Abstract
Rationale
Deficits in amygdala-related stimulus-reward learning are produced following 18 drug-free days of cocaine self-administration or its passive delivery in rats exposed during adulthood. No deficits in stimulus-reward learning are produced by cocaine exposure initiated during adolescence.
Objectives
To determine if age of initiating cocaine exposure differentially affects behavioral functioning of an additional memory system linked to cocaine addiction, the orbitofrontal cortex.
Materials and methods
A yoked-triad design (n=8) was used. One rat controlled cocaine delivery and the other two passively received cocaine or saline. Rats controlling drug delivery (1.0 mg/kg) self-administered cocaine from either P37–P59 or P77–P99, and then underwent 18 drug-free days (P60–P77 vs. P100–P117). Rats next were tested for acquisition of odor-delayed win-shift behavior conducted over 15 sessions (P78–P96 vs. P118–P136).
Results
Cocaine self-administration did not differ between adults and adolescents. During the test phase of the odor-delayed win-shift task (relatively difficult task demands), rats from both drug-onset ages showed learning deficits. Rats with cocaine self-administration experience committed more errors and had longer session latencies compared to rats passively receiving saline or cocaine. Rats with adolescent-onset cocaine self-administration experience showed an additional learning deficit by requiring more sessions to reach criterion levels for task acquisition compared to same-aged passive saline controls or rats with adult-onset cocaine self-administration experience. Rats passively receiving cocaine did not differ from the passive saline control from either age group.
Conclusions
Rats with adolescent-onset cocaine self-administration experience were more impaired in an orbitofrontal cortex-related learning task than rats with adult-onset cocaine self-administration experience.
Keywords: Adolescents, Adults, Cocaine, Non-spatial working memory, Orbitofrontal cortex, Self-administration
Adolescence in rodents and humans is marked by shifts in neurotransmission and distinct cellular level changes in cortical and subcortical regions (Casey et al. 2008). The age range for adolescence in male rats starts at approximately postnatal day 28 (P28) and extends to approximately P55; rats are considered young adults on approximately P60 (for review, see Spear 2000). Notably, in prefrontal cortex of rats, density of dopaminergic fibers increases continuously until approximately P60 (Kalsbeek et al. 1988). Additionally, dopamine (DA) D1 receptor density in prefrontal cortex peaks at approximately P40 and then declines into adulthood and stabilizes at approximately P100 (Andersen et al. 2000). In contrast, D2 and D4 receptor densities in prefrontal cortex rise continuously until approximately P35 and then remain stable through adulthood (Tarazi et al. 1998a). In striatum and nucleus accumbens, DA D1, D2 and D4 receptor densities peak at approximately P28, and then decline to adult levels by approximately P60 (Tarazi and Baldessarini 2000). This reorganization of the DA neurotransmitter system may render adolescent and adult rats differentially sensitive to a drug such as cocaine, which potently blocks reuptake of DA (Heikkila et al. 1975).
Preclinical studies have demonstrated age-dependent variations in sensitivity to the psychomotor stimulant effects of cocaine. Acute cocaine injections (10 mg/kg) produce more locomotor activation in adult than adolescent rats (Laviola et al. 1995). Cocaine challenge injections (15 mg/kg) following chronic cocaine treatment produce locomotor sensitization in adult but not in adolescent rats (Collins and Izenwasser 2002). At higher acute dosing (20 mg/kg), adolescent rats do display sensitivity to the locomotor activating effects of cocaine (Badanich et al. 2008), and show greater sensitization than adult rats to cocaine challenge injections (20 mg/kg) following chronic cocaine treatment (Frantz et al. 2007). Additionally, adolescent rats may be more sensitive than adult rats to the conditioned rewarding effects of cocaine, as conditioned place preference is produced at lower doses of cocaine in adolescent compared to adult rats (Badanich et al. 2006; Zakharova et al. 2009). Despite these age-related differences in cocaine-induced locomotion and conditioned reward, several studies have indicated that adult and adolescent rats acquire and maintain cocaine self-administration to the same degree (Belluzzi et al. 2005; Frantz et al. 2007; Kantak et al. 2007; Kerstetter and Kantak 2007; Leslie et al. 2004), suggesting that the reinforcing effects of cocaine are comparable in adult and adolescent rats. Although there is little difference in self-administration behavior maintained by cocaine under these conditions, cocaine-induced changes in neurocognition may be different when cocaine use begins during adulthood vs. adolescence because adult and adolescent brains are in different states of development with respect to DA neurotransmission.
Previous research in adult male rats, either trained to self-administer cocaine or passively receiving cocaine in a yoked manner, demonstrated deficits in an amygdala-related stimulus-reward learning task either within 30 min of the cocaine self-administration sessions ending (Udo et al. 2004) or after an 18-day cocaine-free period (Kerstetter and Kantak 2007). In comparison, rats with adolescent-onset cocaine exposure that preceded an 18-day cocaine-free period did not exhibit any deficits in this same task (Kerstetter and Kantak 2007). There is evidence, though, that adult and adolescent rats may both be sensitive to cocaine-induced impairment of prefrontal cortex-related neurocognitive functions. Adult male rats were shown to have deficits in spatial working memory and sustained attention measured either within 30 min of the cocaine self-administration sessions ending (Kantak et al. 2005) or 1 day but not 7 days after the cocaine self-administration sessions ended (Dalley et al. 2005). In adolescent rats, experimenter-delivered injections of cocaine produced deficits in spatial working memory (Santucci et al. 2004) and attentional set-shifting (Black et al. 2006) following a 10- or 24-day cocaine-free period. Spatial working memory, sustained attention and attentional set-shifting are functions of the medial prefrontal cortex (Floresco and Magyar 2006) and collectively these results suggest that dysfunction of this memory system by cocaine may be more prolonged in adolescent than adult rats.
The primary aim of the present study was to extend the limited research comparing the effects of adolescent-onset and adult-onset cocaine self-administration on the functioning of another prefrontal cortex subregion, the orbitofrontal cortex, because this subregion is known to be dysfunctional in cocaine addicts (Volkow and Fowler 2000). Previously, adult male rats were shown to have deficits in reversal learning during a go, no-go odor discrimination task following 2–4 weeks of abstinence from cocaine self-administration or chronic experimenter-delivered cocaine (30 mg/kg/d) injections (Calu et al. 2007; Schoenbaum et al. 2004). Interestingly, the number of reversal learning errors was greater in rats trained to self-administer cocaine than those receiving experimenter-delivered injections. Reversal learning in this task selectively requires intact functioning of the orbitofrontal cortex (Schoenbaum et al. 2003). Another learning task that requires intact functioning of the orbital but not prelimbic subregion of rat prefrontal cortex is the odor-delayed win-shift task, which utilizes non-spatial working and reference memory for task acquisition (Di Pietro, et al. 2004). Using a yoked-triad design in adult male rats tested within 30 min of the cocaine self-administration sessions ending, it was demonstrated that only contingent cocaine self-administration produced deficits in the odor-delayed win-shift task (Kantak et al. 2005). In the current study, it was determined if rats with adult-onset or adolescent-onset cocaine experience exhibited deficits in the odor-delayed win-shift task following an 18-day drug-free period. In order to assess any potential differences in the effects of contingent and non-contingent cocaine delivery, a yoked-triad design was used. A secondary aim of the present study was to compare effects of cocaine exposure in adult and adolescent rats on behavior mediated by the orbitofrontal cortex with behavior mediated by the amygdala (Kerstetter and Kantak 2007).
Materials and methods
Subjects
Adult (n = 24; arrival on P65, 275–300 g) and adolescent (n = 24; arrival on P25, 60–80 g) non-littermate male rats of the Wistar strain (Crl(WI)BR, Charles River Laboratories, Portage, MI, USA) were individually housed in plastic cages (24 cm × 22 cm ×20 cm) within a temperature- (21–23°C) and light-(08:00 hours on ; 20:00 hours off) controlled vivarium. During self-administration sessions, rats were allowed ad libitum access to food and water except for two days prior to and two days following initiation of self-administration sessions when food was restricted to approximately16 g per day. Food was again restricted during the 18-day drug-free period and during the 15 sessions of the odor-delayed win-shift task. During this second period of food restriction, approximately 16g of food per day was provided, which maintained rats at no less than 85–90% of free-feeding body weight. Rats had unlimited access to water in their home cages between experimental sessions. All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington DC, 1996) as well as specific national laws. The Boston University Institutional Animal Care and Use Committee approved this study.
Apparatus
Experimental chambers (Model ENV-008CT, Med Associates, Georgia, VT, USA) described previously in detail (Kantak et al. 2002) were used for self-administration. The odor-delayed win-shift task was performed in a modular eight-arm radial maze (Model ENV-538, Med Associates), also described previously in detail (Kantak et al. 2001).
Drugs
Cocaine hydrochloride (gift from NIDA, Bethesda, Maryland, USA) was dissolved in 0.9% sterile saline containing 3 IU heparin per mL to a final concentration of 2.68 mg/mL. Throughout self-administration sessions, a unit dose of 1.0 mg/kg was delivered intravenously by infusing the 2.68 mg/mL solution at a rate of 1.8 mL/min. In order to attain a dose of 1.0 mg/kg, the infusion duration was adjusted for each animal’s daily body weight (1.2 s/100 g). Yoked rats passively exposed to saline received an infusion of the saline/heparin solution, which was delivered at the same rate as the cocaine infusion.
Lever Shaping and Surgery
Four days following arrival, food was removed from the home cages in the morning and that same evening, animals were autoshaped overnight in the experimental chambers to respond on either lever under a fixed-ratio 1 (FR1) schedule of food reinforcement (45 mg chocolate-flavored precision pellets; Bio-Serv, Frenchtown, NJ, USA). Following autoshaping, food was available ad libitum in the home cages, and catheter surgery took place two to three days later. Jugular vein catheters were implanted, as described previously in detail (Kantak et al. 2000), in both adult and adolescent rats using back mounts as the site of catheter attachment. Furazolidone powder spray (Veterinary Products Laboratories, Phoenix, AZ, USA) was used to treat wounds until healed. Rats received a subcutaneous injection of 0.05 mg/kg Buprenorphine (Reckitt Benckiser Pharmaceuticals, Richmond, VA) each day for 2 days, and 5 mL Children’s Acetaminophen (CVS Pharmacy, Woonsocket, RI, USA) and 1.5 mL of 22.7 mg/mL Baytril (Bayer Health Care, Leverkusen, Germany) were provided per 250 mL drinking water for ten days following surgery to alleviate any post-surgical discomfort and reduce the risk of infection. All rats were given five to six days to recover from surgery prior to initiating self-administration sessions.
Catheters were maintained Monday through Friday by flushing them once daily with 0.1 mL of a 0.9% saline solution containing 3 IU heparin (LymphoMed, Rosemont, IL, USA) and 6.7 mg of the antibiotic tricarcillin with clavulanic acid (SmithKline Beecham Pharmaceuticals, Philadelphia, PA, USA). A locking solution that consisted of glycerol and undiluted (1000 IU/ml) heparin (3:1) was used to fill the catheter dead space on Fridays. The locking solution was removed on Mondays and replaced with the saline/heparin solution prior to beginning experimental sessions. Catheters were also checked for functionality each week by infusing 1.0 mg/0.1 mL methohexital sodium (Eli Lilly, Indianapolis, IN, USA) and noting the presence of rapidly induced sedation.
Self-Administration Procedures
Since the dopamine transporter does not reach adult levels of density until approximately P35 in rats (Tarazi et al. 1998b), the first self-administration session took place on P37 for adolescent rats. This was implemented to ensure that any observed neurocognitive differences were not a result of age-related differences in cocaine binding at the dopamine transporter during self-administration sessions. Self-administration sessions in adult rats began on P77. Self-administration was conducted during the light phase for 18 sessions, with five sessions per week (Monday through Friday). Each session was 2 h in duration. Prior to initiating self-administration sessions, adult and adolescent rats each were divided into triads (n=8 triads for each age). In each triad, one rat controlled cocaine delivery and the other two rats passively received either cocaine or saline. Rats self-administering cocaine (1.0 mg/kg) were initially trained under an FR1 reinforcement schedule with a 20-s timeout (TO) period after each infusion. A stimulus light located above the active lever was illuminated during the infusion and the 20-s TO period for each member of the triad. The TO period was accompanied by the offset of the house light. The active lever was counterbalanced to the left or right. There were no scheduled consequences for responses made on the inactive lever. Response requirements were gradually increased to a terminal FR5 20-s TO reinforcement schedule during which rats self-administering cocaine pressed the active lever five times to receive a cocaine infusion. Adult rats reached the FR5 response requirement after an average of 9.4 ± 0.7 sessions (age range from P85 to P92) and adolescent rats after an average of 8.6 ± 0.2 sessions (age range from P46 to P49). Rats remained at this terminal schedule for the remainder of the sessions. Responses made on either the active (light-paired) or inactive (no-light-paired) lever by the yoked rats passively receiving cocaine or saline were recorded but had no scheduled consequences. Adolescent rats completed the 18th self-administration session on P59 and adult rats on P99. Following these sessions, rats underwent an 18-day drug-free period in their home cages.
Odor- Delayed Win-Shift Task
Following the 18-day drug-free period (P60–P77 vs. P100–P117), rats were tested in the radial-arm maze for acquisition of the odor-delayed win-shift task as previously described (Di Pietro et al. 2004; Kantak et al. 2005). Prior to daily acquisition sessions, rats were given 10 training trials per day for 4 days to learn to dig in an unscented sand cup for a hidden Froot Loop (Kellogg’s, Battle Creek, MI, USA) reinforcer. Acquisition sessions for the odor-delayed win-shift task consisted of a training phase and a test phase that were separated by a 5-min delay. The training phase is a period when task demands are relatively easy, as rats must discriminate among four odors in four arms, and avoid digging in sand cups containing an odor cue for which the reinforcer was already retrieved. During the training phase, four arms were randomly selected (no more than two adjacent arms) and baited with a Froot Loop reinforcer that was buried 1cm below the sand in a clear plastic cup (6.5 cm diameter × 6.5 cm height) that contained 125 g of sand mixed with 5 g of an odor cue (allspice, basil, celery seed, or dill weed). Rats were placed into the central hub of the maze and given free access to the four cups, which were placed 17 cm inside the selected arms. The training phase concluded when rats dug for and retrieved all 4 reinforcers or after 5 min had elapsed. During the 5-min delay between the training and test phases, rats were placed back into their home cages and the maze arms and cups used during the training phase were cleaned to eliminate residual rat odors. The test phase is a period when task demands are relatively difficult, as rats must discriminate among eight odors in eight arms, and after a 5-min delay, avoid digging in sand cups containing the four previously reinforced odors as well as avoid digging in sand cups containing an odor cue for which the reinforcer was already retrieved. For the test phase, four arms again were randomly selected (no more than two adjacent arms) and four different sand cups scented with paprika, thyme, cinnamon or marjoram were baited with a reinforcer and placed into the selected arms. The scented cups used in the training phase were placed in the remaining arms without any reinforcers. During the test phase, rats were placed into the central hub of the maze and given access to all eight cups. The test phase ended when all four reinforcers were retrieved or after 5 min had elapsed.
Errors were recorded if a rat dug in the sand of a cup in which the reinforcer had already been retrieved (within-phase or working memory error) or of an un-baited cup (between-phase or reference memory error). Thus, non-spatial working memory errors are exhibited during the training and test phases and non-spatial reference memory errors are exhibited only during the test phase. Maze arms that contained reinforced cups during training and test phases were randomly selected each day for each phase and each triad. The odor-delayed win-shift task was conducted Monday-Friday for 15 sessions (P78–P96 vs. P118–P136). Acquisition criterion was set at 1 or fewer total errors (working + reference) on three out of four consecutive sessions. If a rat did not acquire the task within 15 sessions, then a value of 16 was assigned as the sessions to criterion for that rat for statistical purposes.
Data Analysis
The self-administration data were analyzed to determine if behavior during sessions was similar or different for adolescent and adult rats. Prior to analysis, responses on active (light-paired) and inactive (no light-paired) levers were averaged in individual subjects across sessions conducted under the FR5 contingency (approximately 9 sessions for each age group). Response data were analyzed by a three-factor (drug-onset age X administration condition X lever) ANOVA, with repeated measures on the lever factor. Infusion data were analyzed over all 18 sessions by a two-factor (drug-onset age X session number) ANOVA, with repeated measures on the session number factor. Post-hoc Tukey tests were used where appropriate. For the odor-delayed win-shift task, the dependent measures consisted of the number of sessions to reach the learning criterion during training and test phases, the cumulative number of non-spatial working memory errors made during the training or test phases, the cumulative number of non-spatial reference memory errors made during the test phase, and the average latency to complete sessions during the training or test phases. Data were analyzed via two-factor (drug-onset age X administration condition) ANOVA. Post-hoc Tukey tests were used for follow-up comparisons. It should be noted that 1–2 rats from each of the three administration conditions within the two age groups never moved from the central hub during the first three training and test phase sessions, thereby committing no working or reference memory errors. Since committing no errors by virtue of not digging vs. committing no errors by being 100% accurate with digging choices are not identical measures of performance, the non-spatial working and reference memory errors were summed from session 4 through session 15 in each subject prior to analyses. During sessions 4 though 15, all rats completed the retrieval of the four reinforcers within 5 min during the training and test phases of the task, thus providing meaningful data for comparisons of the cumulative number of working memory and reference memory errors across the age groups and administration conditions.
Results
Self-Administration Behavior
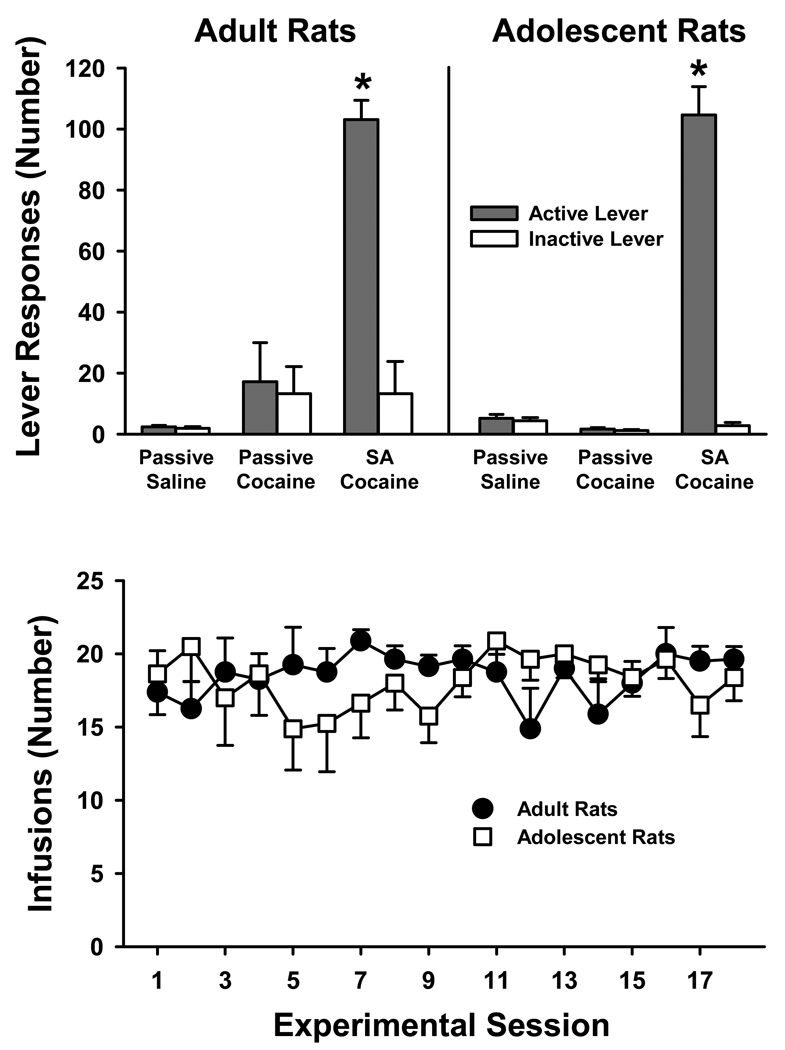
Cocaine self-administration behavior did not differ significantly in adult and adolescent rats. A three-factor ANOVA of lever responses revealed significant main effects of administration condition (F [2,42]=101.0, p≤0.001) and lever (F [1,42]=68.7, p≤0.001), and a significant administration condition X lever interaction (F [2,42]=63.0, p≤0.001). Post-hoc testing of the administration condition X lever interaction indicated that differences were a due to a greater number of active than inactive lever responses being emitted by rats self-administering cocaine under the FR5 contingency (p≤0.001). The number of active and inactive lever responses was not significantly different in rats passively receiving either cocaine or saline. Neither the drug-onset age X lever condition interaction nor the drug-onset age X administration condition interaction was significant, indicating that responding was similar across age groups, on each lever and for each administration condition (Fig. 1, top panel). Additionally, the number of infusions earned by rats self-administering cocaine did not differ significantly between age groups across the 18 self-administration sessions (Fig. 1, bottom panel). A two-factor ANOVA revealed that infusions were not significantly different over sessions, and that there was no drug-onset age X session number interaction. Adult rats earned an overall average of 18.5 ± 0.7 infusions per session and adolescent rats earned an overall average of 18.1 ± 1.5 infusions per session.
Figure 1.
Behavior during 2-h self-administration sessions in rats with adult-onset and adolescent-onset cocaine or saline experience. Values are the mean ± SEM number of active and inactive lever responses averaged over all sessions under the FR5 contingency (top panel) and infusions earned for each of 18 self-administration sessions (bottom panel). Rats either contingently self-administered cocaine (SA cocaine) or non-contingently received cocaine (passive cocaine) or saline (passive saline) in a yoked manner. * p≤0.001 compared to the passive cocaine and saline groups and to the inactive lever.
Odor-Delayed Win-Shift Task
Training Phase
During the training phase of the odor-delayed win-shift task (relatively easy task demands), ANOVA revealed that there were no significant differences due to drug-onset age, administration condition or their interaction for trials to criterion, cumulative non-spatial working memory errors made or average latency to complete the sessions. Rats from the three administration conditions within the two age groups reached criterion in approximately 6–8 sessions and made a cumulative total of approximately 4–8 non-spatial working memory errors on average. The latency to complete the daily training sessions averaged between 116 and 151 sec in all six groups.
Test Phase
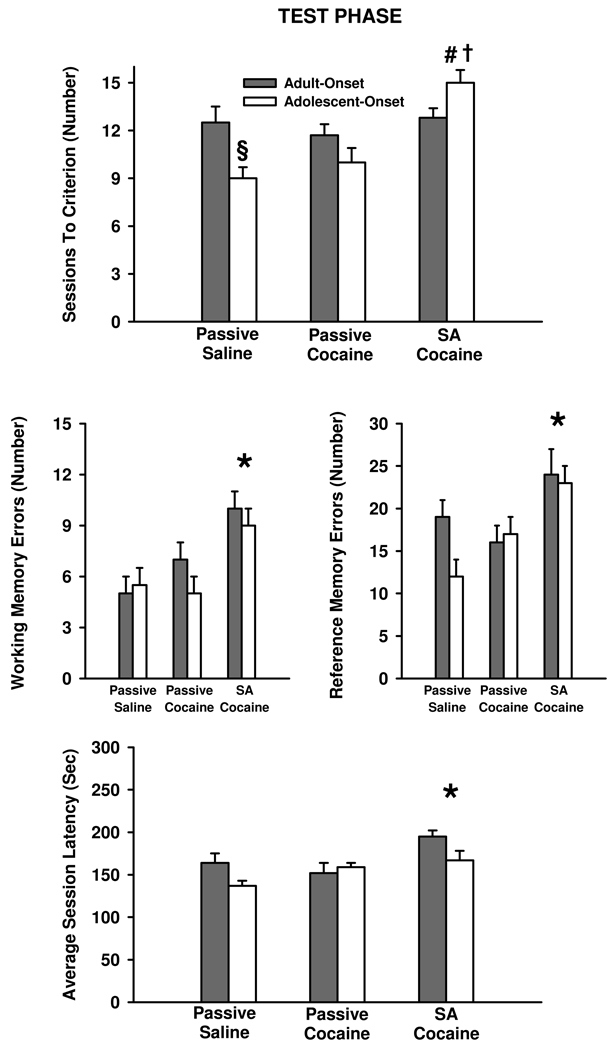
Analysis of sessions to criterion revealed a significant main effect of administration condition (F [2, 42]=6.6, p≤0.003) and a significant drug-onset age X administration condition interaction (F [2, 42]=3.5, p≤0.04) during the test phase (relatively difficult task demands). Post-hoc testing of the drug-onset age X administration condition interaction demonstrated that during the test phase, there were no significant differences in the number of sessions to reach criterion in rats with adult-onset cocaine experience (contingent or non-contingent) relative to rats passively receiving saline (Fig. 2, top panel). However, in rats with adolescent-onset cocaine self-administration experience, the number of sessions to reach criterion was greater than the number required in rats passively receiving either saline (p≤0.001) or cocaine (p ≤ 0.003), which did not differ from each other (p≤0.92) (Fig. 2, top panel). Within the 15 sessions allotted during the test phase, six of eight rats with adolescent-onset cocaine self-administration experience never reached criterion levels of accuracy. In contrast, all eight rats in the same-aged passive saline group and seven of eight rats in the same-aged passive cocaine group reached criterion levels of accuracy. Among rats with adult-onset cocaine or saline experience, one of eight rats from the passive saline and passive cocaine groups did not reach criterion levels of accuracy and two of eight rats from the cocaine self-administration group did not reach criterion levels of accuracy within 15 sessions during the test phase. Between-age comparisons for sessions to criterion during the test phase revealed that more sessions were required in rats with adolescent-onset than adult-onset cocaine self-administration experience (p ≤0.04). In contrast, fewer sessions were required in rats with adolescent-onset than adult-onset passive saline exposure (p ≤ 0.04).
Figure 2.
Behavior during the test phase (relatively difficult task demands) of the odor-delayed win-shift task in rats with adult-onset and adolescent-onset cocaine or saline experience. Values are the mean ± SEM number of sessions to criterion (top panel), cumulative number of non-spatial working memory errors (middle left panel), cumulative number of non-spatial reference memory errors (middle right panel), and average session latency in seconds (bottom panel). Rats either contingently self-administered cocaine (SA cocaine) or non-contingently received cocaine (passive cocaine) or saline (passive saline) in a yoked manner. § p≤0.04 compared to adult rats passively receiving saline; # p≤0.003 compared to the same-aged passive saline and passive cocaine groups; † p≤0.04 compared to adult rats self-administering cocaine; * p≤0.05 compared to rats passively receiving saline or cocaine across both drug-onset age groups.
Although rats with adult-onset cocaine self-administration experience did not differ from same-aged passive saline and passive cocaine groups for the number of sessions to reach criterion levels of accuracy, the analysis of non-spatial working memory and reference memory errors as well as average session latencies demonstrated that they exhibited deficits in these latter measures during the test phase that were similar in magnitude as the deficits exhibited by rats with adolescent-onset cocaine self-administration experience. Analysis of non-spatial working memory errors revealed a significant main effect of administration condition (F [2, 42]= 3.9, p≤0.020) that did not interact significantly with drug-onset age. Post-hoc testing of the administration condition main effect indicated that rats self-administering cocaine made more non-spatial working memory errors than rats passively receiving saline (p≤0.04), but not passively receiving cocaine (p≤0.11), across both drug-onset age groups (Fig. 2, middle left panel). However, passive cocaine exposure was not significantly different from passive saline exposure for non-spatial working memory errors (p≤0.88). In addition, analysis of non-spatial reference memory errors revealed a significant main effect of administration condition (F [2, 42]=6.1, p≤0.005) that did not interact significantly with drug-onset age. Post-hoc testing of the administration condition main effect indicated that rats self-administering cocaine made more non-spatial reference memory errors than rats passively receiving saline (p≤0.004) or cocaine (p≤0.006) across both drug-onset age groups (Fig. 2, middle right panel). Passive cocaine exposure was not significantly different from passive saline exposure for non-spatial reference memory errors (p≤0.83).
Similar to non-spatial working and reference memory errors, analysis of the average session latencies during the test phase revealed a significant main effect of administration condition (F [2, 42]=4.7, p≤0.01) that did not interact significantly with drug-onset age. Post-hoc testing of the administration condition main effect showed that this difference was a result of rats with cocaine self-administration experience taking longer to complete the daily sessions than rats passively receiving saline (p ≤ 0.02) or cocaine (p ≤ 0.05), across the two drug-onset age groups (Fig. 2, bottom panel). Passive cocaine exposure was not significantly different from passive saline exposure for average session latencies (p ≤ 0.85).
Body Weight Changes with Food Restriction
Self-Administration Phase
Prior to beginning the initial 4-day food restriction period, adult rats had an average free-feeding (baseline) body weight of 331.6 ± 7.7 g and adolescent rats had an average free-feeding (baseline) body weight of 127.1 ± 2.8 g. At the end of 4 days of moderate food restriction (after the second self-administration session), body weights in adult rats increased an average of 2.6 ± 1.9 % (339.5 ± 8.6 g) and body weights in adolescent rats increased an average of 7.3 ± 1.5 % (139.5 ± 2.7 g) from baseline body weight values. At the end of the 18 self-administration sessions, while on ad libitum food, body weights in adult rats corresponded to a 36.6 ± 2.7 % (450.7 ±9.4 g) increase from baseline and body weights in adolescent rats corresponded to a 140.1 ± 6.4 % (303.5 ± 7.9 g) increase from baseline. Thus, the 4 days of food restriction affected relative body weight changes in an age-appropriate manner. The larger relative increase in body weight of adolescent rats compared to adult rats during the initial food restriction and free-feeding periods is consistent with the rapid growth rates expressed during the adolescent developmental period (Wilmouth and Spear 2009).
Odor-Delayed Win-Shift Task Phase
Prior to beginning the second food restriction period that extended from the end of the self-administration phase through the completion of the odor-delayed win-shift task, rats with adult-onset cocaine or saline exposure had an average free-feeding (baseline) body weight of 450.7 ± 9.4 g and rats with adolescent-onset cocaine or saline exposure had an average free-feeding (baseline) body weight of 303.5 ± 7.9 g. At the start of the odor-delayed win-shift task (after 18 days of moderate food restriction), body weights in rats with adult-onset cocaine or saline exposure decreased an average of 5.5 ± 1.8 % (423.5 ± 7.5 g) and body weights in rats with adolescent-onset cocaine or saline exposure decreased an average of 5.8 ± 1.3 % (284.8 ± 6.5 g) from baseline body weight values. Upon completion of the odor-delayed win-shift task 15 sessions later, body weights in rats with adult-onset exposures corresponded to a 15.8 ± 1.5 % (377.9 ± 7.7 g) decrease from baseline body weight and body weights in rats with adolescent-onset exposures corresponded to a 9.5 ± 1.9 % (272.0 ± 3.9 g) decrease from baseline body weight. The similar relative decreases in body weight upon a more prolonged period of moderate food restriction indicate that rats (all now adults) from both drug-onset age groups were not differentially affected by food restriction that could potentially influence performance in the odor-delayed win-shift task.
Discussion
Previous epidemiological studies have suggested that the consequences of cocaine consumption may be different for individuals at distinct developmental stages (Chen and Kandel 2002; Reboussin and Anthony 2006). Presented here is preclinical evidence showing that although adult and adolescent male rats self-administered similar amounts of cocaine, the consequences of cocaine consumption on an orbitofrontal cortex-related learning task were not exactly the same.
Although there is potential for uncontrolled and differential levels of shipping stress in newly weaned rats vs. adult rats that could impact later response to drugs (Wiley and Evans, 2009), in the current study cocaine self-administration behavior was not affected by age of drug-onset. A finding of no age differences in cocaine self-administration is consistent with numerous preclinical studies that have compared self-administration behavior of adult and adolescent male rats and revealed that rats of both ages consume similar amounts of cocaine and emit similar numbers of responses for cocaine delivery (Belluzzi et al. 2005; Frantz et al. 2007; Kantak et al. 2007; Kerstetter and Kantak 2007; Leslie et al. 2004). In contrast, prior cocaine self-administration experience did differentially impact performance during the relatively more difficult test phase of the odor-delayed win-shift task. This difference in rats trained to self-administer cocaine is more likely related to age of drug-onset than to potential differential shipping stress because the behavior of rats from the different age groups and administration conditions were not affected during the relatively less difficult training phase of the odor-delayed win-shift task.
The test phase of the odor-delayed win-shift requires both non-spatial working memory and reference memory to learn the task. Three indices of learning were measured: (1) the number of sessions needed to acquire the task, (2) the cumulative number of errors made, and (3) the length of time it took to retrieve the reinforcers during daily sessions. These three measures of learning are the dependent variables typically analyzed in a variety of radial arm maze tasks (e.g., McDonald and White, 1993; Floresco et al., 1997). Accordingly, rats with adult-onset cocaine self-administration experience exhibited deficits in two of three indices of learning by demonstrating an increase in the cumulative number of working and reference memory errors made and an increase in the latency to complete daily sessions. In contrast, deficits in all three indices of learning were exhibited by rats with adolescent-onset cocaine self-administration experience, including an increase in the number of sessions to acquire the task. Indeed, the magnitude of this latter deficit becomes even more apparent if consideration is given to the finding that the younger rats acquired the task faster than the older rats when passively exposed to saline, and acquired the task more slowly than the older rats when contingently exposed to cocaine. These findings suggest that the rate of learning is different in the two age groups, which may account for the age differences in the number of sessions to reach criterion, but similar cumulative numbers of working and reference memory errors in rats actively self-administering cocaine. Thus, it appears that overall performance in an orbitofrontal cortex-related learning task was poorer in rats with adolescent-onset than adult-onset cocaine self-administration experience.
One possibility to account for the greater overall impairment in rats with adolescent-onset cocaine self-administration experience is that the adult and adolescent rats were exposed to cocaine at distinct stages of prefrontal cortex development. Recent testing in human subjects has revealed age-related differences in activity of the orbitofrontal cortex during reward processing (Galvan et al. 2006). Specifically, while there was no difference in the extent of voxel-based reward-related activity in the nucleus accumbens of teens compared to adults, teens were shown to have a greater extent of voxel-based reward-related activity in the orbitofrontal cortex than adults, suggesting more diffuse processing in teens. The lack of difference in the extent of reward-related activity in the nucleus accumbens of teens and adults is compatible with the similar rates of cocaine self-administration in adult and adolescent rats, as the nucleus accumbens is an important substrate for cocaine reinforcement (Pontieri et al., 1995). However, if the extent of reward-related activity in the orbitofrontal cortex were found to be greater in adolescent than adult rats, then contingent cocaine exposure could cause a relatively more diffuse disturbance in orbitofrontal cortex activity directed toward the processing of non-drug rewards (such as those used in the odor-delayed win-shift task) in adolescent rats.
Kalivas and colleagues (2005) suggest that cocaine addicts have prefrontal cortex-related neurocognitive deficits because they have reduced motivation to respond to non-drug-related stimuli due to a predominance of D1 signaling. Since the orbitofrontal cortex of cocaine addicts is activated only by strong motivational stimuli (such as stimuli arising from contingent association with cocaine), drug-seeking behavior is maintained prepotently at the expense of normal learning and memory functioning of this site, particularly when cognitive demand is high (Kalivas et al. 2005). Such hijacking of the orbitofrontal cortex may help explain why neurocognitive deficits were observed in rats contingently self-administering cocaine and not in rats passively receiving cocaine. Importantly, passive (non-contingent) cocaine exposure did not produce deficits in any of the learning measures analyzed in either adult or adolescent rats. This finding suggests that the deficits observed in rats with cocaine self-administration experience are not due exclusively to the pharmacological effect of cocaine, but may be related also to the expectancy and/or controllability of cocaine delivery. Previous research has shown that separate populations of neurons in the orbitofrontal cortex encode reward expectancy and behavioral responses directed toward reward delivery (Furuyashiki et al. 2008). Thus, the orbitofrontal cortex may be more engaged in rats contingently self-administering cocaine than in those receiving it passively, and therefore more vulnerable to hijacking under the contingent cocaine condition. Interestingly, contingent cocaine self-administration caused impairments only in the relatively more cognitively demanding test phase of the odor-delayed win-shift task after 18 drug-free days, as was observed previously when rats were tested within 30 min of the cocaine self-administration sessions ending (Kantak et al. 2005). This time course suggests that deficits in orbitofrontal cortex-related neurocognitive functioning are not immediately reversible when cocaine is no longer consumed, which is consistent with deficits reported in human cocaine addicts (Di Sclafani et al. 2002).
Potentially important to understanding a basis for the overall poorer performance in the odor-delayed win-shift task in rats with adolescence-onset cocaine self-administration experience are findings from research showing that chronic cocaine induces a greater expression of delta FosB in the orbitofrontal cortex of adult rats contingently self-administering cocaine than of adult rats receiving cocaine passively (Winstanley et al. 2007). Delta FosB expression following cocaine involves D1 and cyclic adenosine monophosphate (cAMP) signaling cascades (Andersson et al. 2001). These findings suggest that cocaine self-administration may increase other important molecular targets in the orbitofrontal cortex arising from DA D1 receptor stimulation and cAMP signaling that are not produced by passive cocaine exposure. One molecular target of interest is hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which are very responsive to cAMP and are expressed in pyramidal neurons in prefrontal cortex (Wang et al., 2007). When activated by cAMP, HCN channels alter the firing pattern of neurons by reducing their capacity for temporal summation of excitatory post-synaptic glutaminergic currents (Day et al. 2005; Pedarzani and Storm 1995).
Wang and colleagues (2007) demonstrated that an increase in cAMP is disruptive to spatial working memory through activation of HCN channels in the medial prefrontal cortex. Such a mechanism could underlie the variety of cocaine-induced deficits in medial prefrontal cortex-related neurocognitive functioning observed in adolescent (Black et al. 2006; Santucci et al. 2004) and adult (Dalley et al. 2005; Kantak et al. 2005) rats. Although an association between activation of HCN channels and orbitofrontal cortex-related neurocognitive functioning is yet to be reported, distribution studies show that there are large numbers of HCN1 channels spread throughout layer V of prefrontal cortex, which encompasses a large portion of the orbitofrontal cortex (Monteggia et al. 2000). If activation of HCN1 channels by cAMP in the orbitofrontal cortex underlies cocaine self-administration-induced deficits in the odor-delayed win-shift task, this may explain the overall poorer performance found in rats with adolescence-onset experience, as chronically administered cocaine causes an upregulation of cAMP (Unterwald et al. 1996) and cAMP activity is higher in the brains of adolescent than adult rats (Andersen et al. 2002). This would also suggest that changes in HCN channel function might be persistent after cocaine self-administration sessions are terminated.
In conclusion, these findings address the role that stimulant drug use plays in the developing brain (Kalechstein et al. 2008). That is, amygdala function appears to be insensitive to disruption following adolescent-onset cocaine use (Kerstetter and Kantak 2007). However, functioning of the later-to-develop orbitofrontal cortex (Casey et al. 2008) appears to be more impaired in rats with adolescent-onset cocaine use even when the substance is no longer being self-administered. Collectively, these findings suggest that there is a differential vulnerability across brain regions during the adolescent phase of development. An increased vulnerability in the cortical domain would mitigate the reduced vulnerability observed for amygdala-related learning in rats exposed to cocaine during adolescence. Developmental plasticity does not protect one from all learning and memory deficits associated with adolescent cocaine use, and it remains important for adolescents to refrain from actively seeking and taking cocaine.
Acknowledgments
This research was supported in part by CELEST, a National Science Foundation Science of Learning Center (SBE-0354378; S. Grossberg, PI) and by the National Institute on Drug Abuse (DA11716; K.M. Kantak, PI).
References
- Andersen SL. Changes in the second messenger cyclic AMP during development may underlie motoric symptoms in attention deficit/hyperactivity disorder (ADHD) Behav Brain Res. 2002;130:197–201. doi: 10.1016/s0166-4328(01)00417-x. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–169. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Andersson M, Konradi C, Cenci MA. cAMP response element-binding protein is required for dopamine-dependent gene expression in the intact but not the dopamine-denervated striatum. J Neurosci. 2001;21:9930–9943. doi: 10.1523/JNEUROSCI.21-24-09930.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Badanich KA, Maldonado AM, Kirstein CL. Early adolescents show enhanced acute cocaine-induced locomotor activity in comparison to late adolescent and adult rats. Dev Psychobiol. 2008;50:127–133. doi: 10.1002/dev.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Wang R, Leslie FM. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology. 2005;30:705–712. doi: 10.1038/sj.npp.1300586. [DOI] [PubMed] [Google Scholar]
- Black YD, Maclaren FR, Naydenov AV, Carlezon WA, Jr, Baxter MG, Konradi C. Altered attention and prefrontal cortex gene expression in rats after binge-like exposure to cocaine during adolescence. J Neurosci. 2006;26:9656–9665. doi: 10.1523/JNEUROSCI.2391-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem. 2007;14:325–328. doi: 10.1101/lm.534807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A. The adolescent brain. Dev Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68:65–85. doi: 10.1016/s0376-8716(02)00086-8. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Laane K, Pena Y, Theobald DE, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology (Berl) 2005;182:579–587. doi: 10.1007/s00213-005-0107-3. [DOI] [PubMed] [Google Scholar]
- Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro NC, Black YD, Green-Jordan K, Eichenbaum HB, Kantak KM. Complementary tasks to measure working memory in distinct prefrontal cortex subregions in rats. Behav Neurosci. 2004;118:1042–1051. doi: 10.1037/0735-7044.118.5.1042. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Magyar O. Mesocortical dopamine modulation of executive functions: beyond working memory. Psychopharmacology (Berl) 2006;188:567–585. doi: 10.1007/s00213-006-0404-5. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz KJ, O'Dell LE, Parsons LH. Behavioral and neurochemical responses to cocaine in periadolescent and adult rats. Neuropsychopharmacology. 2007;32:625–637. doi: 10.1038/sj.npp.1301130. [DOI] [PubMed] [Google Scholar]
- Furuyashiki T, Holland PC, Gallagher M. Rat orbitofrontal cortex separately encodes response and outcome information during performance of goal-directed behavior. J Neurosci. 2008;28:5127–5138. doi: 10.1523/JNEUROSCI.0319-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila RE, Orlansky H, Cohen G. Studies on the distinction between uptake inhibition and release of (3H)dopamine in rat brain tissue slices. Biochem Pharmacol. 1975;24:847–852. doi: 10.1016/0006-2952(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Jacobs EH, Smit AB, de Vries TJ, Schoffelmeer AN. Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci. 2003;24:566–573. doi: 10.1016/j.tips.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Jentsch JD, Kantak KM. Stimulant-associated cognitive abnormalities: mechanisms and impact on reward-related behavior and addiction. Drug Alcohol Depend. 2008;97:276–280. doi: 10.1016/j.drugalcdep.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. J Comp Neurol. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Black Y, Valencia E, Green-Jordan K, Eichenbaum HB. Dissociable effects of lidocaine inactivation of the rostral and caudal basolateral amygdala on the maintenance and reinstatement of cocaine-seeking behavior in rats. J Neurosci. 2002;22:1126–1136. doi: 10.1523/JNEUROSCI.22-03-01126.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Goodrich CM, Uribe V. Influence of sex, estrous cycle, and drug-onset age on cocaine self-administration in rats (Rattus norvegicus) Exp Clin Psychopharmacol. 2007;15:37–47. doi: 10.1037/1064-1297.15.1.37. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Green-Jordan K, Valencia E, Kremin T, Eichenbaum HB. Cognitive task performance after lidocaine-induced inactivation of different sites within the basolateral amygdala and dorsal striatum. Behav Neurosci. 2001;115:589–601. doi: 10.1037//0735-7044.115.3.589. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Udo T, Ugalde F, Luzzo C, Di Pietro N, Eichenbaum HB. Influence of cocaine self-administration on learning related to prefrontal cortex or hippocampus functioning in rats. Psychopharmacology (Berl) 2005;181:227–236. doi: 10.1007/s00213-005-2243-1. [DOI] [PubMed] [Google Scholar]
- Kerstetter KA, Kantak KM. Differential effects of self-administered cocaine in adolescent and adult rats on stimulus-reward learning. Psychopharmacology (Berl) 2007;194:403–411. doi: 10.1007/s00213-007-0852-6. [DOI] [PubMed] [Google Scholar]
- Laviola G, Wood RD, Kuhn C, Francis R, Spear LP. Cocaine sensitization in periadolescent and adult rats. J Pharmacol Exp Ther. 1995;275:345–357. [PubMed] [Google Scholar]
- Leslie FM, Loughlin SE, Wang R, Perez L, Lotfipour S, Belluzzi JD. Adolescent development of forebrain stimulant responsiveness: insights from animal studies. Ann N Y Acad Sci. 2004;1021:148–159. doi: 10.1196/annals.1308.018. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. A triple dissociation of memory systems: hippocampus, amygdala, and dorsal striatum. Behav Neurosci. 1993;107:3–22. doi: 10.1037//0735-7044.107.1.3. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Storm JF. Protein kinase A-independent modulation of ion channels in the brain by cyclic AMP. Proc Natl Acad Sci USA. 1995;92:11716–11720. doi: 10.1073/pnas.92.25.11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the "shell" as compared with the "core" of the rat nucleus accumbens. Proc Natl Acad Sci USA. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reboussin BA, Anthony JC. Is there epidemiological evidence to support the idea that a cocaine dependence syndrome emerges soon after onset of cocaine use? Neuropsychopharmacology. 2006;31:2055–2064. doi: 10.1038/sj.npp.1301037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci AC, Capodilupo S, Bernstein J, Gomez-Ramirez M, Milefsky R, Mitchell H. Cocaine in adolescent rats produces residual memory impairments that are reversible with time. Neurotoxicol Teratol. 2004;26:651–661. doi: 10.1016/j.ntt.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci. 2004;19:1997–2002. doi: 10.1111/j.1460-9568.2004.03274.x. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Ramus SJ. A systems approach to orbitofrontal cortex function: recordings in rat orbitofrontal cortex reveal interactions with different learning systems. Behav Brain Res. 2003;146:19–29. doi: 10.1016/j.bbr.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D(1), D(2) and D(4) receptors in rat forebrain. Int J Dev Neurosci. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine D4-like receptors in rat forebrain regions: comparison with D2-like receptors. Brain Res Dev Brain Res. 1998a;110:227–233. doi: 10.1016/s0165-3806(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ. Postnatal development of dopamine and serotonin transporters in rat caudate-putamen and nucleus accumbens septi. Neurosci Lett. 1998b;254:21–24. doi: 10.1016/s0304-3940(98)00644-2. [DOI] [PubMed] [Google Scholar]
- Udo T, Ugalde F, DiPietro N, Eichenbaum HB, Kantak KM. Effects of persistent cocaine self-administration on amygdala-dependent and dorsal striatum-dependent learning in rats. Psychopharmacology (Berl) 2004;174:237–245. doi: 10.1007/s00213-003-1734-1. [DOI] [PubMed] [Google Scholar]
- Unterwald EM, Fillmore J, Kreek MJ. Chronic repeated cocaine administration increases dopamine D1 receptor-mediated signal transduction. Eur J Pharmacol. 1996;318:31–35. doi: 10.1016/s0014-2999(96)00841-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Zhu W, Maynard L, Telang F, Vaska P, Ding YS, Wong C, Swanson JM. Expectation enhances the regional brain metabolic and the reinforcing effects of stimulants in cocaine abusers. J Neurosci. 2003;23:11461–11468. doi: 10.1523/JNEUROSCI.23-36-11461.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Evans RL. To breed or not to breed? Empirical evaluation of drug effects in adolescent rats. Int J Dev Neurosci. 2009;27:9–20. doi: 10.1016/j.ijdevneu.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmouth CE, Spear LP. Hedonic sensitivity in adolescent and adult rats: Taste reactivity and voluntary sucrose consumption. Pharmacol Biochem Behav. 2009 doi: 10.1016/j.pbb.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstanley CA, LaPlant Q, Theobald DE, Green TA, Bachtell RK, Perrotti LI, DiLeone RJ, Russo SJ, Garth WJ, Self DW, Nestler EJ. DeltaFosB induction in orbitofrontal cortex mediates tolerance to cocaine-induced cognitive dysfunction. J Neurosci. 2007;27:10497–10507. doi: 10.1523/JNEUROSCI.2566-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198:45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]