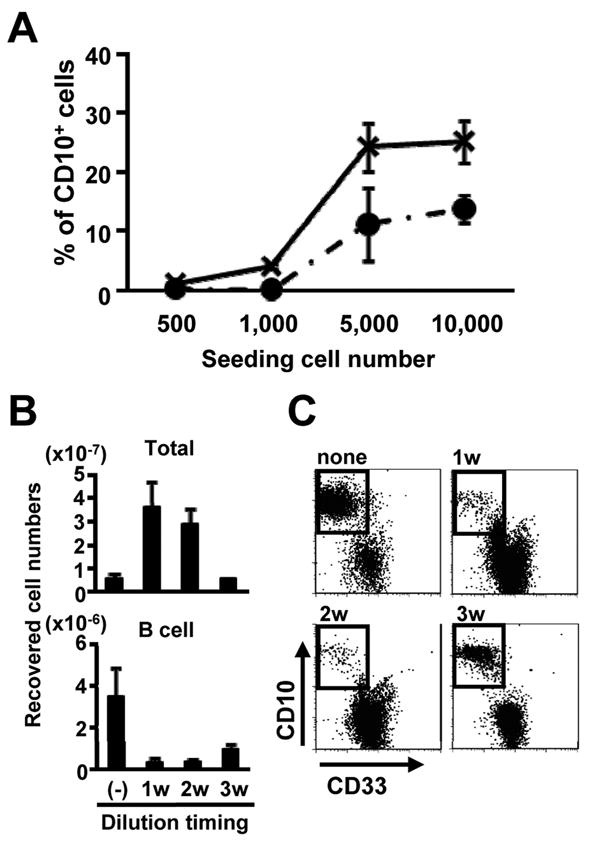
(A) The indicated numbers of CB CD34+ cells were cultured in the presence of SCF and FL with (solid line) or without (dotted line) 10% hMSC supernatant for 4 weeks. Percentages of CD10+ cells recovered were determined by flow cytometry. (B,C) CB CD34+ cells (1×104 cells/ml) were cultured in the presence of SCF, FL and IL-7. The harvested cells were then adjusted back to 1×104 cells/ml on days 7, 14, or 21 of culture, respectively. (B) Numbers of total and CD10 cells generated cells are also shown. (C) Representative flow cytometry results were shown for analyses done after a total of 4 weeks of culture. Similar results were obtained in three independent experiments.
3.4. Addition of recombinant G-CSF promotes lymphopoiesis even with adult marrow progenitors
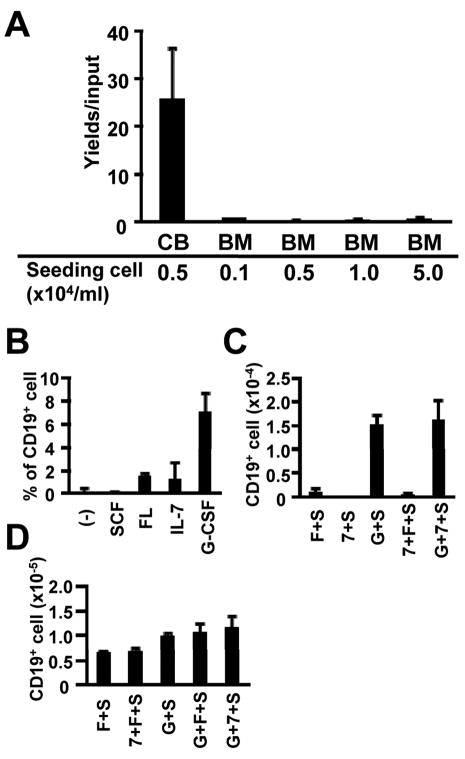
The developmental age of hematopoietic cell donors has a strong influence over their potential to generate lymphocytes in culture (Rossi et al., 2003). That is, conditions that support vigorous lymphopoiesis from CB CD34+ cells yield few lymphocytes with progenitors harvested from adult BM. We observed this phenomenon when cells of the two types were cultured on hMSC or in conditioned medium from hMSC, regardless of the initial cell density (Figure 7A and data not shown). It was reported that G-CSF facilitated lymphopoiesis in co-cultures with murine stromal cells (Nishihara et al., 1998), and we found the same was true in stromal cell-free cultures (Figure 7B and 7C). That is, even CD34+ cells from adult BM generated lymphocytes when this recombinant cytokine was present. Yields of lymphocytes were best when recombinant SCF and G-CSF were added together and refreshed with each weekly medium change. Progenitors from umbilical CB were less fastidious than those from adult marrow (Figure 7A). However, even CB 34+ cells generated more lymphocytes in culture when G-CSF was present (Figure 7D).
Figure 7. Human BM CD34+ cells require G-CSF to generate B lineage cells in stromal cell-free cultures.
(A) 1×104 cells/ml CB CD34+ cells or the indicated concentrations of BM CD34+ cells were cultured in the presence of SCF, FL and IL-7 for 4 weeks. Lymphoid cells were identified by flow cytometry and the results are given as numbers of CD19+ cells generated / input progenitor cell. (B) BM CD34+ cells (5×104 cells/ml) were cultured alone or with the indicated cytokines. After 4 weeks of culture, CD19 expression of the recovered cells was assessed by flow cytometry. (C,D) BM CD34+ cells (5×104 cells/ml) (C) or CB CD34+ cells (1×104 cells/ml) (D) were cultured for 4 weeks in the indicated combinations of SCF (S), FL (F), IL-7 (7) and/or G-CSF (G). The results are given as mean total numbers of CD19+ cells recovered ± S.D. Similar results were obtained in three independent experiments.
4. Discussion
It is believed that bone marrow contains specialized niches where B lineage lymphocytes are generated (Tang et al., 1993; Tokoyoda et al., 2004; Zhu et al., 2007). However, the molecules that comprise these lymphopoietic micro-environments are poorly understood. To our knowledge, this represents the first successful generation of human B lineage lymphocytes in stromal-cell free cultures. Important variables include selection of fetal calf serum lots, appropriate medium, high cell density and addition of G-CSF. Cultures initiated with 1×104 CB CD34+ cells/ml or 5×104 BM CD34+ cells/ml in QBSF®60 contained with 10% selected FCS, SCF, FL, and G-CSF generated CD10+ CD19+ lymphoid cells within 4 weeks. Although far from being an efficient system, it provides an opportunity to systematically screen candidate substances and learn how diffusible factors regulate replenishment of the humoral immune system. Assessment of survival and growth of lymphoid leukemia may represent another application of this new technology.
G-CSF was discovered for its support of myelopoiesis, and while it is not made by hMSC under normal steady-state conditions, the factor is produced by osteoblasts (Eaves et al., 1991; Majumdar et al., 2000; Taichman and Emerson, 1994; Zhang et al., 2004). B lineage progenitors were also mobilized in patients whose stem cells were mobilized by injection of this cytokine (Imamura et al., 2005). In addition, the factor augmented human lymphopoiesis when added to co-cultures supported by murine MS5 stromal cells (Nishihara et al., 1998; Ohkawara et al., 1998). The same investigators reported that lymphoid and non-lymphoid cells have G-CSF receptors. We have now found that G-CSF directly stimulates human CD34+ cells and supports their ability to generate B lymphopoiesis. The effect was most pronounced when stem and progenitors from adult BM was used to initiate cultures. It may be that G-CSF is functioning as a multipoietin, improving the retention of CD34+ cells that subsequently generate lymphocytes. However, we have also found that G-CSF augments lymphocyte formation in cultures initiated with Lin− CD34+ CD38+ CD123− CD45RA+ CD10− lymphoid progenitors (M.I. unpublished observations).
Consistent with many previous studies (Pribyl and Lebien, 1996; Puel et al., 1998), we found no influence of IL-7 on B lymphopoiesis in human cultures and active heat-labile substances released by stromal cells remain undefined. TSLP is a cytokine that preferentially expands fetal lymphopoiesis in mice (Levin et al., 1999; Vosshenrich et al., 2003). Consistent with that, addition of the factor augmented lymphocyte formation in cultures initiated with human cord blood, but not adult marrow progenitors in preliminary experiments (data not shown). However, addition of neutralizing TSLP specific antibodies to our cultures did not identify it as an activity in hMSC supernatants.
High cell densities reportedly improve murine pre-B cell survival in part because of their release of hemokinin-1 (Milne et al., 2004; Zhang et al., 2000). Autocrine lymphocyte-derived factors may also be important for human progenitors, but we never observed changes in cultures treated with Hemokinin-1 or an inhibitor specific for it. Chemokines such as CXCL12 may be recognized by CXCR4 on progenitors and deliver pro-adhesive signals important within bone marrow (Tokoyoda et al., 2004; Zhu et al., 2007), but there was no obvious role for this receptor in our stromal cell-free cultures.
It is noteworthy that B lineage lymphocytes always appeared after the expansion of myeloid cells in all culture conditions. When limiting numbers of CD34+ cells were used to initiate cultures, some wells contained myeloid cells but no lymphocytes. Even highly enriched murine stem cell populations are heterogeneous, and it is believed that subsets differ with respect to lymphopoietic potential. The methods we describe here may suggest experimental approaches to such questions with human stem cells.
Fetal and neonatal CD34+ cells differ from their adult counterparts in multiple ways (Ng et al., 2004; Wang et al., 1997; Weekx et al., 1998). For example, cord blood cells slowly reconstitute adult transplant recipients but smaller numbers are effective (Laughlin et al., 2004; Schmitz and Barrett, 2002). A better understanding of these differences may lead to improved and accelerated transplant recovery as well as reduced incidences of disease relapse. Neonatal cells have also been more robust and less fastidious in previous lymphoid culture studies (Ichii et al., 2008; Rossi et al., 2003). As noted above, G-CSF preferentially influences adult progenitors but lymphopoiesis is still less efficient than when cord blood CD34+ cells are used. Again, experimental approaches involving cultures might be used to seek a molecular basis for neonatal/adult differences.
These findings represent an increment in techniques for observing human B lymphopoiesis in culture. While there is considerable room for further improvement, it is already clear that complex questions about species specific and lymphoid leukemia regulators as well as stem and progenitor cell heterogeneity can now be addressed.
Acknowledgments
The authors thank Shelli Wasson for editorial assistance and Beverly Hurt for assistance with graphics. This work was supported by grant AI020069 (P.W.K.), from the National Institutes of Health. P.W.K. holds the William H. and Rita Bell Endowed Chair in Biomedical Research.
Abbreviations
- HSC
hematopoietic stem cells
- BM
bone marrow
- hMSC
human mesenchymal stem cells
- CB
umbilical cord blood
- FCS
fetal calf serum
- TSLP
thymic stromal lymphopoietin
- G-CSF
granulocyte colony stimulating factor
- SCF
stem cell factor
- FL
flt-3 ligand
- GPA
glycophorin A
- PC5
phycoerythrin 5-succinimidylester
- NOG
NOD/SCID/common γnull
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdallah BM, Kassem M. Human mesenchymal stem cells: from basic biology to clinical applications. Gene Ther. 2008;15:109. doi: 10.1038/sj.gt.3303067. [DOI] [PubMed] [Google Scholar]
- Bertrand FE, Eckfeldt CE, Fink JR, Lysholm AS, Pribyl JAR, Shah N, Lebien TW. Microenvironmental influences on human B-cell development. Immunol Rev. 2000;175:175. [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Eaves CJ, Cashman JD, Kay RJ, Dougherty GJ, Otsuka T, Gaboury LA, Hogge DE, Lansdorp PM, Eaves AC, Humphries RK. Mechanisms that regulate the cell cycle status of very primitive hematopoietic cells in long-term human marrow cultures. II. Analysis of positive and negative regulators produced by stromal cells within the adherent layer. Blood. 1991;78:110. [PubMed] [Google Scholar]
- Hiramatsu H, Nishikomori R, Heike T, Ito M, Kobayashi K, Katamura K, Nakahata T. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/γcnull mice model. Blood. 2003;102:873. doi: 10.1182/blood-2002-09-2755. [DOI] [PubMed] [Google Scholar]
- Ichii M, Oritani K, Yokota T, Nishida M, Takahashi I, Shirogane T, Ezoe S, Saitoh N, Tanigawa R, Kincade PW, Kanakura Y. Regulation of human B lymphopoiesis by the transforming growth factor-β superfamily in a newly established coculture system using human mesenchymal stem cells as a supportive microenvironment. Exp Hematol. 2008;36:587. doi: 10.1016/j.exphem.2007.12.013. [DOI] [PubMed] [Google Scholar]
- Imamura R, Miyamoto T, Yoshimoto G, Kamezaki K, Ishikawa F, Henzan H, Kato K, Takase K, Numata A, Nagafuji K, Okamura T, Sata M, Harada M, Inaba S. Mobilization of human lymphoid progenitors after treatment with granulocyte colony-stimulating factor. J Immunol. 2005;175:2647. doi: 10.4049/jimmunol.175.4.2647. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Kurosaka D, Lebien TW, Pribyl JA. Comparative studies of different stromal cell microenvironments in support of human B-cell development. Exp Hematol. 1999;27:1271. doi: 10.1016/s0301-472x(99)00067-3. [DOI] [PubMed] [Google Scholar]
- Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM, Marks DI, Van Rood JJ, Scaradavou A, Horowitz MM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- LeBien TW. Fates of human B-cell precursors. Blood. 2000;96:9. [PubMed] [Google Scholar]
- Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, Farr AG. Thymic stromal lymphopoietin: A cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol. 1999;162:677. [PubMed] [Google Scholar]
- Majumdar MK, Thiede MA, Haynesworth SE, Bruder SP, Gerson SL. Human marrow-derived mesenchymal stem cells (MSCs) express hematopoietic cytokines and support long-term hematopoiesis when differentiated toward stromal and osteogenic lineages. J Hematother Stem Cell Res. 2000;9:841. doi: 10.1089/152581600750062264. [DOI] [PubMed] [Google Scholar]
- Miller JS, McCullar V, Verfaillie CM. Ex vivo culture of CD34+/Lin−/DR− cells in stroma-derived soluble factors, interleukin-3, and macrophage inflammatory protein-1α maintains not only myeloid but also lymphoid progenitors in a novel switch culture assay. Blood. 1998;91:4516. [PubMed] [Google Scholar]
- Milne CD, Fleming HE, Zhang Y, Paige CJ. Mechanisms of selection mediated by interleukin-7, the preBCR, and hemokinin-1 during B-cell development. Immunol Rev. 2004;197:75. doi: 10.1111/j.0105-2896.2004.0103.x. [DOI] [PubMed] [Google Scholar]
- Muguruma Y, Yahata T, Miyatake H, Sato T, Uno T, Itoh J, Kato S, Ito M, Hotta T, Ando K. Reconstitution of the functional human hematopoietic microenvironment derived from human mesenchymal stem cells in the murine bone marrow compartment. Blood. 2006;107:1878. doi: 10.1182/blood-2005-06-2211. [DOI] [PubMed] [Google Scholar]
- Ng YY, van Kessel B, Lokhorst HM, Baert MR, van den Burg CM, Bloem AC, Staal FJ. Gene-expression profiling of CD34+ cells from various hematopoietic stem-cell sources reveals functional differences in stem-cell activity. J Leukoc Biol. 2004;75:314. doi: 10.1189/jlb.0603287. [DOI] [PubMed] [Google Scholar]
- Nishihara M, Wada Y, Ogami K, Ebihara Y, Ishii T, Tsuji K, Ueno H, Asano S, Nakahata T, Maekawa T. A combination of stem cell factor and granulocyte colony-stimulating factor enhances the growth of human progenitor B cells supported by murine stromal cell line MS-5. Eur J Immunol. 1998;28:855. doi: 10.1002/(SICI)1521-4141(199803)28:03<855::AID-IMMU855>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Nunez C, Nishimoto N, Gartland GL, Billips LG, Burrows PD, Kubagawa H, Cooper MD. B cells are generated throughout life in humans. J Immunol. 1996;156:866. [PubMed] [Google Scholar]
- Ohkawara JI, Ikebuchi K, Fujihara M, Sato N, Hirayama F, Yamaguchi M, Mori KJ, Sekiguchi S. Culture system for extensive production of CD19+IgM+ cells by human cord blood CD34+ progenitors. Leukemia. 1998;12:764. doi: 10.1038/sj.leu.2401004. [DOI] [PubMed] [Google Scholar]
- Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Pribyl JAR, Lebien TW. Interleukin 7 independent development of human B cells. Proc Natl Acad Sci USA. 1996;93:10348. doi: 10.1073/pnas.93.19.10348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puel A, Ziegler SF, Buckley RH, Leonard WJ. Defective IL7R expression in T−B+NK+ severe combined immunodeficiency. Nat Genet. 1998;20:394. doi: 10.1038/3877. [DOI] [PubMed] [Google Scholar]
- Rawlings DJ, Quan SG, Kato RM, Witte ON. Long-term culture system for selective growth of human B-cell progenitors. Proc Natl Acad Sci USA. 1995;92:1570. doi: 10.1073/pnas.92.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi MID, Yokota T, Medina KL, Garrett KP, Comp PC, Schipul AH, Jr, Kincade PW. B lymphopoiesis is active throughout human life, but there are developmental age related changes. Blood. 2003;101:576. doi: 10.1182/blood-2002-03-0896. [DOI] [PubMed] [Google Scholar]
- Schmitz N, Barrett J. Optimizing engraftment--source and dose of stem cells. Semin Hematol. 2002;39:3. doi: 10.1053/shem.2002.29245. [DOI] [PubMed] [Google Scholar]
- Stephan RP, Reilly CR, Witte PL. Impaired ability of bone marrow stromal cells to support B- lymphopoiesis with age. Blood. 1998;91:75. [PubMed] [Google Scholar]
- Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179:1677. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Scott G, Ryan DH. Subpopulations of bone marrow fibroblasts support VLA-4- mediated migration of B-cell precursors. Blood. 1993;82:3415. [PubMed] [Google Scholar]
- Tokoyoda K, Egawa T, Sugiyama T, Choi BI, Nagasawa T. Cellular niches controlling B lymphocyte behavior within bone marrow during development. Immunity. 2004;20:707. doi: 10.1016/j.immuni.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Verfaillie CM. Soluble factor(s) produced by human bone marrow stroma increase cytokine-induced proliferation and maturation of primitive hematopoietic progenitors while preventing their terminal differentiation. Blood. 1993;82:2045. [PubMed] [Google Scholar]
- Von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SEG, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vosshenrich CA, Cumano A, Muller W, Di Santo JP, Vieira P. Thymic stromal-derived lymphopoietin distinguishes fetal from adult B cell development. Nat Immunol. 2003;4:773. doi: 10.1038/ni956. [DOI] [PubMed] [Google Scholar]
- Wang JCY, Doedens M, Dick JE. Primitive human hematopoietic cells are enriched in cord blood compared with adult bone marrow or mobilized peripheral blood as measured by the quantitative in vivo SCID-repopulating cell assay. Blood. 1997;89:3919. [PubMed] [Google Scholar]
- Weekx SFA, Van Bockstaele DR, Plum J, Moulijn A, Rodrigus I, Lardon F, De Smedt M, Nijs G, Lenjou M, Loquet P, Berneman ZN, Snoeck HW. CD34++CD38− and CD34+CD38+ human hematopoietic progenitors from fetal liver, cord blood, and adult bone marrow respond differently to hematopoietic cytokines depending on the ontogenic source. Exp Hematol. 1998;26:1034. [PubMed] [Google Scholar]
- Zhang Y, Li C, Jiang X, Zhang S, Wu Y, Liu B, Tang P, Mao N. Human placenta-derived mesenchymal progenitor cells support culture expansion of long-term culture-initiating cells from cord blood CD34+ cells. Exp Hematol. 2004;32:657. doi: 10.1016/j.exphem.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Lu L, Furlonger C, Wu GE, Paige CJ. Hemokinin is a hematopoietic-specific tachykinin that regulates B lymphopoiesis. Nat Immunol. 2000;1:392. doi: 10.1038/80826. [DOI] [PubMed] [Google Scholar]
- Zhu J, Garrett R, Jung Y, Zhang Y, Kim N, Wang J, Joe GJ, Hexner E, Choi Y, Taichman RS, Emerson SG. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]