Abstract
Natural killer (NK) cells play crucial roles in innate immunity and express CD39 (Ectonucleoside triphosphate diphosphohydrolase 1 [E-NTPD1]), a rate-limiting ectonucleotidase in the phosphohydrolysis of extracellular nucleotides to adenosine. We have studied the effects of CD39 gene deletion on NK cells in dictating outcomes after partial hepatic ischemia/reperfusion injury (IRI). We show in mice that gene deletion of CD39 is associated with marked decreases in phosphohydrolysis of adenosine triphosphate (ATP) and adenosine diphosphate to adenosine monophosphate on NK cells, thereby modulating the type-2 purinergic (P2) receptors demonstrated on these cells. We note that CD39-null mice are protected from acute vascular injury after single-lobe warm IRI, and, relative to control wild-type mice, display significantly less elevation of aminotransferases with less pronounced histopathological changes associated with IRI. Selective adoptive transfers of immune cells into Rag2/common gamma null mice (deficient in T cells, B cells, and NK/NKT cells) suggest that it is CD39 deletion on NK cells that provides end-organ protection, which is comparable to that seen in the absence of interferon gamma. Indeed, NK effector mechanisms such as interferon gamma secretion are inhibited by P2 receptor activation in vitro. Specifically, ATPγS (a nonhydrolyzable ATP analog) inhibits secretion of interferon gamma by NK cells in response to interleukin-12 and interleukin-18, providing a mechanistic link between CD39 deletion and altered cytokine secretion.
Conclusion
We propose that CD39 deficiency and changes in P2 receptor activation abrogate secretion of interferon gamma by NK cells in response to inflammatory mediators, thereby limiting tissue damage mediated by these innate immune cells during IRI.
Cellular components of the innate immune response influence hepatic inflammatory processes. Natural killer (NK) and natural killer T (NKT) cells are components of human and rodent liver lymphoid cell populations that have the potential to respond acutely in various injury models by virtue of inherent cytotoxicity properties with associated release of cytokines. In several recent studies, interferon gamma (IFNγ), secreted by both NK and NKT cells, has been shown to worsen acute ischemia/reperfusion injury (IRI) in the liver and kidney.1,2
Cytokines such as interleukin-12 (IL-12), IL-15, or IL-18 that specifically activate NK cells further exacerbate hepatic injury.3,4 In a manner analogous to cytokines, extracellular nucleotides and nucleosides accumulate at inflammatory sites where they may also serve as metabolokines. In particular, extracellular nucleotides modulate immune reactions through the activation of the specific type-2 purinergic (P2) receptors P2Y and P2X that are expressed on many cell types, including NK cells.5,6
In general, various cell types, including lymphocytes, release cellular adenosine triphosphate (ATP) that accumulates in the pericellular space upon cell stimulation with polyclonal stimuli such as anti-CD37–9 or mitogenic lectins such as concanavalin A.10 Activation of P2 surface receptors regulates lymphocyte and leukocyte functions including cytokine secretion, cell migration, and cell-cell–dependent regulatory effects of a variety of lymphocyte populations such as regulatory T cells or NKT cells.11–14
The ectonucleotidase CD39/ecto-nucleoside triphosphate diphosphohydrolase 1 [E-NTPDase1] hydrolyses extracellular nucleotides and, in concert with CD73/ecto-5′-nucleotidase, generates adenosine.15 NK and NKT cells are second only to the vascular endothelium with regard to the high levels of functional CD39 expression.16
It is clear from other models involving total renal, intestinal, or cardiac IRI with systemic vascular responses that global endothelial CD39 deletion has major deleterious effects.17 Unique among endothelia, the liver vascular sinusoidal endothelial cell layers do not express CD39 under basal conditions.18 Other cells in the hepatic sinusoids, however, do express CD39 ectonucleotidase activity, e.g. NKT cells. Hence, deletion of ectonucleotidase activity may thus limit inflammatory responses such as that induced by concanavalin A–mediated tissue injury where NKT cells undergo rapid rates of apoptosis, precluding major progression of cell-mediated injury.14
In a comparable manner, we show here that CD39 deletion limits warm partial liver IRI by decreasing proinflammatory function of NK cells in an interferon gamma (IFNγ)-dependent manner.
Materials and Methods
Animal Model
Animals were housed in accordance with the guidelines from the American Association for Laboratory Animal Care. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committees (IACUC) approved all research protocols. C57BL/6 backcrossed (for more than six generations) strains of wild-type (Taconic, Germantown, NY) and CD39-null mice were studied.15 IFNγ-null mice (B6.129S7-Ifngtm1Ts/J) and recombination activation gene 1 (Rag1)-null mice (B6.129S7-Rag1tm1Mom/J) were purchased from the Jackson Laboratory (Bar Harbor, ME). Rag2/common gamma-null mice were kindly provided by the National Institute of Allergy and Infectious Diseases19 and purchased from Taconic (Germantown, NY). Mice had free access to standard mouse chow. For adoptive transfer experiments, intravenous injections were performed into the left saphenous vein in animals of 8–10 weeks of age under anesthesia using xylazine 10 mg/mL and ketamine 80 mg/kg. A total of 5 × 105 sorted splenic NK (NK1.1-positive, CD49b-positive, CD3-negative) cells were injected in 100 µL of phosphate-buffered saline with a 29-gauge needle. At the time of sacrifice, mice were anesthetized, blood was taken from the inferior vena cava, and liver lobes were removed for further processing.
For ischemia and reperfusion experiments, body temperature was continually monitored and maintained at 37°C ± 0.5°C. After oblique incisions, the left hepatic lobe was exposed and a clamp was applied to the portal vein and hepatic artery for 75 minutes. Hepatic veins were not clamped. During the period of ischemia, laparotomy was temporarily closed. After 75 minutes, the clamp was removed and the abdominal cavity was closed in two layers. After specific periods of reperfusion, mice were anesthetized, blood was harvested from the inferior vena cava, and the liver lobes were removed, weighed, and further processed.
Reagents and Antibodies
The following reagents and antibodies were used (conjugates are listed in parentheses): Rabbit anti-mouse CD39 polyclonal antibody,20 fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA), anti-mouse NK1.1 (phycoerythrin [PE], allophycocyanin [APC]), CD3 (FITC), CD4 (Pacific blue, PE), CD8 (PE), CD11b (Pacific blue), CD16 (FITC), CD19 (PE), CD25 (PE), CD27 (PE), CD43 (PE), CD49b (PE, PE-Cy7), CD69 (FITC), CD127 (PE), 2B4 (FITC), CD94 (PE), KLRG1 (PE), NKG2A (PE), NKG2D (PE), Ly49A (PE), Ly49Cl (PE), Ly49G (FITC), Ly49F (PE; eBioscience, San Diego, CA). Alanine aminotransferase (ALT) levels were measured on a Cobas Mira analyzer (GMI Inc., Ramsey, MN) with an ALT reagent (JAS Diagnostics, Miami, FL).
Purification of Liver Mononuclear Cells and Splenocytes
Livers were excised and passed through a 200-gauge stainless steel mesh. The filtrate was centrifuged at 50g for 1 minute and the supernatant was collected. The nonparenchymal cell supernatant fraction was washed once. Cells were resuspended in a 40% Percoll (GE Healthcare) solution and overlaid on a 70% Percoll solution. After centrifugation at 1200g for 20 minutes, the interphase was collected. For adoptive transfer experiments, NK cells were purified from the spleen. Using electromagnetic beads, depletion of CD4-positive, CD8-positive, and CD19-positive (all PE-labeled) cells was performed. For cell sorting with electromagnetic beads, the manufacturer protocol (Miltenyi Biotec Inc., Auburn, CA) was followed. The flow-through was labeled with NK1.1-APC, CD49b-PECγ7, and CD3-FITC for sorting by MoFlo. NK cells were defined as CD3-negative, NK1.1-positive, and CD49b-positive; NKT cells were defined as CD3-positive and NK1.1-positive.
Assessment of NTPDase Activity by Thin Layer Chromatography
The pattern of nucleotide hydrolysis was determined by thin-layer chromatography (TLC) using [2,8-3H]ATP (PerkinElmer, Boston, MA) as substrate, as described previously.21 In brief, NK cells (1 × 105 cells) were incubated with 20 µM [3H]ATP in an initial volume of 120 µL Roswell Park Memorial Institute 1640 (RPMI-1640) medium supplemented with 5 mM β-glycerophosphate. Aliquots of the mixture were periodically applied onto Alugram SIL G/UV254 TLC sheets (Nacherey-Nagel, Duren, Germany) and [3H]ATP and the radiolabeled derivates were separated using an appropriate solvent mixture as previously described.13
Cytokine Measurement by Enzyme-Linked Immunoassay
Commercially available enzyme-linked immunoassay (ELISA) kits were used for determination of INFγ (eBioscience, San Diego, CA). Serum levels of circulating cytokines were determined following the manufacturer instructions.
For the measurement of serum cytokines, samples were analyzed for IL1-β, IL-4, IL-6, IL-10, IL-12, IL-13, IL-18, and IFNγ using the SearchLight Chemiluminescent Protein Array by Pierce (quantitative, plate-based antibody arrays based on traditional ELISA).
Cell Proliferation
For the assessment of cell proliferation, a commercially available MTT (3-(4,5-di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) cell proliferation assay (ATCC, Manassas, VA) was used according to the manufacturer instructions.
Expression of P2 Receptors (Reverse Transcription Polymerase Chain Reaction) by NK Cells
Total RNA was extracted from 106 sorted NKT cells using Trizol (Invitrogen, Carlsbad, CA), chloroform, and precipitated with isopropanol. Between 0.5 and 1 µg RNA was reverse-transcribed to complementary DNA using the Taq-Man Reverse Transcription Kit (Applied Biosystems, Foster City, CA) and 1 µL of the reverse-transcribed product was added to the reaction mixture containing 1 × polymerase chain reaction (PCR) buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl), 1.5 mM MgCl2, 0.2 mM deoxynucleotide triphosphates, 2.5 units of Taq polymerase, and specific primers (see Supporting Methods for list of primers).
Real-time PCR was performed on an Applied Biosystems 7700 system. 18S values were used for normalization
Bone Marrow Transplantation
Wild-type (C57BL/6) mice were exposed to a single dose of 10 Gy (0.28 Gy/minute, 200 kV, 4 mA) γ-ray total body irradiation, using an Andrex Smart 225 (Andrex Radiation Products AS, Copenhagen, Denmark) with a 4-mm aluminum filter, 1 hour before bone marrow transplantation. These animals were used as recipients. The marrow from the femur and tibia of matched CD39-null and wild-type mice were harvested under sterile conditions. The marrow cavity was flushed with RPMI-1640 medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 10% fetal bovine serum and drawn through a 22-gauge needle and then through a 70 µm cell strainer (Fisher Scientific, Pittsburgh, PA) to obtain a suspension of nucleated bone marrow cells. Irradiated recipient mice received 1 × 107 bone marrow cells intravenously. Mice that underwent bone marrow transplantation were housed in sterilized filter-top cages and fed sterilized food and drinking water containing sulfamethoxazole (1 mg/mL) and Trimethoprim (0.2 mg/mL) (Pediatric formulation) for at least 8 weeks before experimentation.
Statistical Analyses
Results are expressed as median and range and mean ± standard deviation. For statistical analyses, the Student t test was used. Significance was defined as P < 0.05.
Results
NK Cells Express CD39 that Efficiently Hydrolyzes ATP
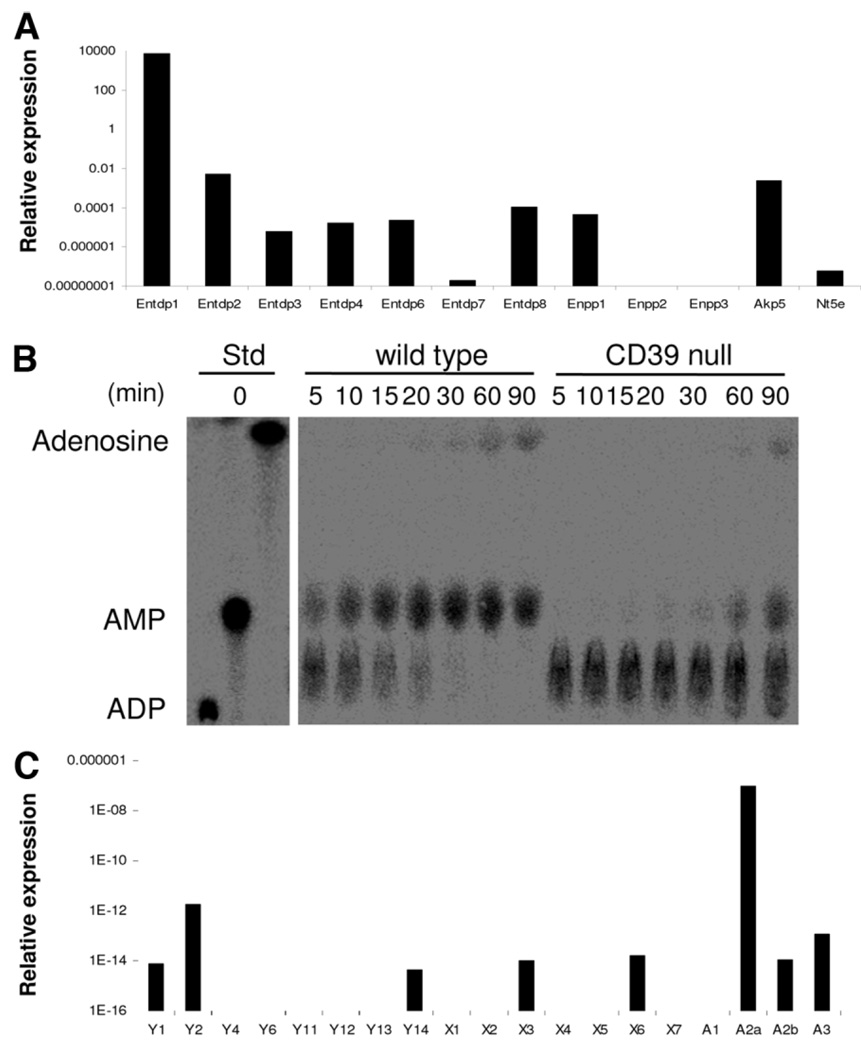
We initially characterized the “purinergic phenotype” of wild-type and quiescent NK cells with regard to the expression of ectonucleotidases and P2 receptors (Fig. 1A,C) using the procedures described previously for NKT cells.14 CD39/E-NTPDase1 expression is dominant among all NK cell ectonucleotidases, including ecto-nucleotide pyrophosphatase/phosphodiesterases (Enpp) and alkaline phosphatases. CD73/5′ ectonucleotidase (Nt5e) is expressed at very low levels by NK cells (Fig. 1A). Hence, sorted hepatic NK cells efficiently hydrolyze adenosine diphosphate (ADP) with respective generation of adenosine monophosphate (AMP); but these cells largely do not generate adenosine (Fig. 1B). Deletion of CD39 can be shown to substantially delay generation of AMP from ADP. These data clearly demonstrate that extracellular nucleotides can be efficiently hydrolyzed to AMP by NK cells from wild-type mice, whereas cells from CD39-null mice show markedly attenuated phosphohydrolysis resulting in decreases in levels of derived AMP and adenosine. In contrast, wild-type NKT cells express both CD39 and CD73 ecto-enzymes at high levels and will generate adenosine as an end-product.14 No compensatory up-regulation of NTPDase activity is observed in NK cells where CD39 has been deleted, as is also the case of NKT cells and other immune cells null for CD39 (Supporting Fig. 1A).8,13–15 We note that expression of P2 receptors in NK cells is restricted to P2Y1, P2Y2, P2Y14, P2X3, and P2X6 (Fig. 1C). NK cells also express P1 receptors with the A2A subtype expressed at the highest level, followed by A3 and A2B at lower levels; NK cells do not express the A1 receptor subtype. Deletion of CD39 does not impact messenger RNA levels of P2-receptors in either quiescent or activated cells (Supporting Fig. 1B).
Fig. 1.
Expression patterns of ectonucleotidases and purinergic receptors on NK cells. Isolated splenic NK (NK1.1-positive, CD49b-positive, CD3-negative) cells from Rag1−/− mice were studied by reverse transcription PCR. (A) Among all the ectonucleotidases tested, the highest expression was noted for CD39/E-NTPDase1. Expression levels of other ectonucleotidases, nucleotide pyrophosphatase (Enpp), and alkaline phosphatase (Akp5) were significantly lower and ecto-5′-ectonucleotidase/CD73 (Nt5e) was near absent. (B) Analysis of NTPDase activity on NK cells was done by TLC. The products of [14C]ADP hydrolysis by purified hepatic NK cells are shown. Extracellular nucle-otides were efficiently hydrolyzed to AMP and adenosine by wild-type cells, whereas cells null for CD39 show substantially delayed hydrolysis of nucleotides and limited production of aden-osine. (C) Isolated NK cells expressed the P2 receptors P2Y1, P2Y2, P2Y14, P2X3, and P2X6; NK also expressed the P1 receptors A2A (at high levels), as well as A2B and A3 but not A1.
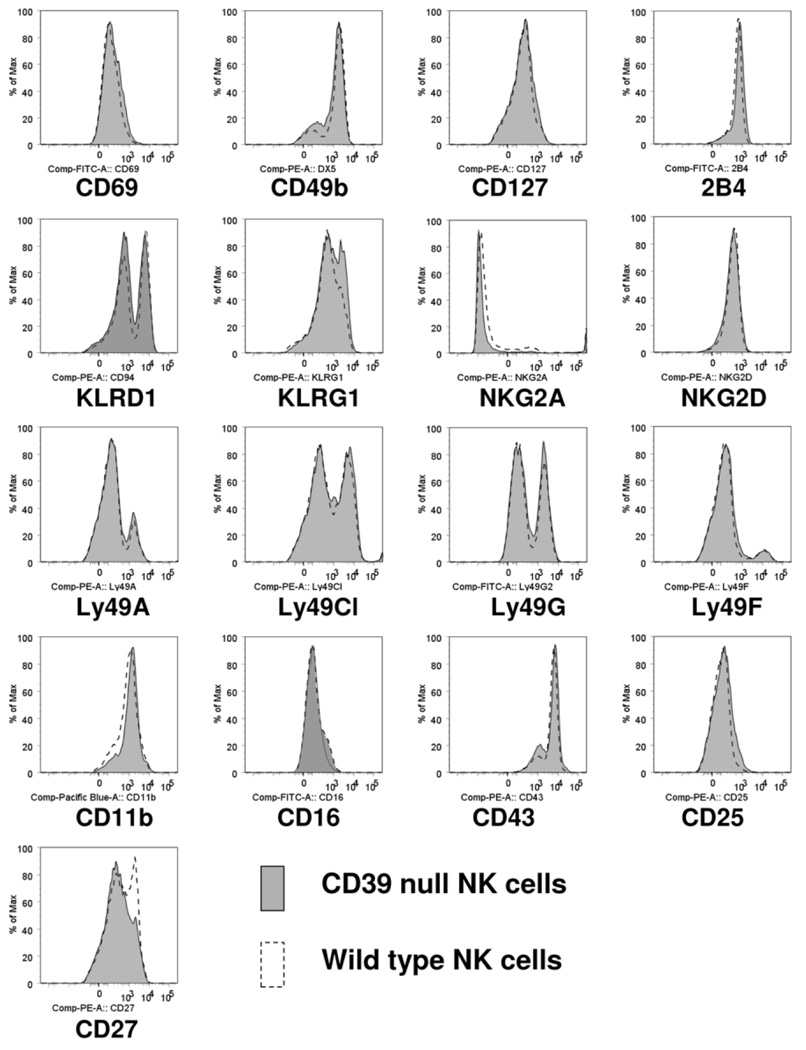
Comparable Expression of NK-Specific Surface Markers in Mice Null for CD39 Compared to Wild-Type Mice
In order to exclude differences in baseline activation of CD39-null versus wild-type NK cells, we compared expression of various surface markers of the two NK cell subtypes isolated from spleens (Fig. 2). The major recognized activation markers did not differ between wild-type and mutant NK cells purified from nonmanipulated wild-type and mutant mice null for CD39. However, CD39-null NK cells did exhibit decreased levels of CD27 with altered patterns of killer cell lectin-like receptor subfamily G, member 1 (KLRG1) expression. The comparable levels in purinergic receptors and the altered levels of CD27 and KLRG1 persisted after activation of NK cells with IL-12 and IL-18 in vitro (data not shown).
Fig. 2.
Expression of NK cell-specific surface markers on sorted NK cells. Isolated splenic NK (NK1.1-positive, CD49b-positive, CD3-negative) cells from wild-type mice and mice null for CD39 were characterized. Surface markers, in general, did not differ between wild-type and mutant NK cells, except KLRG1. Also, CD39-null NK cells expressed decreased levels of CD27 as compared to the wild-type.
Partial Hepatic IRI Is Attenuated in Mice Null for CD39
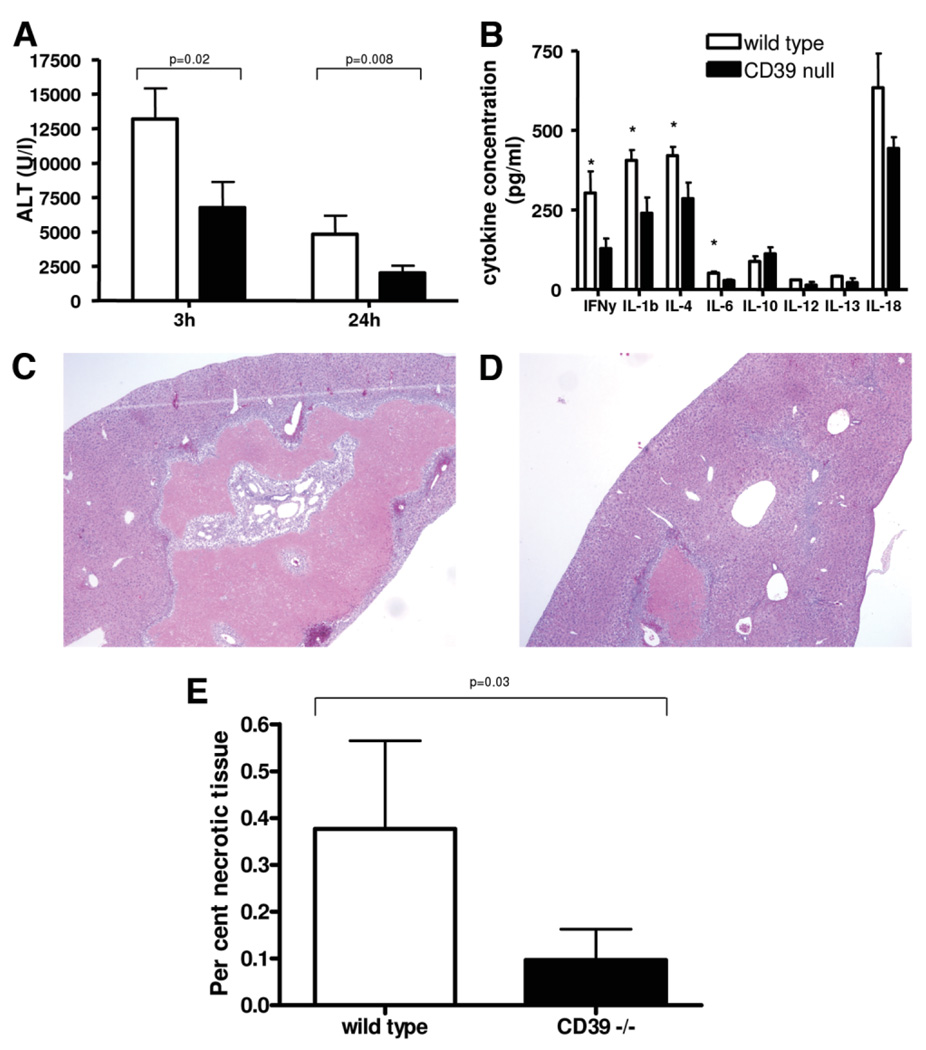
In a standard mouse model of partial, warm hepatic IRI, the left liver lobe was clamped for 75 minutes. Reperfusion was allowed in different groups for 3 hours, 24 hours, and 4 days. In mice null for CD39, liver injury as assessed by ALT levels is significantly decreased after 3 hours and 24 hours of reperfusion (Fig. 3A). Hematoxylin and eosin after 24 hours of reperfusion showed increased hepatocellular injury with swelling and fatty changes in wild-type mice compared to mice null for CD39 (Supporting Fig. 2A,B). Cytokine profiling from serum taken after 3 hours of reperfusion reveals decreased circulating proinflammatory cytokines (Fig. 3B). In order to represent an outcome parameter of potential clinical implication, the area of necrosis was assessed after 4 days of reperfusion.22 Representative images of hematoxylin and eosin staining of liver injury after 4 days of reperfusion are shown in Fig. 3C,D. Long-term effects of liver injury were determined by measurement of the area of liver necrosis at day 4 after ischemia (Fig. 3E).
Fig. 3.
Analysis of liver injury after hepatic ischemia with various periods of reperfusion. (A) Liver injury as assessed by alanine aminotrans-ferase (ALT) levels was significantly decreased in mice null for CD39, when compared to wild-type mice after 3 and 24 hours of reperfusion. (B) Cytokine profiling using arrays after 3 hours of reperfusion revealed decreased levels of circulating cytokines with the exception being IL-10. Representative liver sections of serial hematoxylin & eosin staining after 4 days of reperfusion; (C) wildtype and (D) CD39-null. (E) Results of morphometric analysis of area of necrosis revealed significantly increased area of necrosisin wild-type mice compared to mice null for CD39. Values are means ± standard deviation of at least four animals per time point. Levels of significance were assessed by unpaired t tests. P values are as indicated; asterisks indicate P < 0.05 in (B).
CD39 on Hepatic NK Cells Modulates IRI
CD39 is the dominant ectonucleotidase on the systemic endothelium but is absent on quiescent liver sinusoidal endothelial cells with heightened expression noted with liver injury and regeneration on proliferating cells.18 We undertook to analyze expression patterns of CD39 on sinusoidal nonendothelial cells and to determine how these affected responses after IRI.
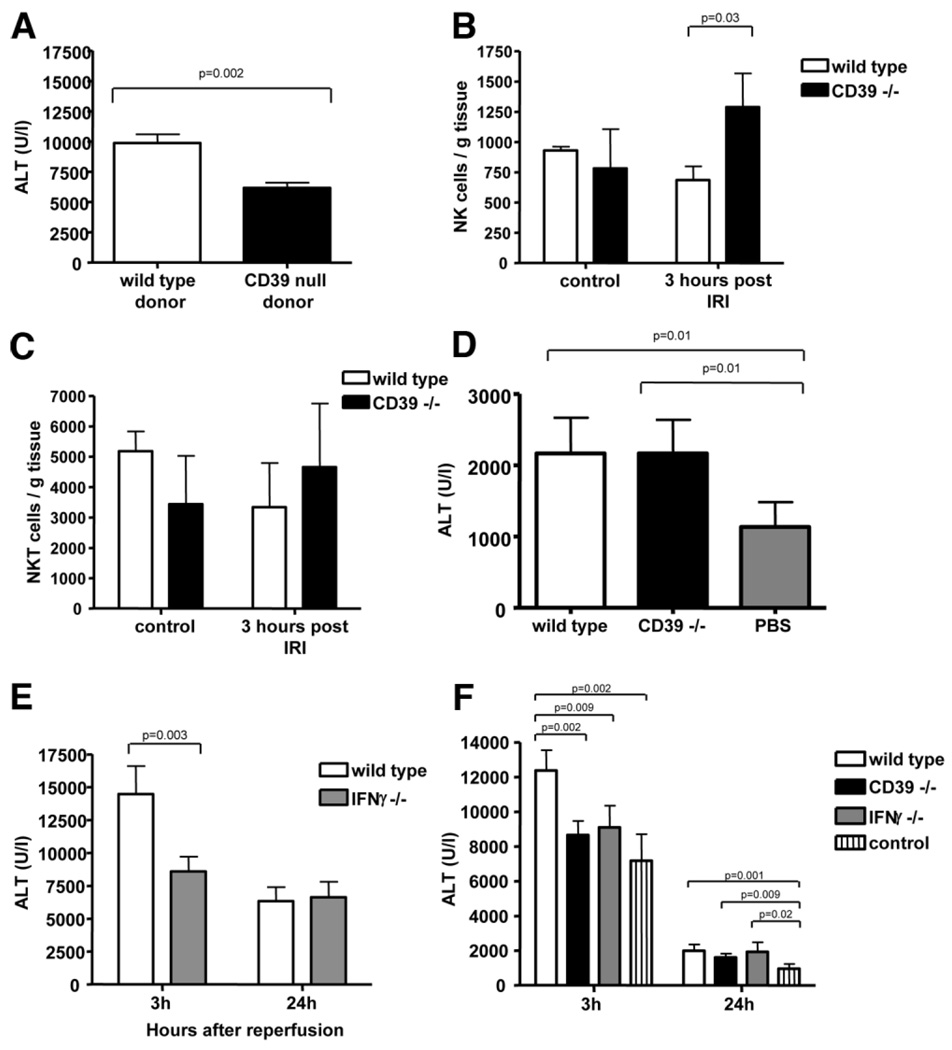
To examine the contributions of the endothelial versus myeloid and immune cell components, wild-type mice were reconstituted with bone marrow from wild-type or CD39-null mice after lethal total body irradiation. Transfer of wild-type bone marrow was associated with significantly more injury after 3 hours of hepatic reperfusion, when compared to transfer of bone marrow from CD39-null mice (Fig. 4A). Expansion of hepatic NK and NKT cells was assessed 3 hours after reperfusion by measuring fractions of intrahepatic lymphocytes. At this time point, expansion of NK cells was observed in wild-type and CD39-null mice (Fig. 4B). The fraction of NK cell population of the entire hepatic mononuclear cells was significantly higher in mice null for CD39. At these same time points, no significant expansion of hepatic NKT cells was observed (Fig. 4C).
Fig. 4.
CD39 on NK cells modulate hepatic IRI. (A) To exclude confounding influences of the hepatic and systemic endothelium, adoptive transfer of wild-type and CD39 bone marrow-derived cells was performed after total body irradiation of wild-type mice. After transfer of CD39-null bone marrow, liver injury was significantly decreased in comparison to transfer of wild-type bone marrow after 3 hours of reperfusion. (B, C) NK cells but not NKT cells expand at 3 hours after reperfusion injury. (D) Performing adoptive transfer of NKT cells into Rag1 null mice revealed decreased injury in the control group and no difference between transfer of wild-type versus CD39-null NKT cells after 24 hours of reperfusion. (E) IRI in mice null for IFNγ was decreased after 3 hours but not after 24 hours. (F) Adoptive transfer of purified NK cells from wild-type mice and mice null for CD39 and null for IFNγ into Rag2/common gamma-null mice. Hepatic injury was significantly decreased after transfer of CD39-null and IFNγ null NK cells after 3 hours of reperfusion as compared to transfer of wild-type NK cells. Values are mean ± standard deviation of at least four animals per time point. Levels of significance were assessed by unpaired t tests. P values are as indicated.
Next, we tested whether CD39 on NKT cells alone can directly modulate hepatic IRI in immunodeficient, reconstituted mice. This was done via adoptive transfer of purified NKT cells (CD3-positive, NK1.1-positive) to Rag1-null mice (which lack T cells, B cells, and NKT cells but contain NK cells). No difference was seen in hepatic injury measured as ALT elevation after adoptive transfer of either CD39-null or of wild-type NKT cells (Fig. 4D). At 24 hours after reperfusion, however, there was significantly less injury in nonreconstituted mice (without previous cell transfer; phosphate-buffered saline used as a vehicle control) when compared to the two reconstituted groups.
IFNγ seems to be crucial in mediating at least some of the manifestations of hepatic and renal IRI.1,2 We noted that in our model only the early phase of injury (3 hours) was substantially influenced by IFNγ; as suggested by the differences between ALT levels of IFNγ knockout mice and wild-type animals (Fig. 4E).
To evaluate the role of CD39 in NK cells, adoptive transfer of sorted NK cells from CD39-null and IFNγ null mice into Rag2/common gamma-null mice (deficient of T cells, B cells, and NK cells) was performed (Fig. 4F). ALT plasma levels used as a parameter of liver injury were significantly decreased in the absence of NK cells, as assessed in Rag2/common gamma-null mice without prior adoptive transfer (designated as control) compared to the same mice after transfer of wild-type NK cells. Hepatic injury was substantially decreased after transfer of NK cells from IFNγ-null (Fig. 4E) or of CD39-null NK (Fig. 4F) animals after reperfusion.
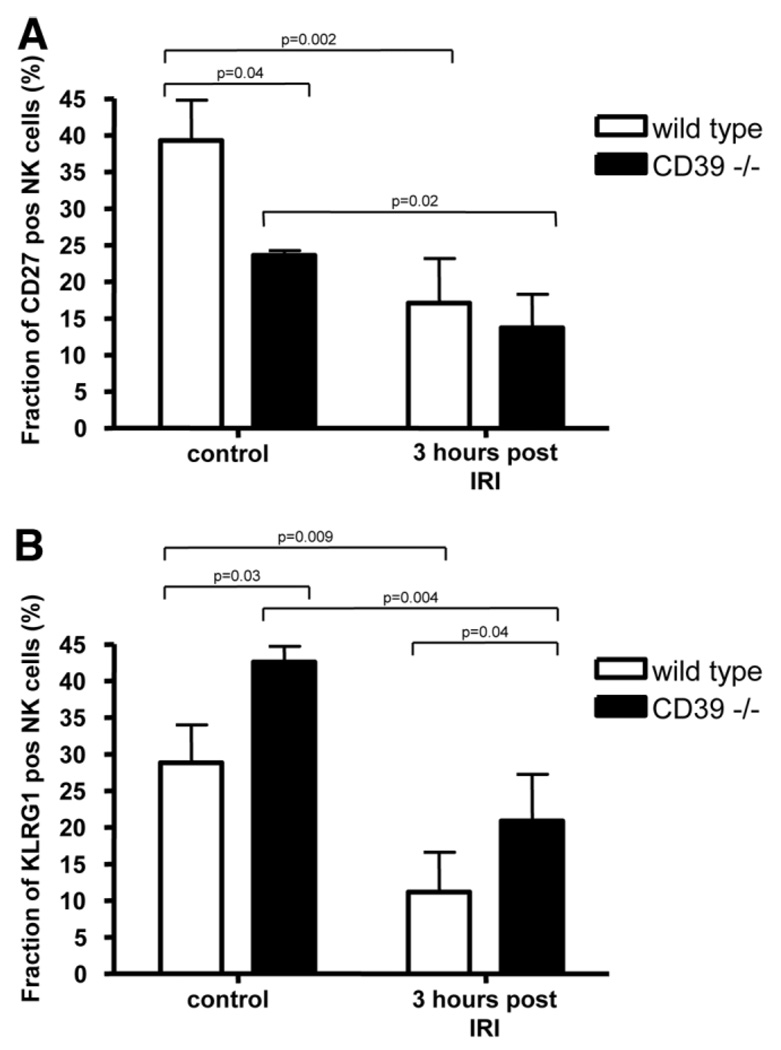
Differences in expression pattern for CD27 and KLRG1 as demonstrated in quiescent cells in vitro were further assessed in vivo (Fig. 5A,B). Hepatic NK cells were purified from control and mutant mice under basal conditions as well as after IRI. Significantly decreased levels of CD27-positive cells were observed in CD39-null mice prior to ischemic injury. After IRI, numbers of CD27-positive NK cells significantly decreased in both wild-type and mutant mice. Conversely, levels of KLRG1-positive cells appeared increased in mutant mice under basal conditions as well as after IRI.
Fig. 5.
Differential numbers of NK cell subsets after IRI in vivo. Hepatic mononuclear cells were isolated in control animals from wild-type and CD39-null mice at baseline (n = 3 each) and at 3 hours after hepatic IRI (n = 4 each). These were gated for NK1.1-positive, CD3-negative, (A) CD27-positive, and (B) KLRG1-positive cells. Significantly decreased levels of CD27-positive cells were observed in CD39-null murine livers prior to injury. After IRI, numbers of CD27-positive NK cells significantly decreased in wild-type and mutant mice. Conversely, levels of KLRG1-positive cells were significantly increased in mutant mice, both under basal conditions and after IRI. Values are mean ± standard deviation. Levels of significance were assessed by unpaired t tests. P values are as indicated.
CD39 Modulates IFNγ Secretion and Expansion of NK Cells In Vitro
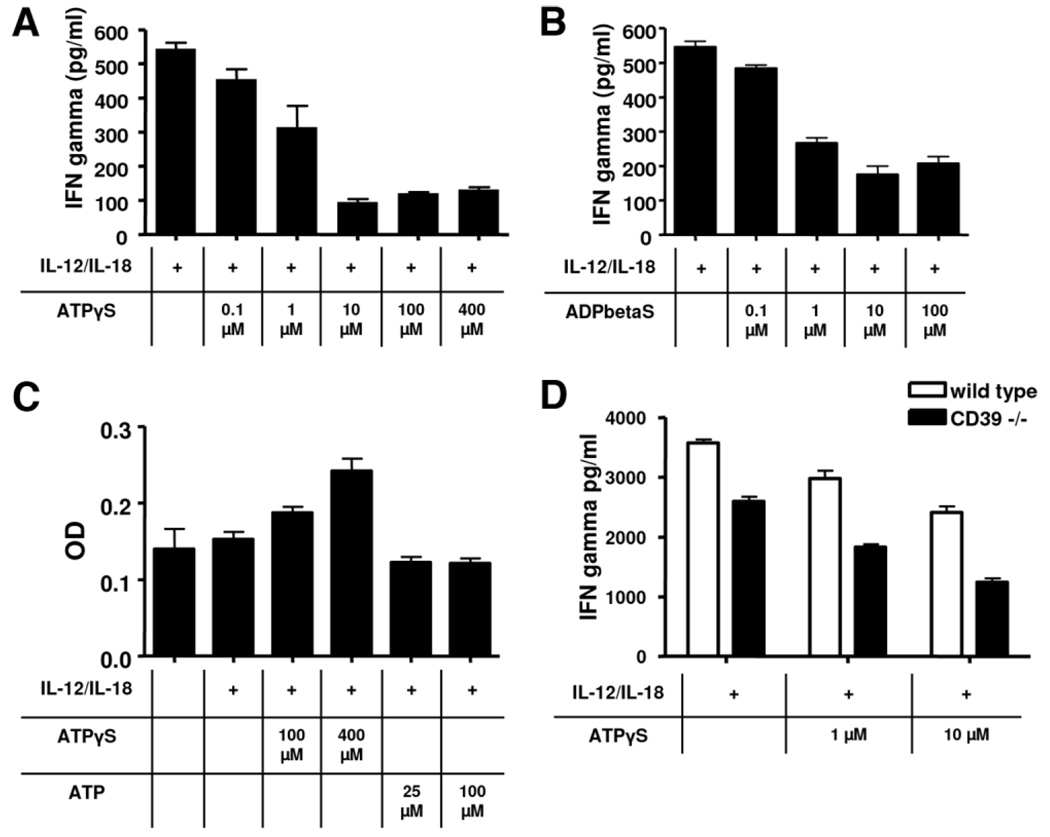
Splenic NK cells were isolated from wild-type mice. To evaluate the role of P2 receptors in regulating the secretion of IFNγ, NK cells were stimulated with the cytokines IL-12 and IL-18 in the presence or absence of extracellular nucleotides. These two selected cytokines are potent activators of NK cells and have been shown to be associated with hepatic IRI.4,23,24 Although the secretion of IFNγ was unaffected by ATP (not shown), this was significantly decreased in response to nonhydrolyzable nucleotides ATPγS and ADPβS; this occurred in a dose-dependent manner (Fig. 6A,B). No inhibition of IFNγ secretion was observed in response to uridine triphosphate gamma S (UTPγS; data not shown).
Fig. 6.
Secrrtion of INFγ by NK cells in vitro. Splenic NK cells were isolated from mice null for CD39 and matched the wild-types. Secretion of IFNγ was induced by administration of IL-12 and IL-18 for 24 hours. (A,B) Addition of ATPγS or ADPβS (nonhydrolyzable ATP/ADP analogs) significantly decreased secretion of IFNγ in a dose-dependent manner. (C) Analysis of cell viability after 36 hours of incubation using a MTT assay: Administration of ATPγS resulted in a dose-dependent increase of optical density. (D) Comparison of IFNγ secretion of wildtype versus CD39-null NK cells. Deletion of CD39 is associated with significantly reduced secretion of IFNγ. Graphs are representative of at least five experiments. Data are given as mean ± standard deviation.
In order to exclude toxic effects of extracellular nucleotides in vitro, cell viability was assessed using an MTT assay (Fig. 6C). In the presence of increasing concentrations of ATPγS, viability in fact increased. Interestingly, this effect occurred in direct response to exposure of cells to ATPγS or UTPγS and it was independent of exposure to IL-12 and/or IL-18 (not shown). Comparisons of wild-type NK cells versus CD39-null NK cells demonstrated decreased levels of IFNγ secretion in the mutant mice (Fig. 6D). This effect was independent of the increasing concentrations of ATPγS.
Discussion
During partial hepatic IRI, the functional expression of CD39 alters levels of extracellular nucleotides and influences the generation of adenosine, thus affecting tissue injury and survival outcomes. The extent of injury in this model, as assessed by elevation of ALT levels and the degree of hepatic necrosis, is substantially decreased in mice null for CD39 when compared to wild-type mice. Cytokine arrays reveal concomitantly decreased levels of proinflammatory cytokines in mice null for CD39 in the post-IRI setting.
CD39 is the dominant ectonucleotidase in NK cells and thereby plays the predominant role in regulating levels of pericellular nucleotide concentrations. Unlike NKT cells, NK cells do not express CD73 and cannot efficiently generate adenosine and primarily mediate ATP/ADP hydrolysis to AMP alone.14 However, low levels of radiolabeled adenosine can still be generated in vitro, possibly due to low-level expression of other ecto-phosphatases by NK or by contaminating cells.25,26
Because NK cells express adenosine (P1) receptors, predominantly of the A2A receptor subtype, the cellular functions of NK cells are most likely inhibited by adenosine generated in the extracellular space for example by ubiquitous CD73.25,26 We show that the repertoire of P2 receptors on NK cells is limited to P2Y1, P2Y2, P2Y14, P2X3, and P2X6. Thus, this P2 receptor expression pattern likely modulates the effects of extracellular nucleotides on NK cell function.
Analysis of expression of cell-specific surface markers revealed enrichment of CD27low and KLRG1high NK cells from mice null for CD39, both after in vitro manipulation and in vivo after IRI. CD27low NK cells secrete less IFNγ and have been further shown to be associated with the expression of KLRG1.27,28 It is considered that this subset of NK cells exhibits less potent effector properties.
Adoptive transfer experiments performed in our study suggest a role for CD39 expression by NK cells, but not by NKT cells, in this model of tissue injury. Curiously, NKT cells per se, in the absence of exogenous adenosine agonists, negatively influence hepatic IRI after 24 hours of reperfusion (Fig. 4D); but not after 3 hours of reperfusion (not shown). It has been shown that NKT cell–derived IFNγ mediates vascular injury in hepatic IRI.1 Blockade of such proinflammatory cytokine secretion, however, is dependent on the activation of the P1 adenosine receptor A2A. On the basis of our experimental data, we propose that activation of P2 receptors on NKT cells does not directly influence hepatic IRI in this model.
NK cell–dependent IFNγ seems to modulate in part the early response to IRI. In the tested model, a distinct early accumulation of NK cells was observed that was dependent on CD39 expression. However, despite higher numbers of NK cells in CD39-null mice, the secretion of IFNγ was markedly diminished overall. As shown in other studies, IFNγ seems to affect ALT levels after hepatic IRI.1 Subsequently, we also noted significant decreases in necrosis up to 4 days after the initial reperfusion in the CD39-null setting. This effect might be increased due to impaired healing and abnormal regeneration in the absence of CD39, as seen in other models.29,30
Importantly, in other organs, such as the kidney,31 CD39 expression has been shown to be protective in IRI, possibly due to high levels of expression by endothelial cells. In the liver, CD39 is not expressed by quiescent sinusoidal endothelial cells.18 Conversely in the kidney or the heart, CD39 is highly expressed by the endothelium where the biological effects exert both local and systemic protective properties.
The liver is distinctive in that the sinusoids contain higher numbers of resident immune mononuclear cells compared to other organs such as the heart and kidney. Beneficial effects have been also noted with adenosine-2A receptor stimulation of NKT cells in the limited lobar, warm hepatic IRI model we have used, as previously studied by Lappas and colleagues.1 This model of partial hepatic ischemia was chosen here to minimize effects mediated by shock and secondary effects on the systemic vasculature. We were able to establish a modulatory role for NK cells in this system where regulation of IFNγ by P2 receptor responses to extracellular nucleotides appears very relevant.
Other studies with IFNγ mutant mice have yielded conflicting data suggesting both beneficial and deleterious effects in IRI models. Different interferons such as interferon alpha and beta have been shown to contribute to hepatic IRI at later time points.32 Decreased injury was observed 6 hours after reperfusion in mice null for the IFNβ receptor, but no significant differences were noted in mice null for IFNγ receptor. In our study, we noted differences at an earlier time point (3 hours) and we used NK cells from mice that have a defect in IFNγ secretion but not in IFN receptor function. Hence, our data reflect differences in release of IFNγ with effects on recipient cells that clearly express the relevant receptors.
We show that IL-12/IL-18–stimulated in vitro secretion of IFNγ by NK cells is decreased by extracellular ATPγS at low (physiologic) concentrations. Interestingly, at a higher concentration (100 µM), ATPγS again elevated levels of IFNγ secretion in wild-type NK cells. Direct toxicity or apoptosis induced in response to stimulation of P2X7 receptors was considered unlikely. First, unlike in NKT cells, the proapoptotic purinoreceptor P2X7 is not expressed in NK cells; second, cell counts were boosted by extracellular nucleotides in a dose-dependent manner. Further, we have shown that extracellular ATP does not directly induce apoptosis in NK cells unlike in NKT cells that rapidly undergo apoptosis in response to extracellular ATP.14
In this study, we show that NK cells influence endorgan injury in hepatic IRI in a process determined by purinergic responses. Regulated pericellular ATP levels on NK cells are required for regulated IFNγ secretion and, thereby, modulation of tissue injury. Future studies will be required to dissect the relative impact of CD39 and other regulatory factors in purinergic signaling and on the other cell types involved in tissue damage and vascular injury resulting from hepatic vascular injury.
Supplementary Material
Acknowledgments
Financial support: S.C.R. acknowledges grant support from National Institutes of Health (NIH) grants HL57307, HL63972, and HL076540. G.B. and Y.B. thank the Swiss National Research Foundation for grant support (PASMA-115700, PBBEB-112764, and PBBEB-112760). W.G.J. acknowledges support from NIH grants GM-51477, GM-60475, and from the DOD/CDMRP PR043034.
Glossary
Abbreviations
- ADP
adenosine diphosphate
- ADPβS
adenosine diphosphate beta S
- ALT
alanine aminotransferase
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- ATPγS
adenosine triphosphate gamma S
- cAMP
cyclic adenosine monophosphate
- CD39/E-NTPDase1
ecto-nucleoside triphosphate diphosphohydrolase 1
- CD73
ecto-5′-ectonucleotidase
- IFN γ
interferon gamma
- IL
interleukin
- IRI
ischemia and reperfusion injury
- NK cell
natural killer cell
- NKT cell
natural killer T cell
- NTPDase
nucleoside triphosphate diphosphohydrolase
- PCR
polymerase chain reaction
- Rag1
recombination activation gene 1
- UTPγS
uridine triphosphate gamma S
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Lappas CM, Day YJ, Marshall MA, Engelhard VH, Linden J. Adenosine A2A receptor activation reduces hepatic ischemia reperfusion injury by inhibiting CD1d-dependent NKT cell activation. J Exp Med. 2006;203:2639–2648. doi: 10.1084/jem.20061097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feng L, Cheng F, Ye Z, Li S, He Y, Yao X, et al. The effect of renal ischemia-reperfusion injury on expression of RAE-1 and H60 in mice kidney. Transplant Proc. 2006;38:2195–2198. doi: 10.1016/j.transproceed.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Kato A, Graul-Layman A, Edwards MJ, Lentsch AB. Promotion of hepatic ischemia/reperfusion injury by IL-12 is independent of STAT4. Transplantation. 2002;73:1142–1145. doi: 10.1097/00007890-200204150-00023. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi D, Yoshidome H, Kato A, Ito H, Kimura F, Shimizu H, et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. HEPATOLOGY. 2004;39:699–710. doi: 10.1002/hep.20117. [DOI] [PubMed] [Google Scholar]
- 5.Friedman DJ, Rennke HG, Csizmadia E, Enjyoji K, Robson SC. The vascular ectonucleotidase ENTPD1 is a novel renoprotective factor in diabetic nephropathy. Diabetes. 2007;56:2371–2379. doi: 10.2337/db06-1593. [DOI] [PubMed] [Google Scholar]
- 6.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32:2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Yegutkin GG, Mikhailov A, Samburski SS, Jalkanen S. The detection of micromolar pericellular ATP pool on lymphocyte surface by using lymphoid ecto-adenylate kinase as intrinsic ATP sensor. Mol Biol Cell. 2006;17:3378–3385. doi: 10.1091/mbc.E05-10-0993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mizumoto N, Kumamoto T, Robson SC, Sevigny J, Matsue H, Enjyoji K, et al. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med. 2002;8:358–365. doi: 10.1038/nm0402-358. [DOI] [PubMed] [Google Scholar]
- 9.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, Inoue Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23:1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87:8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314:1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 12.Langston HP, Ke Y, Gewirtz AT, Dombrowski KE, Kapp JA. Secretion of IL-2 and IFN-gamma, but not IL-4, by antigen-specific T cells requires extracellular ATP. J Immunol. 2003;170:2962–2970. doi: 10.4049/jimmunol.170.6.2962. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beldi G, Wu Y, Banz Y, Nowak M, Miller L, Enjyoji K, et al. Natural killer T cell dysfunction in CD39-null mice protects against concanavalin A-induced hepatitis. HEPATOLOGY. 2008;48:841–852. doi: 10.1002/hep.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5:1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 16.Kaczmarek E, Koziak K, Sevigny J, Siegel JB, Anrather J, Beaudoin AR, et al. Identification and characterization of CD39/vascular ATP diphospho-hydrolase. J Biol Chem. 1996;271:33116–33122. doi: 10.1074/jbc.271.51.33116. [DOI] [PubMed] [Google Scholar]
- 17.Robson SC, Wu Y, Sun X, Knosalla C, Dwyer K, Enjyoji K. Ectonucle-otidases of CD39 family modulate vascular inflammation and thrombosis in transplantation. Semin Thromb Hemost. 2005;31:217–233. doi: 10.1055/s-2005-869527. [DOI] [PubMed] [Google Scholar]
- 18.Beldi G, Wu Y, Sun X, Imai M, Enjyoji K, Csizmadia E, et al. Regulated catalysis of extracellular nucleotides by vascular CD39/ENTPD1 is required for liver regeneration. Gastroenterology. 2008;135:1751–1760. doi: 10.1053/j.gastro.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao X, Shores EW, Hu-Li J, Anver MR, Kelsall BL, Russell SM, et al. Defective lymphoid development in mice lacking expression of the common cytokine receptor gamma chain. Immunity. 1995;2:223–238. doi: 10.1016/1074-7613(95)90047-0. [DOI] [PubMed] [Google Scholar]
- 20.Sevigny J, Sundberg C, Braun N, Guckelberger O, Csizmadia E, Qawi I, et al. Differential catalytic properties and vascular topography of murine nucleoside triphosphate diphosphohydrolase 1 (NTPDase1) and NTP-Dase2 have implications for thromboregulation. Blood. 2002;99:2801–2809. doi: 10.1182/blood.v99.8.2801. [DOI] [PubMed] [Google Scholar]
- 21.Yegutkin GG, Henttinen T, Jalkanen S. Extracellular ATP formation on vascular endothelial cells is mediated by ecto-nucleotide kinase activities via phosphotransfer reactions. FASEB J. 2001;15:251–260. doi: 10.1096/fj.00-0268com. [DOI] [PubMed] [Google Scholar]
- 22.Nocito A, Georgiev P, Dahm F, Jochum W, Bader M, Graf R, et al. Platelets and platelet-derived serotonin promote tissue repair after normothermic hepatic ischemia in mice. Hepatology. 2007;45:369–376. doi: 10.1002/hep.21516. [DOI] [PubMed] [Google Scholar]
- 23.Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, et al. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–1453. doi: 10.1002/hep.510300615. [DOI] [PubMed] [Google Scholar]
- 24.Day YJ, Li Y, Rieger JM, Ramos SI, Okusa MD, Linden J. A2A adenosine receptors on bone marrow-derived cells protect liver from ischemia-reperfusion injury. J Immunol. 2005;174:5040–5046. doi: 10.4049/jimmunol.174.8.5040. [DOI] [PubMed] [Google Scholar]
- 25.Osborne FN, Kalsi KK, Lawson C, Lavitrano M, Yacoub MH, Rose ML, et al. Expression of human ecto-5’-nucleotidase in pig endothelium increases adenosine production and protects from NK cell-mediated lysis. Am J Transplant. 2005;5:1248–1255. doi: 10.1111/j.1600-6143.2005.00868.x. [DOI] [PubMed] [Google Scholar]
- 26.Lokshin A, Raskovalova T, Huang X, Zacharia LC, Jackson EK, Gorelik E. Adenosine-mediated inhibition of the cytotoxic activity and cytokine production by activated natural killer cells. Cancer Res. 2006;66:7758–7765. doi: 10.1158/0008-5472.CAN-06-0478. [DOI] [PubMed] [Google Scholar]
- 27.Hayakawa Y, Huntington ND, Nutt SL, Smyth MJ. Functional subsets of mouse natural killer cells. Immunol Rev. 2006;214:47–55. doi: 10.1111/j.1600-065X.2006.00454.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilk E, Kalippke K, Buyny S, Schmidt RE, Jacobs R. New aspects of NK cell subset identification and inference of NK cells’ regulatory capacity by assessing functional and genomic profiles. Immunobiology. 2008;213:271–283. doi: 10.1016/j.imbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Kunzli BM, Nuhn P, Enjyoji K, Banz Y, Smith RN, Csizmadia E, et al. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134:292–305. doi: 10.1053/j.gastro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, et al. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104:3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 31.Lu B, Rajakumar SV, Robson SC, Lee EK, Crikis S, d’Apice AJ, et al. The impact of purinergic signaling on renal ischemia-reperfusion injury. Transplantation. 2008;86:1707–1712. doi: 10.1097/TP.0b013e31819022bc. [DOI] [PubMed] [Google Scholar]
- 32.Zhai Y, Qiao B, Gao F, Shen X, Vardanian A, Busuttil RW, et al. Type I, but not type II, interferon is critical in liver injury induced after ischemia and reperfusion. Hepatology. 2008;47:199–206. doi: 10.1002/hep.21970. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.