Abstract
AIM:
To assess whether there was an increased risk of bleeding with enoxaparin in patients with a creatinine clearance (CCT) of less than 30 mL/min.
METHODS:
Patients with a CCT of less than 30 mL/min who were given enoxaparin 1 mg/kg/day were included. Antifactor Xa levels (peak and trough) were measured after three doses (days) of enoxaparin. The peak antifactor Xa levels were measured 4 h after the third enoxaparin dose, and the trough levels of antifactor Xa were measured 12 h and 24 h after the third enoxaparin dose. Basic demographic data such as age, sex, race, diagnosis and creatinine values were assessed at baseline. Adverse events were monitored and recorded. Domain-specific review board approval was obtained before the present study began.
RESULTS:
A total of 15 patients were recruited for the present study. Three patients dropped out of the study; therefore, 12 patients were analyzed. The mean age of the 12 patients was 69.25 years (range 41 to 89 years). There were six men and an equal number of women. There were eight Chinese patients, three Malay patients and one Indian patient. The indication for anticoagulation was deep vein thrombosis in seven patients, non-ST elevation myocardial infarction in four patients and atrial fibrillation in one patient. There were no adverse events noted in any patient.
CONCLUSION:
It is safe to administer enoxaparin once a day to patients with renal impairment and a CCT of less than 30 mL/min.
Keywords: Antifactor Xa, Creatinine clearance, Enoxaparin, Peak, Trough
Low molecular weight heparins (LMWHs), such as enoxaparin, are widely used as anticoagulants for deep vein thrombosis, atrial fibrillation and non-ST elevation myocardial infarction. The side effects of LMWH include bleeding, and it must be administered as a subcutaneous injection. Peak antifactor Xa is used for monitoring the status of anticoagulation in patients on LMWHs. LMWH therapy is recommended in patients who are pregnant, have renal impairment, pulmonary embolism or are obese with a weight of more than 150 kg. The recommended dose of enoxaparin, when creatinine clearance (CCT) is less than 30 mL/min, is 1 mg/kg/day. It is advisable to maintain peak antifactor Xa levels between 0.5 U/mL and 1 U/mL (measured 3 h to 4 h after the enoxaparin dose). In pregnant or obese patients, it is recommended to keep the trough levels between 0.2 U/mL and 0.4 U/mL (measured 12 h after dosing), and less than 0.2 U/mL (measured 24 h after the enoxaparin dose) (1–4). However, there are no recommendations for trough antifactor Xa in patients with a CCT of less than 30 mL/min.
The objectives of the present study were to determine whether there was an increased risk of bleeding with enoxaparin in patients with a CCT of less than 30 mL/min, the correlation between bleeding and antifactor Xa levels, and whether the enoxaparin dose in patients with a CCT of less than 30 mL/min was correct or needed adjustment as per the antifactor Xa levels.
METHODS
Patients with a CCT of less than 30 mL/min who were given enoxaparin 1 mg/kg/day for obvious indications such as non-ST elevation myocardial infarction, venous thromboembolism or any other cause were included. Patients were excluded if they had received previous warfarin anticoagulation with or without LMWH within the previous two weeks.
Blood was collected for measurement of antifactor Xa levels (peak and trough) following three doses (days) of enoxaparin. The peak antifactor Xa levels were measured 4 h after the third enoxaparin dose, and the trough levels of antifactor Xa were measured 12 h and 24 h after the third enoxaparin dose.
Subgroup analysis was performed for patients with a CCT of less than 15 mL/min and a CCT between 15 mL/min and 30 mL/min to determine whether there was a difference in the levels of antifactor Xa. There were six patients in each group. A dedicated laboratory technician was assigned to take blood from these patients and transport it to the laboratory to prevent the activation of the coagulation cascade. Basic demographic data, including age, sex, race, diagnosis and creatinine values, were assessed at baseline. CCT was calculated using the Cockcroft-Gault equation (1). The adverse events (bleeding) were monitored. Domain-specific review board (ethics committee) approval was obtained before the present study. Written informed consent was obtained from each patient.
Statistical analysis
The peak and trough values of antifactor Xa of enoxaparin at 4 h, 12 h and 24 h were analyzed using the χ2 test or Fisher’s exact test to determine whether there were any statistically significant differences at each time point. Data analysis was performed using Stata version 10.2 (StataCorp, USA). The level of significance was set at 5%.
RESULTS
A total of 15 patients were recruited for the present study. Three patients dropped out of the study after giving consent because they refused to give any blood for the study. In total, 12 patients were analyzed. Demographic data and clinical characteristics are provided in Table 1.
TABLE 1.
Demographic data and clinical characteristics (n=12)
| Mean age (range), years | 69.25 (41–89) |
| Sex, n | |
| Male | 6 |
| Female | 6 |
| Ethnicity, n | |
| Chinese | 8 |
| Malay | 3 |
| Indian | 1 |
| Indication for anticoagulation, n | |
| Deep vein thrombosis | 7 |
| Non-ST elevation myocardial infarction | 4 |
| Atrial fibrillation | 1 |
| Adverse events | Nil |
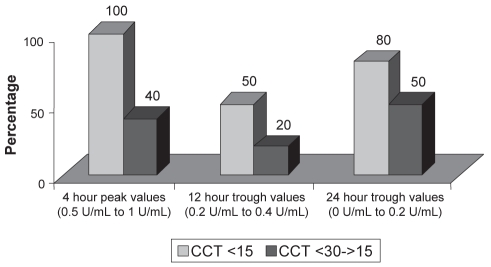
The peak antifactor Xa in all patients was noted to be either normal or subnormal, but not high. This may have been the reason that adverse events, such as bleeding, were not noted. In patients with a CCT of less than 15 mL/min, the peak anti-factor Xa levels were within the therapeutic range; however, in patients with a CCT between 15 mL/min and 30 mL/min, the peak antifactor Xa levels were in the subtherapeutic range. The trough levels were in the subtherapeutic range in both subgroups (Figure 1).
Figure 1).
Percentage of antifactor Xa levels falling within the optimal range. CCT Creatinine clearance in mL/min
DISCUSSION
Peak antifactor Xa monitoring is a blood test recommended for patients who are on LMWH and who have renal impairment (CCT of less than 30 mL/min), pulmonary embolism, are pregnant, or are obese with a weight of more than 150 kg (2–5). LMWH blocks factor X on the intrinsic coagulation pathway compared with unfractionated heparin, which blocks factor 2. The assessment of antifactor Xa is, therefore, more specific than partial thromboplastin time when patients are given LMWH (6).
It is recommended in patients with a CCT of less than 30 mL/min to reduce the enoxaparin dose from 1 mg/kg twice a day to 1 mg/kg/day to avoid accumulation of LMWH (7–9). There are data to suggest that, in patients with a CCT of less than 30 mL/min, there is a two- to threefold increased risk of bleeding. Our data showed that peak antifactor Xa remained within the normal or subtherapeutic range in the present group of patients on enoxaparin; this might be the reason that none of the patients had bleeding.
It is recommended to maintain peak antifactor Xa levels between 0.5 U/mL and 1 U/mL (measured 3 h to 4 h after the enoxaparin dose). In elderly patients, monitoring antifactor Xa levels is still controversial. However, in elderly patients with decreased body weight, the role of antifactor Xa should be monitored (2–5,10,11).
In our group of patients, when the CCT was less than 15 mL/min, the peak antifactor Xa levels were within the therapeutic range in all patients. When the CCT was between 15 mL/min and 30 mL/min, the peak antifactor Xa levels were within the therapeutic range in only 40% of the patients. In 60% of the patients, the peak antifactor Xa levels were subtherapeutic.
In pregnant or obese patients, it is recommended to keep the trough levels between 0.2 U/mL and 0.4 U/mL (measured 12 h after dosing), and less than 0.2 U/mL (measured 24 h after the enoxaparin dose) (2–5). However, there are no recommendations for trough antifactor Xa in patients with a CCT of less than 30 mL/min. When compared with the trough levels recommended for pregnant or obese patients, the trough levels of antifactor Xa when CCT was less than 15 mL/min were therapeutic in only 50% of the patients at 12 h and 80% at 24 h. When the CCT was between 15 mL/min and 30 mL/min, the trough levels of antifactor Xa were therapeutic in only 20% of the patients at 12 h and 50% at 24 h.
Limitations of the present study
The number of patients was limited in the present study. This was for the two following reasons: the present study is a pilot study and, therefore, we wanted some preliminary data before analyzing these parameters in a larger group; and budget constraints. The cost of a single blood test in the laboratory is 150 Singapore dollars. Each patient required three blood tests and a dedicated laboratory technician to collect and transport blood, which was an additional cost.
Strengths of the study
The present prospective pilot study examined both peak and trough levels of antifactor Xa in patients with a CCT of less than 30 mL/min. The number of patients recruited was planned before the study; there were no deviations from the protocol. A dedicated laboratory technician collected blood and transported it to the laboratory to avoid activation of the coagulation cascade.
CONCLUSION
It is safe to administer enoxaparin once a day to patients with a CCT of less than 30 mL/min. In patients with a CCT of less than 30 mL/min on enoxaparin, the peak antifactor Xa values were not high, nor was there an increased risk of bleeding. The peak and trough levels were low, particularly in the subgroup of patients with a CCT between 15 mL/min and 30 mL/min. An increased dose of enoxaparin may improve antifactor Xa levels in this group of patients. However, further studies are needed to explore this option.
Acknowledgments
This study was awarded and funded by the National Healthcare Group – National University Hospital clinical leadership program in March 2008.
REFERENCES
- 1.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 2.Lim MD, Francesco D, John WE, et al. Meta-analysis: Low-molecular-weight heparin and bleeding in patients with severe renal insufficiency. Ann Intern Med. 2006;144:673–84. doi: 10.7326/0003-4819-144-9-200605020-00011. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin G, Nicolas S, Jerome JS, et al. Pharmacokinetics of the low molecular weight heparin enoxaparin during 48 h after bolus administration as an anticoagulant in haemodialysis. Nephrol Dial Transplant. 2003;18:2348–53. doi: 10.1093/ndt/gfg396. [DOI] [PubMed] [Google Scholar]
- 4.Michael WK, Jeremy JL. Retrospective evaluation of a pharmacokinetic program for adjusting enoxaparin in renal impairment. Am Heart J. 2004;148:582–9. doi: 10.1016/j.ahj.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Adam JD, Michael JP, Serdar HU, et al. Antithrombotic therapy and pregnancy: Consensus report and recommendations for prevention and treatment of venous thromboembolism and adverse pregnancy outcomes. Am J Obstet Gynecol. 2007;457:e1–21. doi: 10.1016/j.ajog.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 6.Gailani D, Renne T. Intrinsic pathway of coagulation and arterial thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2507–13. doi: 10.1161/ATVBAHA.107.155952. [DOI] [PubMed] [Google Scholar]
- 7.Green B, Greenwood M, Saltissi D, et al. Dosing strategy for enoxaparin in patients with renal impairment presenting with acute coronary syndromes. Br J Clin Pharmacol. 2004;59:281–90. doi: 10.1111/j.1365-2125.2004.02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klingel R, Schaefer M, Schwarting A, et al. Comparative analysis of procoagulatory activity of haemodialysis, haemofiltration and haemodiafiltration with a polysulfone membrane (APS) and with different modes of enoxaparin anticoagulation. Nephrol Dial Transplant. 2004;19:164–70. doi: 10.1093/ndt/gfg459. [DOI] [PubMed] [Google Scholar]
- 9.Bruno R, Baille P, Retout S, et al. Population pharmacokinetics and pharmacodynamics of enoxaparin in unstable angina and non-ST-segment elevation myocardial infarction. Br J Clin Pharmacol. 2003;56:407–14. doi: 10.1046/j.1365-2125.2003.01904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazineta A, Almanrica K, Bruneta C, et al. Dosage of enoxaparin among obese and renal impairment patients. Thromb Res. 2005;116:41–50. doi: 10.1016/j.thromres.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Dinwoodey DL, Ansell JE. Heparins, low-molecular-weight heparins, and pentasaccharides. Clin Geriatr Med. 2006;22:1–15. doi: 10.1016/j.cger.2005.09.007. [DOI] [PubMed] [Google Scholar]