Abstract
BACKGROUND:
Contrast-induced nephropathy (CIN) continues to be a common cause of acute renal failure in high-risk patients undergoing radiocontrast studies. However, there is still a lack of consensus regarding the most effective measures to prevent CIN.
METHODS:
One hundred eighteen patients with diabetes mellitus and/or renal insufficiency, scheduled for coronary angiography or intervention, were randomly assigned to one of four treatment groups: intravenous (IV) 0.9% NaCl alone, IV 0.9% NaCl plus N-acetylcysteine (NAC), IV 0.9% sodium bicarbonate (NaHCO3) alone or IV 0.9% NaHCO3 plus NAC. All patients received IV hydration as a preprocedure bolus and as maintenance. Iso-osmolar contrast was used in all patients. CIN was defined as an increase of greater than 25% in the serum creatinine concentration from baseline to 72 h.
RESULTS:
The overall incidence of CIN was 6%. There was no statistically significant difference in the incidence of CIN among the groups. There was a CIN incidence of 7% in the NaCl only group, 5% in the NaCl/NAC group, 11% in the NaHCO3 only group and 4% in the NaHCO3/NAC group (P=0.86). The maximum increase in serum creatinine was 14.14±12.38 μmol/L in the NaHCO3 group, 10.60±29.14 μmol/L in the NaCl only group, 9.72±13.26 μmol/L in the NaCl/NAC group and 0.177±15.91 μmol/L for the NaHCO3/NAC group (P=0.0792).
CONCLUSION:
CIN in high-risk patients may be effectively minimized solely through the use of an aggressive hydration protocol and an iso-osmolar contrast agent. The addition of NaHCO3 and/or NAC did not have an effect on the incidence of CIN.
Keywords: Cardiac catheterization, Contrast-induced nephropathy, N-acetylcysteine, Prevention, Sodium bicarbonate
Contrast-induced nephropathy (CIN) is the third most common cause of hospital-acquired acute renal failure, and has become a significant source of morbidity and mortality (1). CIN is commonly defined as an increase in serum creatinine of greater than 25% or 44.2 μmol/L (greater than 0.5 mg/dL) within three days of intravascular contrast medium administration in the absence of an alternative cause (2,3). CIN has been shown to be associated with an increased risk for a prolonged hospital stay, increased health care costs, potentially irreversible reduction in renal function and death (4).
The incidence of CIN has been reported to be as high as 9% to 50% in patients with pre-existing renal impairment or certain risk factors for acute renal dysfunction following exposure to contrast media, such as diabetes, congestive heart failure, advanced age and concurrent administration of nephrotoxic drugs (4–9). The primary intervention for preventing CIN is hydration. However, the most efficacious regimen regarding the minimally effective length of time, optimal rate, and fluid composition of intravenous hydration required before and after contrast medium administration is still unclear (10).
N-acetylcysteine (NAC) and sodium bicarbonate (NaHCO3) infusion are two other prophylactic strategies that have been evaluated. The proposed mechanisms of CIN prevention by NAC are antioxidation via glutathione production or as a direct free radical scavenger, prevention of apoptotic cell death mediated by the reduced generation of oxygen free radicals, and vasodilation (11,12). Several studies (11,13–18) have evaluated the effects of NAC; however, conflicting results have been reported. The postulated mechanism of CIN prevention by NaHCO3 is through the reduction of oxygen free radical formation by increasing the pH of renal flow. The Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL) (19) revealed that volume supplementation with NaHCO3 plus NAC was superior to the combination of normal saline with NAC in preventing CIN in at-risk patients.
The current study was performed to further evaluate whether NaHCO3 and/or high-dose NAC provides an effective option as prophylaxis of CIN in high-risk patients when added to an aggressive hydration protocol and the use of an iso-osmolar contrast medium.
METHODS
Patient population
The present single-centre, randomized study compared four different strategies for preventing CIN in patients with renal insufficiency and/or diabetes mellitus who underwent diagnostic coronary angiography or coronary angioplasty from January 2007 to January 2008 at Beth Israel Medical Center (New York, USA). During this time period, eligible participants included ambulatory or hospitalized patients who were scheduled for invasive coronary angiography or percutaneous coronary intervention for the evaluation and treatment of coronary artery disease. Eligible patients who were willing to participate in the study, and were able to understand and provide informed written consent, were included. Inclusion criteria were patients older than 18 years of age, with renal insufficiency defined by elevated serum creatinine (greater than 132.6 μmol/L in men, and greater than 114.9 μmol/L in women) or reduced calculated creatinine clearance (less than 1.002 mL/s) using the Cockcroft-Gault formula [20]), and/or diabetes mellitus on oral antiglycemic or insulin therapy. Exclusion criteria were pregnancy or lactation; acute myocardial infarction; clinical signs of heart failure (or documented ejection fraction of less than 35%); cardiogenic shock; hypertrophic or restrictive cardiomyopathy; contrast medium exposure within one week before the procedure; previous serious reactions to contrast medium; renal transplantation; dialysis; severe comorbid illness; use of dopamine, mannitol or fenoldopam; newly discovered uncontrolled diabetes mellitus; or the inability to obtain informed consent or follow-up.
Protocol
After written, informed consent was obtained, patients were randomly assigned to one of four regimens: normal intravenous (IV) 0.9% saline hydration alone (NaCl), normal IV 0.9% saline hydration with NAC (NaCl/NAC), IV 0.9% NaHCO3 hydration alone (NaHCO3), or IV 0.9% NaHCO3 hydration with NAC (NaHCO3/NAC). Patients received a weight-adjusted preprocedure bolus as well as a maintenance intravenous regimen of an isotonic solution of either NaHCO3 (154 mL of 1000 mEq/L NaHCO3 to 846 mL of 5% dextrose, slightly diluting the dextrose concentration to 4.23%) or NaCl (154 mEq/L NaCl in 5% dextrose), at an infusion rate of 3 mL/kg/h for 1 h before contrast, and continued at 1 mL/kg/h during the procedure and for 6 h following contrast exposure. Patients who were randomly assigned to one of the NAC regimens also received an intravenous bolus of 1200 mg of NAC 1 h before intervention and 1200 mg orally twice daily for 48 h after intervention.
All patients received a nonionic, iso-osmolar, dimeric contrast medium – iodixanol (320 mg iodine/mL; 290 mOsm/kg water [Visipaque, GE Healthcare, USA]). The volume of contrast medium used was left to the discretion of the operator, and was not standardized due to variation among patients (eg, differing coronary anatomy, need of obtaining additional views to exclude eccentric coronary artery stenosis, and technical and patient characteristics related to the length of the procedure).
The follow-up period was seven days. Baseline clinical status and serum creatinine were measured before examination (0 h) and at 24 h, 72 h and 168 h after the procedure. The central laboratory at Beth Israel Medical Center performed all laboratory tests for inpatients. Individuals who were discharged before reaching the laboratory time points were instructed to go to a standard laboratory near their home or place of work, where a blood sample was drawn; serum creatinine test results were forwarded to Beth Israel Medical Center.
The protocol was approved by the institutional review board at Beth Israel Medical Center.
Study end points
The primary outcome measure was the development of CIN, defined as an increase of greater than 25% in serum creatinine concentration from baseline to 72 h after administration of the contrast media. A secondary end point was the maximum absolute increase in serum creatinine during the study period.
Statistical analysis
Treatment assignment among the four groups was determined by randomization in a 1:1:1:1 ratio. To ensure that almost equal numbers of patients received each of the four treatments, a randomization block was used. Continuous variables are represented as mean ± SD. ANOVA or the Kruskal-Wallis test was used to determine differences between the groups. Categorical variables were reported as percentages and were analyzed using the χ2 test or Fisher’s exact test. To compare the rate of CIN (the primary outcome) in the treatment groups versus the rate of CIN in the control group, tests for significance were performed using Fisher’s exact test for categorical variables. ANOVA was used to test and compare mean creatinine levels between groups. ANCOVA was also performed for changes in serum creatinine levels (controlling for baseline creatinine and contrast volume) to determine whether a group effect was present. All tests were two tailed, with differences reported as significant if P<0.05.
RESULTS
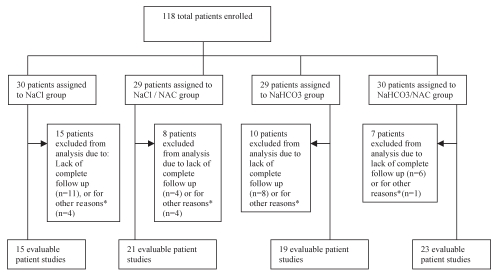
Between January 2007 and January 2008, 118 patients were randomly assigned to the four treatment groups (Figure 1). A total of 78 patients (66%) completed the study. Twenty-nine patients were excluded because they did not complete their follow-up laboratory tests at 72 h after being discharged. The other 11 patients were excluded for various reasons: cancelled procedure (n=1), refusal of procedure (n=5), repeat catheterization the following day (n=4) and development of shortness of breath before receiving fluids (n=1). The statistical analysis was based on the 78 evaluable patient studies.
Figure 1).
Flow chart of trial progress. *Other reasons include: cancelled procedure (n=1), refusal of procedure (n=5), repeat catheterization the following day (n=4) and development of shortness of breath before receiving fluids (n=1). NAC N-acetylcysteine; NaHCO3 Sodium bicarbonate
The baseline clinical and biochemical characteristics of the patients in the four groups are shown in Tables 1 and 2. There were no statistically significant differences in the baseline patient characteristics among the groups with regard to age, sex, height, weight, ethnicity, blood pressure, medications or procedure time. The only variable that significantly differed was contrast volume (P=0.024). Regardless of whether patients were included in the study because of diabetes mellitus, renal insufficiency or both, there were no significant differences between the subgroups in the number of patients fitting each criteria (P=0.313).
TABLE 1.
Baseline clinical characteristics of the patients in each study arm
| Characteristics | All patients (n=78) | NaCl alone (n=15) | NaCl plus NAC (n=21) | NaHCO3alone (n=19) | NaHCO3plus NAC (n=23) | P |
|---|---|---|---|---|---|---|
| Age, years | 66±11 | 64±10 | 65±11 | 67±11 | 65±12 | 0.853 |
| Male sex, % | 60 | 60 | 52 | 58 | 70 | 0.701 |
| Weight, kg | 82±21 | 81±22 | 77±24 | 84±18 | 85±18 | 0.6 |
| Height, cm | 165±10 | 166±8 | 161±10 | 168±10 | 166±12 | 0.225 |
| Ethnicity, % | ||||||
| African-American | 33 | 27 | 33 | 44 | 29 | |
| Asian | 19 | 20 | 24 | 17 | 14 | 0.951 |
| Caucasian | 13 | 20 | 10 | 6 | 14 | |
| Hispanic | 36 | 33 | 33 | 33 | 43 | |
| Blood pressure, mmHg | ||||||
| Systolic | 138±20 | 131±11 | 143±25 | 143±24 | 134±19 | 0.193 |
| Diastolic | 74±10 | 70±11 | 76±10 | 74±9 | 76±10 | 0.25 |
| Patients on each medication, % | ||||||
| Loop diuretic | 21 | 20 | 24 | 28 | 10 | 0.562 |
| ACEI | 36 | 27 | 43 | 39 | 35 | 0.57 |
| Angiotensin receptor blocker | 20 | 20 | 24 | 22 | 15 | 0.939 |
| Acetylsalicylic acid | 76 | 73 | 95 | 61 | 75 | 0.061 |
| Statin | 77 | 67 | 95 | 72 | 75 | 0.115 |
| Contrast volume, mL | 150±61 | 131±63 | 175±81 | 169±59 | 125±42 | 0.024 |
| Procedure time, min | 49.5±22.3 | 50.1±23.0 | 58.4±23.2 | 47.8±24.9 | 41.7±17.9 | 0.107 |
| Inclusion criteria, % | ||||||
| Diabetes mellitus | 59 | 40 | 62 | 68 | 65 | |
| Renal insufficiency | 17 | 33 | 5 | 16 | 13 | 0.313 |
| Both | 24 | 27 | 33 | 16 | 22 | |
Data presented as mean ± SD unless otherwise indicated. ACEI Angiotensin-converting enzyme inhibitor; NAC N-acetylcysteine; NaHCO3 Sodium bicarbonate
TABLE 2.
Change in serum creatinine from baseline
| All patients (n=78) | NaCl alone (n=15) | NaCl plus NaC (n=21) | NaHCO3alone (n=19) | NaHCO3plus NaC (n=23) | P | |
|---|---|---|---|---|---|---|
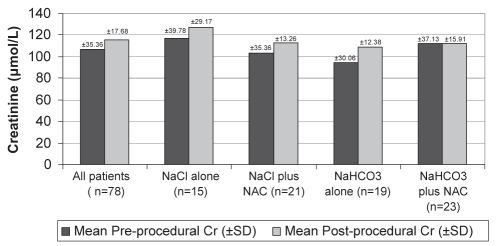
| Baseline serum creatinine, μmol/L | 106.96±35.36 | 116.69±39.78 | 103.43±35.36 | 94.59±30.06 | 112.27±37.13 | 0.273 |
| Change in serum creatinine at 72 h, μmol/L | 5.30±18.56 | 6.19±30.06 | 7.07±13.26 | 7.96±15.03 | –1.77±14.14 | 0.287 |
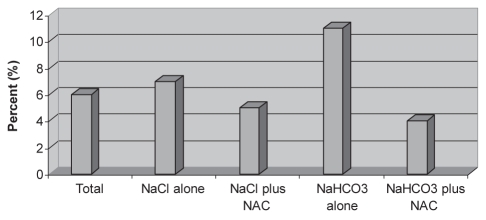
| Patients who developed CIN, n (%) | 5 (6) | 1 (7) | 1 (5) | 2 (11) | 1 (4) | 0.863 |
| Maximum change in serum creatinine, μmol/L | 8.84±17.68 | 10.60±29.17 | 9.72±13.26 | 14.14±12.38 | 0.177±15.91 | 0.079 |
Data presented as mean ± SD unless otherwise indicated. CIN Contrast-induced nephropathy; NAC N-acetylcysteine; NaHCO3 Sodium bicarbonate
Five patients (6%) developed CIN in the present study population (Figure 2). Each group had one patient who developed CIN, except the NaHCO3 group, which had two patients who developed CIN, although the difference was not statistically significant (P=0.863). Results for baseline creatinine, change in creatinine at 72 h, maximum change in creatinine and development of defined CIN in each subgroup were not statistically significant (Table 2). ANOVA was used to calculate any significant difference in 72 h creatinine change or maximum change in creatinine while controlling for contrast volume and baseline creatinine, but there was no difference (F=1.02, P=0.39 and F=1.98, P=0.125, respectively). Finally, there were no serious adverse events from any of the medications given or from the procedure itself.
Figure 2).
Percentage of patients in each group who developed contrast-induced nephropathy. NAC N-acetylcysteine; NaHCO3 Sodium bicarbonate
DISCUSSION
The results of the present study did not show a statistically significant difference in the incidence of CIN among the patients who received periprocedural prophylactic treatment with NaCl alone, NaCl with NAC, NaHCO3 alone or NaHCO3 with NAC. The overall incidence of CIN, across all study groups, was 6%, much lower than expected in our high-risk population. This low incidence may be explained by the aggressive hydration protocol and the use of an iso-osmolar contrast medium alone, independent of any of the other interventions. The amount of fluid given to each patient (infusion rate of 3 mL/kg/h for 1 h before contrast and continued at 1 mL/kg/h during the procedure and for 6 h following contrast exposure) was larger than in most previously performed studies. Although a small study (n=39) (21) showed that slow hydration is superior to bolus hydration, it is still unclear which hydration method is most effective. Furthermore, iso-osmolar contrast media was used for all study participants, which may have further contributed to the low incidence of CIN seen in the present study. The risk difference between iso-osmolar and low-osmolar contrast media is currently debatable because definitive studies are lacking in this area (22–25). A recent meta-analysis (26) concluded that the iso-osmolar contrast medium, iodixanol, was associated with neither a reduction nor an increase in acute kidney injury when compared with low-osmolar contrast media.
While not statistically significant, there were trends in the changes in creatinine values that may indicate an added benefit of NaHCO3 and NAC in the prevention of CIN. The NaHCO3/NAC group was the only one to show a decrease in the average creatinine value at 72 h (−1.77 μmol/L). Also, the average maximum change in creatinine for the NaHCO3/NAC group was much lower (0.177 μmol/L) than in the other three groups (NaCl 10.60 μmol/L, NaCl/NAC 9.72 μmol/L, NaHCO3 alone 14.14 μmol/L; P=0.079) (Figure 3). With more patients, this trend may have reached statistical significance. Because of the low overall incidence of CIN, our study was underpowered for demonstrating that NaHCO3 plus NAC had a significant effect in the prevention of CIN. The number of patients needed to adequately power the study to detect differences was drastically higher than our original estimates. In the current setting, this increase in the number of subjects needed was not feasible, and therefore, the study was prematurely terminated. The REMEDIAL trial (19), with a larger sample size (n=326), was adequately powered to demonstrate a statistically significant benefit of the combination of NAC and NaHCO3. Therefore, more randomized controlled trials are still needed to evaluate the efficacy of NaHCO3 and/or NAC in preventing CIN, above that achieved with the use of aggressive hydration and an iso-osmolar contrast medium.
Figure 3).
Average maximum change in serum creatinine (Cr). NAC N-acetylcysteine; NaHCO3 Sodium bicarbonate
CONCLUSION
The addition of NaHCO3 and/or NAC to an aggressive hydration protocol with the use of an iso-osmolar contrast medium did not affect the incidence of CIN. Even considering the limitations of the present study, we conclude that CIN in high-risk patients may be effectively minimized solely through the use of an aggressive hydration protocol and an iso-osmolar contrast medium. However, although not statistically significant, the trends toward the lowest incidence of CIN and lowest increase in serum creatinine with the combined use of NaHCO3 and NAC still suggest a possible benefit.
REFERENCES
- 1.Manske CL, Sprafka JM, Strony JT, Wang Y. Contrast nephropathy in azotemic diabetic patients undergoing coronary angiography. Am J Med. 1990;89:615–20. doi: 10.1016/0002-9343(90)90180-l. [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Whish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: A prospective study. Am J Med. 1983;74:243–8. doi: 10.1016/0002-9343(83)90618-6. [DOI] [PubMed] [Google Scholar]
- 3.Morcos SK. Contrast media-induced nephrotoxicity – questions and answers. Br J Radiol. 1998;71:357–65. doi: 10.1259/bjr.71.844.9659127. [DOI] [PubMed] [Google Scholar]
- 4.McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: Incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–75. doi: 10.1016/s0002-9343(97)00150-2. [DOI] [PubMed] [Google Scholar]
- 5.Morcos SK, Thomsen HS, Webb JA. Contrast-media-induced nephrotoxicity: A consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR) Eur Radiol. 1999;9:1602–13. doi: 10.1007/s003300050894. [DOI] [PubMed] [Google Scholar]
- 6.Parfrey PS, Griffiths SM, Barrett BJ, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both: A prospective controlled study. N Engl J Med. 1989;320:143–9. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 7.Gruberg L, Mintz G, Mehran R, et al. The prognostic implications of further renal function deterioration within 48h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000;36:1542–8. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 8.Levy E, Viscoli C, Horwitz RI. The effect of acute renal failure on mortality: A cohort analysis. JAMA. 1996;275:1489–94. [PubMed] [Google Scholar]
- 9.Murphy SW, Barrett BJ, Parfrey PS. Contrast nephropathy. J Am Soc Nephrol. 2000;11:177–82. doi: 10.1681/ASN.V111177. [DOI] [PubMed] [Google Scholar]
- 10.Mueller C, Buerkle G, Buettner HJ, et al. Prevention of contrast media-associated nephropathy: Randomized comparison of 2 hydration regimens in 1620 patients undergoing coronary angioplasty. Arch Intern Med. 2002;162:329–36. doi: 10.1001/archinte.162.3.329. [DOI] [PubMed] [Google Scholar]
- 11.Fishbane S, Durham JH, Marzo K, Rudnick M. N-acetylcysteine in the prevention of radiocontrast-induced nephropathy. J Am Soc Nephrol. 2004;15:251–60. doi: 10.1097/01.asn.0000107562.68920.92. [DOI] [PubMed] [Google Scholar]
- 12.Heyman SN, Goldfarb M, Shina A, Karmeli F, Rosen S. N-acetylcysteine ameliorates renal microcirculation: Studies in rats. Kidney Int. 2003;63:634–41. doi: 10.1046/j.1523-1755.2003.00783.x. [DOI] [PubMed] [Google Scholar]
- 13.Webb JG, Pate GE, Humphries KH, et al. A randomized controlled trial of intravenous N-acetylcysteine for the prevention of contrast-induced nephropathy after cardiac catheterization: Lack of effect. Am Heart J. 2004;148:422–9. doi: 10.1016/j.ahj.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 14.Alonso A, Lau J, Jaber BL, Weintraub A, Sarnak MJ. Prevention of radiocontrast nephropathy with N-acetylcysteine in patients with chronic kidney disease: A meta-analysis of randomized, controlled trials. Am J Kidney Dis. 2004;43:1–9. doi: 10.1053/j.ajkd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Birck R, Krzossok S, Markowetz F, Schnülle P, van der Woude FJ, Braun C. Acetylcysteine for prevention of contrast nephropathy: Meta-analysis. Lancet. 2003;362:598–603. doi: 10.1016/S0140-6736(03)14189-X. [DOI] [PubMed] [Google Scholar]
- 16.Liu R, Nair D, Ix J, Moore DH, Bent S. N-acetylcysteine for the prevention of contrast-induced nephropathy. A systematic review and meta-analysis. J Gen Intern Med. 2005;20:193–200. doi: 10.1111/j.1525-1497.2005.30323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tepel M, van der Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic-contrast-agent-induced reductions in renal function by acetylcysteine. N Engl J Med. 2000;343:180–4. doi: 10.1056/NEJM200007203430304. [DOI] [PubMed] [Google Scholar]
- 18.Durham JD, Caputo C, Dokko J, et al. A randomized controlled trial of N-acetylcysteine to prevent contrast nephropathy in cardiac angiography. Kidney Int. 2002;62:2202–7. doi: 10.1046/j.1523-1755.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 19.Briguori C, Airoldi F, D’Andrea D, et al. Renal Insufficiency Following Contrast Media Administration Trial (REMEDIAL): A randomized comparison of 3 preventive strategies. Circulation. 2007;115:1211–7. doi: 10.1161/CIRCULATIONAHA.106.687152. [DOI] [PubMed] [Google Scholar]
- 20.Gault MH, Longerich LL, Harnett JD, Wesolowski C. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- 21.Bader BD, Berger ED, Heede MB, et al. What is the best hydration regimen to prevent contrast media-induced nephrotoxicity? Clin Nephrol. 2004;62:1–7. doi: 10.5414/cnp62001. [DOI] [PubMed] [Google Scholar]
- 22.Aspelin P, Aubry P, Fransson SG, Strasser R, Willenbrock R, Berg KJ, Nephrotoxicity in High-Risk Patients Study of Iso-osmolar and Low-osmolar Non-Ionic Contrast Media Study Investigators Nephrotoxic effects in high-risk patients undergoing angiography. N Engl J Med. 2003;348:491–9. doi: 10.1056/NEJMoa021833. [DOI] [PubMed] [Google Scholar]
- 23.Chalmers N, Jackson RW. Comparison of iodixanol and iohexol in renal impairment. Br J Radiol. 1999;72:701–3. doi: 10.1259/bjr.72.859.10624328. [DOI] [PubMed] [Google Scholar]
- 24.Solomon RJ, Natarajan MK, Doucet S, et al. Cardiac Angiography in Renally Impaired Patients (CARE) study: A randomized double-blind trial of contrast-induced nephropathy in patients with chronic kidney disease. Circulation. 2007;115:3189–96. doi: 10.1161/CIRCULATIONAHA.106.671644. [DOI] [PubMed] [Google Scholar]
- 25.Kelly AM, Dwamena B, Cronin P, Bernstein SJ, Carlos RC. Meta-analysis: Effectiveness of drugs for preventing contrast-induced nephropathy. Ann Intern Med. 2008;148:284–94. doi: 10.7326/0003-4819-148-4-200802190-00007. [DOI] [PubMed] [Google Scholar]
- 26.Reed M, Meier P, Tamhane UU, Welch KB, Moscucci M, Gurm HS. The relative renal safety of iodixanol compared with low-osmolar contrast media: A meta-analysis of randomized controlled trials. JACC Cardiovasc Interv. 2009;2:645–54. doi: 10.1016/j.jcin.2009.05.002. [DOI] [PubMed] [Google Scholar]