Abstract
Background
Many smokers remain unwilling or unable to make a quit attempt. For these smokers, novel strategies to induce quit attempts are necessary to achieve further reductions in smoking prevalence.
Purpose
This article describes the design and methods of an ongoing nationwide telephone-based clinical trial for cessation induction, the principal aim of which is to test the hypothesis that samples of nicotine replacement therapy (NRT), can induce quit attempts among smokers otherwise unmotivated to quit.
Methods
Smokers are recruited proactively through online channels. A ‘behavioral filter’ is used to identify and separate motivated versus unmotivated smokers, the latter of whom (N = 750) are formally entered into the clinical trial. Participants are randomized to one of two treatment conditions designed to promote self-efficacy and motivation to quit: (1) practice quit attempt (PQA) or (2) PQA plus NRT sampling. The primary outcome measure tested over a 6-month follow-up is the incidence of additional quit attempts as well as hypothesized mediators of treatment effects.
Results
This study details the challenges of identifying and treating smokers who are unmotivated to quit. Strengths include a novel treatment approach, tested among a group of proactively recruited smokers nationwide, with a unique method of identifying cessation-resistant smokers.
Limitations
The omission of a true control group, testing the effect of the PQA itself, is an inherent limitation to the study design. Online recruitment presents additional study challenges, all of which are discussed in detail.
Conclusions
The study has translational potential to guide both clinical and policy recommendations for cessation induction. Further, while the focus is on smoking, this trial may serve as an example to researchers and clinicians who focus on other health behaviors, and who themselves are challenged with motivating people who are unmotivated for change.
Introduction
Clinicians are often frustrated by patients who are resistant to health behavior change. Motivating the unmotivated can be a significant challenge, whether the problem behavior is, for example, tobacco or alcohol use, diet, exercise, or screening. Often, a clinician will rely on persuasive messaging, but in the absence of any demonstrable change, that clinician will have few other options at his or her disposal. Cigarette smoking represents a particularly entrenched behavior. Despite vast improvements in tobacco control, most smokers remain either unable or unwilling to quit. Less than 10% of smokers report wanting to quit in the immediate future [1], and 60% of all smokers do not make a quit attempt during any given year [2]. For these change-resistant smokers, novel strategies are necessary for cessation induction, that is, inducing motivation to quit and quit attempts among smokers who otherwise lack both.
Cessation induction trials are common in the smoking literature. Physician-delivered brief advice is an effective approach that reaches many smokers, but meta-analytic reviews suggest only modest effect sizes [3,4]. Another common approach is through interventions tied to stages of change [5]; that is, the idea that smokers at different stages of readiness to quit (precontemplation, contemplation, preparation, action) require different interventions. Across a number of studies, stage-based interventions have been shown to be efficacious [6–8], although two comprehensive reviews questioned the strength of data for stage-based interventions [9,10].
We believe one viable approach to prompt quitting behavior is the notion of sampling cessation medications as a means to learn more about the process of quitting. Despite strong evidence in support of nicotine replacement therapy (NRT) [4], many smokers have enduring misperceptions and strong misgivings about these pharmacotherapies [11–15]. Even as an over-the-counter product, under-use of NRT persists [16,17]. Several studies have shown that provision of NRT samples to smokers often enhances cessation behaviors [18–20], though these were uncontrolled studies and it is not yet entirely clear if sampling per se had any direct effect. Free patch give-away incentives to smokers engaged in a telephone quitline, who presumably may be more motivated to quit, typically yield large increases in call volume and ultimately enhance quit rates [21–23]. Whether a similar NRT sample for unmotivated smokers would have the same effect is unclear, but there is ample rationale for the notion that sampling NRT might motivate smokers towards quitting. Primarily, sampling NRT may increase self-efficacy and familiarization among smokers, particularly those who feel they cannot quit or who have never used NRT previously. A recent test of very brief sampling (i.e., one time use) of NRT demonstrated a positive shift in attitudes towards subsequent use [24], but did not test for subsequent effects on cessation behaviors. Thus, for smokers who are resistant to quitting, sampling NRT for short periods of time could serve as a catalyst for making a quit attempt.
To provide structure for the experience of sampling NRT, we and others [25,26] propose the notion of short periods of ‘trial quitting,’ which itself could serve as a motivating and learning experience to prompt further attempts to quit [25,26]. The notion of a practice quit attempt is conceptually similar to the CBT practice of ‘behavioral experiments’ [27,28] and is partly based on concepts derived from the Great American Smokeout, sponsored by the American Cancer Society. The Great American Smokeout was originally designed for ambivalent smokers to take a brief pause of smoking, if only for a day, to learn about the process of quitting and enhance further resolve. Empirical tests of the Smokeout are lacking, but the presumption is that short periods of abstinence may lead to sustained quitting. Partial evidence for this presumption comes from a small test, which experimentally manipulated brief periods of abstinence among smokers who did not want to quit [29]. Results from that study showed that brief episodes of nonsmoking enhance future abstinence.
Most previous studies have not focused on unmotivated smokers. In this article, we describe the design and methods of a large, ongoing nationwide telephone-based clinical trial to prompt quit attempts among unmotivated smokers (NCT00706979), in which we specifically test whether adding free NRT to brief advice to undertake a practice quit attempt will motivate more smokers to make a serious attempt to stop smoking than brief advice without NRT. A primary focus on cessation would be ideal. Given the study focus on smokers who are unmotivated to quit, the present study was premised on the fact that quit attempts are a necessary precursor to cessation. Thus, it is imperative to first demonstrate that the intervention can induce quit attempts before expending the resources for the much larger study that would be needed to determine the efficacy of the intervention for smoking cessation. Throughout, we describe the sampling method, interventions, and measures of outcome that are perhaps unique to a study of unmotivated smokers. While the focus is on smoking, this trial may serve as an example to researchers and clinicians who focus on other health behaviors, and who themselves are challenged with motivating people who are unmotivated for change.
Methods
Overview of design and study hypotheses
Within a large, single-site randomized clinical trial, smokers not motivated to quit are assigned to one of two treatment groups: (1) practice quit attempt (PQA) or (2) PQA +NRT Sampling. Participants in both groups receive three intervention calls over 6 weeks, and then enter a 6-month follow-up period (assessment only). The incidence of subsequent serious quit attempts is our primary outcome variable. We hypothesize that PQA +NRT sampling will result in a higher incidence of making subsequent, serious quit attempts. We further hypothesize that these effects will be mediated by (a) increased smoking-related self-efficacy, (b) increased belief in the efficacy of NRT, (c) increased social support for not smoking, and (d) less withdrawal distress and craving during the PQA.
General recruitment method and participant eligibility
The recruitment goal for the study is 750 smokers who are unmotivated to quit smoking. Smokers unmotivated to quit smoking do not usually volunteer for smoking research studies, thus constraining typical recruitment strategies that rely on reactive methods (e.g., posting recruitment ads and waiting for smokers to respond). Innovative, proactive methods are necessary to reach out to smokers who are unmotivated to quit [30]. For studies that focus exclusively on ‘unmotivated’ smokers, once a smoker is identified, the challenge then becomes to separate in a reliable and valid manner those smokers who want to quit from those who do not want to quit.
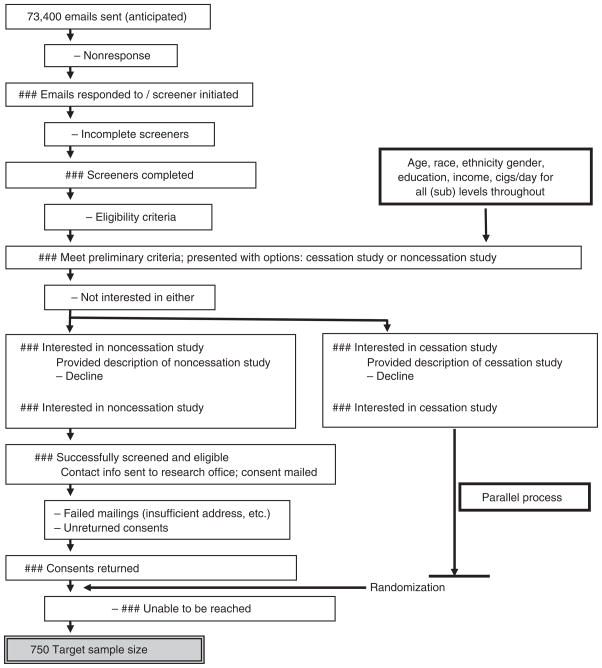
Within the current study, smokers nationwide are identified proactively through an online internet panel, developed and maintained by Harris Interactive, Inc, a large, national market research firm. Based on an established database of individuals who have provided their email address and who have expressed interest in online surveys, an e-mail is sent to individuals inviting them to participate in an online health survey (see Figure 1 for anticipated flow of study recruitment). All individuals in the panel have completed a ‘double opt in’ process, which requires each person to confirm his/her desire to join the panel by clicking on a link within an e-mail that is sent to the registrant’s e-mail address (absence of response disqualifies the individual from further solicitation). This protection mitigates concerns that solicitation through such channels is invasive or unwanted.
Figure 1.
Anticipated recruitment flow
Potential participants in the study are eligible if they are (a) age 18 or above, (b) current cigarette smokers of at least 10 cigarettes per day with no monthly cigar, pipe, smokeless tobacco use, (c) interested in quitting smoking at some time (we wanted even the ‘unmotivated’ smokers to have at least some level of interest in quitting at some future point), (d) no FDA-suggested cautions for nicotine lozenge use (pregnancy, breastfeeding, recent cardiovascular distress, or phenylketonuria), (e) accessible by phone for a 6-month study period, (f) no previous use of NRT, and (g) without a quit attempt of greater than 1 week in the past year (individuals without any quit attempts in past year are eligible). The latter two criteria are imposed with the rationale that NRT-naïve smokers with a minimal history of prior quitting would be most recalcitrant but also possibly most responsive to the intervention.
Once an eligible smoker is identified, additional measures are needed to identify smokers who are truly not currently interested in quitting. In our prior work on unmotivated smokers, we initially relied on self-reported motivation to quit, asking potential study participants on a scale from 0 to 10 how much they wanted to quit in the next 30 days [31]. However, this led to a study sample that was actually more motivated to quit smoking than they reported; quit rates were higher than expected based on national norms. Subsequently, we developed a ‘behavioral filter,’ in which we present all eligible smokers with two options: one study for those who want to quit in the next 30 days and another for those who do not. Both study options are presented simultaneously, and both are described with generally equivalent parameters (e.g., study length, participant compensation) to avoid selection based on study features. By self-selection into either study option, smokers convey their motivation to quit.
We have previously used this behavioral filter to identify unmotivated smokers for a smoking reduction study [32]. Four observations from this prior study indicate that the behavioral filter was successful in reaching unmotivated smokers. First, of all smokers who were interested in joining either study, 15% chose the cessation option and 85% chose the noncessation study, proportions that are consistent with national norms for stages of change [1]. Second, within the noncessation arm alone, more than 95% of the sample was in the stage of change of either precontemplation (not wanting to quit in next 6 months) or contemplation (wanting to quit in next 6 months but not in next month); only 5% were in preparation (wanting to quit in the next month). Third, baseline readiness-to-quit ladder scores were sufficiently low, for both readiness to quit in the next month (mean = 3.0 on 0–10 scale) and next 6 months (mean = 5.0). Finally, the incidence of quit attempts and rates of point prevalent abstinence in the no treatment control condition using this filter (16% and 4%) were very similar to expected findings based on national data from that time (20% and 3%) [33].
We still retain responsibility for those smokers who opt for cessation (enrolling them into a separate study), but the study proper and the remainder of this article focus exclusively on the smokers who are identified as unmotivated to quit, designated by their self-selection into the noncessation study.
Once eligible participants have opted for either study (or neither), they are then mailed a study consent and baseline questionnaire, and are asked to return them to the research office in a preaddressed, prestamped envelope. This presents another departure from traditional in-person studies in which consent is managed interactively and in-person, and the current study is mindful to abide by appropriate IRB considerations (e.g., allowing individuals to ask questions prior to consent via e-mail or through an established toll-free number). Only after a signed consent is returned to the research office is the participant formally enrolled into the study, though attrition can still occur thereafter if the person is unable to be reached via phone for study initiation (Figure 1). The baseline questionnaire assesses participant characteristics (demographics, smoking history) that would be too cumbersome to collect via phone. Upon receipt of the informed consent and baseline questionnaire, each participant is randomized to one of two intervention groups, described below. Randomization does not stratify for common predictors of quit attempts, as the large sample size should equalize both groups on these potential confounds. Randomization is concealed from the study participant; each participant is informed of group assignment upon the first treatment phone call, thus minimizing potential for selection bias. However, neither study personnel nor study participants are blinded to study group.
Interventions
All interventions take place over the phone. The treatment period consists of three phone calls over 6 weeks, after which treatment ceases but follow-up continues for 6 months (Figure 2). Research personnel are trained in instructing on PQAs and NRT use, and are supervised by the principal investigator (MJC). All interventions are semi-scripted but allow for some flexibility. To ensure treatment fidelity, 5–10% of all calls are monitored for quality assurance. Interventions for each group are estimated to be time equivalent. Though our primary focus is on NRT sampling, we first describe the behavioral exercise of a PQA, which serves as a foundation to test our intervention. Readers interested in our treatment protocols may contact the first author.
Figure 2.
Study procedures
Practice quit attempt
The PQA is based on the concept of ‘rehearsal’ for the real experience of quitting. Using analogies along the theme of ‘learning to ride a bike,’ we discuss with the smoker how quitting smoking often requires a bit of practice before one is successful, and that a PQA can be a good opportunity to learn more about the motivations behind smoking and the process of quitting. The central theme of the PQA is to remove the stress and pressure of trying to quit for good, but the intent is to practice different tools to avoid smoking and build confidence for some point in the future when the smoker decides to quit for good. The participant is prompted to set a PQA for whatever duration they wish – a few hours or a few days – or none at all. We debated whether to ask for a specific goal from each participant (based on the rationale that some individuals prefer such structure) versus allowing them to do whatever they wanted (on the rationale that some individuals would be hesitant to commit to the process if we pushed them too far). In the end, we allow either approach, and gauge whether each participant wants to set a firm goal for each PQA or would rather just ‘see how far they could go.’ We emphasize that the duration of the PQA is not essential as much as the learning and confidence that can come from it. A second call takes place 3 weeks later, at which time the counselor reviews success and/or barriers. The participant is prompted to make a second PQA, this time building upon the success of the first. After each of these calls, we mail support materials to aid in the PQA. These include (a) a resource sheet to further describe the rationale of the PQA, (b) a list of coping tools to manage cravings, and (c) a craving tracking form to monitor and document what worked and what did not to manage cravings. At the third and final treatment call, the counselor again reviews progress and reaffirms any success. At this closing point in treatment, the counselor re-orients the participant towards quitting for good and delivers a brief prompt to quit, akin to what would be delivered by physicians in a clinical setting [4].
PQA + NRT sampling (enhanced PQA)
This intervention is based on the similar themes and format as above, but with added support of nicotine lozenge. Just as the PQA is an opportunity to learn more about what it takes to quit, this enhanced PQA is an opportunity to learn more about nicotine replacement – how it works, tastes, and should be used. Our rationale is that sampling NRT may help to familiarize smokers with the lozenge in particular, and with cessation aids in general, and also to dispel any misperceptions regarding safety and side effects. Similar to above, the second call draws upon the success of the first PQA, eliciting and reaffirming any positive experience, particularly with regard to usage of the lozenges, and prompts for a second PQA attempt. In addition to mailings described above, participants in this group receive informational brochures about nicotine lozenges (e.g., FAQs). The third and final call delivers a brief message to quit in earnest as per above. There are no provisions for free NRT beyond the treatment period.
The nicotine lozenge was chosen because it is an over-the-counter product (like the nicotine patch or gum) that can be used ad libitum during periods of high craving (unlike the patch) and generally does not have a disagreeable taste (unlike the gum). For each PQA prompted at either the first or second treatment call, we mail a supply of lozenge. Several studies [34,35], including one of our own [32], have demonstrated that this is feasible and safe. A toll-free hotline has been established for participants to call in the event that they experience any adverse events.
Outcomes
The primary outcome on which the study is powered, like many cessation induction interventions [32,36,37], is further ‘serious’ quit attempts (defined below). We also track the incidence, number, timing, and duration of all quit attempts during the 6-month follow-up.
Secondary outcome measures include abstinence, smoking reduction, adverse events, and use of additional cessation resources (e.g., purchase of (additional) NRT or other pharmacotherapy, use of quitlines). One potential concern that arises through provision of NRT samples to smokers who are not firmly committed to quitting is that they use the nicotine lozenge for noncessation reasons, such as to avoid smoking restrictions. This unintended consequence of sampling is tracked as well.
Another important aspect of this study is its focus on presumed mediators of treatment effects (Figure 2). Sampling NRT could serve to promote self-efficacy or increase familiarization with NRT, which could ultimately promote further quit behaviors. Thus, for these two mechanisms, we include items from a previously established scale on self-efficacy [38] as well as selected items from previous research examining attitudes towards NRT [11,12,39]. Additionally, PQAs, with or without NRT samples, could serve to promote support from others (social support), which, in turn, could provide additional incentive to make additional sustained quit attempts. Enhanced social support during a cessation attempt increases abstinence rates [40]. Thus, we include a short measure of partner support developed internally for use in smoking cessation interventions. Finally, for those who sample the nicotine lozenge, decreased withdrawal during the PQA should inform the smoker that stopping with NRT may be easier than prior quit attempts and thereby increase the likelihood that the smoker will try to stop again; thus, we include a brief measure of withdrawal [41,42].
Follow-up procedures
Beyond the 3-week treatment period, participants in both groups are called three additional times in the 6 months that follow (weeks 4, 12, and 26), for a total of six contacts per participant (Figure 2). We considered one final assessment at Week 26 only but opted against this approach because we did not believe participants could accurately recall smoking and cessation behaviors over a 6-month interval. Interim (Weeks 4 and 12) calls are scheduled on the basis that most outcomes (subsequent quit attempts) would follow shortly after termination of treatment, when motivation and efficacy are increased. One primary challenge of phone-based studies is the collection of assessment measures. Not only must the number of assessments and the duration of each be brief, but they must be simple to deliver, without complicated response options. Alternative, nontelephone-based methods of outcome assessment were considered (mailed surveys, online survey administration), but each were abandoned because of likely problems with nonresponse.
The call schedule amounts to 4500 calls (750 participants × 6 phone calls each). We have developed a computer tracking database to prompt the timing of all calls. These procedures were used in our prior telephone-based study of smoking reduction [32] and resulted in a completion rate of 94%. We make up to five attempts per call before considering it is a missed contact. However, a missed call does not result in forced dropout; participants who miss interviews are eligible for future calls.
During the treatment period, the counselor conducts both the treatment delivery and the assessments. For these calls, assessments are done first and treatment delivery follows, which partially diminishes demand characteristics (i.e., pressure to report quit behavior), which could result if this sequence was reversed. Although bias is still possible, it should be equivalent across groups. Furthermore, as a behavioral intervention, it is nearly impossible to blind either participants or study personnel on group assignment. We considered having a separate therapist and data collector, so that the data collector could be blind to study group. We chose not to because doing so would add additional burden on study participants, asking them to be available for two calls for each ‘study visit.’ We also considered an online data collection system, removing all need for counselors to collect study data. We opted against this approach on the basis that it would likely worsen retention. To enhance data quality, all assessment data are directly entered into a computer database in real time during each call, which both increases efficiency and minimizes data entry errors. All participants are offered payment for each call completed, up to a total of $100 (giftcards sent via mail).
Sample size estimations and data analysis
As noted, our primary outcome is the incidence of ‘serious quit attempts,’ which are operationally defined as attempts that last at least 3 days, for which we estimate a base rate of 14%. We further estimate, based on Cochrane reviews for cessation outcomes [4], that the odds ratio (OR) for the effect of NRT on prompting new quit attempts will be around 1.75–2.0. Using a two-sided alpha of 0.05, to obtain 80% power to detect an OR of 2.0 with a base rate of 14% (i.e., increasing this to 25%) would require 237 participants/group and to detect an OR of 1.75 would require 375 participants per group (i.e., increasing this to 22%). We believe an OR of 1.75 would be significant for clinical and policy practices; thus, we will seek this effect and our total sample size will be 750 (375 × 2) participants.
All analyses are based on an intent-to-treat model. Missing values are imputed as if the participant made no quit attempts, returned to baseline levels of smoking, and had no changes in related mediators (self-efficacy, etc). This conservative approach biases all results towards the null hypothesis. A logistic regression will be used to examine the primary hypothesis that NRT-enhanced PQAs will yield a higher incidence of quit attempts than will the PQA group alone. A similar approach will examine point prevalence abstinence. Mediation will be tested via a series of generalized estimation equations (GEE) [43], with study group as a between-subjects factor and each outcome over time as a within-subjects factor, all adjusted for baseline values of the outcome and other covariates as appropriate.
Discussion
Significance
There are both clinical and policy implications that may be derived from this study. Current practice guidelines from both the USPHS and the American Psychiatric Association [44] do not mention the possibility of PQAs, but rather only a verbal, motivational dialogue (the ‘5 Rs’). However, even when well-trained clinicians implement existing motivational strategies and/or brief advice to unmotivated smokers, at most only half will make a quit attempt [45]. If PQAs are successful in inducing quit attempts and cessation, it may, if replicated, offer clinicians another option in their menu of treatments for this challenging group of smokers.
The policy implications of the current study are equally compelling. Current regulations within the US are such that NRT is indicated only for cessation and not for nonabstinence-based outcomes. Currently, several pharmaceutical companies are developing nonabstinence indications for NRT (including temporary abstinence) to propose to the US FDA. If we found adding NRT to PQAs promotes abstinence, this would provide support for NRT for nonabstinence purposes.
Major methodological questions
Several methodological issues (phone-based treatment, potential for bias through demand characteristics) have been discussed earlier. We consider here the advantages and disadvantages of key methodological issues that may be relevant to the design of future trials.
Study design
Our chief methodological consideration pertained to the number of study arms, and the intervention delivery within those arms. The principal underlying research question that guided the design was the efficacy of NRT sampling in promoting quit attempts. To provide structure for the experience of NRT sampling, we based it within the behavioral strategy of a PQA. Thus, to isolate on the efficacy of NRT sampling in this context, the natural test would be PQA versus PQA + NRT. We strongly considered a true control group (i.e., one without a PQA intervention). This would strengthen the study design by allowing for a direct test of the PQA intervention itself, a question that the present study will be unable to answer. Whereas the scientific rationale for a no-treatment control arm is strong, we opted against this due to concerns about feasibility. Specifically, had we added a no-treatment control group, in which we anticipated an 11% base incidence rate of serious quit attempts (vs. 14% and 22% for our two active groups; see above), this would have required 1907 participants per group, which we deemed unfeasible due to funding constraints. Thus, if our study yields null results (PQA = PQA + NRT), we will be unable to determine if they are equally effective to promote quit attempts, or equally ineffective. We will only be able to make indirect comparisons to what we might expect from the general population. Future studies will be necessary to determine the benefit of the PQA intervention itself.
Definition of study outcome
Our study is primarily designed as a test to induce quit attempts among smokers not motivated to quit. Some might question this outcome, preferring instead to focus exclusively on cessation, which we track as a secondary outcome. The rationale for quit attempts as a primary outcome was two-fold. First, we consider the development of novel interventions for cessation induction to be a two-step process, first to determine if they promote quit attempts, and subsequently to determine if they promote quitting. If an intervention does not pass the first test, then there is no need for a subsequent test of cessation. On the other hand, there is strong evidence that increasing quit attempts increases eventual cessation [46], so if our study is successful, a necessary follow-up will be to examine cessation in greater detail. Second, studies of cessation are large and costly. The very low base rates of abstinence among unmotivated smokers would require a very large sample size. For example, over a 6-month period, less than 1% of such smokers quit for good [47]. Thus, even if our intervention doubled abstinence, we would need to power a study to detect an increase from 1% to 2%, which would require a trial of over 4600 smokers. This would be a very large and costly trial for a novel intervention about which little is presently known.
One challenge of quit attempts as a primary outcome is to distinguish them from PQAs as part of the intervention. Other studies that prompt quit attempts among unmotivated smokers define quit attempts as those lasting at least 24 h [32,36], a definition that is consistent with the CDC [47]. However, we define ‘serious quit attempts’ as those lasting a minimum of 3 days. We recognize this 3-day criterion is arbitrary, and that duration of a quit attempt is not always synonymous with a motivation to quit [48]. We chose this definition for three reasons. First, it is an objective criterion that does not rely solely on participant definitions of ‘serious’ quit attempts. Second, because our intervention is a PQA of ~24 h, we wanted to ensure that our major outcome reflected a quit attempt that was more substantial. Third, this definition is the most stringent outcome (aside from abstinence) and would therefore lead to more conservative findings; that is, we would be more confident in interpreting an intervention effect if we demonstrated group differences, as compared to if we chose a less-stringent outcome.
Biological verification
Because we are primarily interested in induction of quit attempts, we opted against biological verification of smoking behavior. At present, there is no method to biochemically verify quit attempts per se, which by definition are variable in time and transient in length. We do track abstinence as a secondary outcome, and recognize the lack of biochemical verification as a limitation. Our intervention is what most would consider a minimal intervention. Both the Society for Research on Nicotine and Tobacco’s guideline on biochemical verification and other articles suggest that biochemical verification is not necessary with minimal interventions that incur few demand characteristics [49,50].
Nationwide proactive recruitment
The primary rationale for our recruitment strategy was three-fold. First, reactive recruitment (e.g., posting ads/flyers and waiting for smokers to respond) is typically ineffective to reach smokers who are unmotivated to quit [31], and thus proactive methods are necessary. Second, nationwide recruitment should result in a sample that is more representative of the general population compared with local recruitment. Finally, nationwide recruitment is a more feasible approach that allows us to efficiently recruit large numbers of smokers.
Our recruitment method also introduces some limitations, however. Namely, online recruitment could be susceptible to the ‘digital divide,’ in which it is presumed that smokers of lower socioeconomic status would be under-represented. Recruitment bias exists even with traditional clinical trials for smoking, in that most studies are heavily weighted with white, female smokers [51]. Online recruitment as outlined in this study may result in a similarly skewed sample, with additional concerns that smokers recruited through online channels may be of higher socioeconomic status than would otherwise be expected of the general population of smokers. Whereas online recruitment can result in biased samples, this does not necessarily imply a bias in associations between variables [52]. In other words, the potential for a skewed sample through online recruitment may affect external validity, but will have little effect on internal validity. The benefits and limitations of internet-based smoking research, including the potential for recruitment bias, have been documented [53]. A growing body of research that suggests online recruitment and administration of smoking studies is both feasible and effective [54,55], but it is also important to recognize the limitations of this approach.
Conclusion
The stagnant rate of quit attempts over the recent years suggests the need for additional treatment options for smokers who are unmotivated to quit. This article reports on an ongoing randomized clinical trial with the principal aim to examine whether NRT sampling, in combination with a PQA, will motivate more smokers to make a serious attempt to stop smoking than would a PQA alone. Aspects of the study incorporate innovative methodology that can strengthen future population-based studies of cessation induction. The treatment approach is novel and has the potential to change both clinical practice and policy. The significance of the study rests on its potential to provide additional treatment and policy options to move the challenging group of unmotivated smokers towards quitting.
Beyond the current study, this clinical trial may have relevance for researchers and clinicians who focus on other health behaviors, particularly wherein the focus is on individuals who are unmotivated for change. Several procedures described in this study could transfer to clinical trials in other domains. Namely, these include (a) a proactive, population-based recruitment, (b) the use of a behavioral filter to identify truly recalcitrant individuals, and (c) an intervention based on the idea that sampling behaviors may be a viable strategy to motivate people towards health behavior change. It is incumbent upon future research to determine if these study features generalize to studies in other health domains.
Acknowledgments
This study funded through NIDA grants R01DA021619 (MJC), K23DA020482 (MJC), and K12DA000357 (KMG). Partial funding of NRT is provided through GlaxoSmithKline. The authors thank Amy Boatright, Nicola Thornley, Elizabeth Byrd, Diana Rivera, and Dr. Katie Sterba for assistance with study procedures.
Glossary
- CBT
Cognitive-behavioral therapy
- FAQs
Frequently asked questions
- FDA
Food and Drug Administration
- NRT
Nicotine replacement therapy
- PQA
Practice quit attempt
- USPHS
United States Public Health Service
Footnotes
Reprints and permissions: http://www.sagepub.co.uk/journalsPermissions.nav
References
- 1.Wewers ME, Stillman FA, Hartman AM, Shopland DR. Distribution of daily smokers by stage of change: Current Population Survey results. Prev Med. 2003;36:710–20. doi: 10.1016/s0091-7435(03)00044-6. [DOI] [PubMed] [Google Scholar]
- 2.USDHS. Cigarette smoking among adults — United States, 2006. MMWR. 2007;56:1157–61. [PubMed] [Google Scholar]
- 3.Stead LF, Bergson G, Lancaster T. The Cochrane Library. 3. Wiley; Oxford: 2008. Physician Advice for Smoking Cessation (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- 4.Fiore MC, Jaén CR, Baker TB, et al. Clinical Practice Guideline. US Public Health Service; Rockville, MD: 2008. Treating Tobacco Use and Dependence: 2008 Update. [Google Scholar]
- 5.Prochaska JO, Redding CA, Evers KE. The transtheoretical model and stages of change. In: Glanz K, RImer B, Lewis F, editors. Health Behavior and Health Education: Theory, Research, and Practice. Jossey-Bass; San Francisco: 2002. pp. 99–120. [Google Scholar]
- 6.Prochaska JO, Velicer WF, Fava JL, et al. Evaluating a population-based recruitment approach and a stage-based expert system intervention for smoking cessation. Addict Behav. 2001;26:583–602. doi: 10.1016/s0306-4603(00)00151-9. [DOI] [PubMed] [Google Scholar]
- 7.Prochaska JO, Velicer WF, Fava JL, et al. Counselor and stimulus control enhancements of a stage-matched expert system intervention for smokers in a managed care setting. Prev Med. 2001;32:23–32. doi: 10.1006/pmed.2000.0767. [DOI] [PubMed] [Google Scholar]
- 8.Prochaska JO, Velicer WF, Prochaska JM, Johnson JL. Size, consistency, and stability of stage effects for smoking cessation. Addict Behav. 2004;29:207–13. doi: 10.1016/s0306-4603(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 9.Spencer L, Pagell F, Hallion ME, Adams TB. Applying the transtheoretical model to tobacco cessation and prevention: A review of literature. Am J Health Promot. 2002;17:7–71. doi: 10.4278/0890-1171-17.1.7. [DOI] [PubMed] [Google Scholar]
- 10.Riemsma RP, Pattenden J, Bridle C, et al. Systematic review of the effectiveness of stage based interventions to promote smoking cessation. Br Med J. 2003;326:1175–81. doi: 10.1136/bmj.326.7400.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bansal MA, Cummings KM, Hyland A, Giovino GA. Stop-smoking medications: Who uses them, who misuses them, and who is misinformed about them? Nicotine Tobacco Res. 2004;6:S303–10. doi: 10.1080/14622200412331320707. [DOI] [PubMed] [Google Scholar]
- 12.Cummings KM, Hyland A, Giovino GA, et al. Are smokers adequately informed about the health risks of smoking and medicinal nicotine? Nicotine Tobacco Res. 2004;6:S333–40. doi: 10.1080/14622200412331320734. [DOI] [PubMed] [Google Scholar]
- 13.Mooney ME, Leventhal AM, Hatsukami DK. Attitudes and knowledge about nicotine and nicotine replacement therapy. Nicotine Tobacco Res. 2006;8:435–46. doi: 10.1080/14622200600670397. [DOI] [PubMed] [Google Scholar]
- 14.Pederson LL, Nelson D. Literature review and summary of perceptions, attitudes, beliefs, and marketing of potentially reduced exposure products: Communication implications. Nicotine Tobacco Res. 2007;9:525–34. doi: 10.1080/14622200701239548. [DOI] [PubMed] [Google Scholar]
- 15.Vogt F, Hall S, Marteau TM. Understanding why smokers do not want to use nicotine dependence medications to stop smoking: Qualitative and quantitative studies. Nicotine Tobacco Res. 2008;10:1405–13. doi: 10.1080/14622200802239280. [DOI] [PubMed] [Google Scholar]
- 16.Cokkinides VE, Ward E, Jemal A, Thun MJ. Under-use of smoking-cessation treatments: Results from the National Health Interview Survey, 2000. Am J Prev Med. 2005;28:119–22. doi: 10.1016/j.amepre.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34:102–11. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 18.Alberg AJ, Margalit RS, Burke A, et al. The influence of offering free transdermal nicotine patches on quit rates in a local health department’s smoking cessation program. Addict Behav. 2004;29:1763–78. doi: 10.1016/j.addbeh.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Jolicoeur DG, Ahluwalia JS, Richter KP, et al. The use of nicotine patches with minimal intervention. Prev Med. 2000;30:504–12. doi: 10.1006/pmed.2000.0670. [DOI] [PubMed] [Google Scholar]
- 20.Jolicoeur DG, Richter KP, Ahluwalia JS, et al. Smoking cessation, smoking reduction, and delayed quitting among smokers given nicotine patches and a self-help pamphlet. Substance Abuse. 2003;24:101–06. doi: 10.1080/08897070309511538. [DOI] [PubMed] [Google Scholar]
- 21.Fellows JL, Bush T, McAfee T, et al. Cost effectiveness of the Oregon quitline “free patch initiative”. Tobacco Control. 2007;16:i47–52. doi: 10.1136/tc.2007.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bush TM, McAfee T, Deprey M, et al. The impact of a free nicotine patch starter kit on quit rates in a state quit line. Nicotine Tobacco Res. 2008;10:1511–16. doi: 10.1080/14622200802323167. [DOI] [PubMed] [Google Scholar]
- 23.Miller N, Frieden TR, Liu SY, et al. Effectiveness of a large-scale distribution programme of free nicotine patches: A prospective evaluation. Lancet. 2005;365:1849–54. doi: 10.1016/S0140-6736(05)66615-9. [DOI] [PubMed] [Google Scholar]
- 24.Schneider NG, Justice M, Gould JL, et al. Preferences among five nicotine treatments based on information versus sampling. Nicotine Tobacco Res. 2008;10:179–86. doi: 10.1080/14622200701767837. [DOI] [PubMed] [Google Scholar]
- 25.Perkins KA, Stitzer M, Lerman C. Medication screening for smoking cessation: Proposal for new technologies. Psychopharmacology. 2006;184:628–36. doi: 10.1007/s00213-005-0105-5. [DOI] [PubMed] [Google Scholar]
- 26.Brown RA, Lejuez CW, Kahler CW, et al. Distress tolerance and early smoking lapse. Clin Psychol Rev. 2005;25:713–33. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salkovskis P. The importance of behavior in the maintenance of anxiety and panic: A cognitive account. Behav Psychother. 1991;19:6–19. [Google Scholar]
- 28.Bennett-Levy J, Butler G, Fennell M, et al. Oxford Guide to Behavioral Experiments in Cognitive Therapy. Oxford University Press; Oxford, England: 2004. [Google Scholar]
- 29.Heil SH, Alessi SM, Lussier JP, et al. An experimental test of the influence of prior cigarette smoking abstinence on future abstinence. Nicotine Tobacco Res. 2004;6:471–9. doi: 10.1080/14622200410001696619. [DOI] [PubMed] [Google Scholar]
- 30.Paul CL, Wiggers J, Daly JB, et al. Direct telemarketing of smoking cessation interventions: Will smokers take the call? Addiction. 2004;99:907–13. doi: 10.1111/j.1360-0443.2004.00773.x. [DOI] [PubMed] [Google Scholar]
- 31.Carpenter MJ, Hughes JR, Keely JP. Effects of smoking reduction on later cessation: A pilot experimental study. Nicotine Tobacco Res. 2003;5:155–62. doi: 10.1080/146222003100007385. [DOI] [PubMed] [Google Scholar]
- 32.Carpenter MJ, Hughes JR, Solomon LJ, Callas PW. Both smoking reduction with nicotine replacement therapy and motivational advice increase future cessation among smokers unmotivated to quit. J Consult Clin Psychol. 2004;72 :371–81. doi: 10.1037/0022-006X.72.3.371. [DOI] [PubMed] [Google Scholar]
- 33.USDHS. Cigarette smoking among adults – United States, 2003. MMWR. 2005;54:509–13. [PubMed] [Google Scholar]
- 34.Solomon LJ, Scharoun GM, Flynn BS, et al. Free nicotine patches plus proactive telephone peer support to help low-income women stop smoking. Prev Med. 2000;31:68–74. doi: 10.1006/pmed.2000.0683. [DOI] [PubMed] [Google Scholar]
- 35.Solomon LJ, Secker-Walker RH, Flynn BS, et al. Proactive telephone peer support to help pregnant women stop smoking. Tob Control. 2000;9:72. doi: 10.1136/tc.9.suppl_3.iii72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter MJ, Strange C, Jones Y, et al. Does genetic testing result in behavioral health change? Changes in smoking behavior following testing for Alpha-1 Antitrypsin Deficiency. Ann Behav Med. 2007;33:22–8. doi: 10.1207/s15324796abm3301_3. [DOI] [PubMed] [Google Scholar]
- 37.Hughes JR. Motivating and helping smokers to stop smoking. J Gen Intern Med. 2003;18:1053–57. doi: 10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Velicer WF, Diclemente CC, Rossi JS, Prochaska JO. Relapse situations and self-efficacy: An integrative model. Addict Behav. 1990;15:271–83. doi: 10.1016/0306-4603(90)90070-e. [DOI] [PubMed] [Google Scholar]
- 39.Etter JF, Perneger TV. Pharmacoepidemiology and drug utilization: Attitudes toward nicotine replacement therapy in smokers and ex-smokers in the general public. Clin Pharmacol Ther. 2001;69:175–83. doi: 10.1067/mcp.2001.113722. [DOI] [PubMed] [Google Scholar]
- 40.Park E-W, Tudiver F, Schultz JK, Campbell T. Does enhancing partner support and interaction improve smoking cessation? A meta-analysis. Ann Fam Med. 2004;2:170–74. doi: 10.1370/afm.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hughes JR, Gust SW, Skoog K, et al. Symptoms of tobacco withdrawal: A replication and extension. Arch Gen Psychiatry. 1991;48:52–9. doi: 10.1001/archpsyc.1991.01810250054007. [DOI] [PubMed] [Google Scholar]
- 42.Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- 43.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 44.American Psychiatric Association. Practice Guideline for the Treatment of Patients with Nicotine Dependence. American Psychiatric Association; Washington DC: 1996. [DOI] [PubMed] [Google Scholar]
- 45.Kreuter MW, Chheda SG, Bull FC. How does physician advice influence patient behavior? Evidence for a priming effect. Arch Fam Med. 2000;9:426–33. doi: 10.1001/archfami.9.5.426. [DOI] [PubMed] [Google Scholar]
- 46.West R, McEwen A, Bolling K, Owen L. Smoking cessation and smoking patterns in the general population: A 1-year follow-up. Addiction. 2001;96:891–902. doi: 10.1046/j.1360-0443.2001.96689110.x. [DOI] [PubMed] [Google Scholar]
- 47.USDHS. Cigarette smoking among adults — United States, 2007. MMWR. 2008;57:1221–6. [PubMed] [Google Scholar]
- 48.Carpenter MJ, Hughes JR. Defining quit attempts: What difference does a day make? Addiction. 2005;100:257–8. doi: 10.1111/j.1360-0443.2004.00952.x. [DOI] [PubMed] [Google Scholar]
- 49.Benowitz N, Ahijevych K, Hall S, et al. Biochemical verification of tobacco use and cessation. Nicotine Tobacco Res. 2002;4:149–59. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 50.Hughes JR, et al. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tobacco Res. 2003;5 :13–25. [PubMed] [Google Scholar]
- 51.Hughes JR, Giovino GA, Kelvins RM, Fiore MC. Assessing the generalizability of smoking studies. Addiction. 1997;92:469–472. [PubMed] [Google Scholar]
- 52.Etter JF, Perneger TV. A comparison of cigarette smokers recruited through the internet or by mail. Int J Epidemiol. 2001;30:521–25. doi: 10.1093/ije/30.3.521. [DOI] [PubMed] [Google Scholar]
- 53.Etter JF. The internet and the industrial revolution in smoking cessation counselling. Drug Alcohol Rev. 2006;25 :79–84. doi: 10.1080/09595230500459545. [DOI] [PubMed] [Google Scholar]
- 54.Walters ST, Wright JA, Shegog R. A review of computer and Internet-based interventions for smoking behavior. Addict Behav. 2006;31:264–77. doi: 10.1016/j.addbeh.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 55.West R, Gilsenan A, Coste F, et al. The ATTEMPT cohort: A multi-national longitudinal study of predictors, patterns and consequences of smoking cessation; introduction and evaluation of internet recrunitment and data collection methods. Addiction. 2006;101:1352–61. doi: 10.1111/j.1360-0443.2006.01534.x. [DOI] [PubMed] [Google Scholar]