Abstract
One contributing factor in the worldwide decline in amphibian populations is thought to be exposure of eggs to UV light. Enrichment of pigment in the animal hemisphere of eggs laid in the sunlight defends against UV damage, but little is known about the cell biological mechanisms controlling such polarized pigment patterns. Even less is known about how such mechanisms were modified during evolution to achieve the array of amphibian egg pigment patterns. Here, we show that ectopic expression of the γ-tubulin regulator, Shroom2, is sufficient to induce co-accumulation of pigment granules, spectrin, and dynactin in Xenopus blastomeres. Shroom2 and spectrin are enriched and co-localize specifically in the pigmented animal hemisphere of Xenopus eggs and blastulae. Moreover, Shroom2 mRNA is expressed maternally at high levels in Xenopus. By contrast to Xenopus, eggs and blastulae of Physalaemus pustulosus have very little surface pigmentation. Rather, we find that pigment is enriched in the perinuclear region of these embryos, where it co-localizes with spectrin. Moreover, maternal Shroom2 mRNA was barely detectable in Physaleamus, though zygotic levels were comparable to Xenopus. We therefore suggest that a Shroom2/spectrin/dynactin-based mechanism controls pigment localization in amphibian eggs, and that variation in maternal Shroom2 mRNA levels accounts in part for variation in amphibian egg pigment patterns during evolution.
Keywords: Shroom2, Spectrin, pigmentation, melanosome, Physalaemus
Introduction
The pigmentation of amphibian eggs and cleavage stage embryos differs dramatically from species to species, but the species can be generally categorized as having pigmented or unpigmented eggs. The evolutionary transition from pigmented to unpigmented (and vice-versa) has occurred a number of times independently. It has been suggested that this variation represents an adaptation for protection against UV radiation from the Sun, as species that lay their eggs in the open (in ponds and lakes) tend to have darkly-pigmented eggs, but eggs are largely unpigmented in species in which fertilized eggs develop in a concealed location (e.g. undersides of leaves, foam nests, under rocks, in the brood pouch of a female)(Duellman and Trueb, 1986). In pigmented eggs, dark pigmentation is typically restricted to the upward-facing, sunlight-exposed animal pole and absent from the downward-facing vegetal pole (Duellman and Trueb, 1986), and it is thought that the pigment offers protection from the exposure to ultraviolet B (UVB) radiation (Blaustein and Belden, 2003). Indeed, exposure to UV radiation can impair hatching rates in many frogs (Croteau et al., 2008; Duellman and Trueb, 1986). Understanding how and why these pigment patterns differ among species is of interest, as exposure to UV light has been suggested as a possible cause for the current worldwide decline in amphibian populations (Blaustein and Belden, 2003; Croteau et al., 2008; Licht and Grant, 1997).
Essentially nothing is known about the cell biological mechanisms of animal hemisphere pigmentation in amphibians or about how such mechanism may be modified during evolution to achieve the interspecific variation in pigmentation patterns. On the other hand, the development of animal-vegetal asymmetries in amphibian eggs has been extensively studied, because this polarity is central to the normal development of the embryonic axes (Gerhart et al., 1989; Kloc et al., 2001).
Mature oocytes and eggs of amphibians are polarized along the animal-vegetal axis by unequally distributed maternal molecules including ribosomes, mitochondria, certain mRNAs and yolk platelets (Browder, 1991). Xenopus laevis is the most commonly used model amphibian, and in the wild, its clutches of eggs are laid in the open. In Xenopus, pigment is enriched in the animal hemisphere, whereas the vegetal hemisphere is white or cream colored (Passmore and Carruthers, 1979). This asymmetric distribution of pigment granules is established during oogenesis. Pigmentation begins at stage III with pigment uniformly distributed along the animal-vegetal axis. However, at the next stage (stage IV), the oocyte starts exhibiting an obvious color difference between the animal and vegetal hemispheres. Finally, a fully matured oocyte has the distinct pigment polarity (Dumont, 1972).
The specific pathway that directs the accumulation of pigmentation in the animal cortex in Xenopus is not well understood. However, the transport of pigment has been well-studied in pigment-producing cells of mature animals, such as frog melanophores or mammalian melanocytes (Coudrier, 2007; Nascimento et al., 2003; Tuma and Gelfand, 1999). Pigment is contained in melanosomes, which are specialized, membrane-bound organelles derived from the lysosome. In pigment containing cells, melanosomes are transported by motors along the cellular cytoskeleton. Actin-based motors such as MYO5a and MYO7a are unidirectional and move melanosomes over short distances. A cytoplasmic dynein and kinesin II are microtubule-based motors that tightly interact with melanosomes and are used for long distance movements (Tuma and Gelfand, 1999; Wu et al., 1998). Because melanosomes have been shown to bind with both actin-based motor and microtubule-based motors (Rogers and Gelfand, 1998; Rogers et al., 1997), it has been suggested that melanosomes be able to move between actin filaments and microtubules and switch motors for their proper distribution (Brown, 1999; Rodionov et al., 1998; Rogers and Gelfand, 1998; Tuma and Gelfand, 1999). Such a mechanism may underlie melanosome positioning in amphibian eggs, as actin filaments and microtubules are highly polarized along the animal-vegetal axis in Xenopus oocytes (Gard, 1999; Gard et al., 1995).
Another cytoskeletal molecule, spectrin, has also been reported to be involved in melanosome transport (Aspengren and Wallin, 2004; Watabe et al., 2008). Spectrin is a membrane-associated cytoskeletal element that exists in the Golgi and cytoplasmic vesicles (Beck, 2005; Stankewich et al., 1998). Spectrin interacts with dynactin, which links dynein and/or kinesin II to vesicles to control vesicle transport (Deacon et al., 2003; Holleran et al., 2001; Muresan et al., 2001). Recent studies have shown that spectrin binds dynein and two dynactin components p150glued and Arp1 (Aspengren and Wallin, 2004; Holleran et al., 2001; Papoulas et al., 2005). Furthermore, spectrin co-localizes and co-immunoprecipitates with melanosomes in frog melanophores (Aspengren and Wallin, 2004; Watabe et al., 2008), and proteomic analysis shows that dynein and spectrin localize in both premature and mature human melanosomes, indicating that they are involved in melanosome transport (Chi et al., 2006; Watabe et al., 2008). These studies, and the fact that spectrin localizes asymmetrically in the animal hemisphere of Xenopus oocyte and egg (Carotenuto et al., 2000), suggests that spectrin might be involved in the determination of pigment distribution during Xenopus oogenesis.
Here, we suggest that Shroom2 is a key regulator of melanosome transport during Xenopus oogenesis. Shroom2 is required for melanosome biogenesis and localization in the retinal pigment epithelium (RPE) of Xenopus (Fairbank et al., 2006), and Shroom2 binds to MYO7a, a known motor for melanosome transport in RPE (Etournay et al., 2007). We show that ectopic Shroom2 induces spectrin accumulation and pigmentation in Xenopus epithelial cells. We also show that Shroom2 protein co-distributes with spectrin in a polarized manner in Xenopus eggs, being enriched animally. To ask if variations in Shroom2 or spectrin localization may underlie variations in egg pigment patterns in amphibians, we examined Physalaemus pustulosus, which has “unpigmented” eggs that are deposited in foam nests (Romero-Carvajal et al., 2009). Pigment is concentrated in the perinuclear region of Physalaemus blastula, where it co-localizes with spectrin, and in Physalaemus eggs and blastulae, Shroom2 mRNA levels are very low compared to those in Xenopus. We therefore propose that changes in the expression level or localization pattern of Shroom2 and spectrin may underlie interspecific variation in amphibian egg pigment pattern.
Materials and Methods
Preparation of oocytes and embryos
Xenopus oocytes were isolated from a Xenopus laevis female as described previously (Lee et al., 2009). The oocytes were fixed with 3.7 % formaldehyde in OR2 buffer (82.5 mM NaCl, 2.5 mM Kcl, 1 mM MgCl2, 1 mM NaH2PO4, 5 mM Hepes, 3.8 mM NaOH, pH 7.8). Physalaemus eggs and embryos in foam nest were de-jellied with 3% cysteine in 1/3X MMR and were incubated until proper developmental stage.
mRNAs for Xenopus Shroom1, mouse Shroom2, Xenopus Shroom3 and human Shroom4 were transcribed using mMESSAGE mMACHINE kit (Ambion) and 1 ng of mRNAs were injected into 2 dorsal cells at 4-cell stage Xenopus embryo. For ectopic expression of Shroom 2 and 3 in epidermis, 0.5 ng plasmid DNA containing Shroom2 or Shroom3 was injected into one ventral cell at 4-cell stage and the injected embryos were fixed at stage 14.
In situ hybridization and immunostaining
In situ hybridization was performed as described previously (Sive et al., 2000). To make probe against Physalaemus Shroom2 mRNA, partial cDNA was cloned using FirstChoice RLM-RACE Kit (Ambion). Probe against Xenopus Shroom2 was synthesized from NIBB clone XL031d13.
Fixed embryos were immunostained as described before (Lee et al., 2007). Monoclonal anti-myc antibody (1:300 dilution, abcam 9E10 and abcam ab9106), rabbit polyclonal anti-spectrin antibodies (1:300 dilution, Abcam ab11182 and Sigma S1515), rabbit polyclonal anti-MYO5A (1:300 dilution, ab11094, abcam) and mouse anti-p150glued (1:300 dilution, BD Bioscience, #610473) were used for primary antibodies. Mouse anti-Xenopus Shroom2 antibody was raised against the Xenopus tropicalis peptide sequence, SVPPENDRYHLEKKYFESE (amino acid 1249-1267). Alexa fluor-488 or 555 goat anti-mouse or rabbit IgG was used for secondary antibody (Invitrogen, 1:300 dilution). Propidium iodide (20 μg/ml, Sigma) was incubated with secondary antibody).
The images were obtained by using a stereomicroscope (Leica MZ16FA) and a Zeiss LSM5 Pascal confocal microscope.
RT-PCR (reverse transcriptase PCR)
Total RNA was prepared from 5 embryos of Xenopus and Physalaemus at each different stage. RT-PCR was performed with following primers: for Physalaemus Shroom2, 5′-CTTGAGCAGCGGGAACTG-3′ and 5′-GAGATCAGACTTGCGGTC-3′; for Xenopus Shroom2, 5′-TCCTACTCCCGATTTTGTGC-3′ and 5′-CTGCTCCTGCATGTCTTTCA-3′; for ODC (ornithine decarboxylase), 5′-GGCAAGGAATCACCCGAATG-3′ and 5′-GGCAACATAGTATCTCCCAGGCTC-3′.
Results
Ectopic expression of Shroom proteins causes pigment accumulation on the surface of Xenopus blastomeres
Previously, we reported that the overexpression of Shroom2 and Shroom3 induces rapid pigment accumulation on the surface of Xenopus blastomeres (Fairbank et al., 2006; Haigo et al., 2003; Lee et al., 2007). These findings raised the possibility that there might be a general relationship between Shroom family proteins and pigmentation. We therefore examined whether all Shroom family proteins are able to induce pigmentation when expressed in Xenopus blastomeres. mRNA encoding each of the other four Shroom family proteins (Hagens et al., 2006) was microinjected into two blastomeres of four-cell stage Xenopus embryos and the embryos were raised until the mid-blastula stage (just before the onset of zygotic transcription). In all cases, pigmentation is induced, although the intensity of pigment accumulation differs for each Shroom family member. Shroom2 and Shroom3 induce very strong pigment on the entire apical surface of expressing cells, while the pigmentation in Shroom1 and 4 expressing cells is comparatively weak and is concentrated at the cell edges (Fig. S1 and Fig. 1b). The consistent pigment accumulation in Shroom-family protein expressing cells supports the idea that these proteins are involved in pigmentation.
Figure 1. Ectopic expression of Shroom2 induces pigment, spectrin and dynactin on apical surface of Xenopus blastomere and epidermal tissue.
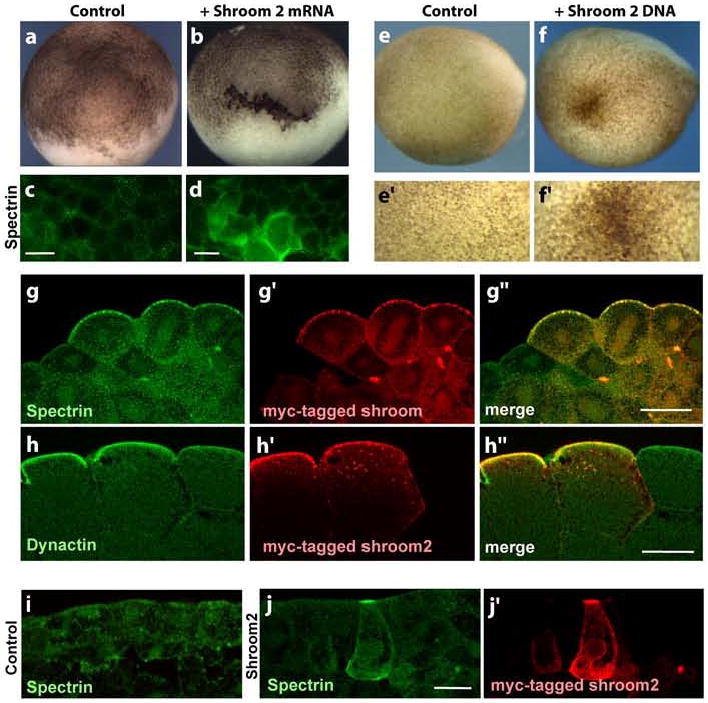
(a-b) Lateral views of blastula (a) Control (b) Shroom2 expressing blastula (c-d) Surface views of blastula (stage 8) stained with spectrin (green) antibody, scale bar = 100 μm. (c) Control (d) Shroom2 expressing cells (e-f) Lateral view of neurula, anterior to the left and dorsal to the top, bottom panels are high magnification views of top panels. (e) Control (f) Ectopic Shroom2 expressing neurula. (g) Cross-section view of ectopic Shroom2 expressing blastula (stage 8) stained with myc-tag Shroom2 (red) and spectrin (green) antibodies, scale bar = 50 μm. (h) Cross-section view of ectopic Shroom2 expressing blastula (stage 8) stained with myc-tag Shroom2 (red) and p150glued (green) antibodies, scale bar = 50 μm. (i-j) Cross-section view of neurula epidermis stained with with myc-tag Shroom2 (red) and spectrin (green) antibodies, scale bar = 20 μm. (d) Control (e) Ectopic Shroom2 expressing epidermis.
Ectopic Shroom expression induces apical spectrin accumulation as well as pigment accumulation
We next sought to further understand the mechanism by which Shroom proteins bring about pigment accumulation. The ectopic expression of Shroom2 induces the apical accumulation γ-tubulin, a minus-end organizer of microtubules (Fairbank et al., 2006). Spectrins are known to link melanosomes and other vesicles to the minus-end directed motor, dynein, in a variety of cell types, including pigment cells of Xenopus skin (Aspengren and Wallin, 2004; Watabe et al., 2008), and a physical interaction between Shroom1 and spectrin has been reported (Zuckerman et al., 1999).
To test if spectrin is involved in the pigment accumulation induced by Shroom family proteins, the distribution of spectrin was observed by immunostaining in cells expressing myc-tagged Shroom family proteins. Spectrin is accumulated dramatically at the apical surface in Shroom2 and Shroom3 expressing cells. This spectrin induction is more obvious in a surface view (Figs. 1d and S2b). As shown in Figs. 1g and S2c, a number of spectrin particles are detected in Shroom expressing cells compared to neighboring cells. These data suggest that the functions of Shroom family proteins include controlling spectrin distribution.
To confirm this, we expressed shroom family proteins in another epithelial population, epidermal cells of neurula stage, using plasmid DNA injection. In Shroom2 and Shroom3 DNA injected embryos, ectopic apical pigment concentration is found in epidermal cells (Figs. 1f and S2e) and spectrin is also accumulated at the apical surface of Shroom expressing cells. Furthermore, it seems that spectrin co-colocalizes with ectopic Shroom2 and Shroom3 (Figs. 1j and S2g). Together, these data suggest that Shroom family proteins are involved both in the pigmentation and the regulation of spectrin distribution in Xenopus.
Shroom2-mediated pigment accumulation correlates with enriched dynactin at the blastomere surface
Spectrin governs the localization of melanosomes by facilitating their interaction with the minus-end directed microtubule motor protein, dynein (Aspengren and Wallin, 2004; Muresan et al., 2001). Spectrin binds directly to two subunits of the dynactin complex, which are essential for dynein motor function (Aspengren and Wallin, 2004; Holleran et al., 2001). We therefore asked if dynactin localization was changed in blastomeres overexpressing Shroom2. Indeed, p150glued, a subunit of dynactin, is accumulated at the apical surface of cells expressing ectopic Shroom2 (Fig. 1h). Together, these data suggest that Shroom2 acts via a spectrin-dynein-dynactin complex to transport melanosomes to the cell surface.
Shroom2 localizes in animal hemisphere during oogenesis and early development in Xenopus
The animal hemisphere of eggs from many amphibian species is darkly pigmented, but the cellular mechanism by which this pigmentation is controlled remains unknown. Shroom3 and Shroom 4 are not present maternally in Xenopus, however Shroom1 and Shroom2 are expressed maternally and their mRNAs localize in the pigmented animal hemisphere of Xenopus (Lee et al., 2009). Because Shroom2 has been reported to control the biogenesis and transport of melanosomes in the retinal pigment epithelium of Xenopus (Fairbank et al., 2006), we hypothesized that Shroom2 may be involved in the pigment polarity of Xenopus oocytes and eggs.
To test this idea, we asked if Shroom2 protein localizes asymmetrically in the animal region of Xenopus oocytes and embryos using immunostaining with an anti-Xenopus Shroom2 peptide antibody. Stage III Xenopus oocytes have little polarity in pigmentation. Within these oocytes, Shroom2 protein is mainly concentrated around the germinal vesicle (GV) and is also detected along the entire surface of the oocytes. Some protein is detected in between the GV and oocyte surface, which seems to indicate that the protein moves from the GV to oocyte surface (Fig. 2a). However, in a fully-grown oocyte (stage VI), which has obviously polarized pigment, Shroom2 protein is distributed mostly in the cortex of animal hemisphere (Fig. 3b). The difference in protein levels is most apparent when comparing the animal and vegetal cytosol. Animal cytosolic Shroom2 is localized in streams to the surface, while vegetal cytosolic Shroom2 forms small clusters (Fig. 2b and c). The streams of animally-localized Shroom2 are reminiscent of streams of spectrin protein that localize to the animal hemispere of Xenopus oocytes, described previously (Carotenuto et al., 2000).
Figure 2. Shroom2 localizes in animal hemisphere during late oogenesis and early development in Xenopus.
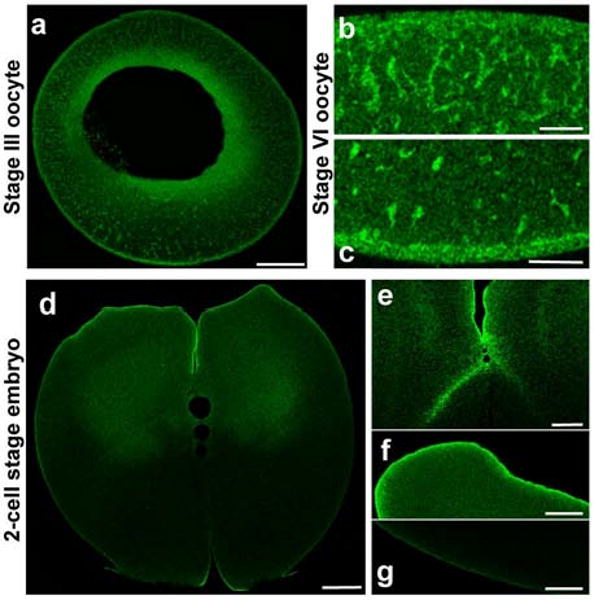
(a-g) Shroom2 peptide antibody staining (green) (a) stage III oocyte, sagittal section, animal to the top, scale bar = 100 μm. (b-c) Stage VI oocyte, sagittal section, scale bar = 20 μm. (b) Animal cortex (c) Vegetal cortex (d-g) 2 cell stage embryo, sagittal section, animal to the top (d) Whole embryo, scale bar = 100 μm. (e) High magnification of cleavage furrow, scale bar = 50 μm (f) Animal pole, scale bar = 50 μm. (g) Vegetal pole, scale bar = 50 μm.
Figure 3. Shroom2 colocalizes with spectrin in Xenopus oocyte and early blastula.
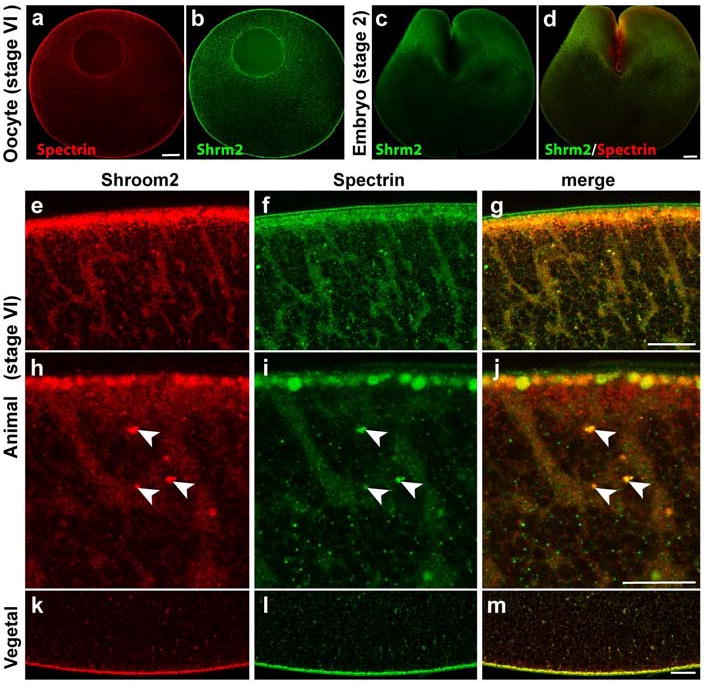
(a-l) Shroom2 (green) and spectrin (red) proteins are immunostained at stage VI oocyte and 2-cell stage embryo. (a-b) Stage VI oocyte, sagittal section views, animal to the top, scale bar = 100 μm. (a) Spectrin (b) Shroom2 (c-d) 2-cell stage embryo, sagittal section view, scale bar = 100 μm. (c) Shroom2 (d) Merge view (e-m) Sagittal section views of stage VI oocyte (e-g) Animal cortex, animal to the top, scale bar = 20 μm. (e) Shroom2 (f) Spectrin (g) Merge (h-j) High magnification views of E-G, scale bar = 10 μm. (h) Shroom2 (i) Spectrin (j) Merge (k-m) Vegetal cortex, vegetal to the bottom, scale bar = 20 μm. (k) Shroom2 (l) Spectrin (m) Merge.
After fertilization, the Shroom2 protein remains mostly in the animal hemisphere but not in the vegetal hemisphere, even at the cortex (Fig. 2d-g).
Shroom2 partially colocalizes with spectrin in Xenopus oocyte and early blastula
Spectrin protein localizes asymmetrically in Xenopus oocytes and embryos to the animal hemisphere (Carotenuto et al., 2000). Since spectrin has a similar localization pattern to Shroom2, we tested whether Shroom2 colocalizes with spectrin during oogenesis and early development. Like Shroom2, spectrin also localizes mostly in the cytosol of the animal pole, but rarely in the vegetal cytosol. Spectrin protein also forms streams in the animal hemisphere (Fig. 3f and 3i) and clusters in the vegetal hemisphere (Fig. 3l), as seen in Shroom2 localization (Fig. 3e, h and k).
In 2-cell stage embryos, spectrin protein localizes predominantly to the animal hemisphere, as does Shroom2 (Fig. 3c and d). Since we found that Shroom2 and spectrin localization seems to overlap, we used high-magnification microscopy to examine whether Shroom2 and spectrin colocalize in vesicles during oogenesis. We observed that Shroom2 overlaps with spectrin in some, but not all vesicles. These vesicles in which Shroom2 and spectrin colocalize are distinguished from other vesicles by their larger size (Fig. 3h-j, arrowhead). These observations suggest that Shroom2 and spectrin are shared components governing transport of a certain type of vesicle, possibly melanosomes.
The absence of Shroom2 function may affect the pigment localization in Physalaemus
Little is known about the evolution of the cell biological mechanisms that produce the variety of pigmentation patterns observed in amphibian eggs. Based on our data from Xenopus, we hypothesized that changes to the localization of Shrooom2 or spectrin may be one mechanism underlying changes in pigment patterns during amphibian evolution. To test this hypothesis, we examined the eggs and embryos of Physalaemus pustulosus. This species lays its eggs in protected foam nests, and in contrast to Xenopus (Fig. 4b), the embryos and eggs of this species have no pigment on the surface (Fig. 4a), and thus appear “unpigmented”.
Figure 4. Maternal levels of Shroom2 mRNA are very high in Xenopus embryos, but very low in Physaleamus.
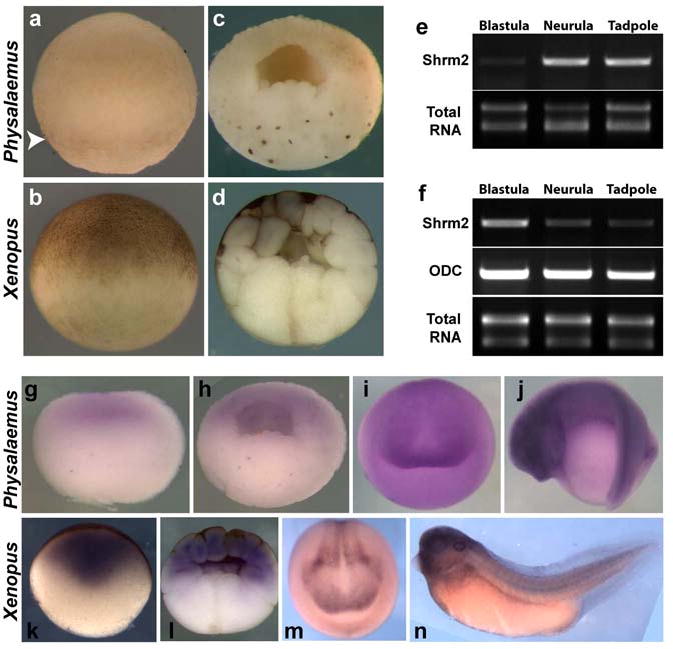
(a-b) Lateral view of early blastula, animal to the top (a) Physalaemus, arrowhead indicates pigment in the Physalaemus egg, (b) Xenopus (c-d) Sagittal section of early blastula, animal to the top (c) Physalaemus (d) Xenopus (e-f) Comparison of Shroom2 expression levels between Xenopus and Physalaemus by RT-PCR (reverse transcriptase PCR) (e) Physalaemus (f) Xenopus (g-j) In situ hybridization against shroom2 in Physalaemus (g) Sagittal section of egg, animal to the top (h) Sagittal section of blastula, animal to the top (i) Anterior view of neurula, dorsal to the top (j) Lateral view of tailbud stage embryo, dorsal to the top (k-n) In situ hybridization against shroom2 in Xenopus (k) Sagittal section of egg, animal to the top (l) Sagittal section of blastula, animal to the top (m) Anterior view of neurula, dorsal to the top (n) Lateral view of tailbud stage embryo, dorsal to the top.
However, pigment is produced in Physalaemus eggs and embryos. A weak band of pigment can be seen in the eggs, just below the equator (Fig. 4a, arrowhead). Moreover, very dark pigment is detected surrounding the nuclei of vegetal blastomeres in hemi-sectioned cleavage stage embryos (Fig. 4c). Thus, pigment is produced by these eggs, but it is not transported to the surface of the animal hemisphere. We hypothesize that this pigment localization difference might be explained by an absence of the Shroom2 function in these embryos.
Based on the sequence of Xenopus Shroom2, a 3′ fragment of Physalamus Shroom2 mRNA was cloned and reverse transcription PCR (RT-PCR) was performed using primers based on the obtained fragment. In Xenopus, Shroom2 is highly expressed at blastula stages as compared to the lower expresion levels at neurula or tadpole stage embryos (Fig. 4f). By contrast, Physalaemus Shroom2 transcripts are detected at only a very low level in blastula stage embryos, as compared to the higher levels seen in neurula or tadpole stage embryos (Fig. 4e). Moreover, in situ hybridization confirms that Physalaemus Shroom2 is barely expressed in the egg (Fig. 4g) or in blastula stage embryos (Fig. 4h). Neurula and tadpole stage expression patterns for Shroom2 were comparable between Xenopus and Physaleamus. (Fig. 4j, m and n).
These data support the idea that Shroom2 may play a role in facilitating melanosome transport in oocytes of some amphibians and that a lack of Shroom2 expression may contribute to a perinuclear pigment localization in other species. To test this idea more directly we used mRNA injection to ectopically express Shroom2 in the animal pole of Phyasleamus embryos. Injection of Shroom2 mRNA resulted in the accumulation of pigment on the otherwise unpigmented animal surface of injected blastomeres (Fig. 5b). This pigment accumulation was modest as compared to that observed in Xenopus, suggesting that control of Shroom2 levels, while important, is only one of multiple mechanisms by which egg pigmentation patterns are varied between amphibian species.
Figure 5. Ectopic Shroom2 expression induces pigment accumulation at the apical surface in Physalaemus embryo.
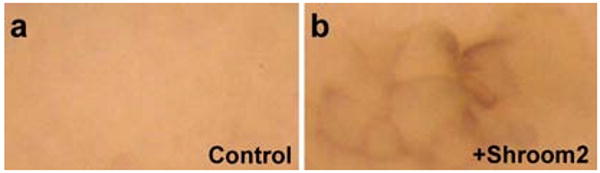
These embryos are around stage 8 (staged according to the X. laevis table of stages). Animal views (a) Control (b) Ectopic shroom2 expressing embryo.
Spectrin is concentrated at nuclei in Physalaemus, but concentrates in cytoplasmic aggregates in Xenopus
We hypothesized that spectrin, as well as Shroom2, plays a role in polarized transport of pigment granules during Xenopus oogenesis. To test this idea, we compared the distribution of spectrin in Physalaemus embryos with that in Xenopus embryos. As mentioned previously, distribution of spectrin protein is asymmetric along the animal-vegetal axis in Xenopus oocyte (Carotenuto et al., 2000). This distribution persists until mid-blastula stage where spectrin is abundant in the animal hemisphere but less so in the vegetal hemisphere (data not shown). In addition, a large number of spectrin puncta are detected in cytoplasm of both animal and vegetal hemispheres, although the number of speckles differs between the two hemispheres (Fig. 6c and d). We hypothesize that these puncta are vesicles that are transported to and from the Golgi, possibly by spectrin (Beck, 2005; Stankewich et al., 1998). However, such puncta are barely present in the cytoplasm of Physalaemus embryos (Fig. 6a and b). Instead most of the spectrin is highly concentrated in the perinuclear region of vegetal pole cells (Fig. 6b). This pattern is very similar to the patten of pigment localization in these embryos (Fig. 4c). This result suggests that the localization of spectrin in perinuclear regions reflects the localization of pigment granules in this region.
Figure 6. Spectrin distribution is distinguishable, but not that of MYO5a between Physalaemus and Xenopus.
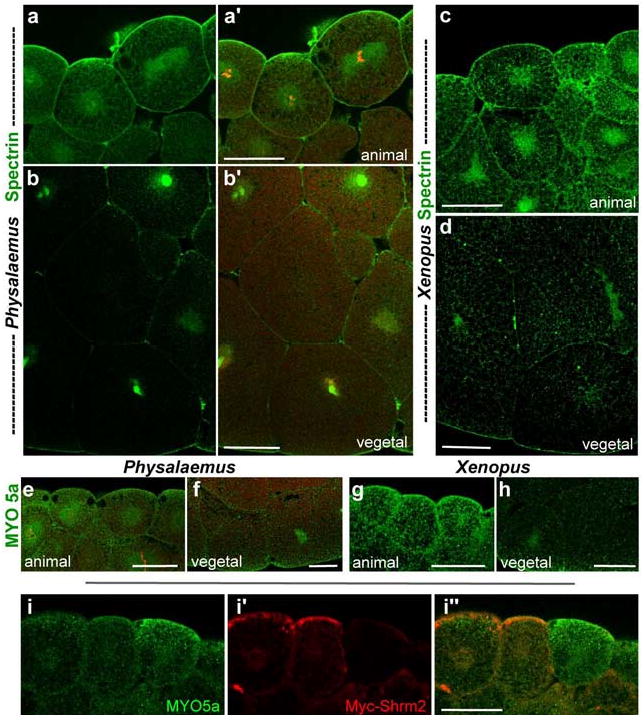
(a-d) Distribution of spectrin (green) in early blastula, sagittal section, animal to the top (a, a′) Animal cortex of Physalaemus blastula, (b, b′) Vegetal cortex of Physalaemus blastula. The nuclei were stained with propidium iodide (red) in a′ and b′. (c) Animal cortex of Xenopus blastula (d) Vegetal cortex of Xenopus (e-h) Distribution of myosin Va (MYO5a, green) in early blastula, sagittal section, animal to the top (e) Animal cortex of Physalaemus blastula (f) Vegetal cortex of Physalaemus blastula (g) Animal cortex of Xenopus blastula (h) Vegetal cortex of Xenopus (i) MYO5a (green) distribution in Shroom2 (red) expressing cells, sagittal section. Scale bar = 50 μm.
Finally, we examined the distribution of the MYO5a, an actin-based motor that also controls localization of melanosomes. MYO5a is recruited to melanosomes by Rab27, which has been shown to be involved in pigmentation by Shroom2, so it was conceivable that Shroom2 cooperates with myosin family motors to transport melanosomes (Etournay et al., 2007; Fairbank et al., 2006; Seabra and Coudrier, 2004). Moreover, in skin pigment cells lacking MYO5a, melanosomes accumulate abnormally in the perinuclear region, reminiscent of pigment localization in Physalaemus (Wu et al., 1998). We observed MYO5a protein by immunostaining, but MYO5a distribution is not observably different between Physalaemus and Xenopus. In both, MYO5a forms small puncta and is distributed uniformly over all cells, indicating that MYO5a is not a key motor in determining the species differences in melanosome distribution between these two frogs (Fig. 6e-h). Furthermore, in ectopic Shroom2 expressing cells, MYO5a is not accumulated at the apical surface, although pigment is (Fig. 6i).
Discussion
Here, we present a comparative analysis of the cell biological basis of ooocyte and egg pigmentation in two frog species, Xenopus laevis and Physalaemus pustulosus. Unlike the Xenopus egg, which has polarized pigment along its animal-vegetal axis, the Physalaemus egg has no external pigment. We show that the expression level and distribution pattern of two molecules, Shroom2 and spectrin, is significantly different between Xenopus and Physalaemus.
Shroom2 is an actin binding protein and γ-tubulin regulator (Dietz et al., 2006; Fairbank et al., 2006) involved in eye pigmentation in Xenopus. Shroom2 is expressed in the retinal pigment epithelium of Xenopus and down-regulation of Shroom2 levels causes defects in eye pigmentation (Fairbank et al., 2006). We hypothesized that Shroom2 is involved in pigmentation of amphibian oocytes and egge. It should be noted, however, that Shroom1 is present maternally (Lee et al., 2009) and can drive pigment accumulation (Fig. S1). It is therefore possible that Shroom1 acts in concert with Shroom2. Several points of data support a role for Shroom proteins in egg pigment patterning. First, a large quantity of Shroom2 is expressed maternally in Xenopus and both Shroom2 protein and mRNA localize to the animal hemisphere of oocytes and eggs, where pigment is polarized (Lee et al., 2009). However, this is not the case in all frog species; in Physalaemus, which lack animal surface pigmentation, only a very small amount of Shroom2 is maternally expressed (Fig. 4e, g and h).
Another line of evidence suggesting a role for Shroom2 in oocyte and egg pigmentation is that ectopic expression of Shroom2 in the early blastula induces strong pigmentation on the surface of the embryo before the mid-blastula transition, the starting point of zygotic transcription (Fairbank et al., 2006). Third, pigmentation by Shroom2 depends on the activity of Rab27a, a critical factor for actin-based melanosome transport (Fairbank et al., 2006; Seabra and Coudrier, 2004). Rab27a functions in MYO7a-dependent melanosome transport, linking melanosomes to actin motors (Seabra and Coudrier, 2004). Finally, Shroom2 directly binds with the c-terminal domain of MYO7a (Etournay et al., 2007), a motor protein essential for pigment granule transport in RPE (Futter et al., 2004; Gibbs et al., 2004; Liu et al., 1998a). Of further interest is the fact that in MYO7a mutant mice, RPE pigment fails to move apically and accumulates in the perinuclear region (Liu et al., 1998b). A similar phenomenon is observed in Physalaemus embryos (Fig. 4c), consistent with our hypothesis that pigment granules accumulate in the perinuclear region in Physalaemus embryos due to a lack of maternally-supplied Shroom2. Unfortunately, due to the lack of effective MYO7a antibodies for amphibians, the co-localization of MYO7a and Shroom2 in embryos has not been tested.
Although Shroom2 is an actin binding protein and associated with an actin-based motor, MYO7a (Coudrier, 2007; Dietz et al., 2006), it may be also involved in microtubule-based movement of pigment granules. Indeed, ectopic expression of Shroom2 in epidermis induces polarized microtubule assembly along apico-basal axis of individual cells (Lee et al., 2009). Supporting our hypothesis, Shroom1, Shroom2, and Shroom3 are each sufficient to alter the distribution of γ-tubulin, a minus-end microtubule nucleator (Fairbank et al., 2006; Lee et al., 2007). Moreover, Shroom2 also induces the accumulation of dynactin which forms a complex with dynein, a minus-end microtubule motor (Fig. 6j). These data together suggest that Shroom2 may affect the structure and polarization of microtubules, thus allowing melanosomes to move along these microtubules in a dynein-dependnent manner.
Indeed, microtubules are highly polarized along the animal-vegetal axis in Xenopus oocytes. During stage IV and V, microtubule begins to be polarized along animal-vegetal axis, resulting in dense microtubule bundles in animal hemisphere and less dense arrays in the vegetal hemisphere (Gard, 1999; Gard et al., 1995). This timing matches that of pigment polarization. In addition, it has been observed that over 90% of microtubules are oriented with minus ends toward the cell cortex in Xenopus oocytes (Pfeiffer and Gard, 1999) and that γ-tubulin is localized to the cortex (Gard, 1994). These observations give rise to the possibility that Shroom2 controls the polarity of γ-tubulin distribution and microtubule orientation, and eventually generates the pigment polarity through this microtubule alignment during Xenopus oogenesis.
Spectrin is another cytoskeletal molecule that is polarized in the oocyte and early blastula of Xenopus. Like Shroom2, spectrin is abundant in the animal hemisphere (Carotenuto et al., 2000). Furthermore, overexpression of Shroom2 induces spectrin accumulation as well as pigment in Xenopus blastomeres, and spectrin co-localized with Shroom2 in Xenopus blastomeres (Fig. 1b, d and g). Since spectrin is involved in the transport of intracellular vesicles, including melanosomes (Aspengren and Wallin, 2004; Beck, 2005; Watabe et al., 2008), we hypothesize that spectrin may play a role in pigment transport with Shroom2 during Xenopus oogenesis. In early Xenopus embryos, spectrin protein is detected in small puncta, possibly vesicles (data not shown); in Shroom2 overexpressing cells the number of these puncta is increased and the protein is accumulated at the cortex where ectopic Shroom2 localizes (Fig. 1g and i). Very interestingly, it has been observed that spectrin concentrates at the nuclear region of vegetal cells in early the Physalaemus embryo, unlike Xenopus embryo (Fig. 6b and d). This localization pattern of spectrin may coincide with that of pigment granules in Physalaemus (Fig. 4c). Dynactin is known to form a complex with spectrin and dynein to control pigment distribution in Xenopus skin melanophores (Aspengren and Wallin, 2004), and we suggest that Shroom2 acts via a similar system in the oocyte and egg. Finally, we suggest that evolutionary changes in maternal Shroom2 mRNA levels impact the deployment of this spectrin/dynactin complex, resulting in variations in egg pigment patterns between species.
Supplementary Material
Acknowledgments
We thank M.J. Ryan and J. Bond for providing Physalaemus eggs and embryos. Also, we thank Esther Kieserman for critical reading of this manuscript. This work was supported by grants from The Burroughs-Wellcome Fund, The March of Dimes, and the NIH/NIGMS.
References
- Aspengren S, Wallin M. A role for spectrin in dynactin-dependent melanosome transport in Xenopus laevis melanophores. Pigment Cell Res. 2004;17:295–301. doi: 10.1111/j.1600-0749.2004.00150.x. [DOI] [PubMed] [Google Scholar]
- Beck KA. Spectrins and the Golgi. Biochim Biophys Acta. 2005;1744:374–82. doi: 10.1016/j.bbamcr.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Blaustein AR, Belden LK. Amphibian defenses against ultraviolet-B radiation. Evol Dev. 2003;5:89–97. doi: 10.1046/j.1525-142x.2003.03014.x. [DOI] [PubMed] [Google Scholar]
- Browder L. Developmental Biology. 3rd. Harcourt Brace College Publishers; 1991. [Google Scholar]
- Brown SS. Cooperation between microtubule- and actin-based motor proteins. Annu Rev Cell Dev Biol. 1999;15:63–80. doi: 10.1146/annurev.cellbio.15.1.63. [DOI] [PubMed] [Google Scholar]
- Carotenuto R, Vaccaro MC, Capriglione T, Petrucci TC, Campanella C. alpha-Spectrin has a stage-specific asymmetrical localization during Xenopus oogenesis. Mol Reprod Dev. 2000;55:229–39. doi: 10.1002/(SICI)1098-2795(200002)55:2<229::AID-MRD13>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chi A, Valencia JC, Hu ZZ, Watabe H, Yamaguchi H, Mangini NJ, Huang H, Canfield VA, Cheng KC, Yang F, Abe R, Yamagishi S, Shabanowitz J, Hearing VJ, Wu C, Appella E, Hunt DF. Proteomic and bioinformatic characterization of the biogenesis and function of melanosomes. J Proteome Res. 2006;5:3135–44. doi: 10.1021/pr060363j. [DOI] [PubMed] [Google Scholar]
- Coudrier E. Myosins in melanocytes: to move or not to move? Pigment Cell Res. 2007;20:153–60. doi: 10.1111/j.1600-0749.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- Croteau MC, Davidson MA, Lean DR, Trudeau VL. Global increases in ultraviolet B radiation: potential impacts on amphibian development and metamorphosis. Physiol Biochem Zool. 2008;81:743–61. doi: 10.1086/591949. [DOI] [PubMed] [Google Scholar]
- Deacon SW, Serpinskaya AS, Vaughan PS, Lopez Fanarraga M, Vernos I, Vaughan KT, Gelfand VI. Dynactin is required for bidirectional organelle transport. J Cell Biol. 2003;160:297–301. doi: 10.1083/jcb.200210066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz ML, Bernaciak TM, Vendetti F, Kielec JM, Hildebrand JD. Differential actin-dependent localization modulates the evolutionarily conserved activity of Shroom family proteins. J Biol Chem. 2006;281:20542–54. doi: 10.1074/jbc.M512463200. [DOI] [PubMed] [Google Scholar]
- Duellman W, Trueb L. Biology of Amphibians. McGraw Hill; New York: 1986. [Google Scholar]
- Dumont JN. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972;136:153–79. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Etournay R, Zwaenepoel I, Perfettini I, Legrain P, Petit C, El-Amraoui A. Shroom2, a myosin-VIIa- and actin-binding protein, directly interacts with ZO-1 at tight junctions. J Cell Sci. 2007;120:2838–50. doi: 10.1242/jcs.002568. [DOI] [PubMed] [Google Scholar]
- Fairbank PD, Lee C, Ellis A, Hildebrand JD, Gross JM, Wallingford JB. Shroom2 (APXL) regulates melanosome biogenesis and localization in the retinal pigment epithelium. Development. 2006;133:4109–18. doi: 10.1242/dev.02563. [DOI] [PubMed] [Google Scholar]
- Futter CE, Ramalho JS, Jaissle GB, Seeliger MW, Seabra MC. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol Biol Cell. 2004;15:2264–75. doi: 10.1091/mbc.E03-10-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL. Gamma-tubulin is asymmetrically distributed in the cortex of Xenopus oocytes. Dev Biol. 1994;161:131–40. doi: 10.1006/dbio.1994.1015. [DOI] [PubMed] [Google Scholar]
- Gard DL. Confocal microscopy and 3-D reconstruction of the cytoskeleton of Xenopus oocytes. Microsc Res Tech. 1999;44:388–414. doi: 10.1002/(SICI)1097-0029(19990315)44:6<388::AID-JEMT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Gard DL, Cha BJ, Schroeder MM. Confocal immunofluorescence microscopy of microtubules, microtubule-associated proteins, and microtubule-organizing centers during amphibian oogenesis and early development. Curr Top Dev Biol. 1995;31:383–431. doi: 10.1016/s0070-2153(08)60234-3. [DOI] [PubMed] [Google Scholar]
- Gerhart J, Danilchik M, Doniach T, Roberts S, Rowning B, Stewart R. Cortical rotation of the Xenopus egg: consequences for the anteroposterior pattern of embryonic dorsal development. Development. 1989;107(Suppl):37–51. doi: 10.1242/dev.107.Supplement.37. [DOI] [PubMed] [Google Scholar]
- Gibbs D, Azarian SM, Lillo C, Kitamoto J, Klomp AE, Steel KP, Libby RT, Williams DS. Role of myosin VIIa and Rab27a in the motility and localization of RPE melanosomes. J Cell Sci. 2004;117:6473–83. doi: 10.1242/jcs.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagens O, Ballabio A, Kalscheuer V, Kraehenbuhl JP, Schiaffino MV, Smith P, Staub O, Hildebrand J, Wallingford JB. A new standard nomenclature for proteins related to Apx and Shroom. BMC Cell Biol. 2006;7:18. doi: 10.1186/1471-2121-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigo SL, Hildebrand JD, Harland RM, Wallingford JB. Shroom induces apical constriction and is required for hingepoint formation during neural tube closure. Curr Biol. 2003;13:2125–37. doi: 10.1016/j.cub.2003.11.054. [DOI] [PubMed] [Google Scholar]
- Holleran EA, Ligon LA, Tokito M, Stankewich MC, Morrow JS, Holzbaur EL. beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem. 2001;276:36598–605. doi: 10.1074/jbc.M104838200. [DOI] [PubMed] [Google Scholar]
- Kloc M, Bilinski S, Chan AP, Allen LH, Zearfoss NR, Etkin LD. RNA localization and germ cell determination in Xenopus. Int Rev Cytol. 2001;203:63–91. doi: 10.1016/s0074-7696(01)03004-2. [DOI] [PubMed] [Google Scholar]
- Lee C, Le M, Wallingford J. The Shroom family proteins play broad roles in the morphogenesis of thickened epithelial sheets. Dev Dyn. 2009;238:1480–91. doi: 10.1002/dvdy.21942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Scherr HM, Wallingford JB. Shroom family proteins regulate gamma-tubulin distribution and microtubule architecture during epithelial cell shape change. Development. 2007;134:1431–41. doi: 10.1242/dev.02828. [DOI] [PubMed] [Google Scholar]
- Licht L, Grant K. The Effects of Ultraviolet Radiation on the Biology of Amphibians. Integr Comp Biol. 1997;37:137–145. [Google Scholar]
- Liu X, Ondek B, Williams DS. Mutant myosin VIIa causes defective melanosome distribution in the RPE of shaker-1 mice. Nat Genet. 1998a;19:117–8. doi: 10.1038/470. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Hope C, Walsh J, Newton V, Ke XM, Liang CY, Xu LR, Zhou JM, Trump D, Steel KP, Bundey S, Brown SD. Mutations in the myosin VIIA gene cause a wide phenotypic spectrum, including atypical Usher syndrome. Am J Hum Genet. 1998b;63:909–12. doi: 10.1086/302026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muresan V, Stankewich MC, Steffen W, Morrow JS, Holzbaur EL, Schnapp BJ. Dynactin-dependent, dynein-driven vesicle transport in the absence of membrane proteins: a role for spectrin and acidic phospholipids. Mol Cell. 2001;7:173–83. doi: 10.1016/s1097-2765(01)00165-4. [DOI] [PubMed] [Google Scholar]
- Nascimento AA, Roland JT, Gelfand VI. Pigment cells: a model for the study of organelle transport. Annu Rev Cell Dev Biol. 2003;19:469–91. doi: 10.1146/annurev.cellbio.19.111401.092937. [DOI] [PubMed] [Google Scholar]
- Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–8. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- Pfeiffer DC, Gard DL. Microtubules in Xenopus oocytes are oriented with their minus-ends towards the cortex. Cell Motil Cytoskeleton. 1999;44:34–43. doi: 10.1002/(SICI)1097-0169(199909)44:1<34::AID-CM3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Rodionov VI, Hope AJ, Svitkina TM, Borisy GG. Functional coordination of microtubule-based and actin-based motility in melanophores. Curr Biol. 1998;8:165–8. doi: 10.1016/s0960-9822(98)70064-8. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Gelfand VI. Myosin cooperates with microtubule motors during organelle transport in melanophores. Curr Biol. 1998;8:161–4. doi: 10.1016/s0960-9822(98)70063-6. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Tint IS, Fanapour PC, Gelfand VI. Regulated bidirectional motility of melanophore pigment granules along microtubules in vitro. Proc Natl Acad Sci U S A. 1997;94:3720–5. doi: 10.1073/pnas.94.8.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Carvajal A, Saenz-Ponce N, Venegas-Ferrin M, Almeida-Reinoso D, Lee C, Bond J, Ryan M, Wallingford J, Pino Ed. Embryogenesis and laboratory maintenance of the foam-nesting tungara frogs, genus Engystomops (=Physalaemus) Dev Dyn. 2009;238:1444–54. doi: 10.1002/dvdy.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC, Coudrier E. Rab GTPases and myosin motors in organelle motility. Traffic. 2004;5:393–9. doi: 10.1111/j.1398-9219.2004.00190.x. [DOI] [PubMed] [Google Scholar]
- Sive HL, Grainger RM, Harland RM. Early Development of Xenopus laevis: A Laboratory Manual. Cold Spring Harbor Press; Cold Spring Harbor, N.Y.: 2000. [Google Scholar]
- Stankewich MC, Tse WT, Peters LL, Ch'ng Y, John KM, Stabach PR, Devarajan P, Morrow JS, Lux SE. A widely expressed betaIII spectrin associated with Golgi and cytoplasmic vesicles. Proc Natl Acad Sci U S A. 1998;95:14158–63. doi: 10.1073/pnas.95.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuma MC, Gelfand VI. Molecular mechanisms of pigment transport in melanophores. Pigment Cell Res. 1999;12:283–94. doi: 10.1111/j.1600-0749.1999.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Watabe H, Valencia JC, Le Pape E, Yamaguchi Y, Nakamura M, Rouzaud F, Hoashi T, Kawa Y, Mizoguchi M, Hearing VJ. Involvement of dynein and spectrin with early melanosome transport and melanosomal protein trafficking. J Invest Dermatol. 2008;128:162–74. doi: 10.1038/sj.jid.5701019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman JB, Chen X, Jacobs JD, Hu B, Kleyman TR, Smith PR. Association of the epithelial sodium channel with Apx and alpha-spectrin in A6 renal epithelial cells. J Biol Chem. 1999;274:23286–95. doi: 10.1074/jbc.274.33.23286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


