Abstract
Hyponatremia is associated with reduced survival in patients with cirrhosis awaiting orthotopic liver transplantation (OLT). However, data are sparse regarding the impact of hyponatremia on outcome following OLT. We investigated the effect of hyponatremia at the time of OLT on mortality and morbidity following the procedure. The study included 2,175 primary OLT recipients between 1990 and 2000. Serum sodium concentrations obtained immediately prior to OLT were correlated with subsequent survival using proportional hazards analysis. Morbidity associated with hyponatremia was assessed, including length of hospitalization, length of intensive care unit (ICU) admission, and occurrence of central pontine myelinolysis (CPM). Out of 2,175 subjects, 1,495 (68.7%) had normal serum sodium (>135 mEq/L) at OLT, whereas mild hyponatremia (125–134 mEq/L) was present in 615 (28.3%) and severe hyponatremia (<125 mEq/L) in 65 (3.0%). Serum sodium had no impact on survival up to 90 days after OLT (multivariate hazard ratio = 1.00, P = 0.99). Patients with severe hyponatremia tended to have a longer stay in the ICU (median = 4.5 days) and hospital (17.0 days) compared to normonatremic recipients (median ICU stay = 3.0 days, hospital stay = 14.0 days; P = 0.02 and 0.08, respectively). There were 10 subjects that developed CPM, with an overall incidence of 0.5%. Although infrequent, the incidence of CPM did correlate with serum sodium levels (P < 0.01).
Conclusion
Pre-OLT serum sodium does not have a statistically significant impact on survival following OLT. The incidence of CPM correlates with hyponatremia, although its overall incidence is low. Incorporation of serum sodium in organ allocation may not adversely affect the overall post-OLT outcome.
Impairment in body water homeostasis and hyponatremia are often seen in patients in advanced stages of endstage liver disease (ESLD).1,2 Dilutional hyponatremia results from a higher rate of renal retention of water despite increased total body sodium due to antidiuretic hormone-mediated reduction in free water clearance.3–6 Hyponatremia is often seen in cirrhotic patients with advanced portal hypertension and liver dysfunction. Hyponatremia in this setting has been correlated with increased mortality.1,7–14
Because the model for endstage liver disease (MELD) was established as a predictor of short-term mortality, serum sodium (Na) has been shown to provide additional prognostic information in patients with ESLD.14–19 Although those data suggest that incorporation of Na into the organ allocation decisions may decrease waitlist outcome, concerns may be raised that giving a high priority to hyponatremic patients may be detrimental to the overall outcome after OLT, as hyponatremic patients may be at risk of increased mortality or morbidity related to central pontine myelinolysis (CPM).18–23
In this work we evaluate the effect of recipient serum sodium on the outcome of OLT, including mortality and morbidity, the latter including the length of overall hospitalization and stay in an intensive care unit (ICU), as well as incidence of CPM.
Patients and Methods
Patients and Clinical Information
This study was based on two multicenter OLT databases. First, the National Institute of Diabetes and Digestive and Kidney Diseased Liver Transplantation Database (NIDDK-LTD) enrolled patients who underwent OLT from 1990 through 1994 at the Mayo Clinic (Rochester, MN), Baylor University (Dallas, TX), the University of California at San Francisco (San Francisco, CA), and the University of Nebraska (Omaha, NE). Second, another database (Models for Optimal Liver Transplant Outcomes, MOLTO) included OLT recipients from 1994 through 2000 at the Mayo Clinic and Baylor University. From these databases, all adult (>16 years) recipients of primary OLT since 1990 were included in this analysis. Given the focus of this investigation, patients with fulminant liver disease or malignancy including hepatocellular carcinoma were excluded.
Patient demographics, etiology of liver disease, and serum sodium were recorded immediately prior to OLT. In line with previous studies, patients were divided into three groups according to serum sodium values: normal (more than 135 mEq/L), mild hyponatremia (125–134 mEq/L), and severe hyponatremia (<125 mEq/L).2,24,25 Patients were further classified into the following diagnostic categories: alcoholic, cholestatic, viral, and other liver disease. In the event of multiple diagnoses, patients were assigned to the diagnosis that was deemed most likely to influence their prognosis (e.g., patients with hepatitis C and alcohol were classified as hepatitis C). All patients with primary biliary cirrhosis, primary sclerosing cholangitis, and other biliary diseases were assigned to the cholestatic category.
CPM was identified by being listed as either a complication or a cause of death. If available, medical records were used to verify the diagnosis.
Statistical Analyses
Following OLT, several outcome variables were evaluated, including mortality within 30 and 90 days of OLT, number of days in ICU and hospital, and incidence of CPM. Data necessary for these analyses were extracted from the databases. These included the dates of OLT, dismissal from ICU and hospital, and the last follow-up and the vital status at the time of last follow-up.
Survival was calculated by the interval between the dates of OLT and last follow-up; living patients were censored at the date of last follow-up. Patients undergoing retransplantation were censored at the time of retransplantation. The Kaplan-Meier method was used to estimate recipient survival after OLT and outcome of patients with CPM. Proportional hazards regression analysis was used to evaluate the effect of serum sodium on survival. As a patient’s pre-OLT condition primarily affects early post-OLT outcome, we focused our analysis on the outcome within the first 90 days after OLT. Covariates at the time of OLT such as age, gender, total bilirubin, creatinine, and diagnostic categories were also taken into account. Because renal physiology and sodium handling have been studied primary in noncholestatic liver disease and post-OLT outcome of patients with cholestatic liver disease is usually better than that of patients with another etiology, we also conducted a sensitivity analysis, in which patients with cholestatic liver disease were excluded.2,26–28 All analyses were performed stratifying on the transplant center.
The relationship between serum sodium and hospital and ICU days was examined by analysis of variance. Linear and logistic regression analysis was used to compare the incidence of CPM according to serum sodium. A two-tailed P-value less than 0.05 was used for statistical significance in all analyses.
Results
Patient Characteristics
There were 2,321 recipients that met the inclusion criteria, of whom serum sodium data at the time of OLT was not available in 146 patients (6.3%). Thus, this analysis is based on 2,175 patients (1,173 from the NIDDK-LTD database and 1,002 from the MOLTO database). Table 1 describes patient characteristics at the time of OLT. The mean age was 50.1 years (range, 16.1–77.5 years). The mean serum sodium concentration (±standard deviation) was 136.2 ± 5.4 mEq/L (range, 115–153 mEq/L), total bilirubin 7.2 ± 10.4 mg/dL (range, 0.2–80.0 mg/dL), serum creatinine 1.4 ± 1.2 mg/dL (0.3–11.2 mg/dL), and prothrombin time 15.2 ± 5.4 seconds (8.3–114.0 seconds). The etiology of liver disease was viral in 33.6%, cholestatic in 24.9%, alcoholic in 15.1%, and other in 26.3%.
Table 1.
Recipient Characteristics at Time of Transplantation
| Overall | Severity of Hyponatremia (mEq/L) |
P value | |||
|---|---|---|---|---|---|
| Na < 125 | 125≤ Na < 135 | Na ≥ 135 | |||
| N | 2175 | 65 (3.0%) | 615 (28.3%) | 1495 (68.7%) | |
| Serum sodium (mEq/L) | 136.2 ± 5.4 | 121.8 ± 2.3 | 130.7 ± 2.7 | 139.1 ± 3.0 | NA |
| Age (years) | 50.1 ± 10.8 | 49.6 ± 11.0 | 50.6 ± 10.6 | 50.0 ± 10.9 | 0.37 |
| % male | 55.7 | 63.1 | 59.8 | 53.7 | 0.02 |
| Total bilirubin (mg/dL) | 7.2 ± 10.4 | 9.3 ± 9.1 | 8.4 ± 11.3 | 6.6 ± 10.0 | <0.01 |
| Serum creatinine (mg/dL) | 1.4 ± 1.2 | 1.8 ± 1.3 | 1.5 ± 1.2 | 1.3 ± 1.1 | <0.01 |
| Prothrombin time(seconds) | 15.2 ± 5.3 | 16.2 ± 4.5 | 16.4 ± 7.0 | 14.7 ± 4.4 | <0.01 |
| Etiology | <0.01 | ||||
| Alcohol | 329 (15.1%) | 15 (23.1%) | 124 (20.2%) | 190 (12.7%) | |
| Cholestatic | 542 (24.9%) | 10 (15.4%) | 113 (18.4%) | 419 (28.0%) | |
| Viral | 731 (33.6%) | 21 (32.3%) | 212 (34.5%) | 498 (33.3%) | |
| Other | 573 (26.3%) | 19 (29.2%) | 166 (27.0%) | 388 (26.0%) | |
| Multiorgan | 48 (2.4%) | 0 (0%) | 14 (2.6%) | 34 (2.5%) | 0.45 |
The majority of the patients (68.7%) had normal sodium value (Na ≥ 135 mEq/L), whereas 31.3% had hyponatremia, including 3.0% with severe hyponatremia. Patients with severe hyponatremia were more predominantly male than other groups (P = 0.02). Patients with hyponatremia had higher serum total bilirubin, prothrombin time, and serum creatinine compared with normonatremic patients. The proportion of patients with alcoholic liver disease was higher in the hyponatremic group than others (P < 0.01).
Outcome After Liver Transplantation
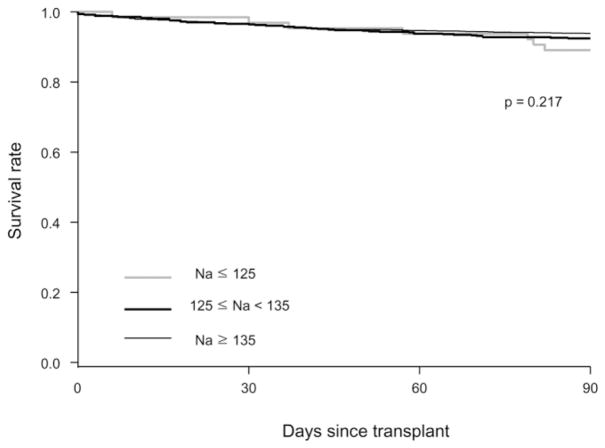
In all, 144 (6.6%) of the 2,175 subjects died within the first 90 days after OLT, including 78 (3.6%) who died within the first 30 days. Figure 1 compares survival after OLT by the serum sodium level. There was no statistically significant difference in survival among the three groups (P = 0.22), as evidenced by the extensive overlap of the curves. We also considered defining hyponatremia at a serum sodium of 130 mEq/L, in keeping with previous studies.3,22 Again, there was no difference in post-OLT survival with that cutoff value (P = 0.89).
Fig. 1.
Kaplan-Meier survival, grouped by sodium.
Table 2 describes the results of a multivariate proportional hazards analysis predicting mortality after post-OLT. In the primary analysis, patients of all diagnosis were included, whereas the sensitivity analysis excluded patients with cholestatic liver disease. In both cases, serum sodium had no impact on overall mortality. Variables that were associated with 90-day mortality included: age and total bilirubin (hazard ratio [HR] 1.04 and 1.47, respectively, P < 0.01). When included in the primary analysis, cholestatic etiology was associated with a decreased risk of mortality (HR 0.37, P < 0.01).
Table 2.
Results of a Multivariate Proportional Hazards Regression (HR) Analysis Predicting 90-Day Mortality Post-OLT
| Variable | Primary Analysis* (n=2175) |
Sensitivity Analysis† (n=1633) |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.04 | 1.03–1.06 | < 0.01 | 1.04 | 1.02–1.06 | <0.01 |
| % male | 1.02 | 0.72–1.46 | 0.91 | 1.10 | 0.75–1.61 | 0.64 |
| Serum sodium (mEq/dL) | 1.00 | 0.97–1.03 | 0.99 | 0.99 | 0.96–1.02 | 0.62 |
| Total bilirubin (mg/dL) | 1.47 | 1.22–1.77 | <0.01 | 1.51 | 1.23–1.85 | <0.01 |
| Serum creatinine (mg/dL) | 1.14 | 0.84–1.54 | 0.41 | 1.10 | 0.60–2.81 | 0.56 |
| Prothrombin time (seconds) | 1.47 | 0.71–3.06 | 0.30 | 1.29 | 0.60–2.81 | 0.51 |
| Diagnosis (reference: viral) | ||||||
| Alcohol | 0.77 | 0.47–1.26 | 0.29 | 0.78 | 0.47–1.28 | 0.32 |
| Other | 0.91 | 0.61–1.36 | 0.64 | 0.94 | 0.63–1.41 | 0.77 |
| Cholestatic | 0.37 | 0.21–0.65 | <0.01 | NA | NA | NA |
All subjects (with all diagnosis) were included in the analysis.
Subjects with cholestatic liver disease were excluded.
Table 3 summarizes morbidity outcomes following OLT by serum sodium. The median number of days in ICU (interquartile range) after OLT was 3 days (2–5 days) and the median length of hospitalization after OLT was 14 days (10–21 days). Although there was a trend toward increased length of hospital stay in patients with lower sodium, the differences did not reach statistical significance (P = 0.08). Patients with severe hyponatremia had a longer length of stay in ICU compared with patients with other groups (P = 0.02). Out of the 2,175 patients, a total of 10 developed CPM (0.5%). Of those, eight patients were hyponatremic at the time of OLT including three who were severely hyponatremic. The association between hyponatremia and CPM was statistically significant (P < 0.01).
Table 3.
Morbidity Outcome According to Serum Sodium
| Severity of Hyponatremia |
Total (n = 2175) | P value | |||
|---|---|---|---|---|---|
| Na < 125 (n = 65) | 125 ≤ Na < 135 (n = 615) | Na ≥ 135 (n = 1495) | |||
| Days in hospital (median) | 17.0 | 15.0 | 14.0 | 14.0 | 0.08 |
| Days in ICU (median) | 4.5 | 3.0 | 3.0 | 3.0 | 0.02 |
| CPM | 3 (4.6%) | 5 (0.8%) | 2 (0.1%) | 10 (0.5%) | <0.01 |
Characteristics and Outcome of Patients with CPM
Table 4 compares characteristics of patients with and without CPM. CPM was not associated with age, gender, etiology of OLT, or 30-day mortality. The presence of CPM was associated with lower serum sodium and higher serum total bilirubin and creatinine and higher prothrombin time. The lengths of hospitalization and ICU stay were higher in the CPM group. Of the 10 patients with CPM, four (40.0%) died within 3 months after transplant. The cause of death was central nervous system complication in three patients and infection in the last. CPM patients had poorer survival than those without CPM (P < 0.01).
Table 4.
Characteristics of Patients with CPM
| Characteristics | CPM Present (n = 10) | CPM Absent (n = 2165) | P value |
|---|---|---|---|
| Age (years) | 51.6 ± 13.6 | 50.1 ± 10.8 | 0.50 |
| % male | 70.0 | 55.7 | 0.36 |
| Serum sodium (mEq/L) | 128.8.1 ± 6.9 | 136.3 ± 5.4 | <0.01 |
| Total bilirubin (mg/dL) | 21.3 ± 18.4 | 7.1 ± 10.3 | <0.01 |
| Serum creatinine (mg/dL) | 1.8 ± 0.9 | 1.4 ± 1.2 | 0.06 |
| Prothrombin time (sec) | 18.3 ± 6.6 | 15.2 ± 5.3 | 0.04 |
| Etiology | 0.75 | ||
| Alcohol | 1 (10.0%) | 328 (15.2%) | |
| Cholestatic | 2 (20.0%) | 540 (24.9%) | |
| Viral | 5 (50.0%) | 726 (33.5%) | |
| Other | 2 (20.0%) | 571 (26.4%) | |
| Days of ICU (median) | 13.5 | 3.0 | <0.01 |
| Days of hospital (median) | 36.0 | 14.0 | <0.01 |
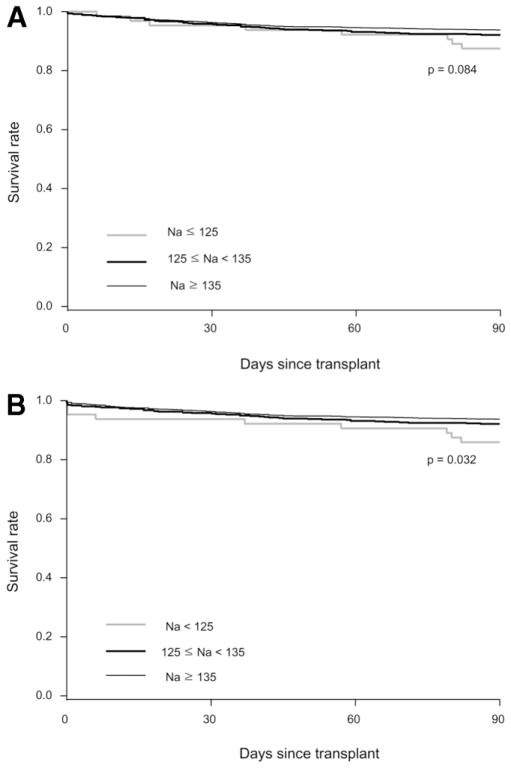
Given the devastating nature of CPM, Fig. 2 considers CPM as an outcome (equivalent to death). Figure 2A is based on the date of the diagnosis of CPM, whereas Fig. 2B assumes that CPM occurred on the day of OLT. As expected, the survival curve of the severely hyponatremic group was affected most. The shift in the survival curve in Fig. 2B resulted in a difference that reached statistical significance (P = 0.03).
Fig. 2.
(A) Kaplan-Meier survival treating CPM as equivalent to death at the time of the diagnosis. (B) Kaplan-Meier survival assuming that CPM occurs on the day of OLT.
Discussion
The main observation in this study is that, from the standpoint of survival, hyponatremia at the time of OLT did not affect the outcome afterward. As shown in Fig. 1, the survival curves completely overlapped among the groups, excluding the possibility of a type II error. On the other hand, hyponatremia may affect morbidity following OLT: Pre-OLT hyponatremia was associated with prolonged ICU stay and a trend to increased length of hospitalization. However, because hyponatremic patients had more advanced hepatic decompensation, the degree to which that effect is purely attributable to hyponatremia alone remains uncertain. Finally, we found a significant correlation between hyponatremia and the occurrence of CPM. However, the overall incidence was low, especially in patients whose hyponatremia was not severe.
It is now well established that hyponatremia is an important prognostic indicator in patients with ESLD.6,8,12 Recently, we and others have shown that serum sodium carries prognostic information independent of MELD and that the effect of Na varies depending on the MELD.14,17–19 For example, in patients with low MELD hyponatremia is a much more serious prognostic marker than in those with high MELD, in whom the mortality risk is already adequately captured by MELD.
Although hyponatremia and MELD are very helpful indicators of the risk of mortality prior to (or without) OLT, pre-OLT variables, in general, have not been found as useful in predicting post-OLT survival or complications. For example, the MELD score at the time of OLT had little impact on post-OLT survival except at the extreme high range.29,30 Although physiologic integrity prior to OLT is important for successful outcome of the procedure, random events that occur in the perioperative and postoperative periods are by definition not predictable and reduce the correlation between pre-OLT status and post-OLT outcome. Further, one may postulate this correlation to be even poorer in highly skilled transplant centers, as patient selection and quality of care at those centers may negate the tendency for a poor outcome in the sickest patients undergoing OLT. One such example may be that in our multivariate model, serum creatinine had only a small and nonsignificant effect on 90-day mortality, in contrast to previous reports that renal function is an important determinant of post-OLT survival. It may be that the adverse effects attributable to pre-OLT renal dysfunction may be overcome at experienced transplant centers.
Similarly, the lack of effect of hyponatremia on post-OLT survival shown in this analysis may be construed as just an example of the disconnect between the pre-OLT condition and post-OLT outcome. As such, this finding is in contradiction with previous reports.20,22 In a multi-center study based on a registry of 5,150 OLT recipients in the UK and Ireland, serum sodium <130 mEq/L was associated with a 55% increase in 90-day mortality after adjusting for other factors that may affect post-OLT survival.20 In another study of 241 patients from a single center in Spain, the 90-day mortality in hyponatremic (Na < 130 mEq/L, n = 19) patients was 16% compared with 5% in those with normonatremia.22 In both of these studies, as in our study, hyponatremic patients had clearly more advanced hepatic decompensation. Again, we postulate that the difference between these and our studies may be in part explained by the fact that this study was conducted at select, academic, high-volume centers. This may include a selection process in which patients who are deemed at too high a risk were not offered a transplant. We are unable to address the magnitude of the impact of this selection process, as the databases used in this study did not include patients who were denied transplantation.
Although pre-OLT hyponatremia did not have an appreciable effect on post-OLT survival, it increased post-OLT morbidity, specifically CPM. CPM has been reported to occur in 1%–2% of liver transplant recipients and is thought to be mainly related to a rapid correction of hyponatremia in the perioperative period.23,31,32 The results of the current study indicate that patients with hyponatremia along with impairment of kidney and liver function at OLT are at an increased risk of developing CPM. Development of CPM was associated with longer ICU and hospital stay, and poor survival within 3 months after OLT. In the most pessimistic analysis (shown in Fig. 2B) in which all CPM patients were considered to have died intraoperatively, serum Na < 125 mEq/L was associated with a significant decrease in survival. However, our data also show that not all severely hyponatremic patients developed CPM and not all patients with CPM had been hyponatremic. Clearly, a better understanding of the pathogenesis, risk factors, and prevention of CPM is necessary.
The authors recognize several limitations of the study. The databases used in this study were designed to track post-OLT events and their determinants and, thus, lack detailed information on pretransplant events. As stated above, we have no information about patients who were excluded from transplantation for being too sick, a feature of which may have been hyponatremia. Similarly, detailed information about pretransplant management, such as hypertonic saline infusion or use of pharmacologic agents, is not available. It may have been possible that some hyponatremic patients underwent correction of their hyponatremia and thus were classified as normonatremic at the time of OLT, which will have the effect of negating a potential difference in survival. We believe that is unlikely because severe hyponatremia that would have prompted saline administration, for example, was infrequent and misclassification of that small number of patients into the normonatremic group would have resulted in a minimal shift in the survival curve. Finally, it may be possible that the databases used in this study may not have recorded CPM cases with a minor degree, and thus the incidence of CPM was underestimated in this study. Our databases included a series of variables designated for neurologic complications, yet CPM was not a discrete variable in and of itself. We supplemented our data collection by searching through the causes of death, in which CPM was one of the variables as a specific cause. When uncertain, we reviewed available medical records for verification of the diagnosis of CPM. Thus, although we are confident that we captured all cases with clinically important CPM (i.e., those resulted in death or left with sequelae), cases with minor symptoms that resolved may have existed. Of note, the incidence in this study is not too different from a previous report (10/2,175 versus 3/344).23
In conclusion, the present study demonstrated that pretransplant hyponatremia, a strong indicator of waitlist mortality, does not affect mortality following liver transplantation. These data suggest that incorporation of serum sodium in determining organ allocation priority should not diminish survival outcome after OLT. The largest impact of pretransplant hyponatremia was seen in CPM: hyponatremic patients are at an increased, albeit low, risk of CPM. A better understanding of the pathogenesis, risk factors, and prevention of CPM in this setting is warranted.
Acknowledgments
Supported entirely by a grant from the National Institute of Diabetes, Digestive and Kidney Disease (DK-34238).
Abbreviations
- CPM
central pontine myelinolysis
- ESLD
endstage liver disease
- ICU
intensive care unit
- MELD
model of end-stage liver disease
- NIDDK-LTD
the National Institute of Diabetes and Digestive and Kidney Disease — Liver Transplantation Database
- OLT
orthotopic liver transplantation
Footnotes
Potential conflict of interest: Nothing to report.
References
- 1.Arroyo V, Rodes J, Gutierrez-Lizarraga MA, Revert L. Prognostic value of spontaneous hyponatremia in cirrhosis with ascites. Am J Dig Dis. 1976;21:249–256. doi: 10.1007/BF01095898. [DOI] [PubMed] [Google Scholar]
- 2.Angeli P, Wong F, Watson H, Gines P. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 3.Gines P, Berl T, Bernardi M, Bichet DG, Hamon G, Jimenez W, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851–864. doi: 10.1002/hep.510280337. [DOI] [PubMed] [Google Scholar]
- 4.Bichet D, Szatalowicz V, Chaimovitz C, Schrier RW. Role of vasopressin in abnormal water excretion in cirrhotic patients. Ann Intern Med. 1982;96:413–417. doi: 10.7326/0003-4819-96-4-413. [DOI] [PubMed] [Google Scholar]
- 5.Epstein M. Derangements of renal water handling in liver disease. Gastroenterology. 1985;89:1415–1425. doi: 10.1016/0016-5085(85)90664-x. [DOI] [PubMed] [Google Scholar]
- 6.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 7.Llach J, Gines P, Arroyo V, Rimola A, Tito L, Badalamenti S, et al. Prognostic value of arterial pressure, endogenous vasoactive systems, and renal function in cirrhotic patients admitted to the hospital for the treatment of ascites. Gastroenterology. 1988;94:482–487. doi: 10.1016/0016-5085(88)90441-6. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Esparrach G, Sanchez-Fueyo A, Gines P, Uriz J, Quinto L, Ventura PJ, et al. A prognostic model for predicting survival in cirrhosis with ascites. J Hepatol. 2001;34:46–52. doi: 10.1016/s0168-8278(00)00011-8. [DOI] [PubMed] [Google Scholar]
- 9.Porcel A, Diaz F, Rendon P, Macias M, Martin-Herrera L, Giron-Gonzalez JA. Dilutional hyponatremia in patients with cirrhosis and ascites. Arch Intern Med. 2002;162:323–328. doi: 10.1001/archinte.162.3.323. [DOI] [PubMed] [Google Scholar]
- 10.Borroni G, Maggi A, Sangiovanni A, Cazzaniga M, Salerno F. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis. 2000;32:605–610. doi: 10.1016/s1590-8658(00)80844-0. [DOI] [PubMed] [Google Scholar]
- 11.Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993;105:229–236. doi: 10.1016/0016-5085(93)90031-7. [DOI] [PubMed] [Google Scholar]
- 12.Cosby RL, Yee B, Schrier RW. New classification with prognostic value in cirrhotic patients. Miner Electrolyte Metab. 1989;15:261–266. [PubMed] [Google Scholar]
- 13.Shear L, Kleinerman J, Gabuzda GJ. Renal failure in patients with cirrhosis of the liver. I. Clinical and pathologic characteristics. Am J Med. 1965;39:184–198. doi: 10.1016/0002-9343(65)90041-0. [DOI] [PubMed] [Google Scholar]
- 14.Biggins SW, Kim WR, Terrault NA, Saab S, Balan V, Schiano T, et al. Evidence-based incorporation of serum sodium concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 15.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 16.Wiesner R, Edwards E, Freeman R, Harper A, Kim R, Kamath P, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124:91–96. doi: 10.1053/gast.2003.50016. [DOI] [PubMed] [Google Scholar]
- 17.Heuman DM, Abou-Assi SG, Habib A, Williams LM, Stravitz RT, Sanyal AJ, et al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology. 2004;40:802–810. doi: 10.1002/hep.20405. [DOI] [PubMed] [Google Scholar]
- 18.Biggins SW, Rodriguez HJ, Bacchetti P, Bass NM, Roberts JP, Terrault NA. Serum sodium predicts mortality in patients listed for liver transplantation. Hepatology. 2005;41:32–39. doi: 10.1002/hep.20517. [DOI] [PubMed] [Google Scholar]
- 19.Ruf AE, Kremers WK, Chavez LL, Descalzi VI, Podesta LG, Villamil FG. Addition of serum sodium into the MELD score predicts waiting list mortality better than MELD alone. Liver Transpl. 2005;11:336–343. doi: 10.1002/lt.20329. [DOI] [PubMed] [Google Scholar]
- 20.Dawwas MF, Lewsey JD, Neuberger JM, Gimson AE. The impact of serum sodium concentration on mortality after liver transplantation: a cohort multicenter study. Liver Transpl. 2007;13:1115–1124. doi: 10.1002/lt.21154. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Liang TB, Zheng SS, Shen Y, Wang WL, Ke QH. The possible causes of central pontine myelinolysis after liver transplantation [in Chinese] Zhonghua Wai Ke Za Zhi. 2004;42:1048–1051. [PubMed] [Google Scholar]
- 22.Londono MC, Guevara M, Rimola A, Navasa M, Taura P, Mas A, et al. Hyponatremia impairs early posttransplantation outcome in patients with cirrhosis undergoing liver transplantation. Gastroenterology. 2006;130:1135–1143. doi: 10.1053/j.gastro.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Abbasoglu O, Goldstein RM, Vodapally MS, Jennings LW, Levy MF, Husberg BS, et al. Liver transplantation in hyponatremic patients with emphasis on central pontine myelinolysis. Clin Transplant. 1998;12:263–269. [PubMed] [Google Scholar]
- 24.Adrogue HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342:1581–1589. doi: 10.1056/NEJM200005253422107. [DOI] [PubMed] [Google Scholar]
- 25.Hoorn EJ, Lindemans J, Zietse R. Acute and concomitant deterioration of hyponatremia and renal dysfunction associated with heart and liver failure. Clin Nephrol. 2006;65:248–255. doi: 10.5414/cnp65248. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez E, Rimola A, Navasa M, Andreu H, Grande L, Garcia-Valdecasas JC, et al. Liver transplantation in patients with non-biliary cirrhosis: prognostic value of preoperative factors. J Hepatol. 1998;28:320–328. doi: 10.1016/0168-8278(88)80020-5. [DOI] [PubMed] [Google Scholar]
- 27.Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886–897. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 28.Esteva-Font C, Baccaro ME, Fernandez-Llama P, Sans L, Guevara M, Ars E, et al. Aquaporin-1 and aquaporin-2 urinary excretion in cirrhosis: relationship with ascites and hepatorenal syndrome. Hepatology. 2006;44:1555–1563. doi: 10.1002/hep.21414. [DOI] [PubMed] [Google Scholar]
- 29.Desai NM, Mange KC, Crawford MD, Abt PL, Frank AM, Markmann JW, et al. Predicting outcome after liver transplantation: utility of the model for end-stage liver disease and a newly derived discrimination function. Transplantation. 2004;77:99–106. doi: 10.1097/01.TP.0000101009.91516.FC. [DOI] [PubMed] [Google Scholar]
- 30.Ioannou GN. Development and validation of a model predicting graft survival after liver transplantation. Liver Transpl. 2006;12:1594–1606. doi: 10.1002/lt.20764. [DOI] [PubMed] [Google Scholar]
- 31.Wszolek ZK, McComb RD, Pfeiffer RF, Steg RE, Wood RP, Shaw BW, Jr, et al. Pontine and extrapontine myelinolysis following liver transplantation. Relationship to serum sodium. Transplantation. 1989;48:1006–1012. doi: 10.1097/00007890-198912000-00023. [DOI] [PubMed] [Google Scholar]
- 32.Pujol A, Graus F, Rimola A, Beltran J, Garcia-Valdecasas JC, Navasa M, et al. Predictive factors of in-hospital CNS complications following liver transplantation. Neurology. 1994;44:1226–1230. doi: 10.1212/wnl.44.7.1226. [DOI] [PubMed] [Google Scholar]