Abstract
Pregnancy-associated plasma protein-A (PAPP-A), a member of the metalloproteinase superfamily, is an important regulator of mammalian growth and development. However, the role of PAPP-A and its mechanism of action in various cellular processes remain unknown. In this study, we have investigated the role of PAPP-A in skeletal myogenesis using C2C12 myoblasts. Recombinant PAPP-A was purified from the conditioned medium of HT1080 cells overexpressing PAPP-A. Treatment of C2C12 myoblasts with PAPP-A increased their proliferation in a dose- and time-dependent manner. Addition of exogenous PAPP-A also increased the myotube formation and the activity of creatine kinase in C2C12 cultures. Transient overexpression of the full-length PAPP-A-(1–1547), but not truncated protease-inactive N-terminal PAPP-A-(1–920) or C-terminal PAPP-A-(1100–1547), significantly enhanced the proliferation of C2C12 myoblasts. In vitro and in situ experiments demonstrated that PAPP-A cleaves insulin-like growth factor-binding protein (IGFBP)-2, but not IGFBP-3, in the conditioned medium of C2C12 myoblasts. Overexpression of PAPP-A led to degradation of the IGFBP-2 produced by C2C12 myoblasts and increased free IGF-I concentrations without affecting total IGF-I concentrations. Addition of protease-resistant IGFBP-4 completely abolished the PAPP-A-induced proliferation of C2C12 myoblasts. Our results demonstrate that 1) PAPP-A increases the proliferation and differentiation of myoblasts, 2) the stimulatory effect of PAPP-A on myogenesis is governed by its proteolytic activity, and 3) PAPP-A promotes skeletal myogenesis by increasing the amount of free IGFs via specific degradation of IGFBP-2 produced by myoblasts.
Myogenesis is a complex phenomenon that involves the proliferation of myoblasts followed by their morphological, biochemical, and molecular modifications, resulting in the formation of multinucleated myotubes (1–3). Among the few factors that have been shown to promote the myogenic program, the insulin-like growth factors (IGFs)2 I and II potently stimulate proliferation and differentiation of myogenic cells (4). The physiological significance of IGFs in skeletal muscle development has been strongly supported by genetic studies that show that targeted disruption of the Igf-I gene in mice led to a dramatic decrease in both mass and function of skeletal muscles (5, 6). Conversely, overexpression of IGF-I in mice significantly increased muscle mass (7), and Igf-I gene transfer to muscles via adenoviral vectors blocked age-related muscle degeneration (8). Furthermore, muscle-specific expression of IGF-I has been shown to counteract muscle loss in mdx mice, the mouse model of Duchenne muscular dystrophy (9).
The actions of IGFs in vitro and in vivo are modulated by IGF-binding proteins (IGFBPs), which have high affinity and specificity for the IGFs (10, 11). Although some IGFBPs may potentiate IGF action in certain biological systems (10, 12), most of the known IGFBPs inhibit the myogenic function of IGFs (4, 13–17). This suggests that changes in the rate of the synthesis or degradation of the inhibitory IGFBPs may play a pivotal role in the regulation of the myogenic actions of IGFs.
Recent studies from our group and others have demonstrated that PAPP-A is a major protease that degrades IGFBP-4 (18–21). PAPP-A also cleaves IGFBP-5 into fragments with reduced IGF binding activity(22).Additionally, it has been shown that PAPP-A present in bovine and porcine follicular fluid can cleave IGFBP-2 (23). These in vitro studies demonstrate that PAPP-A serves as a proteolytic enzyme for selected IGFBPs, namely, IGFBP-2, IGFBP-4, and IGFBP-5. Recently, the physiological significance of PAPP-A has been strongly supported by a study by Conover et al. (24) that showed that PAPP-A and Igf-II knock-out mice exhibit similar phenotypes, characterized by a 40% reduction in birth weight and impaired bone development. Based on these in vitro and in vivo findings, we have proposed that PAPP-A may enhance the bioavailability of IGFs (the amount of free IGFs) by degrading selected IGFBPs, subsequently releasing IGFs from IGF•IGFBP complexes to act on target tissues.
Although significant progress has been made toward biochemical characterization and identification of in vivo function of PAPP-A, the role and mechanisms of action of PAPP-A in various cellular processes, including myogenic differentiation, have not been determined. In addition to the protease domain, PAPP-A contains several other functional domains, such as the five complement control protein (CCP1–5) modules and two Lin12-Notch repeats (25). However, the roles of these additional functional domains regarding PAPP-A function remain enigmatic. Using C2C12 cells (a mouse myoblast cell line), we have investigated the potential role, mechanism of action, and functional determinants of PAPP-A in myogenesis. Our data show that the stimulatory effect of PAPP-A on proliferation and differentiation of myoblasts is caused by increased IGF bioavailability, which occurs as a consequence of IGFBP-2 degradation. Furthermore, our results suggest that the proteolytic activity of PAPP-A is required for the induction of the myogenic program.
EXPERIMENTAL PROCEDURES
Materials
Dulbecco’s modified Eagle’s medium (DMEM) was purchased from Invitrogen. The His6-tagged recombinant IGFBP-4 and amino acid 121–142-deleted IGFBP-4 peptides were prepared as previously described (26). Purified polyclonal anti-human PAPP-A IgG and normal IgG produced in rabbits were purchased from DAKO Corp. (Carpinteria, CA). Heparin-agarose was purchased from Bio-Rad. M2 FLAG antibody, M2 FLAG antibody-agarose, FLAG peptide, horse serum, and fetal calf serum (FCS) were from Sigma. Mouse monoclonal MF20 antibody specific to myosin heavy chain-fast twitch (MyHCf) protein was obtained from the Developmental Studies Hybridoma Bank of the University of Iowa. Creatine kinase assay kit was obtained from Stanbio Laboratory (Boerne, TX). Recombinant human IGF-I and IGF-II were purchased from GroPep. Other chemicals and reagents were of reagent grade and were obtained from Sigma.
Cell Culture
C2C12 and HT1080 cell lines were obtained from American Type Culture Collection (Rockville, MD). Cells were grown at 37 °C in a CO2 incubator in DMEM containing 10% FCS. Differentiation of C2C12 myoblasts was induced by replacing the medium with differentiation medium (DM) (2% heat-inactivated horse serum in DMEM) for 96 h. All culture media were also supplemented with 100 units/ml penicillin and 100 µg/ml streptomycin.
PAPP-A Expression Plasmids
Plasmids expressing the full-length PAPP-A-(1–1547), C-terminal PAPP-A-(1100–1547), and N-terminal PAPP-A-(1–920) were constructed in the pFLAG-CMV-1 vector (Sigma) as described previously (26). When transfected into mammalian cells, these plasmids led to expression of N-terminal FLAG epitope-tagged PAPP-A peptides.
Purification of Recombinant Human PAPP-A
HT1080 cells were seeded in 6-well plates and co-transfected with linearized pcDNA3.1 vector (0.2 µg) and PAPP-A-(1–1547)/pFLAG plasmid DNA (2 µg). After 48 h, the cells were trypsinized and selected in the presence of G418 (400 µg/ml). Expression of recombinant PAPP-A in G418-resistant HT1080 cells was confirmed by Western blot analysis of the conditioned medium (CM), using either PAPP-A or FLAG antibodies. Serum-free CM (200–400 ml) was collected from cultured HT1080/PAPP-A cells, centrifuged to remove cellular debris, adjusted to pH 7.5, and applied to a heparin-agarose affinity column (20 ml of resin). After washing the column with 150 ml of 50 mm Tris-Cl (pH 7.5) containing 300 mm NaCl, bound proteins were eluted with 50 mm Tris-Cl (pH 7.5) containing 1 m NaCl. Eluted proteins were concentrated with an Amicon filtration unit (10 kDa cutoff), diluted with 50 mm Tris-Cl (pH 7.5) to 150 mm NaCl, and applied to a M2 FLAG monoclonal antibody-agarose column (1 ml of resin). After washing the column with 30 ml of Tris-Cl (pH 7.5) containing 150 mm NaCl, bound proteins were eluted with 5 ml of 50 mm Tris-Cl (pH 7.5) containing 150 mm NaCl and 100 µg/ml FLAG peptide (Sigma). FLAG peptides (<1 kDa) in the purified PAPP-A were removed by an Amicon filtration device (100 kDa cutoff) with extensive washing with phosphate-buffered saline (PBS). Purified PAPP-A was quantitated by Bradford reagents using bovine serum albumin as standard and stored at −75 °C in aliquots.
Proliferation Assay
Proliferation of C2C12 myoblasts was measured using AlamarBlue dye, which measures the metabolic activity of live cells, as described previously (27). C2C12 myoblasts were seeded in DMEM/10% FCS in 24-well plates (10,000 cells/well). After 12–24 h of incubation, the cells were washed once with DMEM and starved in serum-free DMEM for an additional 12–24 h prior to addition of effectors in DMEM containing 5% FCS unless specified. After an appropriate length of incubation, the CM was removed, and 0.5 ml (for 24-well plates) or 1 ml (for 6-well plates) of 10% AlamarBlue (BioSource International, Camarillo, CA) in phenol red-free DMEM was added to each well. After 1–2 h of incubation, 0.2-ml reaction from each well was used to determine fluorescence at the optimal excitation and emission wavelengths of 546 and 590 nm, respectively. The proliferation of myoblasts was also confirmed by measuring the total protein content in cell lysates.
Myogenic Index Determination
As a morphological parameter of muscle differentiation, the myogenic index is defined as the number of nuclei residing in the cells containing three or more nuclei, divided by the total number of nuclei in hematoxylin-stained cells. Cells were washed twice in PBS, fixed with 3.7% formaldehyde in PBS for 10 min, and permeabilized with 0.1% Triton X-100 in PBS for 5 min. Cells were then stained with hematoxylin for 20 s followed by washing in running water. Distribution of nuclei in myoblasts and myotubes was measured by counting the nuclei at 7–10 different locations selected randomly using an inverted microscope with counting grid (Olympus).
Immunocytochemistry
Expression of MyHCf was examined by immunocytochemistry. C2C12 myoblasts were grown in a 24-well plate and were allowed to differentiate into myotubes. The cells were then fixed with 3.7% paraformaldehyde followed by permeabilization with 0.1% Triton-X-100 as described above. After being washed three times (3 min each) with PBS, the cells were blocked with 1% bovine serum albumin in PBS for 1 h and then incubated with MF-20 antibody (specific to MyHCf) at 1:100 dilutions in PBS for 2 h at room temperature. The cells were washed with PBS, incubated with goat anti-mouse IgG-Alexa546 at a 1:100 dilution for 1 h, and counterstained for nuclei with 4′-6-diamidino-2-phenylindole (DAPI) for 5 min. Stained cells were analyzed under a fluorescence microscope (Olympus IX 70). Pictures were captured using Olympus MagnaFire Digital Camera and software.
Creatine Kinase Assay
Creatine kinase activity was measured to assess myogenic differentiation biochemically. Following treatment with recombinant PAPP-A, cells were washed twice in cold PBS and lysed in lysis buffer A (50 Tris-Cl (pH 8.0), 200 mm NaCl, 50 mm NaF, 1 mm dithiothreitol, 0.3% IPEGAL). Lysates were centrifuged for 5 min at 14,000 rpm, and the supernatant collected was used immediately for creatine kinase assay. Protein content in the samples was measured using Bradford reagent. Creatine kinase activity was measured using a spectrophotometrically based kit (Stanbio Laboratory, Boerne, TX). Specific creatine kinase activity was calculated after correction for total protein and expressed as units/mg protein.
Miscellaneous Procedures
Western immunoblot analysis of PAPP-A using either FLAG antibody or PAPP-A antibody was performed as described previously (26, 28). [125I]IGF-II ligand blot analysis was conducted as described (21). Free IGF-I concentrations in the CM samples were determined by a commercial kit using 50 µl of CM (Diagnostic Systems Laboratories, Webster, Texas). Total IGF-I concentrations were measured by radioimmunoassay (29).
Statistical Analysis
Results are expressed as mean ± S.E. and statistically analyzed by Student’s t-test or analysis of variance. A value of p<0.05 was considered statistically significant.
RESULTS
Here we have investigated the role and mechanisms by which PAPP-A modulates skeletal myogenesis. Because the major muscle differentiation steps can be reproduced in vitro with the mouse myoblast cell line C2C12 (30, 31), we used C2C12 myoblasts to investigate the role of PAPP-A in myogenesis.
Purification of Recombinant PAPP-A Peptide
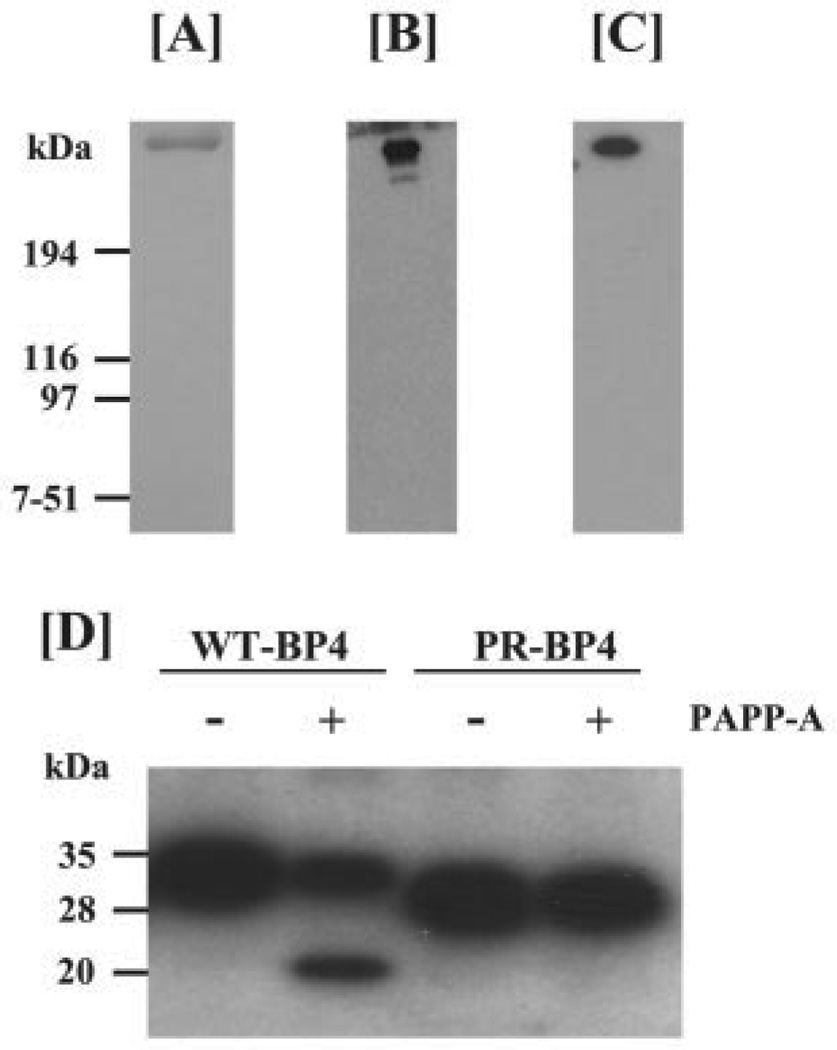
Development of an efficient procedure to produce and purify recombinant PAPP-A peptide is crucial to study cellular responses. We employed the two-step purification protocol (described under “Experimental Procedures”) that has been shown to remove both contaminating proteins and PAPP-A proteolytic fragments, as heparin antibody and FLAG antibody bind to the distal C-terminal region and N terminus of recombinant PAPP-A, respectively (26, 32). Assessment of PAPP-A purity by Coomassie Blue staining revealed the presence of a major single band (Fig. 1A) with the expected molecular mass (~400 kDa). Immunoblot analyses using either polyclonal PAPP-A antibody (Fig. 1B) or monoclonal FLAG antibody (Fig. 1C) demonstrate that the purified PAPP-A peptide contains insignificantly low amounts of PAPP-A proteolytic fragments.
FIGURE 1. Purification of recombinant human PAPP-A peptide.
Recombinant human PAPP-A peptide was sequentially purified by heparin affinity and M2 FLAG antibody affinity chromatography from the CM of HT1080 cells stably overexpressing FLAG-tagged PAPP-A as described under “Experimental Procedures.” A, 0.5 µg of purified recombinant PAPP-A was separated on a 5% SDS-PAGE gel under non-reducing conditions, followed by Coomassie Blue staining. Recombinant PAPP-A peptide migrated as a single major band of expected molecular mass (~450 kDa). B and C, 10 ng of purified PAPP-A was separated on a 5% SDS-PAGE gel, transferred to a nitrocellulose membrane, and subjected to immunoblot analysis with either polyclonal PAPP-A antibody (B) or monoclonalM2anti-FLAG antibody (C).D, a mixture of 100 ng of IGF-II with either 100 ng of the wild-type IGFBP-4 (WT-BP4) or 100 ng of protease-resistant IGFBP-4 (PR-BP4) peptide was incubated for 2 h at 37 °C with 2 ng of purified PAPP-A. Proteolysis of IGFBP-4 peptide was determined by [125I]IGF-II ligand blot analysis. Data presented here show that our purified PAPP-A preparation can cleave WT-BP4 but not PR-BP4.
To determine the activity of the purified recombinant PAPP-A, we performed protease assays using IGFBP-4 as a substrate. Purified PAPP-A effectively cleaved the wild-type IGFBP-4 (WT-BP4) at a rate of ~20 ng of IGFBP-4/ng PAPP-A/h under defined conditions. In contrast, as shown in Fig. 1D, PAPP-A was unable to cleave the protease-resistant IGFBP-4 analog (PR-BP4) lacking a broad sequence (amino acids 121–142) containing the cleavage site (Met-135-Lys-136) (33). Consistent with previous published reports (21, 22), purified recombinant PAPP-A peptide also effectively cleaved IGFBP-5 (data not shown).
PAPP-A Stimulates Proliferation of C2C12 Myoblasts
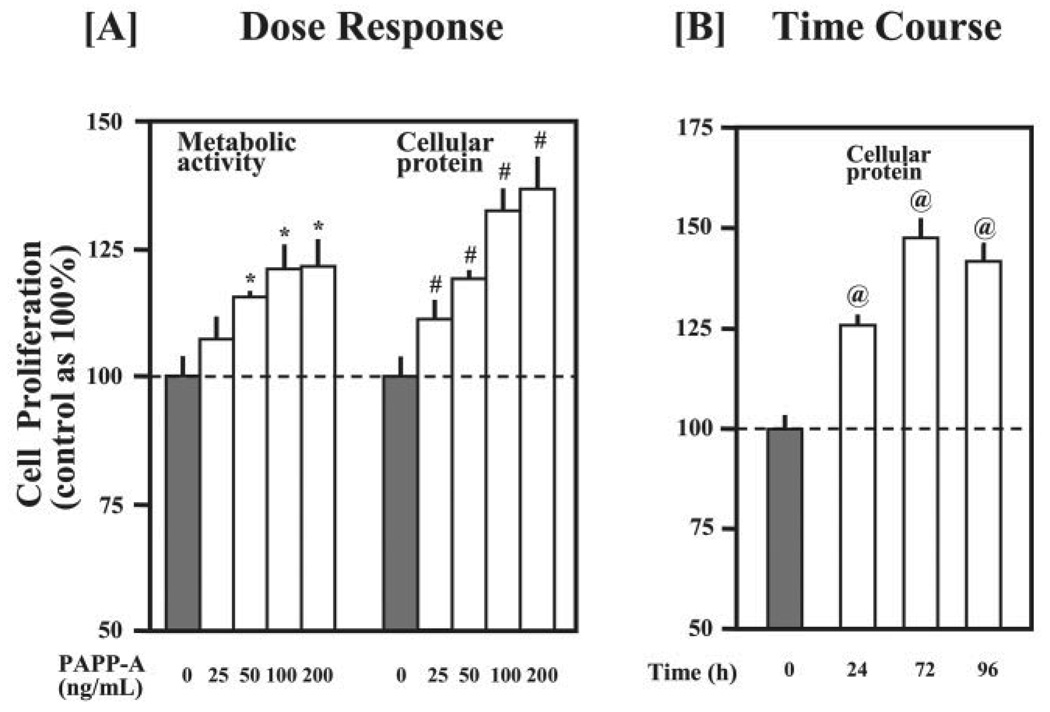
Because C2C12 myoblasts cultured at low cell density in serum-free medium underwent severe apoptosis and did not proliferate, the stimulatory effect of PAPP-A on cell proliferation was less pronounced under serum-free conditions (data not shown). Therefore, we subsequently used 5% FCS medium to study the effect of PAPP-A on cellular proliferation. As shown in Fig. 2A, treatment with PAPP-A increased the proliferation of C2C12 myoblasts in a dose-dependent manner with maximum effect observed at a concentration of 100 ng/ml PAPP-A. Furthermore, the cellular proliferation peaked after 72 h of PAPP-A treatment (Fig. 2B).
FIGURE 2. Effect of PAPP-A on the proliferation of C2C12 myoblasts.
Cellular proliferation was first determined by AlamarBlue assay, followed by quantitation of cellular proteins by Bradford reagent in the cell lysates as described under “Experimental Procedures.” A, C2C12 myoblasts were cultured for 48 h in DMEM containing 5% FCS and varying amounts of purified recombinant PAPP-A protein. The data presented here show that PAPP-A treatment increases the proliferation of C2C12 myoblasts in a dose-dependent manner (*, p<0.05 versus untreated control and #, p<0.05 versus untreated control). B, C2C12 myoblasts were seeded in 24-well plates (10,000 cells/well) and treated with PAPP-A (100 ng/ml). After PAPP-A treatment, cellular proliferation was measured at the indicated time points. The data show that the maximum proliferation of C2C12 myoblasts was noticed after 72 h of PAPP-A treatment (@, p <0.05 versus 0 h).
PAPP-A Promotes Differentiation of C2C12 Myoblasts into Myotubes
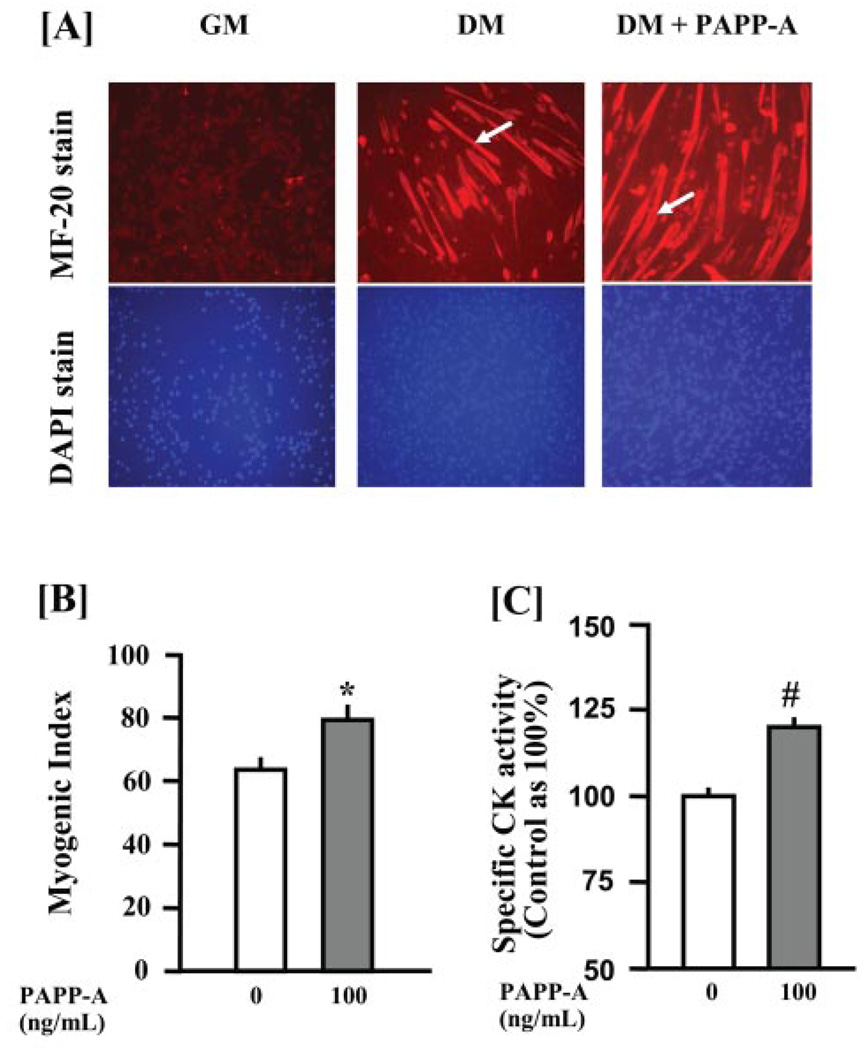
The effect of PAPP-A on differentiation of C2C12 myoblasts was examined. C2C12 myoblasts were incubated in DM (DMEM/2% heat-inactivated horse serum) with or without 100 ng/ml PAPP-A. Myoblasts cultured in growth medium (DMEM/10% FCS) were included as a negative control for myogenic differentiation. After 96 h, myotube formation in C2C12 cultures was studied by immunostaining for MyHCf protein. No fusion of myoblasts into myotubes was observed in C2C12 myoblasts incubated in GM (Fig. 3A, left panel). Incubation of C2C12 myoblasts in DM led to formation of myotubes expressing MyHCf (Fig. 3A, middle panel, arrow). Interestingly, the size and the number of myotubes in C2C12 cultures were significantly increased with PAPP-A treatment (Fig. 3A, right panel, arrow).
FIGURE 3. PAPP-A increases the differentiation of C2C12 myoblast.
C2C12 myoblasts were cultured in growth medium (GM, left panel) or differentiation medium (DM) in the absence (middle panel) or in the presence (right panel) of 100 ng/ml purified recombinant PAPP-A for 96 h. A, cells were labeled with MyHCf-specific MF-20 antibody and then subjected to nuclear staining as described under “Experimental Procedures.” The data presented here show an increased number and size of myotubes in PAPP-A-treated C2C12 cultures as compared with untreated cultures (arrows indicate the difference in the size of myotubes). B, the myogenic index (percentage of total nuclei associated with myotubes) was significantly higher in the PAPP-A-treated C2C12 cultures as compared with the C2C12 cultures incubated in DM alone (*, p <0.05 versus control). C, creatine kinase (CK) activity measured in cell lysates after adjustment for protein concentrations was also significantly higher in PAPP-A-treated C2C12 cultures compared with the untreated C2C12 cultures (#, p <0.05 versus control).
The myogenic index (the fraction of total nuclei residing in cells containing ≥3 nuclei in hematoxylin-stained C2C12 cultures) was also determined in a separate experiment. A significant increase in myogenic index was observed in PAPP-A-treated C2C12 cultures compared with control C2C12 cultures incubated in DM alone (Fig. 3B). Additionally, treatment of C2C12 myoblasts with PAPP-A significantly increased the activity of creatine kinase (Fig. 3C), an important biochemical marker for myogenic differentiation (34).
Overexpression of PAPP-A in C2C12 Myoblasts Promotes Their Proliferation and Differentiation
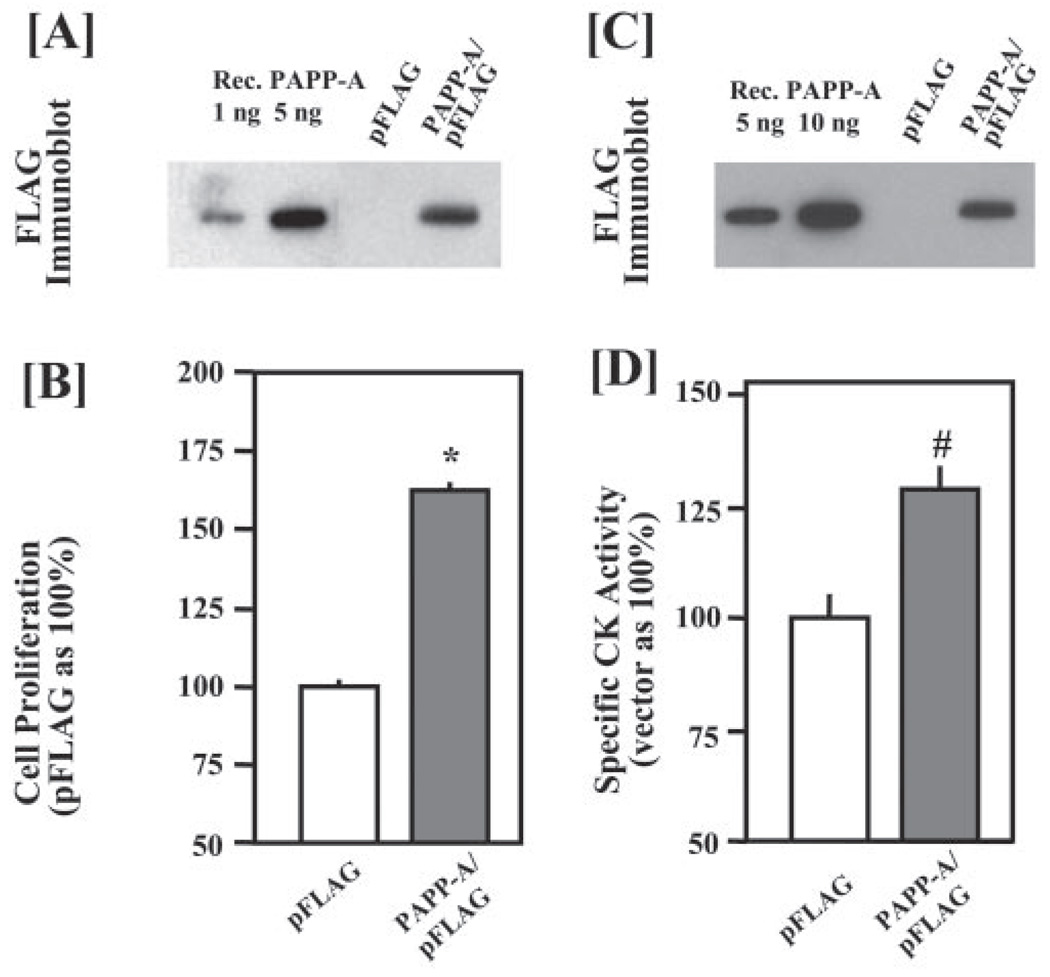
Based on our assessment of the purity of our recombinant PAPP-A protein (Fig. 1), it is unlikely that the observed effects of PAPP-A on myoblasts result from other contaminating growth factors possibly co-purified with PAPP-A. To completely rule out this possibility, we determined whether direct overexpression of PAPP-A in C2C12 myoblasts could also promote their proliferation and differentiation. C2C12 myoblasts were transfected with 2 µg of either empty pFLAG vector DNA or PAPP-A/pFLAG plasmid DNA. Transfected C2C12 myoblasts were then cultured in either DMEM/5%FCS to study cellular proliferation or in DM to study differentiation. Expression of PAPP-A was confirmed by immunoblot analysis of the CM with FLAG antibody (Fig. 4, A and C). It was estimated that the concentration of PAPP-A in the CM of PAPP-A/pFLAG-transfected C2C12 myoblasts ranged from 75 to 200 ng/ml, similar to the concentration of recombinant PAPP-A used in prior experiments (Fig. 2). Consistent with results from experiments using exogenously added recombinant PAPP-A (Fig. 2), overexpression of PAPP-A in C2C12 myoblasts significantly increased their proliferation (Fig. 4B). Furthermore, overexpression of PAPP-A in C2C12 myoblasts enhanced their differentiation, evident by increased specific creatine kinase activity (Fig. 4D).
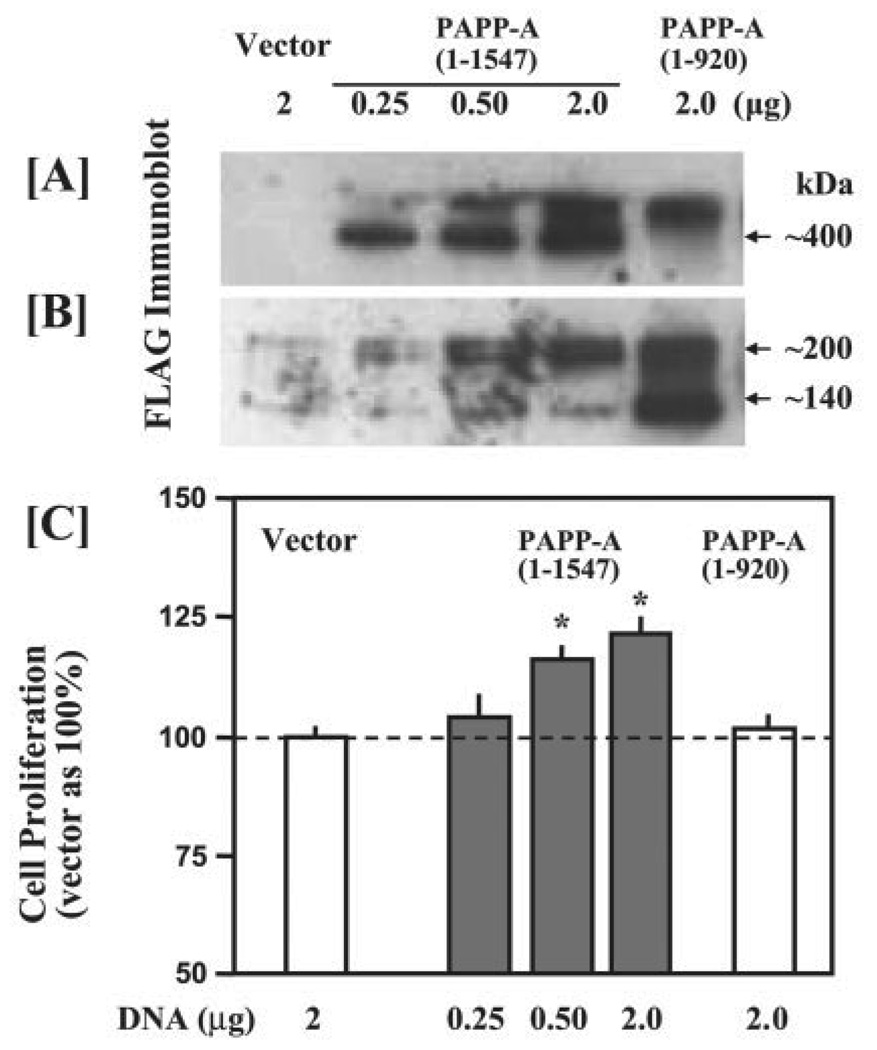
FIGURE 4. Transient overexpression of PAPP-A increases myoblast proliferation and differentiation.
C2C12 myoblasts were transfected with 2 µg/well of either pFLAG empty vector DNA or PAPP-A/pFLAG plasmid DNA. The production of recombinant PAPP-A after transfection in each experiment was measured in culture supernatants and quantified using purified protein (A and C). After 16 h of transfection, the medium was replaced with either DMEM containing 5% fetal calf serum (for proliferation assay) or 2% horse serum (for differentiation study). The proliferation of myoblasts was studied using AlamarBlue dye, whereas the differentiation was monitored by assaying the creatine kinase (CK) activity. The data presented here show an increase in the proliferation (*, p <0.05 versus vector alone) (B) and the differentiation (#, p <0.05 versus vector alone) (D) in PAPP-A-transfected C2C12 cultures as compared with C2C12 cultures transfected with vector alone.
Proteolytic Activity of PAPP-A Is Required for Its Stimulatory Effect on Myoblast Proliferation
Studies were performed to investigate whether the myogenic activity of PAPP-A is determined by its proteolytic activity or is due to other potential functional domains (25). Cell proliferation was compared in C2C12 cells transfected with either the plasmid expressing the full-length PAPP-A-(1–1547) or the N-terminal PAPP-A-(1–920), which exhibits at least two orders of magnitude reduction in IGFBP-4 proteolytic activity (26). Immunoblot analysis with FLAG antibody revealed a dose-dependent increase in PAPP-A production with increasing amounts of PAPP-A/pFLAG plasmids (Fig. 5A). In our previous studies, nonreduced PAPP-A-(1–920) peptide overexpressed in 293T cells was detected as a primary band at 120 kDa and aminor band at >500 kDa (26). Although only the >500-kDa band was detected in the CM of transfected C2C12 cells (Fig. 5A), reduced PAPP-A-(1–920) was detected as a major band of 140 kDa (Fig. 5B) as reported in our previous studies (26). Overexpression of the full-length PAPP-A dose-dependently stimulated C2C12 cell growth, whereas overexpression of the PAPP-A-(1–920) peptide had no significant effect (Fig. 5C). Additionally, overexpression of a protease-inactive C-terminal PAPP-A-(1100–1547) peptide containing the five complement control protein modules (25) had no effect on myoblast proliferation (data not shown). These data demonstrate that the proteolytic activity of PAPP-A is required to promote the myogenic program in C2C12 myoblasts.
FIGURE 5. Effect of overexpression of mutant PAPP-A on the proliferation of C2C12 myoblasts.
C2C12 myoblasts were transfected with either full-length (amino acids 1–1547) or truncated (amino acids 1–920) PAPP-A plasmid as described in Fig. 4A. Empty vector DNA was used to adjust the total amount of DNA to 2 µg/well. 40 µl of CM was treated without (A) or with (B) reducing agent β-mercaptoethanol and subjected to immunoblot analysis with FLAG antibody. C, proliferation of transfected C2C12 myoblasts was determined in 5% fetal calf serum medium using AlamarBlue assay as described under “Experimental Procedures.” The data presented here show that overexpression of full-length PAPP-A, but not truncated PAPP-A, significantly increased the proliferation of C2C12 myoblasts (*, p <0.05 versus vector alone).
PAPP-A Degrades IGFBP-2 Present in the Culture Supernatants of C2C12 Myoblasts
To understand the mechanisms responsible for increased myogenesis in response to PAPP-A, we tested the hypothesis that degradation of IGFBPs in cultured myoblasts by PAPP-A increases the bioavailability of IGFs. IGF-II ligand blot analysis of serum-free CM collected from C2C12 myoblasts revealed the presence of a predominant 30-kDamurine IGFBP-2 (Fig. 6A, mIGFBP-2). It has been reported that mIGFBP-2 is the predominant IGFBP produced by C2C12 myoblast (35, 36). IGF binding activity of mIGFBP-2 in the CM of C2C12 cultures was estimated to be equivalent to >200 ng/ml IGFBP-4. To determine whether mIGFBP-2 could be cleaved by PAPP-A, 40 µl of serum-free CM from C2C12 cultures was incubated with varying amounts of recombinant PAPP-A. As shown in Fig. 6B, the 30-kDa mIGFBP-2 was cleaved by PAPP-A in a dose-dependent manner. Addition of IGF-II (known to promote cleavage of IGFBP-4 by PAPP-A) to protease assays also enhanced the degradation of mIGFBP-2 (Fig. 6B).
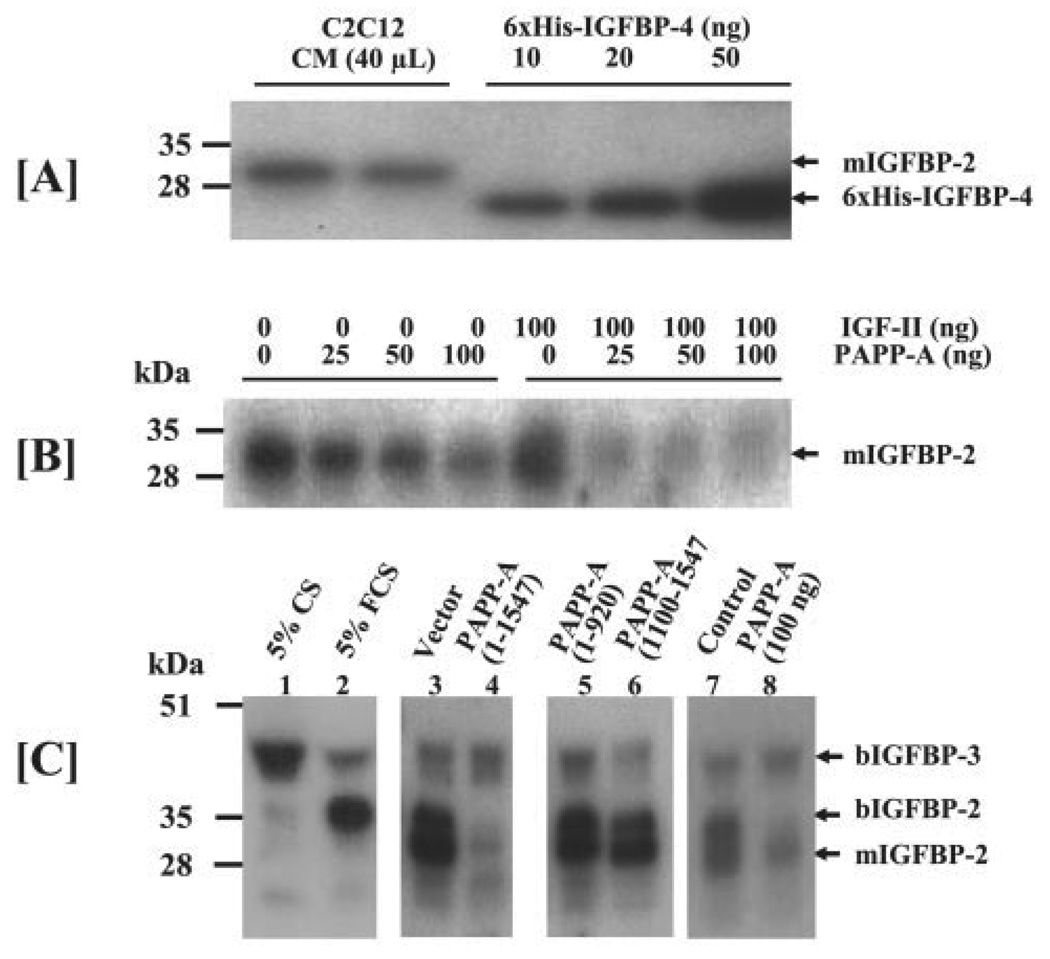
FIGURE 6. [125I]IGF-II ligand blot analysis of the IGFBPs in C2C12 cultures.
A, C2C12 myoblasts were seeded in 10-cm dishes in DMEM containing 10% fetal calf serum. Upon 100% confluence, cells were rinsed twice with DMEM and cultured in DMEM/0.1% bovine serum albumin for an additional 48 h prior to collection of CM. 40 µl of CM was subjected to [125I]IGF-II ligand blot analysis. Recombinant His6-IGFBP-4 was used as a standard to estimate the amount of total IGF binding activity in the CM samples. The 30-kDa IGFBP, previously identified as the murine IGFBP-2 (mIGFBP-2), is the primary IGFBP produced by C2C12 myoblasts. B, 40 µl of serum-free C2C12CM was incubated for 20 h at 37 °C with increasing concentrations of recombinant PAPP-A in the absence or presence of 100 ng of IGF-II. Incubated CM was then subjected to [125I]IGF-II ligand blot analysis. The data presented here show that PAPP-A cleaves the mIGFBP-2 in a dose-dependent manner and the cleavage of mIGFBP-2 is further increased in the presence of IGF-II. C, 40 µl of CM from either C2C12 myoblasts that were treated with 100 ng/ml PAPP-A (pooled from three independent experiments) or transfected with PAPP-A expression plasmid (pooled from two independent experiments) were subjected to IGF-II ligand blot analysis in order to measure proteolysis of IGFBPs. 5% CS or 5% FCS was used to differentiate the exogenous IGFBPs from the endogenous IGFBPs in the CM. The data show degradation of the 30-kDa mIGFBP-2 and 34-kDa bovine IGFBP-2 (bIGFBP-2) in the CM of the C2C12 cultures treated with exogenous PAPP-A or those transfected with PAPP-A-(1–1547). The levels of mIGFBP-2 or bIGFBP-2 in the CM of C2C12 myoblasts transfected with either PAPP-A-(1–920) or PAPP-A-(1100–1547) were not affected.
We also evaluated the degradation of IGFBPs in the CM collected from C2C12 myoblasts either treated with PAPP-A or transfected with PAPP-A/pFLAG plasmid. CM samples, freshly prepared DMEM/5% bovine calf serum (CS), or DMEM/5% bovine FCS were subjected to IGF-II ligand blot analysis to differentiate C2C12 myoblast-produced IGFBPs from those present in added serum. The predominant IGFBP in bovine CS is the ~40-kDa bIGFBP-3 (Fig. 6C, lane 1), whereas the major IGFBP in bovine FCS (lane 2) is the 34-kDa IGFBP, previously determined to be bovine IGFBP-2 (37, 38). Identities of the 34-kDa IGFBP as bovine IGFBP-2 (bIGFBP-2) and the 30-kDa IGFBP as murine IGFBP-2 (mIGFBP-2) were also supported by the finding that the 30-kDa mIGFBP produced by C2C12 cells is recognized by the antibody raised against bIGFBP-2 (39). The reduced level of bIGFBP-2 in bovine CS compared with bovine FCS (Fig. 6C, lane 1 versus lane 2) is consistent with the observation that rodent serum IGFBP-2 levels decrease after birth (40). The majority of the mIGFBP-2 and bIGFBP-2 were cleaved in the CM of C2C12 myoblasts transfected with the full-length proteolytically active PAPP-A-(1–1547) plasmid (Fig. 6C, lane 4 versus lane 3). Overexpression of each mutant PAPP-A peptide had no effect on degradation of IGFBP-2 (Fig. 6C, lanes 5 and 6 versus lane 3). Similar proteolysis of the endogenous or exogenous IGFBP-2 was observed in the CM of C2C12 myoblasts treated with exogenous PAPP-A protein (Fig. 6C, lane 8 versus lane 7). Consistent with our previously published report (21), IGFBP-3 (mainly originating from the added bovine serum) was not degraded by PAPP-A.
PAPP-A Increases the Free IGF Concentration in C2C12 Cultures
The ability of PAPP-A to increase free IGF-I concentrations through enhancing degradation of the IGFBP-2 produced by the myoblasts was investigated. The effect of PAPP-A on proliferation was determined in C2C12 myoblasts cultured in the presence of 5% bovine CS that contained a significantly lower amount of the 34-kDa bovine IGFBP-2 (bIGFBP-2) as compared with 5% bovine FCS (Fig. 6C, lane 1 versus lane 2). Consistent with the results from experiments using 5% bovine FCS, overexpression of PAPP-A significantly increased cell proliferation in the presence of 5% CS (Fig. 7A) and caused a complete degradation of the mIGFBP-2 endogenously produced by the C2C12 myoblasts (Fig. 7B). Free IGF-I in the CM of vector plasmid-transfected cells was below the sensitivity of the assay (<0.1 ng/ml). The concentration of free IGF-I in the CM of C2C12 cells overexpressing PAPP-A was increased to 1.3 ± 0.1 ng/ml, which is ~10% of the total IGF-I (Fig. 7C). A similar increase in free IGF-I concentration was found in the CM of cells treated with recombinant PAPP-A in either DMEM/5% CS or DMEM/5% FCS (data not shown). It should be noted that the free IGF-I enzyme-linked immunosorbent assay measures truly free IGF-I. The IGF-I loosely bound to the N-terminal IGFBP-2 proteolytic fragments may not be detected as free IGF-I but can be released to interact with its receptors more quickly than IGF-I bound to the intact high affinity IGFBP-2.
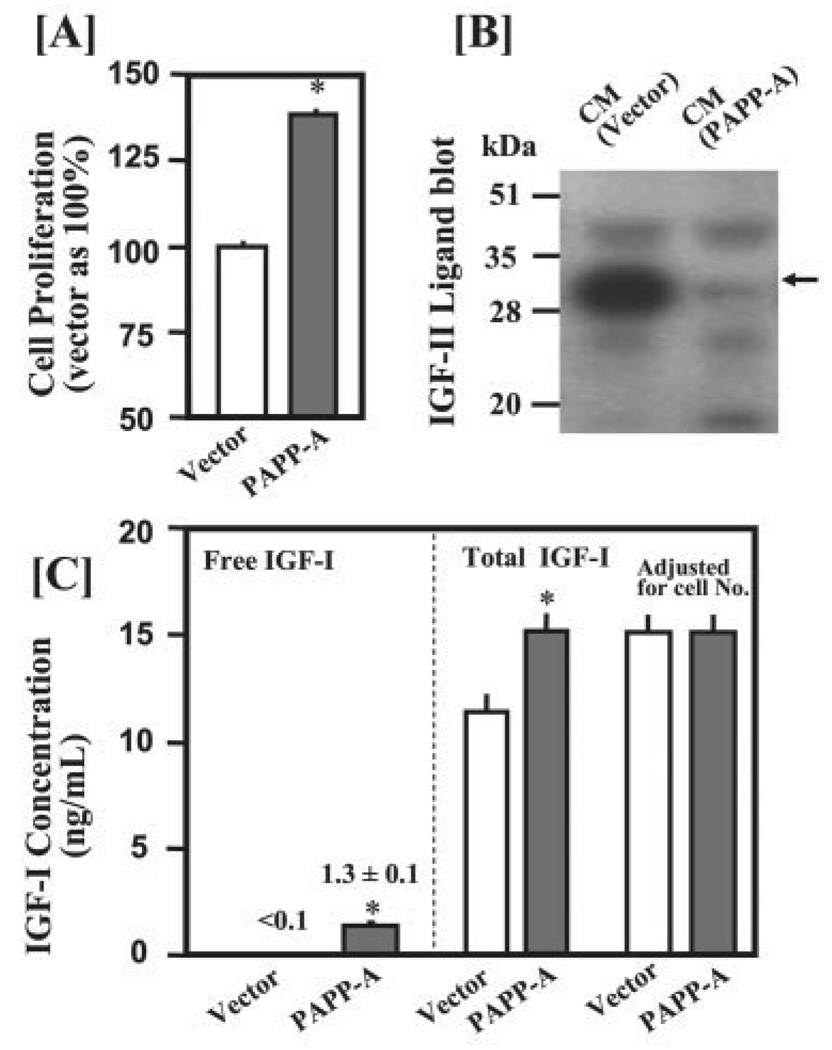
FIGURE 7. PAPP-A overexpression increases free IGF-I concentration primarily through enhancing degradation of the IGFBP-2 produced by C2C12 myoblasts.
C2C12 cells were seeded in 5% CS in 6-well plates at a density of 80,000 cells/well. After 24 h of incubation, cells were transfected with 1 µg of pFLAG empty vector DNA or PAPP-A-(1–1547)/pFLAG plasmids in 1 ml of DMEM containing 5% CS. After 24 h of incubation, medium was replaced with 2 ml of DMEM containing 5% CS. CM samples were collected after an additional 48 h of incubation for determination of IGFBP proteolysis and IGF-I concentrations. A, overexpression of PAPP-A significantly increased cell proliferation as determined by the AlamarBlue assay. B, 40 µl of CM were subjected to IGF-II ligand blot analysis. Overexpression of PAPP-A caused a nearly complete degradation of the mIGFBP-2 (indicated by arrow) produced by C2C12 myoblasts. C, 50 µl of CM was used to determine free IGF-I concentrations by a commercial kit (Diagnostic Systems Laboratories, Webster, Texas). Total IGF-I concentrations were determined by radioimmunoassay (29). Similar results were obtained in another independent experiment. *, p <0.05 versus vector control (n = 6)
To exclude the possibility that increase in free IGF-I concentration is due to increased IGF-I production in response to PAPP-A treatment, we also estimated the total IGF-I (free IGF-I + IGF-I bound to IGFBPs in the culture medium) in the same samples. PAPP-A overexpression significantly increased the total IGF-I concentration in the CM by ~30%. However, no significant difference was observed after normalizing for the cell numbers in control and PAPP-A-transfected cultures (Fig. 7C). In addition, quantitative real-time PCR analysis revealed that treatment of C2C12 cells with PAPP-A did not affect the level of Igf-I mRNA (data not shown). These data suggest that PAPP-A increases free IGF-I concentration through degradation of IGFBP-2 endogenously produced by the C2C12 myoblasts.
PAPP-A Enhances C2C12 Myoblast Proliferation via an IGF-dependent Mechanism
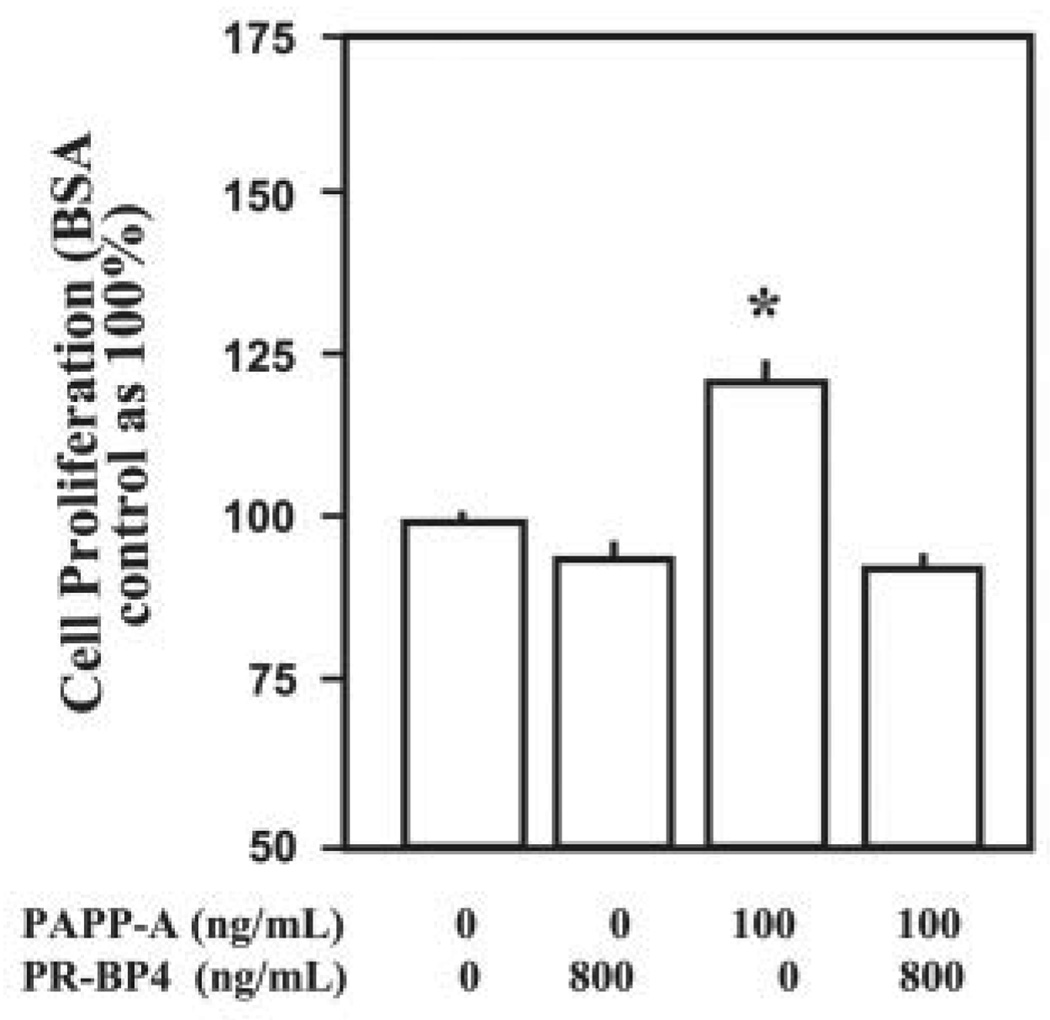
To further confirm that PAPP-A promotes myoblast proliferation by increasing the bioavailability of IGFs, we investigated whether inhibition of the activity of IGFs could block cell proliferation induced by PAPP-A. We have previously shown that the PR-BP4 (protease-resistant IGFBP-4) analog binds to IGFs (IGF-I and IGF-II) with similar affinity as exhibited by the wild-type IGFBP-4 but cannot be cleaved by the IGFBP-4 protease produced by osteoblasts (33, 41). More importantly, this mutant IGFBP-4 peptide cannot be cleaved by PAPP-A (Fig. 1D). Thus, this IGFBP-4 mutant can serve as an ideal IGF inhibitor in the presence of PAPP-A. C2C12 myoblasts were treated with PAPP-A either alone or in combination with PR-BP4 peptide, and the cellular proliferation was measured. Interestingly, PAPP-A-induced myoblast proliferation was completely abolished by the PR-BP4 (Fig. 8). It should be noted that PR-BP4 at the same concentration did not inhibit cell proliferation induced by des-1–3 IGF-I (lacking the first 3 amino acids), which has very low binding affinity with IGFBPs (data not shown).
FIGURE 8. PAPP-A augments C2C12 myoblast proliferation via an IGF-dependent mechanism.
DMEM/5% FCS supplemented with effectors at the indicated concentrations was added to the C2C12 cell cultures, and cellular proliferation was measured using AlamarBlue assay after 48 h of incubation. Data presented here show that PR-BP4 treatment completely blocked the PAPP-A-induced proliferation of C2C12 myoblasts (*, p <0.05 versus PAPP-A alone).
DISCUSSION
Using exogenous recombinant PAPP-A or ectopic expression of PAPP-A in C2C12 myoblasts, we have studied the role and mechanism by which PAPP-A affects myogenesis. Our data clearly show that PAPP-A induces the proliferation and differentiation of C2C12 myoblasts. Increased myogenesis in response to PAPP-A is intrinsic to its proteolytic activity. Furthermore, the results of our in vitro experiments suggest that PAPP-A promotes C2C12 myoblast proliferation by degradation of IGFBP-2, the major IGFBP produced by C2C12 myoblasts. Based on the results of this study, we propose a model summarizing the mode of action of PAPP-A in skeletal myogenesis (Fig. 9).
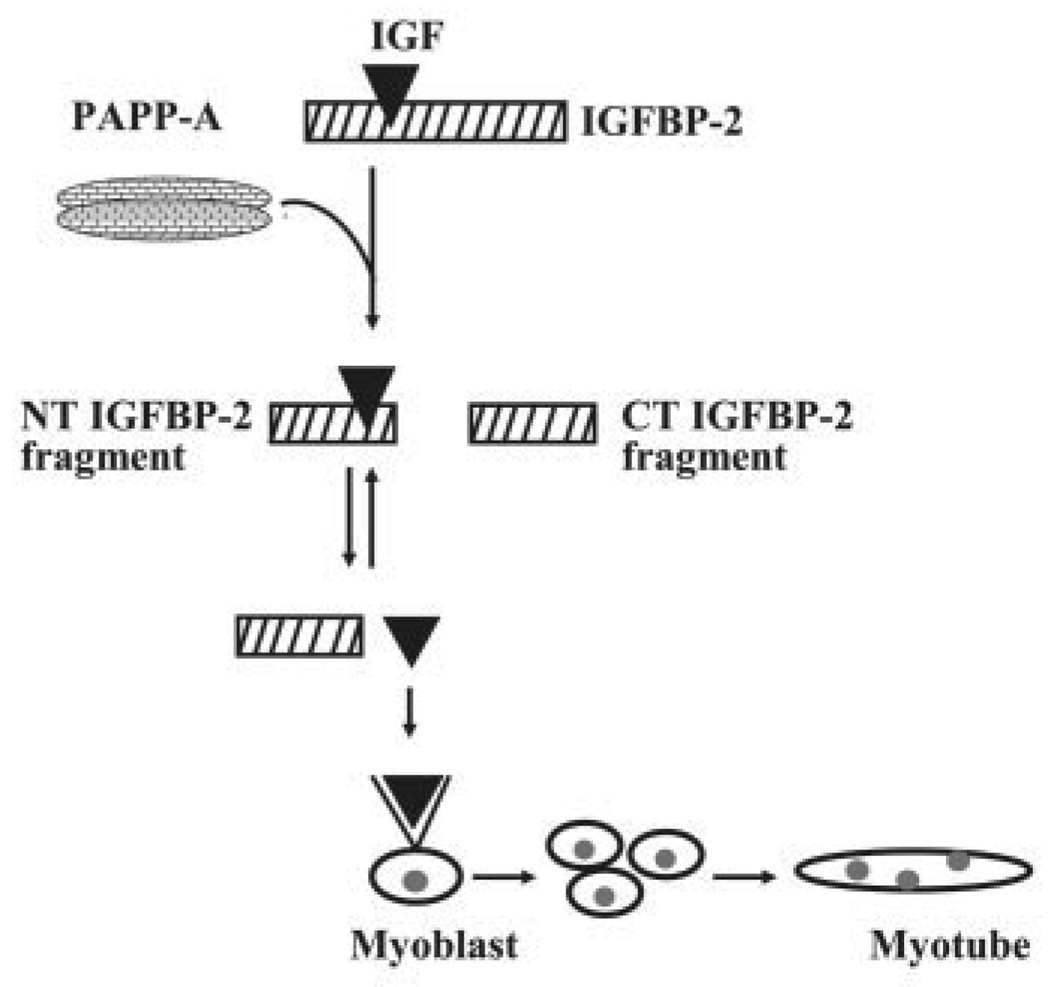
FIGURE 9. Proposed model for mechanism of action of PAPP-A in myogenesis.
PAPP-A cleaves IGFBP-2, leading to generation of a C-terminal (CT) IGFBP-2 fragment and an N-terminal (NT) IGFBP-2 fragment loosely bound to IGFs. The affinity of IGFs with the NT IGFBP-2 fragment is much lower than the affinity of IGFs with intact IGFBP-2 or the affinity of IGFs with type I IGF receptors. Thus, IGFs bound to the NT IGFBP-2 fragment readily dissociate to interact with IGF receptors in myoblasts, thereby leading to enhanced myoblast proliferation and differentiation.
Myogenesis is a developmental program that generates and regenerates skeletal muscle (3). Intensive research in the last decade has led to significant understanding of the complexity of specification and differentiation of skeletal muscle cells in mammals. Myogenic differentiation is regulated by both positive- and negative-acting factors (42). Serum and peptide growth factors, such as transforming growth factor-β and fibroblast growth factor, are potent inhibitors of myoblast differentiation (43, 44). Myostatin, a growth and differentiation factor belonging to the transforming growth factor-β superfamily, is a negative regulator of skeletal muscle mass and myoblast terminal differentiation (45, 46). A number of inflammatory cytokines such as tumor necrosis factor-α, interleukin-1β, and interleukin-6 have also been shown to inhibit the myogenic differentiation through distinct mechanisms (34, 47). In contrast, IGFs promote muscle differentiation and increase muscle mass (4, 48). Our study shows that PAPP-A is a potent regulator of myogenic differentiation, hence adding a new member to the growing list of molecules modulating skeletal myogenesis.
PAPP-A represents the newest member of the complex IGF/IGFBP system that could be involved in the regulation of diverse biological functions. The physiological significance of PAPP-A can be best attested to by a recent report showing that targeted deletion of the Papp-a gene in mice caused a global growth retardation (24). Curiously, we found that PAPP-A not only promotes myoblast proliferation but also induces differentiation of myoblasts into myotubes similar to IGFs. Our group and others have proposed that PAPP-A may increase the bioavailability of IGFs through degradation of selected IGFBPs (18–21, 23). Thus far, direct evidence to support this hypothesis has been lacking. In this report, for the first time, we provide direct evidence that PAPP-A induces the proliferation of C2C12 myoblasts by increasing the free IGF concentration through enhanced degradation of IGFBPs, especially IGFBP-2. Because PAPP-A can cleave multiple IGFBPs whose relative abundance to other non-PAPP-A substrate IGFBPs may vary among tissues/cell types, strategies developed in this study could be employed to further explore the role and mechanism of action of PAPP-A in other systems.
The activity of IGFs in any biological system is determined by the balance between IGFs and IGFBPs. Because intact IGFBPs have higher affinity for IGFs (Kd ~10−10 m) than the type-I IGF receptors (Kd ~10−8–10−9 m), binding of IGFs to IGF receptors is limited when IGFBPs are in excess relative to IGFs (49). In essentially all body fluids, a majority of IGFs exit in the form of IGFBP•IGF complex, which is biologically inactive (50). Among the six high affinity IGFBPs, IGFBP-2, IGFBP-4, and IGFBP-5 can be cleaved by PAPP-A, as supported by the data in this study (Fig. 6) and those reported earlier (18–21, 23).
There are three major IGFBPs with different molecular masses on SDS-PAGE in the C2C12 myoblast culture system: a 30-kDamIGFBP-2 produced by the myoblasts, a 34-kDa bIGFBP-2 originating from exogenously added bovine serum, and a ~40-kDa bIGFBP-3 (Fig. 6C). Our data suggest that IGFBP-2 produced by myoblasts and present in FCS accounts for >80% of the total IGF binding activity and can be effectively cleaved by PAPP-A (Figs. 6 and 7). The importance of endogenously produced IGFBP-2 by myoblasts in mediating the effects of PAPP-A is supported by the data that PAPP-A overexpression significantly increased proliferation of C2C12 myoblasts in the presence of 5% bovine CS that contains very low levels of the bIGFBP-2 (Fig. 6C, left panel). Based on the abundance of IGFBP-2 in the CM of C2C12 cultures, the majority of IGFs would be expected to exist in the IGF•IGFBP-2 complex, which is biologically inactive. Indeed, there is essentially no free IGF-I in control C2C12 cultures (Fig. 7C).
Although proliferation and differentiation are both necessary for myogenesis, they are intrinsically opposing pathways, because most factors that stimulate proliferation inhibit differentiation and vice versa (4, 48). IGFs are rare growth factors, because they are able to stimulate both proliferation and differentiation of myoblasts (4). Our finding that the dual biological functions of IGFs in myogenesis are reproduced by PAPP-A treatment implicates that PAPP-A acts on myoblasts via an IGF-dependent mechanism. This contention is strongly supported by three lines of evidence. First, PAPP-A fragments with extremely low or no IGFBP proteolytic activity (26) are unable to stimulate myoblast proliferation (Fig. 5). Second, PAPP-A overexpression led to significant levels of free IGF-I, which was essentially absent in untreated C2C12 cultures (Fig. 7C). Third, biological activity of PAPP-A on myoblasts was completely abolished by a potent IGF inhibitor, protease-resistant IGFBP-4 analog (Fig. 8), which should neutralize all of the free IGFs from the system.
Studies demonstrate that addition of exogenous IGFBP-2 inhibits proliferation or differentiation of myoblasts induced by exogenous IGFs (51). Consistent with these in vitro data, it has been reported recently that serum IGFBP-2 concentrations are negatively correlated with skeletal muscle strength, physical activity, and bone quality in the elderly (52, 53). Based on our in vitro findings that PAPP-A promotes myogenesis via an IGF-dependent mechanism that involves degradation of IGFBP-2 (Fig. 9), it is conceivable that PAPP-A may play an important role in the regulation of myogenesis in vivo. Future studies involving targeted overexpression of PAPP-A in skeletal muscle or local intramuscular injection of recombinant PAPP-A peptide are needed to define the in vivo role of PAPP-A in skeletal myogenesis.
Acknowledgments
We thank Arun Sivanandam, Christopher Sexton, Tina Boykin, Daniela Guilliam, Dr. Georgy Bagi, and Dr. Sanjay Kapur for excellent technical help. We also thank Gina Risso for proofreading our manuscript. All work was performed at facilities provided by the Jerry L. Pettis Memorial Veterans Affairs Medical Center in Loma Linda, CA.
Footnotes
This work was supported by a Department of Veterans Affairs grant (Merit Review to X. Q.), National Institutes of Health Grants R01 AR45210 (to X. Q.) and R01 AR31062 (to S. M.), and grants from the Muscular Dystrophy Association (to A. K.) and Loma Linda University (to X. Q.). The costs of publication of this article were defrayed in part by the payment of page charges.
The abbreviations used are: IGF, insulin-like growth factor; IGFBP, IGF-binding protein; mIGFBP, murine IGFBP; bIGFBP, bovine IGFBP; CM, conditioned medium; CS, calf serum; DMEM, Dulbecco’s modified Eagle’s medium; DM, differentiation medium (DMEM supplemented with 2% horse serum); FCS, fetal calf serum; MyHCf, myosin heavy chain-fast twitch; PAPP-A, pregnancy-associated plasma protein; PBS, phosphate-buffered saline; PR-BP4, PAPP-A-resistant IGFBP-4.
REFERENCES
- 1.Perry RL, Rudnick MA. Front. Biosci. 2000;5:D750–D767. doi: 10.2741/perry. [DOI] [PubMed] [Google Scholar]
- 2.Charge SB, Rudnicki MA. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 3.Yun K, Wold B. Curr. Opin. Cell. Biol. 1996;8:877–889. doi: 10.1016/s0955-0674(96)80091-3. [DOI] [PubMed] [Google Scholar]
- 4.Florini JR, Ewton DZ, Coolican SA. Endocr. Rev. 1996;17:481–517. doi: 10.1210/edrv-17-5-481. [DOI] [PubMed] [Google Scholar]
- 5.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A. Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- 6.Powell-Braxton L, Hollingshead P, Warburton C, Dowd M, Pitts-Meek S, Dalton D, Gillett N, Stewart TA. Genes Dev. 1993;7:2609–2617. doi: 10.1101/gad.7.12b.2609. [DOI] [PubMed] [Google Scholar]
- 7.Mathews LS, Hammer RE, Behringer RR, D’Ercole AJ, Bell GI, Brinster RL, Palmiter RD. Endocrinology. 1988;123:2827–2833. doi: 10.1210/endo-123-6-2827. [DOI] [PubMed] [Google Scholar]
- 8.Barton-Davis ER, Shoturma DI, Musaro A, Rosenthal N, Sweeney HL. Proc. Natl. Acad. Sci. U. S. A. 1998;95:15603–15607. doi: 10.1073/pnas.95.26.15603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton ER, Morris L, Musaro A, Rosenthal N, Sweeney HL. J. Cell Biol. 2002;157:137–148. doi: 10.1083/jcb.200108071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen CJ, Donahue LR, Hunter SJ. Proc. Soc. Exp. Biol. Med. 1994;206:83–102. doi: 10.3181/00379727-206-43726. [DOI] [PubMed] [Google Scholar]
- 11.Jones JI, Clemmons DR. Endocr. Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 12.Clemmons DR. Cytokine Growth Factor Rev. 1997;8:45–62. doi: 10.1016/s1359-6101(96)00053-6. [DOI] [PubMed] [Google Scholar]
- 13.Haugk KL, Wilson HM, Swisshelm K, Quinn LS. Endocrinology. 2000;141:100–110. doi: 10.1210/endo.141.1.7235. [DOI] [PubMed] [Google Scholar]
- 14.Xi G, Kamanga-Sollo E, Pampusch MS, White ME, Hathaway MR, Dayton WR. J. Cell. Physiol. 2004;200:387–394. doi: 10.1002/jcp.20068. [DOI] [PubMed] [Google Scholar]
- 15.Ewton DZ, Coolican SA, Mohan S, Chernausek SD, Florini JR. J. Cell. Physiol. 1998;177:47–57. doi: 10.1002/(SICI)1097-4652(199810)177:1<47::AID-JCP5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Damon SE, Haugk KL, Birnbaum RS, Quinn LS. J. Cell. Physiol. 1998;175:109–120. doi: 10.1002/(SICI)1097-4652(199804)175:1<109::AID-JCP12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 17.Salih DA, Tripathi G, Holding C, Szestak TA, Gonzalez MI, Carter EJ, Cobb LJ, Eisemann JE, Pell JM. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4314–4319. doi: 10.1073/pnas.0400230101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence JB, Oxvig C, Overgaard MT, Sottrup-Jensen L, Gleich GJ, Hays LG, Yates JR, III, Conover CA. Proc. Natl. Acad. Sci. U. S. A. 1999;96:3149–3153. doi: 10.1073/pnas.96.6.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin X, Byun D, Lau KH, Baylink DJ, Mohan S. Arch. Biochem. Biophys. 2000;379:209–216. doi: 10.1006/abbi.2000.1872. [DOI] [PubMed] [Google Scholar]
- 20.Qin X, Sexton C, Byun D, Strong DD, Baylink DJ, Mohan S. Growth Horm. IGF Res. 2002;12:359–366. doi: 10.1016/s1096-6374(02)00046-1. [DOI] [PubMed] [Google Scholar]
- 21.Byun D, Mohan S, Yoo M, Sexton C, Baylink DJ, Qin X. J. Clin. Endocrinol. Metab. 2001;86:847–854. doi: 10.1210/jcem.86.2.7223. [DOI] [PubMed] [Google Scholar]
- 22.Laursen LS, Overgaard MT, Soe R, Boldt HB, Sottrup-Jensen L, Giudice LC, Conover CA, Oxvig C. FEBS Lett. 2001;504:36–40. doi: 10.1016/s0014-5793(01)02760-0. [DOI] [PubMed] [Google Scholar]
- 23.Monget P, Mazerbourg S, Delpuech T, Maurel MC, Maniere S, Zapf J, Lalmanach G, Oxvig C, Overgaard MT. Biol. Reprod. 2003;68:77–86. doi: 10.1095/biolreprod.102.007609. [DOI] [PubMed] [Google Scholar]
- 24.Conover CA, Bale LK, Overgaard MT, Johnstone EW, Laursen UH, Fuchtbauer EM, Oxvig C, van Deursen J. Development. 2004;131:1187–1194. doi: 10.1242/dev.00997. [DOI] [PubMed] [Google Scholar]
- 25.Kristensen T, Oxvig C, Sand O, Moller NP, Sottrup-Jensen L. Biochemistry. 1994;33:1592–1598. doi: 10.1021/bi00172a040. [DOI] [PubMed] [Google Scholar]
- 26.Sivanandam AS, Mohan S, Kapur S, Kita H, Lau KH, Bagi G, Baylink DJ, Qin X. Arch. Biochem. Biophys. 2004;423:343–350. doi: 10.1016/j.abb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Miyakoshi N, Richman C, Kasukawa Y, Linkhart TA, Baylink DJ, Mohan S. J. Clin. Investig. 2001;107:73–81. doi: 10.1172/JCI10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sivanandam AS, Mohan S, Kita H, Kapur S, Chen ST, Linkhart TA, Bagi G, Baylink DJ, Qin X. Biochem. J. 2004;379:57–64. doi: 10.1042/BJ20030937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohan S, Libanati C, Dony C, Lang K, Srinivasan N, Baylink DJ. J. Clin. Endocrinol. Metab. 1995;80:2638–2645. doi: 10.1210/jcem.80.9.7545694. [DOI] [PubMed] [Google Scholar]
- 30.Tomczak KK, Marinescu VD, Ramoni MF, Sanoudou D, Montanaro F, Han M, Kunkel LM, Kohane IS, Beggs AH. FASEB J. 2004;18:403–405. doi: 10.1096/fj.03-0568fje. [DOI] [PubMed] [Google Scholar]
- 31.Delgado I, Huang X, Jones S, Zhang L, Hatcher R, Gao B, Zhang P. Genomics. 2003;82:109–121. doi: 10.1016/s0888-7543(03)00104-6. [DOI] [PubMed] [Google Scholar]
- 32.Laursen LS, Overgaard MT, Weyer K, Boldt HB, Ebbesen P, Christiansen M, Sottrup-Jensen L, Giudice LC, Oxvig C. J. Biol. Chem. 2002;277:47225–47234. doi: 10.1074/jbc.M209155200. [DOI] [PubMed] [Google Scholar]
- 33.Qin X, Byun D, Strong DD, Baylink DJ, Mohan S. J. Bone Miner. Res. 1999;14:2079–2088. doi: 10.1359/jbmr.1999.14.12.2079. [DOI] [PubMed] [Google Scholar]
- 34.Langen RC, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- 35.Ernst CW, McFarland DC, White ME. Differentiation. 1996;61:25–33. doi: 10.1046/j.1432-0436.1996.6110025.x. [DOI] [PubMed] [Google Scholar]
- 36.Ernst CW, White ME. J. Endocrinol. 1996;149:417–429. doi: 10.1677/joe.0.1490417. [DOI] [PubMed] [Google Scholar]
- 37.Funston RN, Moss GE, Roberts AJ. Endocrinology. 1995;136:62–68. doi: 10.1210/endo.136.1.7530196. [DOI] [PubMed] [Google Scholar]
- 38.Skaar TC, Baumrucker CR, Deaver DR, Blum JW. J. Anim. Sci. 1994;72:421–427. doi: 10.2527/1994.722421x. [DOI] [PubMed] [Google Scholar]
- 39.Ernst CW, McCusker RH, White ME. Endocrinology. 1992;130:607–615. doi: 10.1210/endo.130.2.1370791. [DOI] [PubMed] [Google Scholar]
- 40.Orlowski CC, Brown AL, Ooi GT, Yang YW, Tseng LY, Rechler MM. Endocrinology. 1990;126:644–652. doi: 10.1210/endo-126-1-644. [DOI] [PubMed] [Google Scholar]
- 41.Qin X, Strong DD, Baylink DJ, Mohan S. J. Biol. Chem. 1998;273:23509–23516. doi: 10.1074/jbc.273.36.23509. [DOI] [PubMed] [Google Scholar]
- 42.Husmann I, Soulet L, Gautron J, Martelly I, Barritault D. Cytokine Growth Factor Rev. 1996;7:249–258. doi: 10.1016/s1359-6101(96)00029-9. [DOI] [PubMed] [Google Scholar]
- 43.Vaidya TB, Rhodes SJ, Taparowsky EJ, Konieczny SF. Mol. Cell. Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olson EN, Sternberg E, Hu JS, Spizz G, Wilcox C. J. Cell Biol. 1986;103:1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas M, Langley B, Berry C, Sharma M, Kirk S, Bass J, Kambadur R. J. Biol. Chem. 2000;275:40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 46.McPherron AC, Lawler AM, Lee SJ. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 47.Langen RC, Van Der Velden JL, Schols AM, Kelders MC, Wouters EF, Janssen-Heininger YM. FASEB J. 2004;18:227–237. doi: 10.1096/fj.03-0251com. [DOI] [PubMed] [Google Scholar]
- 48.Florini JR, Ewton DZ, Magri KA. Annu. Rev. Physiol. 1991;53:201–216. doi: 10.1146/annurev.ph.53.030191.001221. [DOI] [PubMed] [Google Scholar]
- 49.Hwa V, Oh Y, Rosenfeld RG. Endocr. Rev. 1999;20:761–787. doi: 10.1210/edrv.20.6.0382. [DOI] [PubMed] [Google Scholar]
- 50.Rajaram S, Baylink DJ, Mohan S. Endocr. Rev. 1997;18:801–831. doi: 10.1210/edrv.18.6.0321. [DOI] [PubMed] [Google Scholar]
- 51.Gustafsson T, Andersson P, Arnqvist HJ. J. Endocrinol. 1999;161:245–253. doi: 10.1677/joe.0.1610245. [DOI] [PubMed] [Google Scholar]
- 52.van den Beld AW, Blum WF, Pols HA, Grobbee DE, Lamberts SW. Eur. J. Endocr. 2003;148:627–634. doi: 10.1530/eje.0.1480627. [DOI] [PubMed] [Google Scholar]
- 53.Amin S, Riggs BL, Atkinson EJ, Oberg AL, Melton LJ, III, Khosla S. J. Bone Min. Res. 2004;19:1075–1083. doi: 10.1359/JBMR.040301. [DOI] [PubMed] [Google Scholar]