Abstract
Cellular and tissue regeneration in the gastrointestinal tract and liver depends on stem cells with properties of longevity, self-renewal and multipotency. Progress in stem cell research and the identification of potential esophageal, gastric, intestinal, colonic, hepatic and pancreatic stem cells provides hope for the use of stem cells in regenerative medicine and treatments for disease. Embryonic stem cells and induced pluripotent stem cells have the potential to give rise to any cell type in the human body, but their therapeutic application remains challenging. The use of adult or tissue-restricted stem cells is emerging as another possible approach for the treatment of gastrointestinal diseases. The same self-renewal properties that allow stem cells to remain immortal and generate any tissue can occasionally make their proliferation difficult to control and make them susceptible to malignant transformation. This Review provides an overview of the different types of stem cell, focusing on tissue-restricted adult stem cells in the fields of gastroenterology and hepatology and summarizing the potential benefits and risks of using stems cells to treat gastroenterological and liver disorders.
Introduction
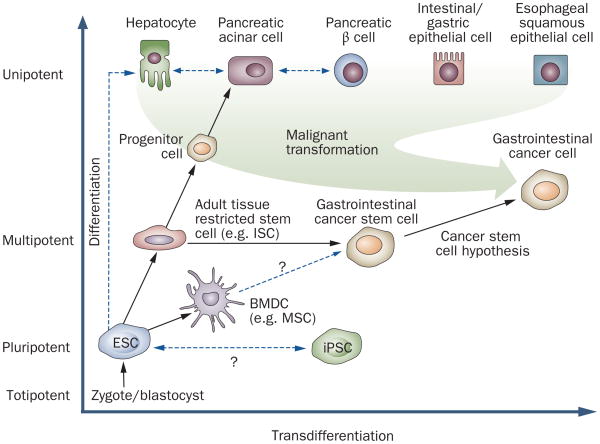
The ability to regenerate and replace cells is vital for the viability and maintenance of most epithelial tissues, including the gastrointestinal tract. Cellular regeneration typically depends on stem cells: primitive and relatively unspecialized cells in fetal and adult tissues that have properties of longevity, self-renewal and multipotency (Table 1).1 All stem cells are capable of self-renewal through the generation of daughter stem cells, and of differentiation into a variety of mature cell types. These processes of self-renewal and differentiation can occur through either symmetric or asymmetric cellular division.2 Stem cells are now thought to range from the totipotent cells of the zygote to pluripotent embryonic stem cells (ESCs) and the more tissue-restricted adult stem cells (Figure 1).
Table 1.
The role and definition of stem cells in gastroenterology
| Stem cell type | Potency | Differentiation | Origin | Function in gastroenterology |
|---|---|---|---|---|
| Embryonic stem cells | Pluripotent | Endoderm, mesoderm, ectoderm | Blastocyst |
In vitro hepatocyte-like or β-cell-like cell differentiation possible In vitro differentiation into intestinal or gastric cells not possible yet Not patient specific Potential role in organ replacement Immunogenic potential |
| Induced pluripotent stem cells | Pluripotent | Endoderm, mesoderm, ectoderm | Somatic cells | Differentiation potential not fully understood Patient specific Not immunogenic |
| Mesenchymal stem cells | Multipotent | Mesoderm (and perhaps ectoderm, endoderm) | Bone marrow | Differentiation into myo_broblasts, pericytes, endothelial cells Transdifferentiation into intestinal epithelial cells controversial Anti-inflammatory potential Contribution to tissue repair and regeneration |
| Tissue stem cells | Multipotent | Tissue-restricted lineage only | Adult tissue | Give rise to multiple tissue-specific lineages Potential therapeutic target for tissue repair and regeneration |
Figure 1.
Schematic illustration of the potency and differentiation status of the different stem cells and progenitor cells or differentiated tissue cells that are relevant to gastroenterology. The differentiation status of cells ranges from completely undifferentiated, totipotent cells to fully differentiated, unipotent cells. Stem cells, which range from pluripotent to multipotent, can be classified as embryonic or adult, and within the gastrointestinal tract they can be further subdivided (for example, hepatic, pancreatic and intestinal). Excluding the zygote or blastocyst, the ESC is the most potent cell and can give rise to any tissue cell of the body. Whether the rare, artificially iPSCs are identical to ESCs has yet to be defined. Adult tissue-restricted stem cells, such as gastrointestinal tissue stem cells, lack cell-specific patterns of expression but give rise to so-called progenitor cells. These, in turn, produce cellular descendants that have a more restricted lineage potential. MSCs and tissue-restricted stem cells are multipotent and both might give rise to potential gastrointestinal cancer stem cells. Abbreviations: BMDC, bone marrow-derived stem cell; ESC, embryonic stem cell; IPSC, induced pluripotent stem cell; ISC, intestinal stem cell; MSC, mesenchymal stem cell.
Stem cells can be roughly classified as embryonic or adult and within the gastrointestinal tract they can be further subdivided into esophageal, gastric, intestinal, colonic, hepatic and pancreatic stem cells. Adult stem cells, such as gastrointestinal tissue stem cells, lack cell-specific patterns of expression but give rise to so-called progenitor cells. These, in turn, produce cellular descendants that have a more restricted lineage potential.3 There is an ongoing debate about how many intermediate cell entities, such as progenitor cells, exist.4 Nevertheless, the presence of adult stem-like cells in the gastrointestinal tract was first postulated by Charles LeBlond 60 years ago,5 well before they were recognized in other organ systems. In addition, over the past 5 years, remarkable progress has been made in the identification and understanding of adult gastrointestinal stem cells.
The recent advances in stem cell research over the past 5 years have generated great interest in the potential therapeutic applications of stem cells in the treatment of gastrointestinal and hepatic disorders. While tissue regeneration could theoretically be accomplished with tissue-restricted adult stem cells, given the limited availability of, and difficulties isolating, these cells, most of the focus has been on the potential of using ESCs (Box 1) and similar induced pluripotent stem cells (iPSCs) (Box 2).6,7 In the fields of gastroenterology and hepatology, stem cells could be used to restore tissue function to patients who have failure of the liver, small intestine or pancreas. Similar to whole organ transplantation of the liver or pancreas, the application of those stem cells that are restricted to an endodermal lineage could in theory be used to regenerate most gastrointestinal or hepatic tissues and thus restore organ function. Stem cells can also support tissue repair, without giving rise to epithelial lineages, when they function as different stromal cells or directly modulate immune function.7 Work since 2007 indicates that mesenchymal stem cells (MSCs) (Box 3) may ameliorate diseases such as IBD or liver failure by modulating immune function.8 Finally, the in vivo stimulation of tissue-restricted adult stem cells or use of their ex vivo expanded and differentiated progenies is emerging as a promising approach to treating digestive diseases.
Box 1. Embryonic stem cells.
Embryonic stem cells (ESCs) are defined as pluripotent cells derived from the inner cell mass of the preimplantation embryo that can self-renew and generate all the cell types of the body in vivo and in vitro (Table 1 and Figure 1).115 The isolation of human ESCs in 1998 generated tremendous interest in the possible use of ESCs for cell therapy.116 For example, genes could potentially be manipulated in ESCs to correct genetic deficiencies before therapeutic implantation. In addition, while most adult stem cells have limited proliferative capacity and can give rise to cell types within one particular lineage only, ESCs treated with the antidifferentiation cytokine leukemia inhibitory factor (LIF) can proliferate indefinitely in cell culture and retain their potential to form all the tissues of the developing organism.2,117 Patient-specific ESCs could in theory also be developed by somatic cell nuclear transfer, whereby a nucleus from a donor somatic cell is reimplanted into an enucleated oocyte to generate a cloned embryo, as was the case with Dolly the sheep.118 Although experiments in animals have shown that nuclear cloning combined with gene and cell therapy represents a valid strategy for treating genetic disorders,119 this is unlikely to be an efficient approach in humans. Some of the extracellular signals and a number of the molecular pathways required for differentiation of ESCs have been identified using both in vitro and in vivo systems. However, although moderate success has been achieved for differentiation of ESCs into ectodermal and mesodermal tissues, progress has been somewhat limited for differentiation of ESCs into endodermal tissues, such as in most gastrointestinal organ systems.
Box 2. Induced pluripotent stem cells.
Induced pluripotent stem cells (iPSCs) are the product of reprogramming a somatic cell into an embryonic stem cell (ESC)-like state,120 providing a new approach to the generation of ESC-like cells. This pioneering method was first described in 2007 by Yamanaka and colleagues using mouse fibroblasts, in which the retroviral-mediated introduction of four genes encoding human transcription factors (octamer-binding transcription factor 3/4 [OCT3/4], SRY-related high-mobility group box protein-2 [SOX2], the oncoprotein c-MYC and Kruppel-like factor 4 [KLF4]) induced pluripotency.121 To date, iPSCs seem to be identical to ESCs,122 although the risks associated with using the oncogene c-MYC and retroviral vectors limit the use of iPSCs in a clinical setting. Another limitation has been the relatively low efficiency of generating iPSCs. However, many variations to this protocol have been described, including the use of nonretroviral vector approaches (adenovirus,123,124 plasmids,124 transposons,125,126 chemical compounds127), and the technique has been applied to several types of mouse and human somatic cells.128 Studies have shown that different combinations of other factors can substitute for the oncoproteins c-MYC and KLF4 and even produce iPSCs with as few as one factor (OCT3/4 or KLF4) in mouse neural stem cells.129 The finding that continued expression of the exogenously introduced genes is not required, and that the factors activate epigenetic reprogramming of somatic cells into an ESC-like state, offers hope that the methodology will continue to improve. In theory, all of the necessary factors could be introduced using one vector,130 which would be removed after reprogramming.126 Thus, iPSC technology seems to be a viable method for generating iPSCs, without the controversy surrounding use of embryonic cells. However, similar to the case with ESCs, much additional work is needed to define the methodologies necessary to achieve liver-specific and gut-specific differentiation.
Box 3. Mesenchymal stem cells.
Mesenchymal stem cells (MSCs) are bone marrow-derived stem cells that were originally defined by their adherence to plastic dishes when cultured. Although MSCs lack specific and unique markers, there is now a general consensus that human MSCs do not express hematopoietic markers (protein tyrosine phosphatase CD45, hematopoietic progenitor antigen CD34 and monocyte differentiation antigen CD14), but do express variable levels of endoglin (CD105), 5′-nucleotidase (CD73), CD44, THY1 membrane glycoprotein (CD90), transferrin receptor protein 1 (TfR1; CD71), and CD271 (tumor necrosis factor receptor superfamily member 16), and are recognized by the monoclonal antibody STRO-1.131 MSCs typically give rise to many mesodermal tissues such as bone, cartilage, smooth muscle and fat. MUC18 (CD146) is an in situ MSC marker of human bone marrow,132 and is also expressed on circulating endothelial progenitors and pericytes. As pericytes can differentiate into osteoblasts, chondrocytes, adipocytes and smooth muscle cells, there has been speculation that pericytes and MSCs may be one and the same cell type.133 A unique subpopulation of MSCs that originates in the mesenchymal compartment of the bone marrow are multipotent adult progenitor cells (MAPCs), which seem to have greater multipotency than most MSCs.95 MSCs can also differentiate into several endodermal or neuroectodermal cell lineages including gastric epithelial cells,134 hepatocytes,135 pneumocytes,136 pigment epithelial cells137 and astrocytes.138 As a result of their plasticity, MSCs could in theory contribute to tissue regeneration139 and studies have suggested a role for MSCs in wound healing. Some of the effects of MSCs are probably independent of tissue engraftment or epithelial differentiation139,140 and might promote engraftment of transplanted organs and reduce graft-versus-host disease.141,142 In 2009, we found a specific subset of MSCs (lineage negative [Lin-], CD44 high expressing, SCA1 negative, c-KIT positive and CD34 negative) that reduces the progression of early gastric tumorigenesis in mice, indicating that a defined subset of MSCs could be used therapeutically.143
There is a dark side to the presence of stem cells in the gastrointestinal tract and liver that also needs to be considered: the same self-renewal properties that allow stem cells to remain immortal and generate thousands of progeny can occasionally make their proliferation difficult to control and make them susceptible to malignant transformation. Indeed, the cancer stem cell theory is an emerging paradigm that suggests that most cancers are sustained by aberrant stem cells that lack the normal ability to undergo terminal differentiation (Figure 1).1,9 Studies published over the past 10 years have linked cancer stem cells and carcinogenesis to tissue-specific stem cells, and to the accumulation of genetic alterations that occur in these tissue stem cells as they age and respond to chronic inflammation.10
In this Review, the different types of stem cell are discussed, and the adult stem/progenitor cell types and their niches in the field of gastroenterology are briefly characterized. To date, most work has been done in mice and only a little is known about tissue-restricted stem cells in humans. The therapeutic potential of stem cells in human gastroenterological and hepatic disorders is also summarized and insight is provided into the potential risks of stem cell therapies.
Tissue-restricted stem cells
To date, the hematopoietic stem cell (HSC), which was initially isolated from mouse bone marrow in 1988, is by far the best characterized multipotent stem cell.11 Other tissue-restricted stem cells that have been reasonably well characterized include those in the peripheral and central nervous system.12,13 Until 2007, few other adult stem cell populations outside the bone marrow had been well characterized or understood. For a number of gastrointestinal organ systems, such as the liver and pancreas, the identity and even existence of a resident stem cell population is still debated. One exception is the intestinal stem cell, for which there has long been solid evidence,5 and in the past few years this has represented an area of rapid and remarkable progress.11,14
Tissue-restricted stem cells are generally difficult to identify morphologically and are not easily distinguished from other epithelial cells by any consistent set of markers, except for perhaps their ability to divide and self renew.15,16 Tissue stem cells or progenitor cells are thought to reside within a ‘niche’—an area with extracellular substrates that provide an optimal micro-environment for normal differentiation.17 In most tissues, stem cells within a niche are assumed to be present in relatively small numbers. These cells are thought to remain largely quiescent or undergo division at a very slow rate,1 such that they are generally negative for most proliferation markers. Typically, proliferation markers such as Ki67, proliferating cell nuclear antigen (PCNA) or 5-bromo-2-deoxyuridine (BrdU) will label progenitor cells, which are immediate descendents of stem cells and are located adjacent to the stem cells.
Progenitor cells divide quickly and are responsible for the bulk of cell division, but seem to have a limited lifespan and are replaced periodically by descendents of the true stem cell. The relative quiescence associated with long-lived stem cells has been attributed to the stem cell niche.17 Thus, alterations in the stem cell niche, such as those associated with chronic inflammation, could be responsible in part for the eventual transformation of stem or progenitor cells to cancer stem cells.18,19 However, data suggest that some classes of intestinal stem cell may be actively dividing with a high cell turnover rate, and may be able to undergo differentiation only in cell culture with the artificial replacement of niche growth factors.20 Some single stem cells can initiate the formation of crypt-villus organoids as self-organizing structures in the absence of a nonepithelial cellular niche and maintained stem cell hierarchy.21 It was thought for a long time that stem cells usually divide asymmetrically, producing one identical quiescent daughter cell and one progenitor cell. However, under regenerative states they can occasionally undergo symmetric division to expand the pool of stem cells. This mechanism of maintaining the stem cell in a relatively dormant state was, in theory, assumed to protect the genome from mutation. Nevertheless, it has to be considered that there are two different types of stem cell—quiescent and fast dividing—that adjust their cell-cycle properties depending on their microenvironment.22
Although tissue-restricted stem cells are thought to be less multipotent and less plastic than other stem cells, they nonetheless require serious consideration with respect to regenerative therapy. The presence of endogenous stem cells in many tissues that participate in maintenance and repair of damaged tissue offers an opportunity to modulate the signals that regulate the behavior of these stem cells, and this may require a deeper understanding of the stem cell microenvironment or niche.
Intestinal stem cells
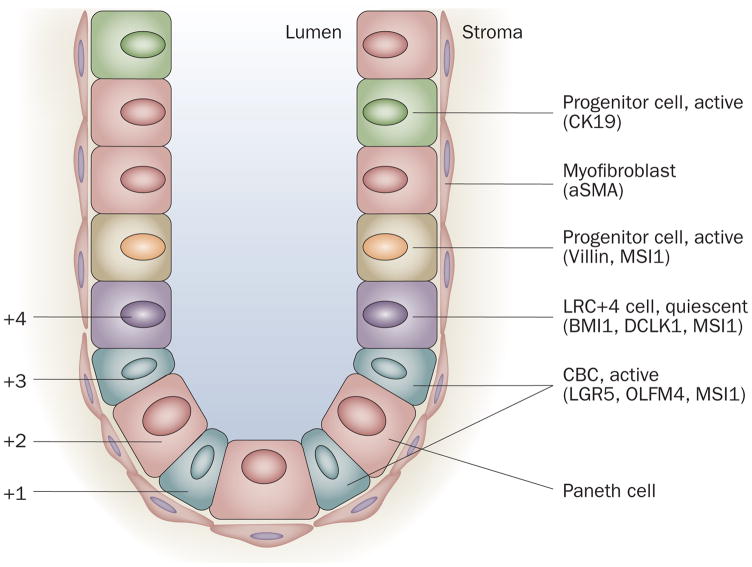
Stem cells in the intestine are located in specific sites within the epithelium adjacent to areas of rapid proliferation and high cell turnover. In the small intestine, proliferation occurs at the base of intestinal crypts; most of the cells migrate up from the crypts to the villi, while some of the cells migrate below the stem cells to form Paneth cells. A few enteroendocrine, mucus and columnar cells might also migrate downward from the common origin into cell positions 1–4 (Figure 2).23 In the colon, the same concept of basally located stem cells has been proposed, although bidirectional migration may also occur here.19
Figure 2.
Schematic illustration of the location of putative intestinal stem cells and/or progenitor cells and their markers in the crypt of the intestine. Quiescent stem cells may be located at position +4, the more active stem cells (crypt base columnar cells [CBCs]) are located anywhere from position +1 to +4 scattered between the Paneth cells. The intestinal glands are surrounded by stromal cells (niche cells), such as myofibroblasts. Modified from Quante, M. & Wang, T. C. Physiology (Bethesda) 23, 350–359 (2008).
The initial location for the intestinal stem cell was deemed to be position +4 (+4 label-retaining cell [LRC]) based on the presence of slowly cycling cells at the fourth cell position from the bottom of the crypt that show label-retention of BrdU. That is, long-term administration of BrdU is followed by a long cessation time to wash out the BrdU in the fast dividing cells, which have to be replaced by the quiescent stem cells occasionally (Figure 2).24 However, other studies suggested that basal crypt cells, known as crypt base columnar cells (CBCs), could also be stem cells. In 2007, a single marker, LGR5 (also called GPR49; Table 2), a leucine-rich orphan G protein-coupled receptor, was identified in lineage-tracing studies to specifically label stem cells in the mouse small intestine, such as the CBCs between the Paneth cells.14 Lineage tracing is a technique whereby the specific expression of Cre endonuclease in stem cells or progenitor cells is used to activate a reporter gene in the cells from which the rest of the tissue, such as the crypts, originates. This research has reactivated the debate about the location of intestinal stem cells. Some LGR5-positive cells seem to be multipotent and are able to form all mature intestinal epithelial cells. They seem to undergo self-renewal, to persist for several months and to be resistant to irradiation. Thus, these rapidly proliferating cells with intestinal stem cell characteristics have challenged the previously held belief that all adult stem cells are generally quiescent or slowly cycling (Figure 3).25 In 2009 lineage-tracing studies of adult prominin-1 (also called CD133; a pentaspan transmembrane glycoprotein that localizes to membrane protrusions) showed that some prominin-1-positive cells are located at the base of crypts in the small intestine, co-express LGR5 and can generate the entire intestinal epithelium, and therefore seem to be small intestinal stem cells as well (Figure 2).26,27 Moreover, olfactomedin 4 (OLFM4), which was identified in a gene expression profile for LGR5-positive cells, has been shown to be highly expressed in CBCs in the human small intestine and colon and may therefore be a marker for human intestinal and colon stem cells.28 Sangiorgi and Capecchi characterized the progeny of crypt BMI1-positive cells (Figure 2) and make the argument in support of the +4 LRCs as a population of stem cells within the small intestine.29 BMI1 encodes a chromatin remodeling protein of the polycomb group that has essential roles in self-renewal of hematopoietic and neural stem cells. BMI1 seems to consistently mark long-lived cell clones (>12 months) populated by all intestinal lineages and serves as a specific marker of a cell population located at the +4 position of the crypt. Furthermore, ablation of BMI1+ cells by targeted expression of the diphtheria toxin depletes the epithelium of the genetically marked crypts (known as whole crypt units). Thus expression of BMI1 also identifies intestinal stem cell candidates.
Table 2.
Gastrointestinal tissue stem cell markers
| Marker | Characteristics of cells | Reference |
|---|---|---|
| Esophagus | ||
| SP (Hoechst 33342)/CD34+ | Slow cycling label-retaining cells in the basal layer | Kalabis et al. (2008)53 |
| Stomach | ||
| Villin transgene | Scattered basal cells in the antrum that give rise to the antral crypt cells (lineage tracing) but no villin expression | Qiao et al. (2007)46 |
| DCLK1 | Rarely expressed in the isthmus of crypts in the corpus and scattered in the antrum (no lineage tracing) | M. Quante & T. C. Wang, unpublished data |
| Intestine | ||
| LGR5 | Active cycling crypt base columnar cells that give rise to all intestinal lineages (lineage tracing) | Barker et al. (2007)14 |
| Prominin-1 | Active cycling crypt base columnar cells that give rise to all intestinal lineages (lineage tracing), overlaps with LGR5 | Zhu et al. (2009),26 Snippert et al. (2009),27 Vermeulen et al. (2008)87 |
| BMI1 | Quiescent cells around position 4+ that give rise to all intestinal lineages (lineage tracing) | Sangiorgi and Capecchi(2008)29 |
| DCLK1 | Expression around position 4+ (no lineage tracing) | Giannakis et al. (2006),35 May et al. (2008)36 |
| Label retaining (BrdU) | Quiescent cells at position 4+ | Potten et al. (1974)24 |
| CCK-BR | Probably present on, but not speci_c for, colonic stem cells or progenitor cells | Jin et al. (2009)37 |
| Liver | ||
| Prominin-1 | Potential marker for murine oval cells (no lineage tracing) | Rountree et al. (2007)57 |
| FOXL1 | Bipotential precursor during oval cell activation (lineage tracing) | Sackett et al. (2009)58 |
| Pancreas | ||
| Neurogenin-3 | Probably endocrine progenitor β cells | Xu et al. (2008)70 |
| BMI1 | Subpopulation of acinar cells capable of self-renewal | Sangiorgi and Capecchi(2009)71 |
Abbreviations: CCK-BR, cholecystokinin-2 receptor; DCLK1, doublecortin CaM kinase-like 1; SP, side population.
Figure 3.
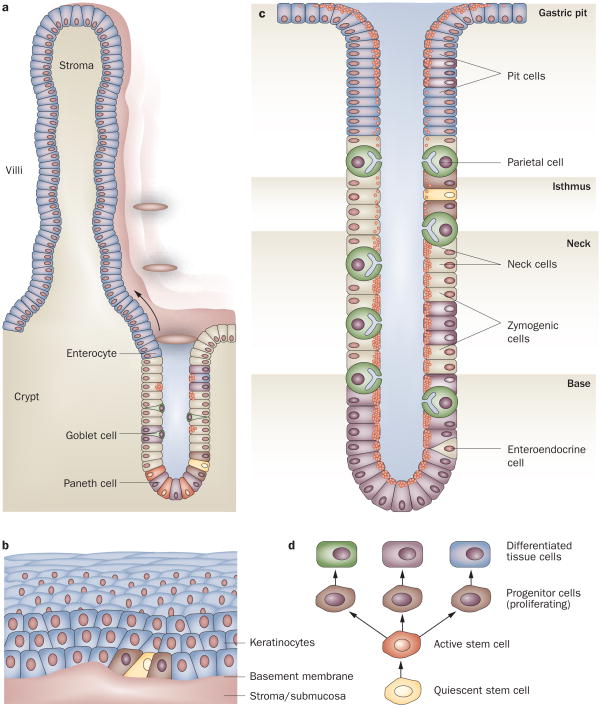
Schematic illustration of the different location and structural organization of stem cells in the gut. The a | intestine, b | esophagus and c | stomach are shown. d | Quiescent stem cells through asymmetric division probably give rise to more rapidly dividing active stem cells, which then produce progenitor cells, while losing their multipotency and ability to proliferate. All of these progeny cells have defined positions in the different organs. To maintain its function the stem cell can give rise to another stem cell at the same position (symmetric division).
In addition to LGR5 and prominin-1, other potential stem cell markers have been identified for which lineage tracing has not yet been completed. For example, musashi 1 (MSI1) expressing cells include +4 LRCs and CBCs30,31 (Figure 2) and sFRP5, a Wnt signaling antagonist known to be expressed in quiescent skin stem cells, is also present at the mRNA level in +4 LRCs.32 In addition, PTEN (phosphatase and tensin homolog) and AKT1, as well as β-catenin, are predominantly expressed in +4 LRCs.33,34 A promising new putative stem cell marker, doublecortin CaM kinase-like-1 (DCLK-1), a microtubule-associated kinase that is known to be expressed in neurons, was discovered in gut epithelial progenitor cells,35 but to date this has not been confirmed through lineage tracing. A 2008 study identified DCLK1 in the intestinal stem cell zone (+4 LRCs) and observed apoptotic stem cells and mitotic DCLK1-expressing cells 24 h after irradiation, indicating the importance of these cells for tissue renewal.36 We have also found that cholecystokinin 2 receptor (CCK-BR) is expressed in colon crypt cells adjacent to the proliferative zone, and that increased levels of progastrin lead to an expansion of the CCK-BR-expressing cells. Inactivation of CCK-BR reduced proliferation and the number of DCLK1-positive or LGR5-positive cells in progastrin-overexpressing hGAS/+ mice, suggesting that CCK-BR expression is likely to be present on colonic stem and progenitor cells.37 The cytokeratin, CK19, which is a bile duct marker, may also mark intestinal stem cells, since lineage tracing marked almost the complete epithelium, indicating that crypts are monoclonal and arise from one progenitor cell within these labeled crypts.29,38
Similar human experiments are not possible because mutagenesis or exogenous marker introduction are not generally feasible, although there is evidence of stem cell lineage in human colon crypts after therapeutic radiation.39 Instead, spontaneous changes that occur during genome replication can be used to distinguish cell kinetics. Two studies published in 2009 traced inactivating mutations in mitochondrial genes encoding cytochrome C oxidase,40,41 a method that has considerable potential for lineage tracing and for the subsequent identification of human stem cells and their niches. One of these studies suggested that the stem cell niche is located at the base of the human colonic crypt and above the Paneth cell zone in the small intestine.40
The existence of quiescent intestinal cells that seem to show many of the criteria of stemness has raised the possibility of more than one type of intestinal stem cell. In a review, Scoville et al. proposed a model of two types of intestinal stem cells: quiescent stem cells at the traditional +4 locations in a prolonged quiescent state, reflecting their inhibitory microenvironment, and the active Lgr5-positive stem cells, representing a population of stem cells able to respond to stimulating signals generated from adjacent mesenchymal cells.42 A similar model of active and quiescent stem cells seems to function in the hematopoetic system.43 Interestingly, the concept of mobile, fast dividing cancer stem cells, which transiently develop from stationary quiescent cancer stem cells, reflects a similar model in carcinogenesis.44 However, further work is needed to clarify the relationship between these different progenitor cell types. In our opinion, a quiescent stem cell is located near the bottom of the intestinal crypts and might therefore be identified as +4 LRCs. All stem cell markers so far identified by lineage-tracing studies seem to mark this LRC but also mark early proliferating progenitor cells and therefore may not be specific for the intestinal tissue stem cell.
Gastric stem cells
In the gastric oxyntic glands, the proliferative zone encompassing the putative gastric stem cell has been localized to the isthmus, the middle portion of the tubule. From the isthmus, cells are thought to migrate bidirectionally to differentiate into gastric surface mucus cells that coat the gastric pits, and gastric parietal and zymogenic cells that comprise the base of the gland (Figure 3).45 Although the gastric stem cell has been the subject of investigation for several decades, it has not yet been identified, as none of the markers discussed above, with the exception of DCLK1,35 label any specific cells within the gastric isthmus. Lineage tracing of cells positive for villin expression has allowed the identification of a multipotent progenitor located in the lower third of the antral glands and which exists in a subset of antral gastric glands.46 Interestingly, LGR5 shows lineage labeling in some antral gastric glands and therefore might mark the antral stem or progenitor cells.14 As described earlier, studies have demonstrated that somatic mitochondrial mutations can be used to identify clonal populations in the human intestine. A recent study extends this technique to human gastric mucosa and similarly identifies gastric subclones. Moreover, whole units of the human gastric oxyntic glands seem to be clonal and contain multiple multipotential stem cells.47
Esophageal stem cells
Esophageal stem cells are thought to reside within the basal layer of the stratified squamous epithelium (Figure 3).48 Although the esophageal squamous epithelium is morphologically similar to the epidermis, there seem to be differences in the location of the stem cell. The label-retaining cells of the esophagus reside in the basal cell compartment and asymmetric cell division has been observed in the interpapillary zone of the basal layer, suggesting the presence of self-renewing stem cells in this compartment.49 The basement membrane of the esophageal epithelium seems to have a central role in controlling esophageal stem cell behavior, by determining the asymmetric orientation of cell division and dictating the overall tissue architecture.49 Recent data from a study of Drosophila embryogenesis suggest a mechanism by which basement membrane and adhesion molecule interactions might influence the orientation of basal cell division.50
The most widely accepted criteria for characterizing keratinocyte stem cells are slow-cycling growth, self-renewal capacity and a high proliferative potential activated by wound healing.51 Some integrin molecules, such as the β1 and α6 subunits, have been suggested to be stem cell markers for keratinocytes but the evidence for this has so far remained quite limited.52 Indeed, the isolation and characterization of esophageal stem cells have remained elusive. Nevertheless, the Hoechst 33342 dye efflux test, which has been proposed to mark stem cells and define hematopoietic stem cells as a side population (SP; a population of cells seen in flow-activated cell sorter analyses), has recently been applied to mouse esophageal epithelium, to characterize a keratinocyte SP that has properties consistent with self-renewal and gives rise to differentiated suprabasal cells in a 3D organotypic culture.53 Furthermore, the CD34-positive fraction of SP cells seems to participate in epithelial regeneration in an innovative murine esophageal mucosal injury model, suggesting that the CD34-positive fraction of the SP cells also has the capacity for self-renewal.53
Hepatic stem cells
While it is generally believed that the adult liver contains liver stem cells, their existence has not been definitively proven and lineage tracing has not been performed. Unlike the gut, the liver has a very slow rate of cell turnover and liver regeneration in most cases depends not on stem or progenitor cells, but rather involves the mitosis of mature cells.54 It has been shown that murine mature polyploid hepatocytes have a stem cell-like regenerative capacity and even human hepatocytes are highly regenerative.55 However, under conditions of chronic or severe liver injury, there does seem to be activation of adult liver progenitor cells. The adult liver harbors facultative bipotential progenitors that give rise to an intermediary cell type, described as ‘oval cells’, which are thought to differentiate into both biliary epithelium and hepato-cytes.54 In the human liver a method to look at somatic mitochondrial mutations highlighted the presence of defined populations of clonal cells apparently originating from the periportal area.40,56 Some studies have proposed prominin-1 as a potential marker of murine oval cells,57 and recent studies of the marker FOXL1 have provided evidence for a bipotential precursor during oval cell activation.58 In the rat, oval cells resemble embryonic hepatoblasts in that they express α-fetoprotein as well as bile duct (CK19) and hepatocyte (albumin) markers.54 Thus, progenitor cell activation in the adult uses some of the same genetic programs used during development.59 For example, cell differentiation is promoted by fibroblast growth factors, which transiently pattern the foregut endoderm60 and later promote the expansion of progenitor cell populations.61 A transcription factor, TBX3, helps expand the hepatoblast population by suppressing CDKN2A,62 and Wnt signaling initially inhibits liver induction63 but in later stages promotes liver bud growth and differentiation.64 However, oval cells present in the adult human liver probably represent a heterogeneous cell population65 and are clearly distinct from fetal hepatoblasts and may not be true stem cells.
Pancreatic stem cells
The epithelial lineages of the pancreas (exocrine, endocrine and ductal) arise from cells that express the transcription factor PDX1 (pancreatic and duodenal homeobox1) during development. After birth, PDX1 expression becomes largely confined to insulin-producing β cells.66 In the endocrine pancreas, the ability of β cells to expand is limited, especially in the adult, and recovery from cell loss, such as occurs in diabetes, is insufficient.67 There is general consensus that in the normal undamaged adult pancreas, the majority of new β cells derive from pre-existing insulin-expressing cells. Lineage tracing has provided evidence that in adult mice most new β cells are derived from pre-existing β cells, rather than from stem or progenitor cells.68 Thus, the restricted regenerative ability of the endocrine pancreas is probably limited by the size of the progenitor cell pool in the adult pancreas.69 The lack of regeneration of β cells in the setting of autoimmune damage has raised considerable interest in the potential of tissue repair by tissue-restricted stem cells, although it remains controversial as to whether mutipotent stem cells exist in the adult pancreas. Nevertheless, recent work has shown that duct ligation can activate neurogenin 3-positive β-cell precursors in the ductal epithelium, suggesting the existence of adult pancreatic progenitor cells, at least in the setting of tissue damage.70 By contrast, the identification of an acinar cell progenitor or stem cell in the pancreas has been more challenging. A study published in 2009 suggested that BMI1 labels a subpopulation of differentiated acinar cells capable of self-renewing for more than 1 year.71 BMI1 seems to be expressed within each acinus in one or more differentiated acinar cells that retain proliferative potential and replace the surrounding dying cells. This study, along with the characterization of BMI1 in the small intestine, suggests a more general role for BMI1 in self-renewal of stem cells as well as in the maintenance of the proliferative ability of differentiated cells.
The dark side of stem cells
Gastrointestinal cancer stem cells
The theory that cancer in adults arises from stem cells represents a modern interpretation of the ‘embryonal rest theory’ developed by Julius Cohnheim in 1867.9 This hypothesis suggests that cancer arises from resident tissue stem cells or their early descendents (for example, restricted progenitors), and that a tumor can be viewed as an aberrant but heterogeneous organ, in which only a small subset of cancer cells, the ‘cancer stem cells’, are capable of extensive proliferation and metastatic spread.1 The contribution of stem cells to tumors was noted in early studies of teratocarcinoma by Pierce et al., and the concepts were later applied to most tumors.72,73 Both normal stem cells and tumorigenic cells give rise to phenotypically heterogeneous cells that exhibit various degrees of differentiation (Figure 1).74 Thus, tumors are derived from cancer stem cells that undergo an abnormal and poorly regulated process of organogenesis analogous to normal stem cells.75,76
Two sets of markers have emerged as the most useful for the identification of cancer stem cells in a variety of systems: CD44 and prominin-1 (Table 3). CD44 is a class I transmembrane glycoprotein that can act as a receptor for extracellular matrices such as hyaluronic acid, and is a known downstream target of the Wnt/β-catenin pathway.77 It was the first marker identified for a solid tumor stem cell found in a study of tumorigenic breast cancer.78 These cancer stem cells expressed CD44, but not CD24, another adhesion molecule, and classical lineage markers (lin). This publication stimulated further studies examining cancer stem cell markers. Pancreatic cancer stem cells, for example, were shown to express CD44, CD24, and ESA (epithelial specific antigen) (0.2–0.8% of pancreatic cancer cells), and had a 100-fold increased tumorigenic potential compared with non-tumorigenic cancer cells.76 Colorectal cancer stem cells were similarly shown to express CD44 and the epithelial cell adhesion molecule EpCAM, and in several colorectal tumors CD166 (ALCAM, known to be expressed in MSCs) could be used for further enrichment of colon cancer stem cells within the EpCAM/CD44-positive population.79 CD44 as well as CD90 and CD45 expression, may serve as sensitive and specific markers for early diagnosis of hepatocellular carcinoma (HCC),80 although in HCC it seems to be CD90, a surface protein expressed by hepatic stem cells or progenitor cells during development, that is primarily upregulated. Findings by our group support the use of CD44 as a gastric cancer stem cell marker. CD44-positive murine cells formed spheroid colonies, xenograft tumors, and also gave rise to CD44-negative cells and exhibited differentiation. Moreover, CD44 expression correlated with the presence of dysplasia in murine and human gastric cancer.77,81
Table 3.
Gastrointestinal cancer stem cell markers
| Marker | Characteristics | Reference |
|---|---|---|
| Stomach | ||
| CD44 | Correlated with the presence of dysplasia in mouse and human gastric cancer | Takaishi et al. (2009),77 Takaishi et al. (2008)81 |
| Colon | ||
| CD44, EpCam, CD166 | CD166 could be used for further enrichment of colon cancer stem cells within the CD44+EpCAM+ population | Dalerba et al. (2007) 79 |
| Prominin-1 | Prominin-1+ and prominin-1− colon cancer cells could initiate tumorigenesis | Ricci-Vitiani et al. (2007),84 O’Brien et al. (2007),85 Shmelkov et al. (2008),86 Vermeulen et al. (2008)87 |
| Liver | ||
| CD44, CD90, CD45 | CD90 is a surface protein expressed by hepatic stem cells and progenitor cells during development | Yang et al. (2008)80 |
| Prominin-1 | Minority (1–5%) of the tumor cell population | Mishra et al. (2009)89 |
| Pancreas | ||
| CD44, C24, ESA | Minority (0.2–0.8%) of pancreatic cancer cells | Li et al. (2007)76 |
| Prominin-1 | 14% overlap between CD44+CD24+ESA+ and prominin-1+ cells | Hermann et al. (2007)90 |
Abbreviations: ESA, epithelial specific antigen.
However, work from a number of laboratories has pointed to prominin-1 as an alternative cancer stem cell marker for many of the very same tumors. Prominin-1 is thought to function in maintaining stem cell properties by suppressing differentiation.82 Although it was first reported as a specific cancer stem cell marker for glioblastoma,83 it was subsequently shown to be a useful cancer stem cell marker for many gastrointestinal tumors including colorectal cancer. Two groups identified human colon cancer-initiating cells using prominin-1 as a marker.84,85 This finding was later challenged by the finding that both prominin-1-positive and prominin-1-negative colon cancer cells could initiate tumorigenesis.86 In 2008 it was shown that a single prominin-1+/CD24+ colon cancer stem cell can self-renew and reconstitute a complete and differentiated carcinoma.87 Interestingly, spheroid cultures of these colon cancer stem cells contain expression of prominin-1, CD166, CD44, integrin β1, signal transducer CD24, LGR5, and nuclear β-catenin, which have all been suggested to mark the (cancer) stem cell population.87 Other groups have shown that prominin-1 is a marker for the cancer stem cell population in HCC, being expressed in only a minority (1–5%) of the tumor cell population.88 However, the role of these markers in defining functionally distinct populations of cells from progenitor to differentiated hepatocytes is controversial. Further studies indicate that the loss of transforming growth factor β signaling and an increase in the expression of the transcription factor STAT3 (signal transducer and activator of transcription 3) contribute to the transformation of a normal hepatic stem cell to a cancer stem cell.89 Finally, prominin-1 has also been reported to be a marker for cancer stem cells in primary pancreatic cancers and pancreatic cancer cell lines,90 although in this study there was an approximately 14% overlap between CD44, CD24 and ESA-positive cells, and prominin-1-expressing cells.
Thus, the cancer stem cell field has yet to reach a consensus on the best markers for cancer stem cells in digestive tumors. In addition, much of the cancer stem cell paradigm has been based on tumor formation in immunodeficient animals such as immunodeficient NOD/SCID (nonobese diabetic/severe combined immune deficiency) mice, and differences in tumor initiation may be less striking if more highly immunodeficient NOD/SCID interleukin 2 receptor gamma chain null (Il2rg−/−) mice are used, since no immunogenic selection can be assumed in these models.91 Since the number and phenotype of cancer stem cells are highly dependent on the immunodeficient murine model used, there remains some skepticism as to whether results from these models are just an assay-based phenomenon or whether they reflect the true existence and identity of cancer stem cells. It may also be the case that the cancer stem cell phenotype is not fixed but transient and inducible, since some studies have demonstrated that the induction of epithelial–mesenchymal transition (EMT) in immortalized human mammary epithelial cells results in the acquisition of cancer stem cell markers.92 Thus, further work is needed to define the best markers and model systems used for studies of cancer stem cell populations. Nevertheless, it is thought that specifically targeting cancer stem cells may allow more effective therapy for cancer, a notion supported by studies published in 2009 with pancreatic cancer stem cells, when a combination of blocking both sonic hedgehog and mTOR (mammalian target of rapamycin) signaling and standard chemotherapy seemed to eliminate pancreatic cancer stem cells.93
BMDCs contribute to carcinogenesis
Tissue-restricted adult stem cells have for many years been the obvious candidate as a source of cancer stem cells, since in a chronically inflamed environment it is thought that they may slowly acquire a series of genetic and epigenetic changes that lead to loss of growth control and apoptotic programs, and finally the emergence of cancer stem cells. However, recently BMDCs have been proposed as an alternative candidate for precursors of cancer stem cells. BMDCs, although not pluripotent like ESCs (Table 1), have a somewhat wider range of plasticity than many tissue-restricted stem cells and tend to migrate to peripheral organs as a result of inflammation and tissue injury.1 The differentiation pattern and growth regulation of these migrating BMDCs may depend largely on local environmental signals.18 Several in vitro studies have shown that many tissues contain cells that are not clearly epithelial in origin and that are capable of self-renewal and of giving rise to differentiated cell types. The identification of circulating progenitor cells capable of functioning as lineage-specific stem cells (such as endothelial progenitors) has raised questions as to whether distinct and unique stem cell populations exist for each organ or tissue, or whether a more centralized source of stem cells exists, with the organ-specific niche being the ultimate determinant of stem cell function.94–96
Chronic inflammatory stress and injury can lead to the recruitment of circulating progenitors to the gastric epithelium where they may engraft and contribute to the tumor mass. Bone marrow-derived epithelial cells have been identified in the lung, gastrointestinal tract and skin of mice after transplantation of a single purified hematopoietic BMDC.94 In the gastrointestinal tract of lethally irradiated and bone marrow transplanted mice, engrafted BMDCs were present as rare isolated epithelial cells in the esophagus, the small intestinal villi, the colonic crypt and the gastric pit of the stomach.94 Research conducted in our laboratory found in a mouse model of gastric cancer that BMDCs contribute to at least parts of the neoplastic glands,97 and more recently, to carcinoma-associated fibroblasts (M. Quante and T. C. Wang, unpublished work). This model might be restricted to cancers that arise after destruction of inflammatory tissue and it remains unclear how BMDCs undergo malignant conversion after recruitment to the gastric mucosa. Several studies have suggested that the contribution of BMDCs to the epithelium, and possibly to tumorigenesis, may be explained by fusion between a BMDC and a peripheral tissue cell.98,99 Moreover, a number of reports have shown that BMDCs can contribute to epithelial cancers.100,101
Furthermore, bone marrow-derived endothelial progenitor cells can contribute directly to angiogenesis in tumor formation.102 Malignant transformation and the continued growth of a malignant cell requires a fertile microenvironment. Myofibroblasts and endothelial cells have been shown to derive, in part, from circulating BMDCs.103 Inflammatory cells and carcinoma-associated fibroblasts are important cells within the peritumoral stroma, and help to promote an environment permissive for tumor growth, invasion and angiogenesis. Together with the tumor cells, they release factors responsible for the mobilization of bone marrow-derived endothelial progenitor cells and induce them to migrate and become incorporated into the developing vasculature of the tumor.104
Stem cells for treatment
There is great interest in the possible use of stem cells in regenerative therapy for gastrointestinal and liver diseases, particularly with respect to liver and intestinal failure. While organ transplantation over the past few decades has made tremendous advances, the issue of the limited donor organ supply has highlighted the potential impact of stem cell therapy for treatment of these gastrointestinal diseases. ESCs (Box 2) have received the most attention, given their pluripotency, and could in theory undergo differentiation ex vivo into differentiated cell types (such as hepatocytes) or into stem or progenitor cells (such as oval cells). Nevertheless, this will probably require further advances in our ability to differentiate ESCs along liver, pancreas, and gut lineages. However, the development of hepatocytes from human ESCs (hESCs) or from other human stem cell sources could be a potential treatment for liver disease. In 2009, hESCs were differentiated by sequential culture in fibroblast growth factor 2, activin-A, hepatocyte growth factor and dexamethasone to generate a reasonably high percentage of cells expressing a mature hepatocyte phenotype. When cultured in vitro, the resulting cells secreted significant levels of human albumin and α1-antitrypsin, and after transplantation into rodents, secreted moderate levels of human albumin and α1-antitrypsin into the serum for more than 2 months.105 This approach using hESCs seems promising. However, ESCs are not patient specific and have the potential for immune rejection, such that lifelong immunosuppression would still be required. While therapeutic cloning offers another strategy to generate patient-specific ESCs that can be used for autologous transplantation, it has not yet been achieved for humans and is unlikely to receive widespread support because of ethical considerations.
The discovery of iPSCs (Box 3) has generated great excitement, since this offers an alternative strategy for generating pluripotent ESC-like stem cells that could be used in regenerative medicine. In addition, iPSCs can be generated from the patients’ own cells, providing a number of theoretical advantages, including absence of immune rejection. In addition, the ability to culture the cells ex vivo provides the additional opportunity to manipulate and/or correct genetic deficiencies. The proof-of-principle of the iPSC strategy has already been demonstrated in several elegant studies. For example, murine iPSCs were differentiated into hematopoietic precursor cells and then used to rescue lethally irradiated mice. In addition, iPSCs were successfully derived from a mouse model of sickle cell anemia, then the defective gene was replaced by homologous recombination, and the differentiated hematopoietic precursors from these cells were used successfully to treat the mouse with sickle cell disease.106 Approaches similar to this could be used to treat a variety of genetic liver disorders such as hereditary hemachromatosis, Wilson disease and α-1-antitrypsin disease. In addition, the generation and differentiation of patient-specific iPSCs could be used in high-throughput screens for validating potential therapeutic targets for small molecule drug development. However, while iPSCs seem to be similar in many ways to ESCs, they may not be quite as pluripotent, they are not identical with respect to their genetic or epigenetic profiles, and may have a slightly greater potential for malignant transformation even without using transfection with oncogenic MYC.
Manipulation of tissue-restricted stem cells for the purpose of regenerative therapy offers another possible therapeutic avenue. However, while tissue-restricted stem cells represent a potential source of autologous cells for transplantation therapy, the therapeutic potential of tissue-restricted stem cells seems to be less than that of ESCs, and their availability is also limited since it has not yet been possible to easily expand the numbers of these tissue-restricted stem cells ex vivo. Nevertheless, with the identification of tissue-restricted stem cells that participate in maintenance and repair of the tissue, strategies to activate and expand endogenous stem cells in situ have potentially broad applications.
One area in which regenerative stem cell-based therapy could have a great impact is in the area of gastrointestinal motility disorders, particularly those associated with the aganglionic gut or Hirschsprung disease. One possible approach has been reported in which postnatal human gut mucosal tissue was used to generate enteric nervous system stem cells ex vivo.107 These cells were obtained from human postnatal gut mucosal tissue by endoscopy, cultured to generate neurosphere-like bodies, and on transplantation into models of aganglionic gut the neurosphere-like bodies were capable of colonizing mucosal tissue and differentiating appropriately into enteric nervous system neuronal and glial cells.
MSCs (Box 3) have been shown to be useful in facilitating tissue regeneration and in accelerating tissue healing largely due to their general anti-inflammatory properties. MSCs, with or without mobilizing or proliferating agents such as granulocyte colony-stimulating factor, enhance mobilization of MSCs and facilitate the activation of endogenous liver stem cells, and therefore seem quite promising for tissue regeneration.108 Since BMDCs seem to be physiologically involved in the process of liver repair in humans,109 the possible therapeutic potential of these cells has been investigated by intraportal autologous transplantation, which in some cases has resulted in clinical improvement.110,111 In addition, recent studies suggest that MSCs can be differentiated into hepatocytes and, when infused into murine models of hepatic failure, can contribute to liver regeneration and effectively rescue these mice from death.112
BMDCs have been used to treat both IBD in humans and induced colitis in mice. Early studies with allogeneic or autologous BMDC transplantation showed that these treatments may effectively treat Crohn’s disease and ulcerative colitis and lead to profound remission with a median follow-up of 7 years.8 Although the mechanism for the induction of remission may be due to elimination of aberrant lymphocyte clones, recent studies suggest that BMDCs can directly facilitate mucosal repair in moderate to severe colitis. Studies by Khalil et al. have shown that infused CD34-negative stem cells home to the inflamed colon, contribute to vasculogenesis and can ameliorate induced colitis in mice.113 Additional studies have suggested that MSCs derived from adipose tissue can alleviate experimental colitis in mice by increasing interleukin 10 levels and inducing T-regulatory cells.114
Conclusions
In summary, achievements in stem cell research have provided new impetus and possibilities for the use of stem cells in the treatment of gastrointestinal and liver diseases. The use of iPSCs (and possibly MSCs) for the treatment of gastrointestinal and hepatic disorders has several theoretical benefits, for instance they are easy to access, are in abundant supply and there is a reduced risk of rejection or need for immunosuppressive therapies. However, much additional work is needed to understand the factors required in each instance to achieve the desired cellular differentiation, as well as the safety, efficacy, and tolerability of stem cell-based treatments. While human iPSCs might seem to be the ideal stem cells for cellular therapy and may even replace the use of immunogenic ESCs, their pluripotency and tumorigenic risk have not yet been adequately determined. Although ESCs or iPSCs have the potential to give rise to any cell type in the human body, the use of adult or tissue stem cells in the liver, pancreas and gut is emerging as a promising approach with many important clinical applications. Cell regenerative therapy with resident tissue stem cells would be possible if the signals that regulate the behavior of these stem cells could be regulated, which may require a deeper understanding of the stem cell microenvironment or niche. Despite the great potential of all kinds of stem cells for achieving tissue regeneration in patients with diseased organ systems, these cells also represent the source of cancer stem cells and additional studies will be needed to determine the benefits versus risks of these therapies.
Key points.
Cellular regeneration depends on stem cells, which are primitive and relatively unspecialized cells present in fetal and adult tissues that have properties of longevity, self-renewal and multipotency
Stem cells can be classified as embryonic or adult, and within the gastrointestinal tract they can be further subdivided into esophageal, gastric, intestinal, colonic, hepatic and pancreatic stem cells
Tissue-restricted stem cells are difficult to identify and are distinguished from epithelial cells by their ability to proliferate and self-renew; they reside within a ‘niche’ that provides an optimal microenvironment for growth
The same self-renewal properties that allow stem cells to remain immortal and generate thousands of progeny can occasionally make their proliferation difficult to control and thus susceptible to malignant transformation
In the fields of gastroenterology and hepatology, stem cells could be used to restore tissue function in patients with failure of the liver, small intestine or pancreas
In addition to embryonic stem cells, the recent discovery of induced pluripotent stem cells has led to a potential alternative strategy for the development of patient-specific cell therapy
Review criteria.
PubMed was searched for articles published from 1960 until June 2009 using the terms “stem cells”, “cancer stem cells”, “tissue stem cells”, “induced pluripotent (iPS) cells”, “embryonic stem (ES) cells”, and “mesenchymal stem cells (MSC)” in combination with “intestinal stem cells”, “gastric or stomach stem cells”, “esophageal stem cells”, “pancreatic stem cells”, “hepatic stem cells”, “colon stem cells”, “treatment”, “myofibroblasts” and “stem cell niche”. Relevant English-language articles were reviewed, as were their reference lists, to identify further relevant articles.
Acknowledgments
T. C. Wang is supported by National Institutes of Health grants 1U54CA126513, RO1CA093405 and R01CA120979. M. Quante is supported by a grant from the Mildred-Scheel-Stiftung, Deutsche Krebshilfe, Germany.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 2.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 3.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 4.Chuong CM, Widelitz RB. The river of stem cells. Cell Stem Cell. 2009;4:100–102. doi: 10.1016/j.stem.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leblond CP, Stevens CE, Bogoroch R. Histological localization of newly-formed desoxyribonucleic acid. Science. 1948;108:531–533. doi: 10.1126/science.108.2811.531. [DOI] [PubMed] [Google Scholar]
- 6.Daley GQ, Scadden DT. Prospects for stem cell-based therapy. Cell. 2008;132:544–548. doi: 10.1016/j.cell.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Stappenbeck TS, Miyoshi H. The role of stromal stem cells in tissue regeneration and wound repair. Science. 2009;324:1666–1669. doi: 10.1126/science.1172687. [DOI] [PubMed] [Google Scholar]
- 8.Brittan M, Alison MR, Schier S, Wright NA. Bone marrow stem cell-mediated regeneration in IBD: where do we go from here? Gastroenterology. 2007;132:1171–1173. doi: 10.1053/j.gastro.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 9.Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977;197:461–463. doi: 10.1126/science.560061. [DOI] [PubMed] [Google Scholar]
- 10.Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 12.Stemple DL, Anderson DJ. Lineage diversification of the neural crest: in vitro investigations. Dev Biol. 1993;159:12–23. doi: 10.1006/dbio.1993.1218. [DOI] [PubMed] [Google Scholar]
- 13.Uchida N, et al. Direct isolation of human central nervous system stem cells. Proc Natl Acad Sci USA. 2000;97:14720–14725. doi: 10.1073/pnas.97.26.14720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 15.Booth D, Haley JD, Bruskin AM, Potten CS. Transforming growth factor-B3 protects murine small intestinal crypt stem cells and animal survival after irradiation, possibly by reducing stem-cell cycling. Int J Cancer. 2000;86:53–59. doi: 10.1002/(sici)1097-0215(20000401)86:1<53::aid-ijc8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 16.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brittan M, et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut. 2002;50:752–757. doi: 10.1136/gut.50.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yen TH, Wright NA. The gastrointestinal tract stem cell niche. Stem Cell Rev. 2006;2:203–212. doi: 10.1007/s12015-006-0048-1. [DOI] [PubMed] [Google Scholar]
- 20.Barker N, van de Wetering M, Clevers H. The intestinal stem cell. Genes Dev. 2008;22:1856–1864. doi: 10.1101/gad.1674008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato T, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;14:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 22.Fuchs E. The tortoise and the hair: slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjerknes M, Cheng H. The stem-cell zone of the small intestinal epithelium. III Evidence from columnar, enteroendocrine, and mucous cells in the adult mouse. Am J Anat. 1981;160:77–91. doi: 10.1002/aja.1001600107. [DOI] [PubMed] [Google Scholar]
- 24.Potten CS, Kovacs L, Hamilton E. Continuous labelling studies on mouse skin and intestine. Cell Tissue Kinet. 1974;7:271–283. doi: 10.1111/j.1365-2184.1974.tb00907.x. [DOI] [PubMed] [Google Scholar]
- 25.Barker N, Clevers H. Tracking down the stem cells of the intestine: strategies to identify adult stem cells. Gastroenterology. 2007;133:1755–1760. doi: 10.1053/j.gastro.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Zhu L, et al. Prominin 1 marks intestinal stem cells that are susceptible to neoplastic transformation. Nature. 2009;457:603–607. doi: 10.1038/nature07589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snippert HJ, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2051–2054. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 28.van der Flier LG, Haegebarth A, Stange DE, van de Wetering M, Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kayahara T, et al. Candidate markers for stem and early progenitor cells, Musashi-1 and Hes1, are expressed in crypt base columnar cells of mouse small intestine. FEBS Lett. 2003;535:131–135. doi: 10.1016/s0014-5793(02)03896-6. [DOI] [PubMed] [Google Scholar]
- 31.Murata H, et al. Helicobacter pylori infection induces candidate stem cell marker Musashi-1 in the human gastric epithelium. Dig Dis Sci. 2008;53:363–369. doi: 10.1007/s10620-007-9858-5. [DOI] [PubMed] [Google Scholar]
- 32.Gregorieff A, et al. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 33.He XC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He XC, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 35.Giannakis M, et al. Molecular properties of adult mouse gastric and intestinal epithelial progenitors in their niches. J Biol Chem. 2006;281:11292–11300. doi: 10.1074/jbc.M512118200. [DOI] [PubMed] [Google Scholar]
- 36.May R, et al. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and CaM kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells. 2008;26:630–637. doi: 10.1634/stemcells.2007-0621. [DOI] [PubMed] [Google Scholar]
- 37.Jin G, et al. Inactivating cholecystokinin-2 receptor inhibits progastrin-dependent colonic crypt fission, proliferation, and colorectal cancer in mice. J Clin Invest. 2009;119:2691–2701. doi: 10.1172/JCI38918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Means AL, Xu Y, Zhao A, Ray KC, Gu G. A CK19(CreERT) knockin mouse line allows for conditional DNA recombination in epithelial cells in multiple endodermal organs. Genesis. 2008;46:318–323. doi: 10.1002/dvg.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campbell F, et al. Post-irradiation somatic mutation and clonal stabilisation time in the human colon. Gut. 1996;39:569–573. doi: 10.1136/gut.39.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fellous TG, et al. A methodological approach to tracing cell lineage in human epithelial tissues. Stem Cells. 2009;27:1410–1420. doi: 10.1002/stem.67. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez-Gonzalez L, et al. Analysis of the clonal architecture of the human small intestinal epithelium establishes a common stem cell for all lineages and reveals a mechanism for the fixation and spread of mutations. J Pathol. 2009;217:489–496. doi: 10.1002/path.2502. [DOI] [PubMed] [Google Scholar]
- 42.Scoville DH, Sato T, He XC, Li L. Current view: intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 43.Wilson A, et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell. 2008;135:1118–1129. doi: 10.1016/j.cell.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 44.Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nat Rev Cancer. 2005;5:744–749. doi: 10.1038/nrc1694. [DOI] [PubMed] [Google Scholar]
- 45.Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381–2388. doi: 10.1242/jcs.115.11.2381. [DOI] [PubMed] [Google Scholar]
- 46.Qiao XT, et al. Prospective identification of a multilineage progenitor in murine stomach epithelium. Gastroenterology. 2007;133:1989–1998. doi: 10.1053/j.gastro.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McDonald SA, et al. Mechanisms of field cancerization in the human stomach: the expansion and spread of mutated gastric stem cells. Gastroenterology. 2008;134:500–510. doi: 10.1053/j.gastro.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Leblond CP. Classification of cell populations on the basis of their proliferative behavior. Natl Cancer Inst Monogr. 1964;14:119–150. [PubMed] [Google Scholar]
- 49.Seery JP, Watt FM. Asymmetric stem-cell divisions define the architecture of human oesophageal epithelium. Curr Biol. 2000;10:1447–1450. doi: 10.1016/s0960-9822(00)00803-4. [DOI] [PubMed] [Google Scholar]
- 50.Lu B, Roegiers F, Jan LY, Jan YN. Adherens junctions inhibit asymmetric division in the Drosophila epithelium. Nature. 2001;409:522–525. doi: 10.1038/35054077. [DOI] [PubMed] [Google Scholar]
- 51.Lavker RM, Sun TT. Epidermal stem cells: properties, markers, and location. Proc Natl Acad Sci USA. 2000;97:13473–13475. doi: 10.1073/pnas.250380097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 53.Kalabis J, et al. A subpopulation of mouse esophageal basal cells has properties of stem cells with the capacity for self-renewal and lineage specification. J Clin Invest. 2008;118:3860–3869. doi: 10.1172/JCI35012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117–130. doi: 10.1016/s0925-4773(02)00338-6. [DOI] [PubMed] [Google Scholar]
- 55.Zaret KS, Grompe M. Generation and regeneration of cells of the liver and pancreas. Science. 2008;322:1490–1494. doi: 10.1126/science.1161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fellous TG, et al. Locating the stem cell niche and tracing hepatocyte lineages in human liver. Hepatology. 2009;49:1655–1663. doi: 10.1002/hep.22791. [DOI] [PubMed] [Google Scholar]
- 57.Rountree CB, et al. A CD133-expressing murine liver oval cell population with bilineage potential. Stem Cells. 2007;25:2419–2429. doi: 10.1634/stemcells.2007-0176. [DOI] [PubMed] [Google Scholar]
- 58.Sackett SD, et al. Foxl1 is a marker of bipotential hepatic progenitor cells in mice. Hepatology. 2009;49:920–929. doi: 10.1002/hep.22705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slack JM. Origin of stem cells in organogenesis. Science. 2008;322:1498–1501. doi: 10.1126/science.1162782. [DOI] [PubMed] [Google Scholar]
- 60.Manfroid I, et al. Reciprocal endoderm-mesoderm interactions mediated by fgf24 and fgf10 govern pancreas development. Development. 2007;134:4011–4021. doi: 10.1242/dev.007823. [DOI] [PubMed] [Google Scholar]
- 61.Jung J, Zheng M, Goldfarb M, Zaret KS. Initiation of mammalian liver development from endoderm by fibroblast growth factors. Science. 1999;284:1998–2003. doi: 10.1126/science.284.5422.1998. [DOI] [PubMed] [Google Scholar]
- 62.Suzuki A, Sekiya S, Buscher D, Izpisua Belmonte JC, Taniguchi H. Tbx3 controls the fate of hepatic progenitor cells in liver development by suppressing p19ARF expression. Development. 2008;135:1589–1595. doi: 10.1242/dev.016634. [DOI] [PubMed] [Google Scholar]
- 63.McLin VA, Rankin SA, Zorn AM. Repression of Wnt/beta-catenin signaling in the anterior endoderm is essential for liver and pancreas development. Development. 2007;134:2207–2217. doi: 10.1242/dev.001230. [DOI] [PubMed] [Google Scholar]
- 64.Ober EA, Verkade H, Field HA, Stainier DY. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 2006;442:688–691. doi: 10.1038/nature04888. [DOI] [PubMed] [Google Scholar]
- 65.Dorrell C, et al. Surface markers for the murine oval cell response. Hepatology. 2008;48:1282–1291. doi: 10.1002/hep.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 67.Nishio J, et al. Islet recovery and reversal of murine type 1 diabetes in the absence of any infused spleen cell contribution. Science. 2006;311:1775–1778. doi: 10.1126/science.1124004. [DOI] [PubMed] [Google Scholar]
- 68.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 69.Stanger BZ, Tanaka AJ, Melton DA. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 70.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 71.Sangiorgi E, Capecchi MR. Bmi1 lineage tracing identifies a self-renewing pancreatic acinar cell subpopulation capable of maintaining pancreatic organ homeostasis. Proc Natl Acad Sci USA. 2009;106:7101–7106. doi: 10.1073/pnas.0902508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sell S, Pierce GB. Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Lab Invest. 1994;70:6–22. [PubMed] [Google Scholar]
- 73.Pierce GB. Neoplastic stem cells. Adv Pathobiol. 1977;6:141–152. [PubMed] [Google Scholar]
- 74.Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675–699. doi: 10.1146/annurev.cellbio.22.010305.104154. [DOI] [PubMed] [Google Scholar]
- 75.Kelly PN, Dakic A, Adams JM, Nutt SL, Strasser A. Tumor growth need not be driven by rare cancer stem cells. Science. 2007;317:337. doi: 10.1126/science.1142596. [DOI] [PubMed] [Google Scholar]
- 76.Li C, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 77.Takaishi S, et al. Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells. 2009;27:1006–1020. doi: 10.1002/stem.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dalerba P, et al. Phenotypic characterization of human colorectal cancer stem cells. Proc Natl Acad Sci USA. 2007;104:10158–10163. doi: 10.1073/pnas.0703478104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang ZF, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919–928. doi: 10.1002/hep.22082. [DOI] [PubMed] [Google Scholar]
- 81.Takaishi S, Okumura T, Wang TC. Gastric cancer stem cells. J Clin Oncol. 2008;26:2876–2882. doi: 10.1200/JCO.2007.15.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Weigmann A, Corbeil D, Hellwig A, Huttner WB. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc Natl Acad Sci USA. 1997;94:12425–12430. doi: 10.1073/pnas.94.23.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh SK, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 84.Ricci-Vitiani L, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445:111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 85.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 86.Shmelkov SV, et al. CD133 expression is not restricted to stem cells, and both CD133+ and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111–2120. doi: 10.1172/JCI34401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vermeulen L, et al. Single-cell cloning of colon cancer stem cells reveals a multi-lineage differentiation capacity. Proc Natl Acad Sci USA. 2008;105:13427–13432. doi: 10.1073/pnas.0805706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ma S, et al. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542–2556. doi: 10.1053/j.gastro.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 89.Mishra L, et al. Liver stem cells and hepatocellular carcinoma. Hepatology. 2009;49:318–329. doi: 10.1002/hep.22704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hermann PC, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 91.Quintana E, et al. Efficient tumour formation by single human melanoma cells. Nature. 2008;456:593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mani SA, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mueller MT, et al. Combined targeted treatment to eliminate tumorigenic cancer stem cells in human pancreatic cancer. Gastroenterology. 2009;137:1102–1113. doi: 10.1053/j.gastro.2009.05.053. [DOI] [PubMed] [Google Scholar]
- 94.Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 95.Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 96.LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multinucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- 97.Houghton J, et al. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568–1571. doi: 10.1126/science.1099513. [DOI] [PubMed] [Google Scholar]
- 98.Nygren JM, et al. Myeloid and lymphoid contribution to non-haematopoietic lineages through irradiation-induced heterotypic cell fusion. Nat Cell Biol. 2008;10:584–592. doi: 10.1038/ncb1721. [DOI] [PubMed] [Google Scholar]
- 99.Johansson CB, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Avital I, et al. Donor-derived human bone marrow cells contribute to solid organ cancers developing after bone marrow transplantation. Stem Cells. 2007;25:2903–2909. doi: 10.1634/stemcells.2007-0409. [DOI] [PubMed] [Google Scholar]
- 101.Janin A, et al. Donor-derived oral squamous cell carcinoma after allogeneic bone marrow transplantation. Blood. 2009;113:1834–1840. doi: 10.1182/blood-2008-07-171702. [DOI] [PubMed] [Google Scholar]
- 102.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Orimo A, Weinberg RA. Stromal fibroblasts in cancer: a novel tumor-promoting cell type. Cell Cycle. 2006;5:1597–1601. doi: 10.4161/cc.5.15.3112. [DOI] [PubMed] [Google Scholar]
- 104.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 105.Basma H, et al. Differentiation and transplantation of human embryonic stem cell-derived hepatocytes. Gastroenterology. 2009;136:990–999. doi: 10.1053/j.gastro.2008.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hanna J, et al. Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science. 2007;318:1920–1923. doi: 10.1126/science.1152092. [DOI] [PubMed] [Google Scholar]
- 107.Metzger M, Caldwell C, Barlow AJ, Burns AJ, Thapar N. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–2225. e1–3. doi: 10.1053/j.gastro.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 108.Piscaglia AC, Shupe TD, Oh SH, Gasbarrini A, Petersen BE. Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats. Gastroenterology. 2007;133:619–631. doi: 10.1053/j.gastro.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gehling UM, et al. Partial hepatectomy induces mobilization of a unique population of haematopoietic progenitor cells in human healthy liver donors. J Hepatol. 2005;43:845–853. doi: 10.1016/j.jhep.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 110.am Esch JS, 2nd, et al. Portal application of autologous CD133+ bone marrow cells to the liver: a novel concept to support hepatic regeneration. Stem Cells. 2005;23:463–470. doi: 10.1634/stemcells.2004-0283. [DOI] [PubMed] [Google Scholar]
- 111.Terai S, et al. Improved liver function in liver cirrhosis patients after autologous bone marrow cell infusion therapy. Stem Cells. 2006;24:2292–2298. doi: 10.1634/stemcells.2005-0542. [DOI] [PubMed] [Google Scholar]
- 112.Kuo TK, et al. Stem cell therapy for liver disease: parameters governing the success of using bone marrow mesenchymal stem cells. Gastroenterology. 2008;134:2111–2121. e1–3. doi: 10.1053/j.gastro.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khalil PN, et al. Nonmyeloablative stem cell therapy enhances microcirculation and tissue regeneration in murine inflammatory bowel disease. Gastroenterology. 2007;132:944–954. doi: 10.1053/j.gastro.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 114.Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989. doi: 10.1053/j.gastro.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 115.Rossant J. Stem cells and early lineage development. Cell. 2008;132:527–531. doi: 10.1016/j.cell.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 116.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 117.Smith AG, et al. Inhibition of pluripotential embryonic stem cell differentiation by purified polypeptides. Nature. 1988;336:688–690. doi: 10.1038/336688a0. [DOI] [PubMed] [Google Scholar]
- 118.Wilmut I, Schnieke AE, McWhir J, Kind AJ, Campbell KH. Viable offspring derived from fetal and adult mammalian cells. Nature. 1997;385:810–813. doi: 10.1038/385810a0. [DOI] [PubMed] [Google Scholar]
- 119.Hochedlinger K, Jaenisch R. Nuclear transplantation, embryonic stem cells, and the potential for cell therapy. N Engl J Med. 2003;349:275–286. doi: 10.1056/NEJMra035397. [DOI] [PubMed] [Google Scholar]
- 120.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 121.Takahashi K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 122.Wernig M, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- 123.Stadtfeld M, Nagaya M, Utikal J, Weir G, Hochedlinger K. Induced pluripotent stem cells generated without viral integration. Science. 2008;322:945–949. doi: 10.1126/science.1162494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–953. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 125.Kaji K, et al. Virus-free induction of pluripotency and subsequent excision of reprogramming factors. Nature. 2009;458:771–775. doi: 10.1038/nature07864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Woltjen K, et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature. 2009;458:766–770. doi: 10.1038/nature07863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Markoulaki S, et al. Transgenic mice with defined combinations of drug-inducible reprogramming factors. Nat Biotechnol. 2009;27:169–171. doi: 10.1038/nbt.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim JB, et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature. 2008;454:646–650. doi: 10.1038/nature07061. [DOI] [PubMed] [Google Scholar]
- 129.Kim JB, et al. Oct4-induced pluripotency in adult neural stem cells. Cell. 2009;136:411–419. doi: 10.1016/j.cell.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 130.Shao L, et al. Generation of iPS cells using defined factors linked via the self-cleaving 2A sequences in a single open reading frame. Cell Res. 2009;19:296–306. doi: 10.1038/cr.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 132.Sacchetti B, et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 133.Covas DT, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 134.Okumura T, et al. Identification of a bone marrow-derived mesenchymal progenitor cell subset that can contribute to the gastric epithelium. Lab Invest. doi: 10.1038/labinvest.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee KD, et al. In vitro hepatic differentiation of human mesenchymal stem cells. Hepatology. 2004;40:1275–1284. doi: 10.1002/hep.20469. [DOI] [PubMed] [Google Scholar]
- 136.Ortiz LA, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arnhold S, et al. Adenovirally transduced bone marrow stromal cells differentiate into pigment epithelial cells and induce rescue effects in RCS rats. Invest Ophthalmol Vis Sci. 2006;47:4121–4129. doi: 10.1167/iovs.04-1501. [DOI] [PubMed] [Google Scholar]
- 138.Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 140.Prockop DJ. ‘Stemness’ does not explain the repair of many tissues by mesenchymal stem/multipotent stromal cells (MSCs) Clin Pharmacol Ther. 2007;82:241–243. doi: 10.1038/sj.clpt.6100313. [DOI] [PubMed] [Google Scholar]
- 141.Karlsson H, et al. Mesenchymal stem cells exert differential effects on alloantigen and virus-specific T-cell responses. Blood. 2008;112:532–541. doi: 10.1182/blood-2007-10-119370. [DOI] [PubMed] [Google Scholar]
- 142.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815–1822. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 143.Wang SW, et al. Bone marrow derived Lin-Cd44hisca1-Ckit+CD34− can give rise to mesenchymal stem cells and delay progression to dysplasia in a murine Helicobacter model of gastric cancer. Gastroenterology. 2009;136 (Suppl 1):58. [Google Scholar]