Abstract
Epidemiologic studies suggest a correlation between fat consumption and the risk of developing prostate cancer (PCa). In previous studies, we reported that decreasing dietary fat intake slowed growth of human prostate cancer xenografts in SCID mice and modulated the circulating IGF-axis. In this report, we evaluated if altering dietary fat impacts on prostate cancer development by utilizing the Hi-Myc mouse transgenic prostate cancer model. Hi-Myc mice develop murine prostatic intraepithelial neoplasia (PIN) at as early as 2-4 weeks and invasive adenocarcinoma between 6 to 9 months due to the over-expression of human c-Myc in the mouse prostate. Three-week old male Hi-Myc mice were placed on high-fat (42% Kcal) or low-fat (12% Kcal) diets and equal caloric intake was maintained until euthanasia at 7 months. Blood was collected during the PIN phase (4-months) and at euthanasia. The number of mice that developed invasive adenocarcinoma at 7 months was 27 % less in the low-fat diet group (12/28) compared to the high-fat diet group (23/33, p=0.04). Epithelial cells in PIN lesions in the LF group had a significantly lower proliferative index compared to epithelial cells in the HF group (21.7 % vs. 28.9 %, p<0.05). During the PIN phase of carcinogenesis (4 months), the LF group had higher serum IGFBP-1 levels (19.46 ± 6.78 ng/ ml vs. 3.61 ± 0.70; p=0.01) relative to the HF group. Serum from mice in the LF group had decreased mitogenic effects in vitro on LNCaP and Myc-Cap cells as compared to serum from the HF group. Akt (Ser 473) phosphorylation, Akt kinase activity, and phosphorylation of downstream targets of Akt in prostates were significantly reduced in the LF diet group compared to the HF group.
We conclude that dietary fat reduction delays transition from PIN to invasive cancer in this Myc-driven transgenic mouse model, possibly through suppression of the IGF-Akt pathway and decreased proliferation of PIN epithelial cells. These findings support utilizing dietary fat reduction in future chemoprevention protocols.
Introduction
Epidemiologic studies suggest that environmental factors associated with Western culture may promote the development of clinical prostate cancer. One such factor that has been implicated is dietary fat. A number of case-controlled and cohort studies found that increased intake of dietary fat was associated with a higher risk of developing prostate cancer (1-3), whereas other studies showed no association between dietary fat intake and prostate cancer risk (4, 5). The mechanisms through which dietary fat may impact on the development of prostate cancer remain to be defined and include effects on the insulin-like growth factor (IGF) system, sex hormone metabolism, free radical damage, and fatty acid metabolic pathways (6). Whereas animal feeding studies have demonstrated dietary fat reduction slowed prostate cancer xenograft growth, few studies have evaluated the role of dietary fat in prostate cancer development (7, 8). Reduction of dietary fat intake lowered the incidence of prostate cancer in two rat models of prostate cancer, but these studies did not incorporate controlled feeding protocols to ensure equal caloric intake between feeding groups (9, 10).
Epidemiologic studies have demonstrated that lower serum IGF-1 levels and increased IGF binding protein-3 (IGFBP-3) levels are associated with decreased prostate cancer risk, but no link has yet been established between serum IGFBP-1 and prostate cancer risk(11). There is a paucity of clinical studies evaluating IGFBP-1 and prostate cancer risk, in part due to the fact that IGFBP-1 is nutritionally regulated and serum samples must be obtained in the fasting state. In human studies, dietary fat reduction combined with exercise was associated with increased serum IGFBP-1 and decreased IGF-1 levels and was causally linked to decreased mitogenic effects of human serum on LNCaP cells (12). Likewise, in controlled feeding studies in mice, fat reduction was associated with increased IGFBP-1 levels, decreased IGF-1 levels and decreased prostate cancer xenograft growth (8). To date however, no controlled-feeding studies have evaluated the effects of dietary fat intake on prostate cancer prevention and IGF-axis parameters. To study the chemo-preventive role of dietary fat reduction in prostate cancer, we utilized a transgenic mouse model that over-expresses the human c-myc oncogene in a prostate-specific manner from the ARR2/probasin promoter (13). These transgenic mice (Hi-Myc mice) develop PIN as early as 2-4 weeks of age and invasive adenocarcinoma of the prostate between 6 and 9-months. The Myc-mouse prostate gene expression signatures share features seen in human prostate cancer development and progression (13). The primary aim of the present study was to evaluate the calorie-independent effects of dietary fat reduction on chemoprevention of prostate cancer.
Materials and Methods
Animal husbandry and feeding protocol
The experimental protocol was approved by the UCLA Chancellor's Animal Research Committee, and the animals were cared for in accordance with Institutional guidelines. The transgenic mice used in this study (Hi-Myc-mice) in which the prostate specific expression of human c-Myc is driven by the rat probasin promoter with two androgen response elements, were a generous gift from Katharine Ellwood-Yen and Charles L. Sawyers (13). Mice were weaned at 21 days post birth, randomly assigned to the low-fat (LF) or the high-fat (HF) diet prepared by DYETS, Inc. (Bethelheim, PA), and placed one mouse per cage to control and accurately monitor caloric intake. The compositions of the diets are listed in the supplemental Table 1. Initially a palatability study with ad libidum intake demonstrated that the LF group consumed fewer calories than the HF group. Thus, the LF mice were fed ad libidum throughout the experiment, and the average daily caloric intake of the LF group was determined once per week. The HF group was given HF food to match the average daily caloric intake of the LF group three times per week (Monday, Wednesday, and Friday). This modified pair feeding technique has provided equal caloric intake in previous isocaloric feeding studies (8). Body weight was measured weekly.
Blood and Prostate Tissue Collection
Blood was collected from the retro-orbital vein in all mice at four-months of age after a 14 hour fast. The serum was separated and frozen at -80 °C. All mice were euthanized at seven-months (after a 14 hour fast). Blood was collected, and urogenital organs were harvested en block, immediately rinsed and placed in ice-cold PBS. Using a dissecting microscope, the ventral, dorsal, lateral, and anterior prostate lobes were dissected from one side and frozen in liquid nitrogen. The remainder of each prostate was fixed in 10 % buffered formalin for 12 hours, washed in running water, and transferred to 50 % ethanol before embedding in paraffin-blocks.
Pathology and Immunohistochemistry
Sections (4 micron) were obtained from the paraffin-embedded blocks and stained with hematoxylin and eosin (H & E). Histopathological analysis to determine presence or absence of PIN, and presence of invasive adenocarcinoma was performed in a blinded fashion by a single pathologist (JS). Laminin and α–smooth muscle actin immunostaining were used to define invasion through the basement membrane and fibromuscular layer. Ki-67 immunostaining was performed as previously described (14). A total of 400 cells, one hundred cells per field, were counted for each mouse and the number of Ki-67 positive cells was scored by a single pathologist. TUNEL (Terminal nucleotidyl transferase-mediated nick end labeling) assays were performed as described in the Apop Tag Peroxidase In Situ Apoptosis Detection Kit from Chemicon (Temecula, CA). A total of eight hundred cells were counted from four fields for each mouse and the number of positive nuclei was scored as apoptosis. The images were acquired with an Olympus BX50 microscope with UplanFI objectives using Metamorph software.
Ex Vivo Bioassay
LNCaP cells were obtained from American Type Culture Collection (Manassa, VA) and grown in RPMI medium without phenol red (Omega Scientific, Tarzana., CA) supplemented with 10% fetal bovine serum (FBS), 100 IU penicillin, 100 μg/ml streptomycin, and 4mM L-glutamine (Omega Scientific, Tarzana, CA). Myc-CaP cells were a generous gift of Phil Watson and Katharine Ellwood-Yen and grown in DMEM supplemented with 10 % FBS (Omega Scientific, Tarzana, CA). LNCaP and Myc-Cap cultures were maintained at 37°C and supplemented with 5% CO2 in a humidified incubator. The mitogenic effect of mouse serum on LNCaP and Myc-CaP proliferation was studied using an in-house bioassay. The cells were plated at 5 ×103 cells/well in 96-well plate and incubated for 24 hours before changing to fresh media containing 10 % mouse serum or 10 % FBS. Each serum sample from individual mouse was tested in triplicate. The cell proliferation in media containing mouse serum was measured by using the CellTiter 96 AQueous One solution Cell Proliferation Assay (Promega Corporation, Madison, WI) as previously described (8, 14) after 48 hour incubation for LNCaP and 24 hour for Myc-CaP at 37 °C. The inter-assay and intra-assay coefficient of variation for the Ex Vivo Bioassay is 6.95 and 2.98 respectively.
Measurement of mouse serum IGF-1 and IGFBPs
The levels of murine IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 were measured using in-house mouse-specific enzyme-linked immunoassays (ELISA) that have been described previously (15, 16) employing mouse specific antibodies and recombinant mouse IGF and IGFBP standards. The mIGF-I assay has a sensitivity of 0.1 ng/ml and no cross reactivity with mIGF-II or hIGF-I. The intra-assay and inter-assay coefficient of variations were <10% in the range from 1 to 10 ng/ml. The mouse IGFBP-1, IGFBP-2 and IGFBP-3 assays have sensitivities of 0.2 ng/ml and no cross reactivity with other IGFBPs or the human homologues. The intra-assay and inter-assay coefficient of variations were < 6% and <8%, respectively, in the range from 1 to 6 ng/ml.
In vitro Akt kinase assay
Frozen mouse prostate tissues (ventral, dorsal, and lateral lobes) were homogenized in Cell Lysis buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1mM EDTA, 1mM EGTA, 1 % Triton, 2.5 mM sodium pyrophosphate, 1 mM β-Glycerophosphate, 1 mM Na3VO4, 1 μg/ml Leupeptin] with 1 μM PMSF followed by 20 second sonication (50 % power) on ice. The protein concentration of the cleared lysate was determined with the BCA protein assay kit (Pierce, Rockfork, IL) and adjusted to be the same in all samples. This lysate was used for immunoprecipitation with immobilized Akt monoclonal antibody supplied in the Akt Kinase Assay kit from Cell Signaling Technology (Danvers, MA). The Akt-kinase assay was performed according to the manufacture's protocol using GSK-3 Fusion Protein as a substrate and measuring the phosphorylation of GSK-3 by Western blotting with phospho-GSK-3 α/β (Ser21/9) antibody.
Western blot analysis
Mouse prostate lysate (50 μg) was subjected to SDS-PAGE followed by Western blot analysis and densitomeric quantification as previously described (14). Antibodies for Akt, phospho-Akt (Ser473), p70 S6 kinase, phospho-p70 S6 kinase (Thr389), phospho-p70 S6 kinase (Thr421/Ser424), and phospho-GSK-3 α/β (Ser21/9) were from Cell Signaling Technology (Danvers, MA) and used at 1:1000 dilution. The secondary horseradish peroxidase-linked antibody was used at 1:2000 dilution.
Statistical Analysis
Quantitative measures were compared between the groups (LF vs. HF) using the two sample t-test calculated by Prism 3.0 software. The data is presented as mean ± SEM. The proportions of developing invasive cancer were compared between the diet groups with Fisher's exact test. The p-values less than 0.05 were considered significant.
Results
Slower Transition from PIN to Cancer with Dietary Fat Reduction
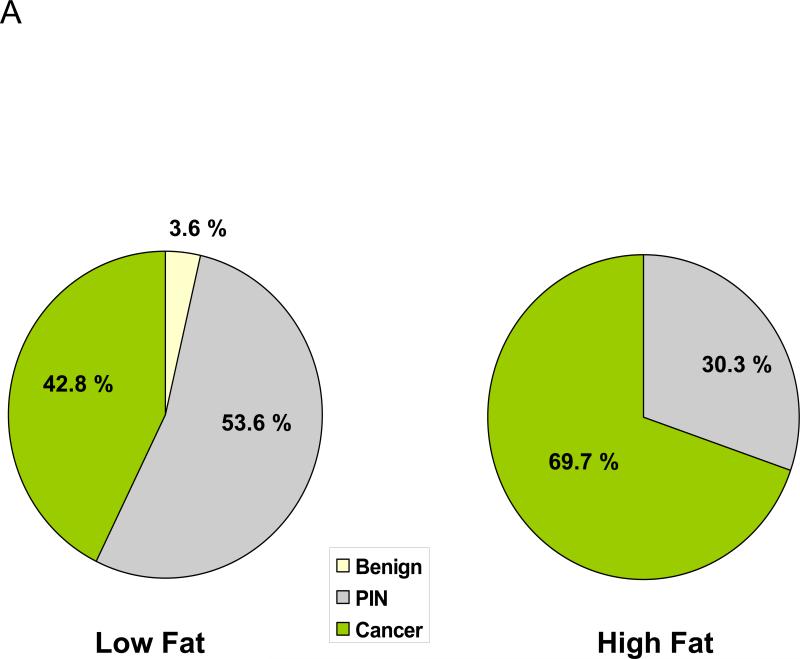
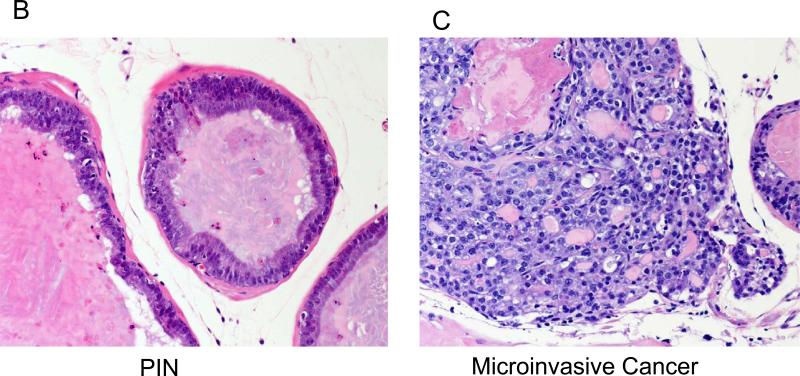
Throughout the 7-month study there was no difference in caloric intakes or mean body weights between the LF and HF diet groups (supplemental Figure 1). Twenty-seven percent fewer mice in the LF group developed invasive prostate cancer by 7-months as compared to the HF group (12/28, 42.8 % vs. 23/33, 69.7%, p<0.05), (Fig. 1A). Conversely, the number of mice with PIN at 7 months was higher in the LF group relative to the HF group (15/28, 53.6 % vs. 10/33, 30.3%, p<0.05), (Figure 1A). The histological appearance of PIN and invasive prostate cancer are demonstrated in Figures 1B & C.
Figure 1. Effect of dietary fat reduction on transition from PIN to cancer in Hi-Hi-Myc-mouse prostate.
A: Reduced incidence of prostate cancer in LF diet group compared to the HF diet group. The prostates dissected from 7 month old Hi-Myc mice were analyzed in H&E stained histologic sections at two different depths by a pathologist blinded to the treatment groups. N=28 for LF group and N=33 for HF group. P=0.042 calculated by the Fisher exact test.
B: Prostate epithelium with high grade prostatic intraepithelial neoplasia (PIN) characterized by tufted epithelial cells with prominent nucleoli.
C: Invasive prostate cancer infiltrating into the adjacent fibroadipose tissue.
Reduced Prostate Cell Proliferation in LF Diet Group PIN
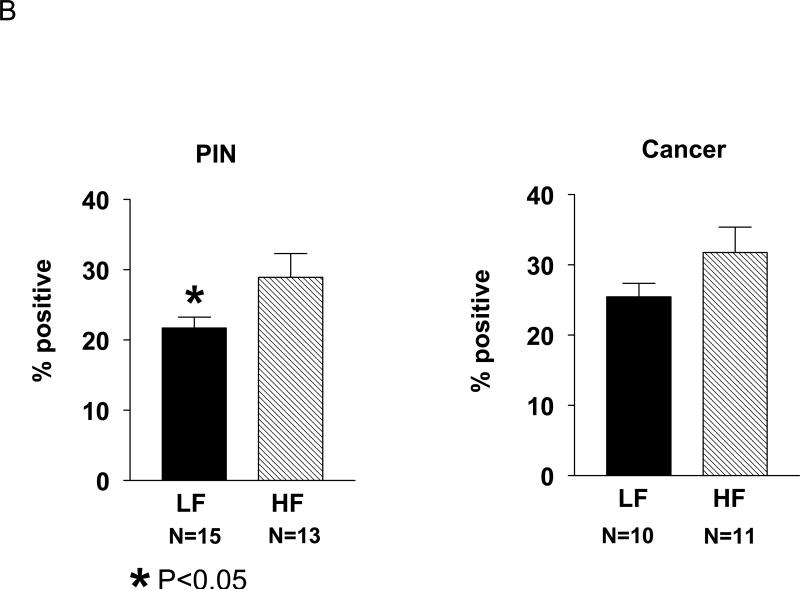
Given that a central mechanism in Myc-induced conversion from PIN to invasive prostate cancer may involve cell proliferation, we investigated whether dietary fat modification affected Hi-Myc-mouse prostate epithelial cell proliferation by Ki-67 immunostaining (Fig. 2). Epithelial cells in PIN lesions in the LF group had a significantly lower proliferative index compared to epithelial cells in the HF group (21.7 % vs. 28.9 %, p<0.05; Fig. 2B). Likewise, the proliferative index of invasive cancer epithelial cells was also lower in the LF group than the HF group (25.4% vs. 31.8%, p=0.07), though the results did not reach statistical significance.
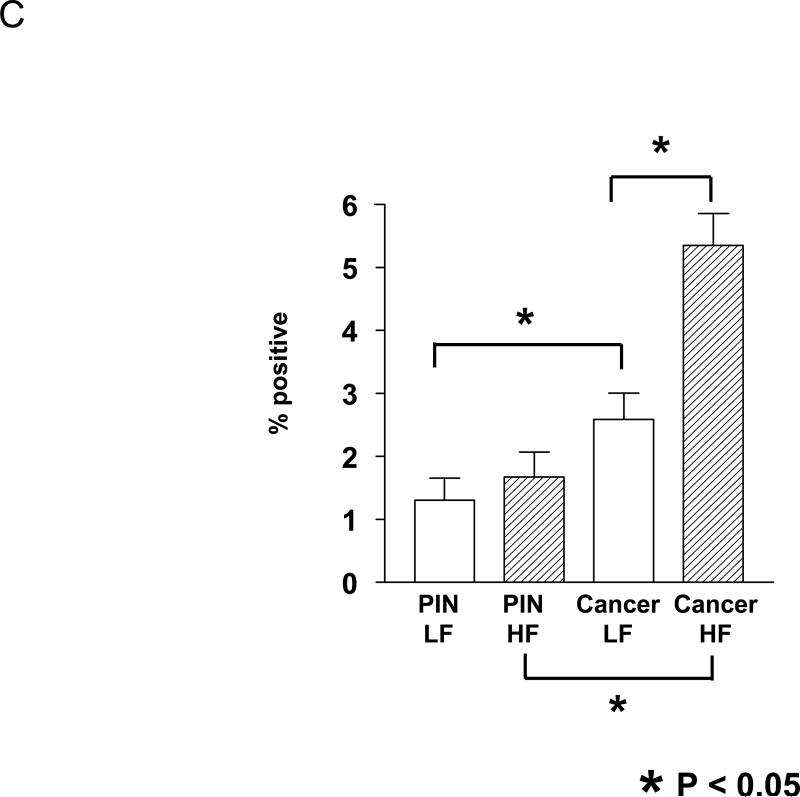
Figure 2. Effect of dietary fat content on Hi-Myc-mouse prostate cell proliferation.
A. Ki-67 immunostaining of PIN and cancer lesions of mouse prostates from the LF and HF groups.
B. Ki-67 immunohistochemistry demonstrated decreased cell proliferation for the LF group in both PIN and cancer lesions. To determine the Ki67 score, one hundred cells were counted in four separate fields per sample, and the average count expressed as a percentage.
C. The percentage of cells with positively stained nuclei by TUNEL assays. A total of eight hundred cells were counted from four fields for each sample and the positive nuclei was scored as apoptosis. *; P<0.05
The percent of apoptotic cells (by TUNEL staining) in PIN lesions was similar between the LF and the HF diet groups (1.3 % vs 1.7 %; Fig. 2C). However, in the cancer lesions the apoptotic index was lower in the LF diet group relative to HF group (2.6 % vs. 5.4 %, p=0.0005; Fig. 2C). Prostate cancer lesions had higher proliferation and apoptosis levels relative to PIN lesions in both diet groups.
Reduced Mitogenicity of LF Group Serum
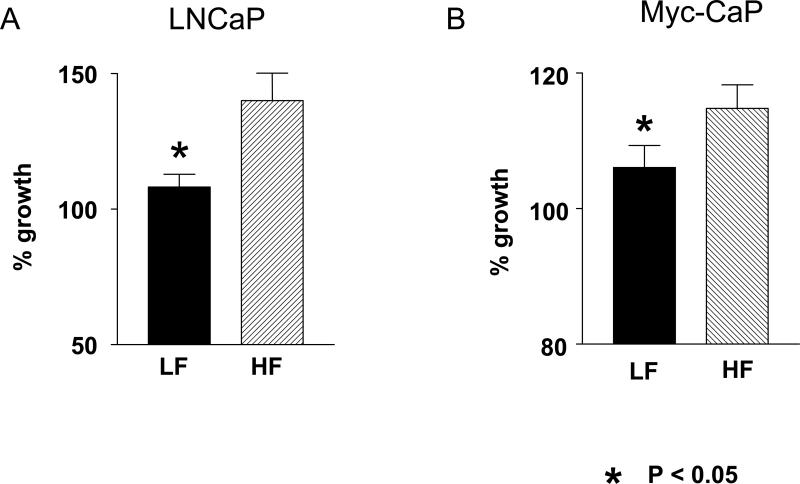
The mitogenicity of LF and HF mouse serum was evaluated by measuring the cell growth of androgen-dependent cell lines, LNCaP and Myc-mouse derived Myc-CAP (17), in media containing 10 % mouse serum from 7 month old Hi-Myc mice. LNCaP and Myc-CaP cell lines had significantly lower proliferation in media containing the LF serum compared to the growth in the media containing the HF serum (32 % and 8 % reduction respectively, p<0.05, Figure 3A and B).
Figure 3. LNCaP and Myc-CaP proliferation in media containing HF and LF serum.
A. LNCaP cells had less proliferation in media containing the LF group mouse serum compared to the cells grown in media containing the HF group serum from Hi-Myc mice. The cell proliferation was measured after 48 hour incubation in the media containing 10 % mouse serum. N=8 for each diet group.
B. Myc-CaP cells grown in media containing 10 % Myc-mouse serum had less proliferation with LF group serum compared to the serum from HF diet group after 24 hour incubation. N= 5 for each group
All experiments were performed in triplicate with the serum from individual mice (not pooled). Data is expressed as a percentage of the cell growth in media containing 10 % FBS. Values are means ± SEM. *P<0.05. Proliferation was determined by measuring colorimetric changes of MTS tetrazolium compound into colored formazan product by NADPH or NADH produced by active cells.
Increased Serum IGFBP-1 in LF Group During the PIN Phase of Cancer Development
To determine if dietary fat affects serum levels of IGF-1 and IGFBP's during the PIN and invasive cancer phases of carcinogenesis, mouse IGF-axis levels were measured at 4-months (PIN phase) and 7 months of age (Table 1). Hi-Myc mice in the LF group had significantly higher fasting serum IGFBP-1 levels relative to the HF group at 4 months (21.0 ± 8.9 ng/ ml vs. 3.2 ± 0.8; p=0.01) and at 7 months (6.2 ± 1.0 vs. 2.0 ± 0.5 ng/ml; p=0.002; Table 1). Fasting serum IGF-1 levels were lower in the LF group relative to the HF group at 4 months (290.2± 35.0 ng/ml vs. 322.8± 33.0 ng/ml; p=0.19) although the differences were not statistically significant. Fasting serum, IGFBP-2, IGFBP-3 and IGF-1 levels were similar in the LF and HF groups at 7 months of age.
Table 1.
Fasting serum IGF-1, IGFBP-1, BP-2, BP-3 concentrations of Hi-Myc mice on LF or HF diet.
| age | (ng/ ml) | LF | HF | P-value |
|---|---|---|---|---|
| 4 mo | mIGF-I | 290.2±35.0 (n=6) | 322.8±33.0 (n=6) | 0.188 |
| 4 mo | mBP1 | 21.0 ± 8.9 (n=7) | 3.24 ± 0.75 (n=8) | *0.027 |
| 7 mo | mIGF-I | 199.8±17.4 (n=8) | 204.8±10.4 (n=8) | 0.404 |
| 7 mo | mBP1 | 6.2±1.0 (n=8) | 2.0±0.5 (n=8) | *0.001 |
| 7 mo | mBP2 | 245.3±26.1 (n-8) | 211.0±32.7 (n=8) | 0.214 |
| 7 mo | mBP3 | 321.8±23.3 (n=8) | 322.9±24.1 (n=8) | 0.487 |
The average values are presented with ± SEM.
n indicates sample size.
indicates statistically significant difference between the two diet groups by student t-test
Down-regulation of Akt Pathway in LF Group Prostate Tissue
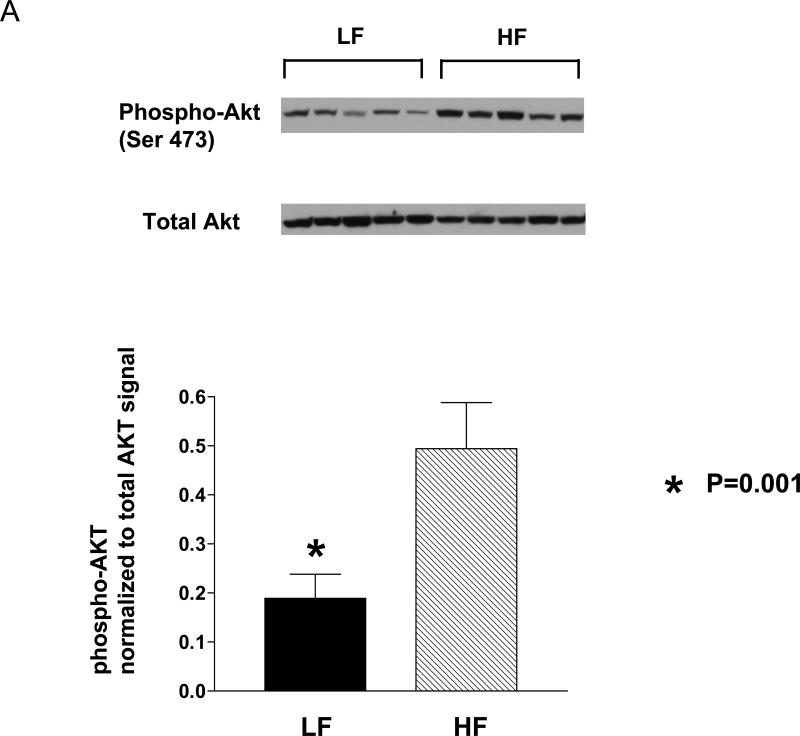
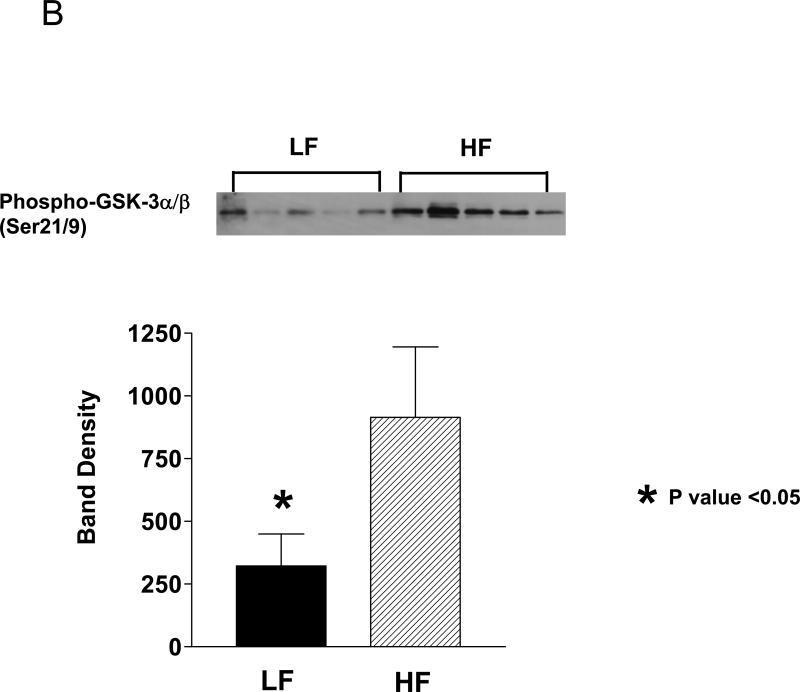
Binding of growth factors such as IGF-1 to their tyrosine kinase receptors leads to autophosphorylation of the receptors and activation of the serine-threonine Akt kinase (protein kinase B) via PI3 kinase. Western blot analyses of the mouse prostate protein showed that the LF group tissue contained 62 % less phospho-Akt (Ser 473) relative to the HF group (p=0.016; Fig 4A). Likewise, there was a 65% reduction in Akt kinase activity in the LF group prostate relative to the HF group as measured by an in vitro kinase assay (P<0.05; Fig.4C). Phosphorylation of downstream targets of Akt were also reduced in the LF group prostate relative to the HF group with a 58% reduction in GSK-3 α/β (Ser 21/9) phosphorylation (p<0.05) and a 90% reduction in p70S6K (Thr 389/421/424) phosphorylation (p<0.05; Figure 5).
Figure 4. Phospho-Akt levels and Akt kinase activity in HF and LF Hi-Myc-mouse prostate tissue.
A. Western Blot analysis of prostate lysate showed marked decrease of phospho-Akt (Ser 473) for the LF group compared to the HF group. The bands on the Western blots were scanned by a densitometer and the phospho-Akt signal was normalized to total Akt bands. N=6 mice for each diet group.
B. in vitro Akt kinase assay. Total Akt was immunoprecipitated from mouse prostate lysate and tested for kinase activity using GSK-3 subunit as a substrate. The resulted phospho-GSK-3 was detected by Western blot. The bands on the Western blots were densitometrically quantitated *; P<0.05. N= 5 for each group.
Figure 5. LF diet prostate contained decreased levels of phosphorylated Akt downstream targets.
A. Western blot analysis of Hi-Myc mouse prostate homogenate for phospho-p70S6K (Thr 389/421/424) and phospho-GSK-3(Ser 21/9).
B. The bands on the Western blots were densitometrically quantitated. The phospho-p70S6K bands were normalized to total p70S6K and phospho-GSK-3 was normalized to β-actin and shown as a histogram. *; P<0.05. N= 5 for each group.
Discussion
In the present study, dietary fat reduction resulted in increased serum IGFBP-1 levels, down-regulation of the Akt-mTOR pathway, and delayed development of invasive murine prostate cancer. In previous studies, we reported that decreasing dietary fat intake slowed growth of the human prostate cancer xenografts in SCID mice and modulated the circulating IGF-axis (8). Xenograft models of prostate cancer are derived from late-stage or metastatic samples of human PCa. Transgenic mouse models are more appropriate to study the efficacy of chemopreventive agents and mechanisms related to early tumor development. Hi-Myc transgenic mice represent a suitable model for human prostate cancer development. Myc gene amplification and overexpression are seen in approximately 30 % of human prostate cancer (18). As well, Hi-Myc mice do not develop neuroendocrine features seen in mouse PCa carcinogenesis driven by the SV40 T-antigen as in TRAMP and LADY mice (19, 20)}.
Mice were housed one mouse per cage and equal caloric intake was maintained throughout the study resulting in similar body weights between the LF and HF diet groups. Whereas two prior feeding studies using rat models of prostate cancer demonstrated dietary fat reduction lowered the incidence of prostate cancer, the animals in these studies were fed ad libidum, and therefore dietary fat intake could not be differentiated from caloric intake (7, 10). In these prior studies, animals in the high fat groups had higher caloric intake and increased body weight. Whereas caloric restriction and caloric excess are known to impact on cancer progression, our study excluded these variables and more clearly isolated out the effect of dietary fat modification on the development of prostate cancer.
IGF-1 is a known mitogen to prostate cancer cells, and epidemiological studies have linked elevated serum levels of IGF-1 in young adulthood with an increased incidence of clinical prostate cancer(21-23). As well, a recent a case-controlled study found a positive association between serum IGF-1 levels at the time of prostate biopsy and the risk of PIN (24). Reduction in IGFBP-1 levels have also been causally linked with prostate cancer progression in experimental systems, although this link has not been established in epidemiologic studies, possibly due to the fact that IGFBP-1 is nutritionally regulated and a previously mentioned epidemiologic study may have lacked sufficient fasting serum specimens for analyses. The present study found that the LF group had increased IGFBP-1 levels (relative to the HF group) at 4-months of age during the pre-malignant stage of tumor development. In addition, the LF mouse serum had reduced mitogenicity on LNCaP and Myc-CaP cells in vitro as compared to the HF serum. Likewise, relative to the HF group, epithelial cells of PIN lesions in the LF group had reduced proliferation as shown by Ki-67 immunostaining. Taken together, these data suggest that dietary fat reduction may attenuate proliferation of prostate epithelial cells in PIN lesions via mechanisms involving the IGF-axis, and ultimately delay the progression to invasive prostate cancer. Given that serum IGFBP-1 levels are modifiable by dietary changes and may affect bioavailable IGF-1, serum levels of IGFBP-1 may potentially serve as a tool for achieving and monitoring therapeutic and prevention goals. Dietary recommendations for the purpose of cancer prevention would be dramatically improved if a serum marker could be followed to assess patient compliance and responsiveness to diet intervention, much like the role of LDL cholesterol for prevention of coronary disease. Future large scale clinical studies will determine to what degree fasting serum IGFBP-1 may serve in such a role.
The LF Myc-mouse prostate tissue contained lower levels of phospho-Akt and Akt kinase activity as compared to the HF group. Overexpression of Akt in the prostate was previously shown to promote PIN in transgenic mice (25). Also, Akt1 deficiency markedly inhibited development of PIN in PTEN +/- mice (26). Studies with human prostate cancer specimens demonstrated increased immunohistochemical staining of Akt (p-S473) in malignant and PIN cells relative to benign epithelial cells, and staining intensity positively correlated with PSA levels, Gleason grade, and biochemical recurrence after radical prostatectomy (27-29)review and references within). Akt promotes cell growth and survival by affecting the activity of various factors such as GSK3, mTOR (TSC2), BAD and FOXO. We report that the LF Myc mouse prostate tissue contained lower levels of phopho-GSK3 and phospho-p70S6k than the HF prostates. P70S6k is a downstream target of mTOR and regulates cap- dependent protein synthesis. Thus reduction of dietary fat may inhibit the transition from PIN to invasive cancer by affecting cell proliferation via the Akt-mTOR pathway.
A number of factors that are implicated in c-Myc mediated transformation are regulated by downstream targets of Akt, and therefore may be affected by dietary fat modification. For example, hypophosphorylation of 4EBP-1 by Akt and mTOR leads to the release of eIF4E (mRNA-cap-binding protein) that is rate-limiting for G1 progression and a target of c-Myc (30). Also Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO protein(31). Activated Akt phosphorylates FoxO proteins resulting in exclusion of FoxO proteins from nuclei and subsequent degradation. In a non-phosphorylated state, FoxO factors inhibit induction of multiple Myc target genes and Myc-induced cell proliferation (31-33). The genes down regulated by FoxO include cyclin D1, of which coexpression with Ki-67 has been reported (34-36).
Myc not only promotes cell proliferation but also sensitizes cells to various apoptotic signals such as hypoxia, DNA damage, glucose starvation, inhibition of translation and transcription, heat shock, and chemotoxin (37, 38). The apoptotic index increases as tumors progress from PIN to invasive cancer in Myc mice (13), in other animal models of prostate cancer (39), and in human prostate cancer (34, 40) In the present report there was no difference in the apoptotic index in PIN lesions in the LF and HF group suggesting dietary fat does not significantly impact on apoptosis during the PIN phase of prostate carcinogenesis. However, of interest, prostate cancer lesions in the LF group had a lower apoptotic index relative to the cancer in the HF group. This finding was the opposite of what we expected to see as anticancer therapies generally are associated with increased apoptosis. As well, prior xenograft and human studies demonstrated decreased apoptosis of prostate cancer cells in response to dietary fat reduction (8). Data in the literature is conflicting with regards to the biologic significance of apoptosis in prostate cancer. A prior retrospective clinical study found that higher apoptotic activity in prostate cancer positively correlated with higher proliferation rates, positive surgical margins, and increased risk of death from prostate cancer (34). Similar observations were made in rat models (39). Further studies are required to determine the mechanism through which fat reduction resulted in lower rates of apoptosis in prostate cancer in the Hi-Myc mouse model.
The fat used in the diets in the present study was from corn oil, which is primarily composed of linoleic acid, an omega-6 polyunsaturated fatty acid. Membrane arachidonic acid (omega-6) derived from linoleic acid is converted by the cyclooxygenase and lipoxygenase pathways to eicosanoids such as prostaglandin E2, leukotrienes, and hydroxyl derivatives of fatty acids. These eicosanoids have been implicated in the pathogenesis of cancer and are believed to play important roles in tumor promotion, progression, and metastasis (41, 42). Whereas the present study focused on the preventative effects of lowering dietary fat in the form of corn oil on the IGF-1-Akt pathway, other pathways may also play a role in the preventive effects of dietary fat modification such as the ratio of omega-6 and omega-3 fatty acids (43). Likewise, further studies should also address if other forms of dietary fats such as monounsaturated and saturated fats impact on the development of prostate cancer.
In summary, our study found that lowering dietary fat intake resulted in; i) higher serum IGFBP-1 and reduced serum mitogenicity; ii) lower proliferation index of prostate epithelial cells in PIN and invasive prostate cancer, iii) lower phospho-Akt protein, Akt kinase activity, and lower levels of phosphorylated GSK3 and p70S6k in murine prostate; and iv) reduced transition from PIN to cancer. Taken together, these studies establish a clear link between dietary fat intake and prostate cancer development, and support the conduct of prospective clinical trials evaluating the chemopreventive effects of dietary fat reduction.
Supplementary Material
Supplemental Figure 1. The mean body weights of Hi-Myc mice on LF or HF diet from three weeks to 7 months of age.
The values are presented as mean ± SE. N=27 for LF group and N=33 for HF group.
There were no statistical differences in mean weights between the LF and HF group (p>0.05).
Acknowledgements
We thank Katharine Ellwood-Yen and Charles Sawyers for the Hi-Myc mice, and George Thomas and Katharine Ellwood-Yen for assisting with the planning phase of this experiment.
Supported by the Department of Veterans Affairs, NIH Grants: Specialized Programs of Research Excellence (SPORE) P50 CA92131-01A1, 1R01CA100938, and UCLA Jonsson Comprehensive Cancer Center Interdisciplinary Grant.
References
- 1.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, Burch JD, Hankin J, Dreon DM, West DW, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J Natl Cancer Inst. 1995;87:652–661. doi: 10.1093/jnci/87.9.652. [DOI] [PubMed] [Google Scholar]
- 2.Giovannucci E, Rimm EB, Colditz GA, Stampfer MJ, Ascherio A, Chute CC, Willett WC. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 3.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 4.Schuurman AG, van den Brandt PA, Dor! ant E, Brants HA, Goldbohm RA. Association of energy and fat intake with prostate carcinoma risk: results from The Netherlands Cohort Study. Cancer. 1999;86:1019–1027. [PubMed] [Google Scholar]
- 5.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int J Cancer. 1997;73:634–638. doi: 10.1002/(sici)1097-0215(19971127)73:5<634::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 6.Sonn GA, Aronson W, Litwin MS. Impact of diet on prostate cancer: a review. Prostate Cancer Prostatic Dis. 2005;8:304–310. doi: 10.1038/sj.pcan.4500825. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456–1462. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 8.Ngo TH, Barnard RJ, Cohen P, Freedland S, Tran C, deGregorio F, Elshimali YI, Heber D, Aronson! WJ. Effect of isocaloric low-fat diet on human LAPC-4 pros! tate cancer xenografts in severe combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–2743. [PubMed] [Google Scholar]
- 9.Pollard M, Luckert PH. Promotional effects of testosterone and high fat diet on the development of autochthonous prostate cancer in rats. Cancer Lett. 1986;32:223–227. doi: 10.1016/0304-3835(86)90123-0. [DOI] [PubMed] [Google Scholar]
- 10.Kondo Y, Homma Y, Aso Y, Kakizoe T. Promotional effect of two-generation exposure to a high-fat diet on prostate carcinogenesis in ACI/Seg rats. Cancer Res. 1994;54:6129–6132. [PubMed] [Google Scholar]
- 11.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 12.Ngo TH, Barnard RJ, Leung PS, Cohen P, Aronson WJ. Insulin-like growth factor I (IGF-I) and IGF binding protein-1 modulate prostate cancer cell g! rowth and apoptosis: possible mediators for the effects of diet and exercise on cancer cell survival. Endocrinology. 2003;144:2319–2324. doi: 10.1210/en.2003-221028. [DOI] [PubMed] [Google Scholar]
- 13.Ellwood-Yen K, Graeber TG, Wongvipat J, Iruela-Arispe ML, Zhang J, Matusik R, Thomas GV, Sawyers CL. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell. 2003;4:223–238. doi: 10.1016/s1535-6108(03)00197-1. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi N, Barnard RJ, Henning SM, Elashoff D, Reddy ST, Cohen P, Leung P, Hong-Gonzalez J, Freedland SJ, Said J, Gui D, Seeram NP, Popoviciu LM, Bagga D, Heber D, Glaspy JA, Aronson WJ. Effect of Altering Dietary {omega}-6/{omega}-3 Fatty Acid Ratios on Prostate Cancer Membrane Composition, Cyclooxygenase-2, and Prostaglandin E2. Clin Cancer Res. 2006;12:4662–4670. doi: 10.1158/1078-0432.CCR-06-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson CS, Bialek P, Anzo M, Khosravi! J, Yee S-P, Han VKM. Elevated Circulating Insul! in-Like G! rowth Factor Binding Protein-1 Is Sufficient to Cause Fetal Growth Restriction. Endocrinology. 2006;147:1175–1186. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- 16.Yakar S, Bouxsein ML, Canalis E, Sun H, Glatt V, Gundberg C, Cohen P, Hwang D, Boisclair Y, LeRoith D, Rosen CJ. The ternary IGF complex influences postnatal bone acquisition and the skeletal response to intermittent parathyroid hormone. J Endocrinol. 2006;189:289–299. doi: 10.1677/joe.1.06657. [DOI] [PubMed] [Google Scholar]
- 17.Watson PA, Ellwood-Yen K, King JC, Wongvipat J, LeBeau MM, Sawyers CL. Context-Dependent Hormone-Refractory Progression Revealed through Characterization of a Novel Murine Prostate Cancer Cell Line. Cancer Res. 2005;65:11565–11571. doi: 10.1158/0008-5472.CAN-05-3441. [DOI] [PubMed] [Google Scholar]
- 18.Nesbit CE, Tersak JM, Prochownik EV. MYC oncogenes and human neoplastic disease. Oncogene. 1999;18:3004–3016. doi: 10.1038/sj.onc.1202746. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan-Lefko PJ!, Chen TM, Ittmann MM, Barrios RJ, Ayala GE, Huss WJ, Maddison LA, Foster BA, Greenberg NM. Pathobiology of autochthonous prostate cancer in a pre-clinical transgenic mouse model. Prostate. 2003;55:219–237. doi: 10.1002/pros.10215. [DOI] [PubMed] [Google Scholar]
- 20.Masumori N, Thomas TZ, Chaurand P, Case T, Paul M, Kasper S, Caprioli RM, Tsukamoto T, Shappell SB, Matusik RJ. A Probasin-Large T Antigen Transgenic Mouse Line Develops Prostate Adenocarcinoma and Neuroendocrine Carcinoma with Metastatic Potential. Cancer Res. 2001;61:2239–2249. [PubMed] [Google Scholar]
- 21.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 22.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello L!B, Lagiou P, Adami HO, Trichopoulos D. Insulin-li! ke growth! factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90:911–915. doi: 10.1093/jnci/90.12.911. [DOI] [PubMed] [Google Scholar]
- 23.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92:1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 24.Nam RK, Trachtenberg J, Jewett MA, Toi A, Evans A, Emami M, Narod SA, Pollak M. Serum insulin-like growth factor-I levels and prostatic intraepithelial neoplasia: a clue to the relationship between IGF-I physiology and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1270–1273. doi: 10.1158/1055-9965.EPI-04-0430. [DOI] [PubMed] [Google Scholar]
- 25.Majumder PK, Yeh JJ, George DJ, Febbo PG, Kum J, Xue Q, Bikoff R, Ma H, Kantoff PW, Golub TR, ! Loda M, Sellers WR. Prostate intraepithelial neoplasia induced by prostate restricted Akt activation: The MPAKT model. PNAS. 2003;100:7841–7846. doi: 10.1073/pnas.1232229100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen M-L, Xu P-Z, Peng X, Chen WS, Guzman G, Yang X, Di Cristofano A, Pandolfi PP, Hay N. The deficiency of Akt1 is sufficient to suppress tumor development in Pten+/- mice. Genes Dev. 2006;20:1569–1574. doi: 10.1101/gad.1395006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun M, Wang G, Paciga JE, Feldman RI, Yuan Z-Q, Ma X-L, Shelley SA, Jove R, Tsichlis PN, Nicosia SV, Cheng JQ. AKT1/PKB{alpha} Kinase Is Frequently Elevated in Human Cancers and Its Constitutive Activation Is Required for Oncogenic Transformation in NIH3T3 Cells. Am J Pathol. 2001;159:431–437. doi: 10.1016/s0002-9440(10)61714-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreisberg JI, Malik SN, Prihoda TJ, Bedolla RG, Troyer DA, Kreisberg S, Ghosh PM. Phosphorylation of Akt! (Ser473) is an Excellent Predictor of Poor Clinical Outcome i! n Prostat! e Cancer.. Cancer Res. 2004;64:5232–5236. doi: 10.1158/0008-5472.CAN-04-0272. [DOI] [PubMed] [Google Scholar]
- 29.Majumder PK, Sellers WR. Akt-regulated pathways in prostate cancer. Oncogene. 24:7465–7474. doi: 10.1038/sj.onc.1209096. [DOI] [PubMed] [Google Scholar]
- 30.Lynch M, Fitzgerald C, Johnston KA, Wang S, Schmidt EV. Activated eIF4E-binding Protein Slows G1 Progression and Blocks Transformation by c-myc without Inhibiting Cell Growth. J. Biol. Chem. 2004;279:3327–3339. doi: 10.1074/jbc.M310872200. [DOI] [PubMed] [Google Scholar]
- 31.Bouchard C, Marquardt J, Bras A, Medema RH, Eilers M. Myc-induced proliferation and transformation require Akt-mediated phosphorylation of FoxO proteins. Embo J. 2004;23:2830–2840. doi: 10.1038/sj.emboj.7600279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramaswamy S, Nakamura N, Sansal I, Bergeron L, Sellers WR. A novel mechanism of gene regulation and tumor suppression by the transcription factor FKHR. Cancer Cell. 2002;2:81–91. doi: 10.1016/s1535-6108(02)00086-7. [DOI] [PubMed] [Google Scholar]
- 33.Schmid! t M, Fernandez de Mattos S, van der Horst A, Klompmaker R, Kops GJ, Lam EW, Burgering BM, Medema RH. Cell cycle inhibition by FoxO forkhead transcription factors involves downregulation of cyclin D. Mol Cell Biol. 2002;22:7842–7852. doi: 10.1128/MCB.22.22.7842-7852.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aaltomaa S, Kärjä V, Lipponen P, et al. Expression of Ki-67, cyclin D1 and apoptosis markers correlated with survival in prostate cancer patients treated by radical prostatectomy. Anticancer research. 2006;26(6C):4873–4878. [PubMed] [Google Scholar]
- 35.Comstock CE, Revelo MP, Buncher CR, Knudsen KE. Impact of differential cyclin D1 expression and localisation in prostate cancer. Br J Cancer. 2007;96:970–979. doi: 10.1038/sj.bjc.6603615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drobnjak M, Osman I, Scher HI, Fazzari M, Cordon-Cardo C. Overexpression of Cyclin D1 Is Associated with Metastatic Prostate Cancer to Bone. Clin Cancer Res. 2000;! 6:1891–1895. [PubMed] [Google Scholar]
- 37.K! lefstrom !J, Verschuren EW, Evan G. c-Myc Augments the Apoptotic Activity of Cytosolic Death Receptor Signaling Proteins by Engaging the Mitochondrial Apoptotic Pathway. J. Biol. Chem. 2002;277:43224–43232. doi: 10.1074/jbc.M206967200. [DOI] [PubMed] [Google Scholar]
- 38.Prendergast GC. Mechanisms of apoptosis by c-Myc. Oncogene. 1999;18:2967–2987. doi: 10.1038/sj.onc.1202727. [DOI] [PubMed] [Google Scholar]
- 39.Xie W, Y. C. W. S. W. T. Correlation of increased apoptosis and proliferation with development of prostatic intraepithelial neoplasia (PIN) in ventral prostate of the noble rat. The Prostate. 2000;44:31–39. doi: 10.1002/1097-0045(20000615)44:1<31::aid-pros5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 40.Ananthanarayanan V, Deaton RJ, Yang XJ, Pins MR, Gann PH. Alteration of proliferation and apoptotic markers in normal and premalignant tissue associated with prostate cancer. BMC Cancer. 2006;6:73. doi: 10.1186/1471-2407-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang MT, Honn KV, Nie D. Cyclooxygenases, prostanoids, and tumor progression. Cancer Metastasis Rev. 2007 doi: 10.1007/s10555-007-9096-5. [DOI] [PubMed] [Google Scholar]
- 43.Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D'Agostino R, Zhang H, Wu H, Kang JX, Chen YQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117:1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. The mean body weights of Hi-Myc mice on LF or HF diet from three weeks to 7 months of age.
The values are presented as mean ± SE. N=27 for LF group and N=33 for HF group.
There were no statistical differences in mean weights between the LF and HF group (p>0.05).