Abstract
The use of illicit prescription drugs is common in cannabis users; however, the effects of few psychoactive drugs have been characterized in this population. In the present study, Δ9-tetrahydrocannabinol (i.e., Δ9-THC), triazolam, hydromorphone and methylphenidate were administered to cannabis users (N=8). Subjects completed the Multiple-Choice Procedure to assess drug reinforcement, as well as self-report questionnaires and performance tasks; physiological assessments were also conducted. Only Δ9-THC increased the crossover point on the Multiple-Choice Procedure, but all of the drugs increased ratings on one or more “positive” drug-effect questionnaire items, as well as items specific for each drug. Triazolam produced the most robust performance impairment, except on a time reproduction task, which was impacted to a greater degree by Δ9-THC. Δ9-THC elevated heart rate and decreased temperature, triazolam increased heart rate, methylphenidate elevated all cardiovascular indices, and hydromorphone reduced respiration. The effects of the drugs tested in the present study were generally consistent with their known pharmacology, although minimal responses to hydromorphone were observed. Future research to directly compare the effects of different psychoactive drugs in cannabis users and non-users would be useful for identifying potential differences in drug effects as a function of use history.
Keywords: marijuana, multiple-choice procedure, subjective effects, time reproduction, repeated acquisition task, digit-symbol-substitution task, cardiovascular, respiration, temperature, human
Introduction
Cannabis remains the most commonly used illicit drug in the United States and worldwide (Leggett, United Nations Office on Drugs and Crime, 2006). Data from the 2007 National Survey on Drug Use and Health (NSDUH; SAMHSA, 2008) indicate that 10% of the U.S. population aged 12 and over (25 million persons) have used cannabis in the past year. For comparison, the combined number of people who have used all other illicit drugs (including diverted prescription drugs such as opioids, benzodiazepines and stimulants) is approximately the same (9%). Of the past year cannabis users, over half (14.4 million persons) reported use in the previous month (SAMHSA, 2008). Moreover, roughly a third (3.9 million persons) of past-month cannabis users meet criteria for cannabis-use disorders (DSM IV-TR; American Psychiatric Association, 2000), with the total number of users meeting criteria for cannabis-use disorders being at least twice that of any other illicit drug (SAMHSA, 2008).
Cannabis users also appear to use other psychoactive drugs more frequently than non-cannabis users. Survey data indicate that the percentage of past-month cannabis users who report past-month use of prescription pain relievers is greater than the number of non-users reporting past-month prescription pain reliever use (14% versus 1.3%; SAMHSA, 2008). A similar pattern emerges for benzodiazepines (5.6% versus 0.4%), prescription stimulants (3.6% versus 0.2%) and cocaine (8.6% versus 0.4%). Likewise, cannabis-use disorders are also associated with abuse and dependence on other psychoactive drugs. For example, the percentage of individuals with cannabis-use disorders is greater than the number of non-users meeting abuse or dependence criteria for pain relievers (9.7 versus 0.4), benzodiazepines (0.8% versus 0.1%), prescription stimulants (4.6% versus 0.1%) and cocaine (9.2% versus 0.4%). Several factors could account for the association between cannabis use and the use of other illicit drugs, including a predisposition to drug use in general stemming from environmental and/or genetic influences, enhanced access to and socialization within illicit drug-use and drug-trade networks, or neurobiological changes that could occur with long-term cannabis use (e.g., Hall and Lynskey, 2005; Rubino and Parolaro, 2008; Vanyukov et al., 2003).
There is little human laboratory research that has tested behavioral effects of drugs other than cannabis or Δ9-THC in cannabis users. One study directly compared the reinforcing, subject-rated, psychomotor performance and physiological effects of another psychoactive drug in cannabis users and non-users (Yajnik et al., 1994). In that study, the effects of nitrous oxide were determined as a function of cannabis-use history. Subjects sampled 40% nitrous oxide in oxygen and 100% oxygen (placebo) across four initial sessions, and were then given the opportunity to choose which gas they wanted to self-administer. Choices did not vary by group, although neither group chose nitrous oxide over oxygen, indicating that it did not function as a reinforcer under those conditions. However, some of the self-reported effects of nitrous oxide (e.g., Spaced Out, High and Carefree) were greater in the cannabis users. These differential drug effects are consistent with animal research demonstrating that the behavioral effects of drugs are modified by the subjects' drug history (e.g., Lile et al., 2000; Nader and Mach, 1996; Young et al., 1981). Furthermore, animal studies that specifically tested the influence of Δ9-THC exposure found that chronic treatment altered the behavioral response to opioids and psychomotor stimulants (Jardinaud et al., 2006; Lamarque et al., 2001; Solinas et al., 2004; Panlilio et al., 2007).
The aim of the present study was to characterize the reinforcing, self-reported, performance and physiological effects of a range of doses of hydromorphone, triazolam and methylphenidate, as well as Δ9-THC, under controlled conditions in subjects reporting weekly cannabis use. These data were collected as part of a larger study evaluating the substitution profile of drugs acting at opioid, GABA and dopamine systems in subjects discriminating oral Δ9-THC (Lile et al, 2009) and are presented here to demonstrate the effects of drugs from other pharmacological classes in cannabis users, in order to contribute to the sparse literature and begin to elucidate both factors contributing to and the consequences of drug use in this population.
Methods
Subjects
Healthy, adult men and women with a history of cannabis use were recruited from the local community to participate in this experiment. All potential subjects completed demographic, drug-use history, medical history and personality questionnaires, as well as medical screens. In addition, because the commercially available preparation of Δ9-THC (Marinol®) is suspended in sesame oil, subjects were screened for an allergy to sesame seeds and sesame oil. Individuals with current or past histories of Axis I psychiatric disorders, including substance dependence disorders (except nicotine), were excluded from participating. All subjects were in good health with no contraindications to the drugs to be administered in the protocol. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document. The study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. All subjects provided sober, written informed consent and the confidentiality of their personal information was maintained throughout.
Eight subjects (4 Caucasian males, 1 Caucasian/Middle-Eastern male, 1 Black male, 2 Caucasian females) completed the experiment. Subjects ranged in age from 20 to 29 years (median = 22 years) and in education from 12 to 20 years (median = 16). Subjects ranged in weight from 65 to 120 kg (median = 73 kg). All subjects reported moderate cannabis use (range of 1-5 times/week; median = 2.5). The duration of cannabis use ranged from 1-12 years (median = 5). It is worth noting that six of the eight subjects met some of the criteria for cannabis-use disorders according to DSM IV criteria (APA, 2000), but did not endorse a sufficient number of items to be diagnosed with dependence. Subjects reported consuming 1 to 24 standard alcohol-containing beverages per week (mean = 11.0). Four subjects reported occasional tobacco cigarette use. Other lifetime drug use included amphetamines, cocaine, benzodiazepines, hallucinogens and opioids. Opioid use was primarily reported as therapeutic, but all other drug use was recreational. The reported frequency of other drug use was low, typically not in the month prior to screening. Urine samples for seven of the eight subjects were positive for Δ9-THC prior to any experimental drug administration. The subject who did not test positive for Δ9-THC had moved to Lexington, KY for the summer, and did not have access to a source of cannabis. In addition, one subject tested positive for cocaine during the medical screening and the first practice session; all subsequent urine samples were negative for cocaine.
General Procedures
Subjects were enrolled as outpatients at the General Clinical Research Center (GCRC) at the University of Kentucky Medical Center. They completed two practice sessions to become familiarized with the behavioral measures and daily laboratory routine. Experimental drugs were not administered during these sessions. Subjects then completed the experiment proper. Compensation for participation was $40 per session with additional performance-based payment as outlined below.
Subjects were informed that during their participation they would receive placebo, Δ9-THC, methylphenidate, hydromorphone and triazolam, administered orally, but were blind to the dose and order of administration. They were told that the purpose of the study was to see how different drugs affect mood and behavior. Subjects were asked to abstain from psychoactive drug use other than cannabis for the duration of the experiment, and to avoid any over-the-counter medication without prior approval, with the exception of non-steroidal anti-inflammatory analgesics. In addition, they were asked to not ingest food or caffeine for 4 hours prior to each experimental session, or alcohol for 12 hours prior to and following each experimental session.
Experimental sessions were conducted daily Monday through Friday, and subjects participated in 1-5 sessions per week. Subjects arrived at the GCRC between 08:00 to 09:00 h on the day of scheduled sessions. Individual subjects were tested separately in a standard hospital room. At the beginning of each session, subjects completed field-sobriety, breath (Alcolyzer, AK Solutions USA, Palisades Park, NJ) and urine tests to assess drug use (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) and possible pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK). All female urine samples were negative for hCG. Subjects then consumed a low-fat snack prior to drug administration.
Outcome measures
Self-reported drug-effect, task performance and physiological data were collected in a fixed order immediately prior to drug administration, and 1, 2, 3, 4 and 5 h after drug administration. The Multiple-Choice Procedure was completed at the end of the 5-h assessment. With the exception of the physiological assessments, data were collected on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA). As noted above, these data were collected as part of a comprehensive study to determine whether oral Δ9-THC would function as a discriminative stimulus in humans and to examine the substitution profile of drugs acting at opioid, GABA and dopamine systems (Lile et al, 2009). The data presented here were not included in that publication, with only a few exceptions noted below.
Multiple-Choice Procedure
The Multiple-Choice Procedure provides a contingency-based assessment of the monetary value of each dose condition and is an efficient and valid method used to assess drug reinforcement in humans (Griffiths et al., 1993, Griffiths et al., 1996). In this procedure, subjects make a series of discrete choices between a drug dose and ascending amounts of money. Each experimental session, nine choices were presented. For each choice, subjects selected between the drug administered that session or a gift card to a local grocery store. The dollar value of this gift card increased from $0.25 to $64 across the nine choices (i.e., each subsequent dollar value was double that of the preceding one). After completing the Multiple-Choice Procedure on the penultimate experimental session, subjects randomly selected one of their previous choices in a lottery. This choice (drug or gift card) was then presented to the subject during a final session. Regardless of the outcome, the final session consisted of the standardized experimental routine. The dependent measure on the Multiple-Choice Procedure is the maximum dollar value at which subjects chose drug over money (i.e., “crossover point”).
Subject-Rated Drug-Effect Questionnaires
Visual Analog Scale (VAS)
Subjects rated 20 items (I feel: any drug effect, a bad drug effect, dizzy, forgetful, a good drug effect, high, hungry, nauseated, restless, a rush, shaky or jittery, stimulated, stoned, suspicious, thirsty; I am having difficulty concentrating; I am seeing or hearing unusual things; I like the drug effect; I would pay for the drug; I would take this drug again) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely”. The data from the items Good Drug Effect, High, and Shaky or Jittery were presented previously in Lile et al., 2009, and are not included here.
Profile of Mood States (POMS)
An experimental 72-item adjective rating scale yields scores on ten mood clusters: Anxiety, Depression, Anger, Vigor, Fatigue, Confusion, Friendliness, Elation, Arousal and Positive Mood. Subjects rate each item by selecting one of five response options: “Not at all,” “A little bit”, “Moderately”, “Quite a bit” and “Extremely.” This version of the POMS consisted of the original 65-item experimental version of the POMS [i.e., described and validated by McNair et al. (1971)] plus an additional seven items (e.g., Fischman et al., 1990). The data from the Fatigue scale were presented previously (Lile et al., 2009) and are not included here.
Performance Tasks
Digit-Symbol-Substitution Test (DSST)
A computerized version of the DSST, which has been described previously (McLeod et al. 1982), was used. Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns displayed on a video screen identified on a given trial. Subjects had 90 s to enter as many geometric patterns as possible. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct), the total number of patterns entered (i.e., trials completed) and the percentage of patterns entered correctly (i.e., percent correct).
Repeated Acquisition of Response Sequences Task (RA task)
This task requires the subject to press 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a new 10-response order (a “chain”). When the first correct key in the sequence was pressed, a “position” counter on the screen increased from 0 to 1. The position counter then increased by one each time the subject pressed the correct key in a given position in the sequence, but did not change if the subject pressed the incorrect key. If any key other than the correct key was pressed, a brief time-out (blank screen) occurred. When the subject pressed the tenth and final key in the sequence, a “points” counter increased by one, and the position counter reset to 0, indicating that the first response in the order was again required. Subjects had 180 s to complete as many chains as possible. A performance version of this task was also included. In that version of the task, the 10-response order remained the same across trials, and subjects had 60 s to complete as many chains as possible. The primary dependent measures for this task were the number of chains completed, the number of errors committed and the percentage of correct responses. For the acquisition version of the task, the index of curvature (IOC) for the number of errors committed was also calculated, which reflects how efficiently the 10-response order was learned (Fry et al., 1960).
Time Reproduction Task
For this task, the time interval to be reproduced was presented on the screen. Subjects responded once to start a timer, and held down the response key until they believed that the required interval has elapsed. Four trials of two time periods, 30 and 60 s were used, and scores were averaged across trials.
Physiological Indices
Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
Respiration
Breathing rates were recorded manually by counting the number of breaths occurring in a 60-s period.
Temperature
An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. A standard oral thermometer was used to measure body temperature. Room temperature was also recorded and statistically analyzed to ensure there were no systematic changes across conditions.
Drug Administration
All drug conditions were administered in a double-blind fashion. During each experimental session, subjects ingested three capsules with water. Methylphenidate (5, 10, 20 and 30 mg; Mallinckrodt, St. Louis, MO), hydromorphone (0.75, 1.5, 3 and 4.5 mg; Ethex Pharmaceuticals, St. Louis, MO) and triazolam (0.0625, 0.125, 0.25, 0.375 mg; Roxane Labs, Columbus, OH) were prepared by encapsulating commercially available generic tablets in an opaque green size 00 capsule. Δ9-THC capsules contained Marinol® (5, 7.5, 15 and 25 mg; Solvay Pharmaceuticals, Marietta, GA) capsules, which consist of Δ9-THC in sesame oil. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch. Capsules were prepared by the University of Kentucky Medical Center Investigational Drug Service Pharmacy.
Data Analyses
Raw data from the self-reported drug-effect questionnaires, performance tasks and physiological measures were analyzed for each drug as the peak-effect (i.e., the mean of the maximum or minimum value observed for each subject 1-5 hr after drug administration) using one-factor, repeated-measures analysis of variance (ANOVA; JMP, SAS Institute Inc., Cary, NC) with Dose (Placebo, Dose 1, Dose 2, Dose 3 and Dose 4) as the factor. For all measures, effects were considered significant for p ≤ 0.05. A priori hypotheses were used to guide the peak effects analyses in an effort to minimize the total number of comparisons conducted. For example, because triazolam was expected to impair performance, the number of errors on the RA task was analyzed as the maximum value but the number of chains completed was analyzed as the minimum value. Crossover point data from the Multiple-Choice Procedure were also analyzed using one-factor, repeated-measures ANOVA, but were first subjected to a square-root transformation because the increase in money values likely violates the assumptions of ANOVA (i.e., the difference due to one additional choice of money over drug could range from $0.25 to $32.00). For all analyses, if the main effect of Dose attained statistical significance, planned comparisons using contrast statements were used to compare active drug doses to placebo.
Results
Δ9-THC
There was a significant main effect of Δ9-THC dose choices for the Multiple-Choice Procedure (F4,28 = 4.0; p < 0.01). Compared to placebo, the 25 mg dose of Δ9-THC increased crossover point, indicating that the drug functioned as a reinforcer.
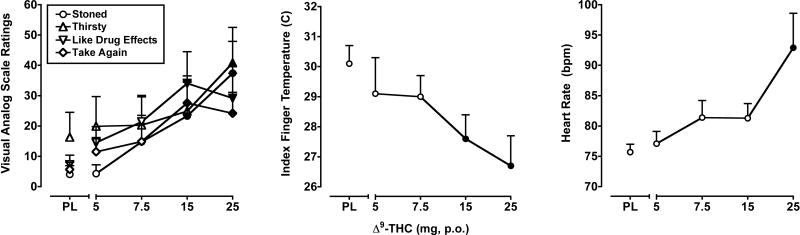
Δ9-THC dose-dependently increased ratings on “positive” drug-effect questionnaire items, as well as some items representing known side effects. Peak effects analysis using maximum ratings from the VAS demonstrated that there was a significant main effect of Δ9-THC dose (F4,28 = 3.2-5.2; p's < 0.05) for six of the 17 VAS items included in this analysis: Any Drug Effect, Like Drug Effects*, Take Again*, Dizzy, Thirsty* and Stoned* The data from VAS items marked with an asterisk* are presented in the left panel of Figure 1. Δ9-THC did not produce significant effects on any of the POMS scales.
Figure 1.
Left Panel: Peak (maximum value) Visual Analog Scale ratings for Δ9-THC on the subject-rated, drug-effect questionnaire items Stoned, Thirsty, Like Drug Effects and Take Again. Middle Panel: Peak (minimum value) effects of Δ9-THC on index finger skin temperature. Right Panel: Peak (maximum value) effects of Δ9-THC on heart rate. Filled symbols indicate values that are significantly different from placebo. The horizontal axis represents the drug dose in mg; PL denotes placebo. Data points show means of 8 subjects. Uni-directional brackets indicate 1 SEM.
Δ9-THC produced only modest and somewhat inconsistent impairment on the DSST and RA Task. Δ9-THC decreased the percentage of correct DSST trials (F4,28 = 3.5; p < 0.01), but had no effect on total or correct trial rate. Compared to placebo, the 5, 7.5 and 15 mg dose of Δ9-THC significantly decreased percentage of correct DSST trials. A significant main effect of Δ9-THC was observed on error rates during both the acquisition and performance components of the RA task (F4,28 = 2.8 and 2.6, respectively; p < 0.05). Compared to placebo, the 5 and 15 mg doses of Δ9-THC significantly increased the number of acquisition component errors, and the 15 mg dose significantly increased the number of performance component errors. A significant main effect of Δ9-THC was observed on chains completed during the performance component of the RA task (F4,28 = 2.6; p < 0.05), with the 5 and 15 mg doses of Δ9-THC significantly decreasing the number of chains completed. A trend towards a decrease in the number of chains completed was also observed for the acquisition component (p = 0.06). Finally, a significant main effect of Δ9-THC was observed on IOC during the acquisition component (F4,28 = 2.8; p < 0.05). with the 15 mg dose of Δ9-THC significantly impairing acquisition efficiency.
There was a significant main effect of Δ9-THC on the Time Reproduction Task for both the 30-s (F4,28 = 3.0; p < 0.05) and 60-s (F4,28 = 2.9; p < 0.05) trials. Following administration of the 7.5 and 15 mg doses of Δ9-THC, subjects under-reproduced the 30-s interval by an average of 4.4 and 5.8 s, respectively and the 60-s interval by 10.0 and 8.2 s.
Δ9-THC increased heart rate (F4,28 = 6.0; p < 0.001) and decreased both oral temperature (F4,28 = 3.1; p < 0.05) and index finger skin temperature (F4,28 = 5.6; p < 0.001). In addition, a trend towards decreased systolic blood pressure was noted (p = 0.06). As shown in the right panel of Figure 1, the 25 mg dose of Δ9-THC significantly elevated heart rate. The 5 and 15 mg doses of Δ9-THC significantly decreased oral temperature, whereas the 15 and 25 mg doses reduced index finger skin temperature (Figure 1, middle panel). Δ9-THC did not alter respiration.
Triazolam
There was no significant main effect of triazolam dose choices for the Multiple-Choice Procedure suggesting that these doses of triazolam did not function as reinforcers.
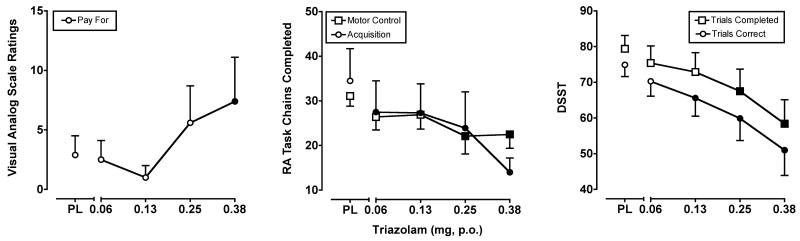
Triazolam dose-dependently increased ratings on “positive” drug-effect questionnaire items, as well as some items consistent with its well-documented sedative effects. A significant main effect of triazolam (F4,28 = 2.7-3.2; p's < 0.05) was observed on three of the VAS items included in this analysis: Take Again*, Pay For* and Stimulated. The data from VAS items marked with an asterisk* are presented in the left panel of Figure 2. Triazolam did not produce significant effects on any of the POMS scales included in the present analysis (but see Lile et al., 2009).
Figure 2.
Left Panel: Peak (maximum value) Visual Analog Scale ratings for triazolam on the subject-rated, drug-effect questionnaire item Pay For. Middle Panel: Peak (minimum value) effects of triazolam on the number of chains completed from the acquisition and performance versions of the RA Task. Right Panel: Peak (minimum value) effects of triazolam on the number of correct trials completed and number of correct trials on the DSST. All other details are as in Figure 1.
Triazolam robustly and dose-dependently impaired performance on the DSST and RA Task. Statistical analyses revealed significant decreases on DSST total trial (F4,28 = 10.9; p < 0.001), and correct trial (F4,28 = 9.8; p < 0.001) rates, as well as on the percentage of correct DSST trials (F4,28= 5.8; p < 0.001). Planned comparisons revealed that the 0.125, 0.25 and 0.375 mg doses of triazolam significantly impaired performance on each of these measures, with the exception of the 0.125 mg dose on total trial rate (p = 0.07). On the acquisition component of the RA Task, triazolam (0.25, 0.375 mg) decreased the number of chains completed (F4,28 = 4.3; p < 0.01) and the percentage of correct responses (F4,28 = 4.9; p < 0.01). In addition, triazolam decreased the number of chains completed (F4,28 = 6.7; p < 0.001) on the performance component, with all triazolam doses impacting performance. Finally, triazolam altered IOC during the acquisition component (F4,28 = 3.4; p < 0.05), with all but the lowest dose impairing acquisition efficiency. The effects of triazolam on total and correct DSST trials, as well as the number of chains completed from both components of the RA Task are shown in the right and middle panels of Figure 2, respectively.
A significant main effect of triazolam was detected on the Time Reproduction Task for the average of the 30-s trials (F4,28 = 6.7; p < 0.001), but not the 60-s trials. Moreover, the average reproduced time interval was significantly lower compared to placebo only at the 0.125 mg triazolam dose.
Triazolam significantly increased heart rate (F4,28 =7.1; p < 0.001) and decreased oral temperature (F4,28 = 3.2; p < 0.05). Planned comparisons demonstrated that the two highest doses of triazolam 0.25 and 0.375 engendered significant effects on these physiological outcomes compared to placebo. Triazolam did not impact blood pressure, index finger skin temperature or respiration.
Methylphenidate
There was no significant main effect of methylphenidate dose choices for the Multiple-Choice Procedure, suggesting that these doses of methylphenidate did not function as reinforcers.
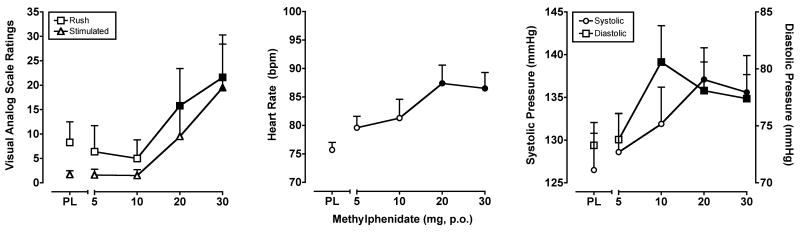
Methylphenidate dose-dependently increased ratings on “positive” drug-effect questionnaire items, as well as some items consistent with its well-documented stimulant effects. A significant main effect of methylphenidate (F4,28 = 2.7-3.0; p's < 0.05) was observed for two of the VAS items from this analysis: Rush and Stimulated. The data from these items are presented in the left panel of Figure 3. A significant main effect of methylphenidate (F4,28 = 2.7-4.5; p's < 0.05) was also found for six of the POMS scales: Anxiety, Vigor, Friendliness, Elation, Arousal and Positive Mood.
Figure 3.
Left Panel: Peak (maximum value) Visual Analog Scale ratings for methylphenidate on the subject-rated, drug-effect questionnaire items Rush and Stimulated. Middle Panel: Peak (maximum value) effects of methylphenidate on heart rate. Right Panel: Peak (maximum value) effects of methylphenidate on systolic and diastolic blood pressure. All other details are as in Figure 1.
Methylphenidate produced performance improvements on the DSST and RA Task. Statistical analyses revealed a significant effect of methylphenidate on correct trial rates (F4,28 = 4.3; p < 0.01) and the percentage of correct trials (F4,28 = 2.9; p < 0.05), but not the total trials. Compared to placebo, all methylphenidate doses significantly improved psychomotor performance, as measured by these outcome measure variables, with the exception of the 5 mg dose on correct trials. A significant main effect of methylphenidate was observed on errors during the performance component of the RA task (F4,28 = 3.4; p < 0.05). Compared to placebo, all doses of methylphenidate significantly reduced the number of errors. No significant effects of methylphenidate were observed during the acquisition component or on Time Reproduction Task performance.
Methylphenidate increased heart rate (F4,28 = 4.9; p < 0.01), systolic blood pressure (F4,28 = 5.2; p < 0.01) and diastolic blood pressure (F4,28 = 5.0; p ≤ 0.01). As shown in the middle panel of Figure 3, planned comparisons revealed that the 20 and 30 mg doses of methylphenidate significantly elevated heart rate compared to placebo. Likewise, these doses increased systolic blood pressure, and the 10, 20 and 30 mg doses increased diastolic blood pressure compared to placebo (Figure 3, right panel). In addition, the 10, 20 and 30 mg doses of methylphenidate decreased index finger skin temperature (F4,28 = 7.4; p < 0.001) and the 10 and 30 mg doses decreased respiration (F4,28 = 3.1; p < 0.05). Methylphenidate did not engender any effects on oral temperature.
Hydromorphone
There was no significant main effect of hydromorphone dose choices for the Multiple-Choice Procedure.
Hydromorphone did not significantly increase ratings on any of the items from the drug-effect questionnaires that were included in the analysis.
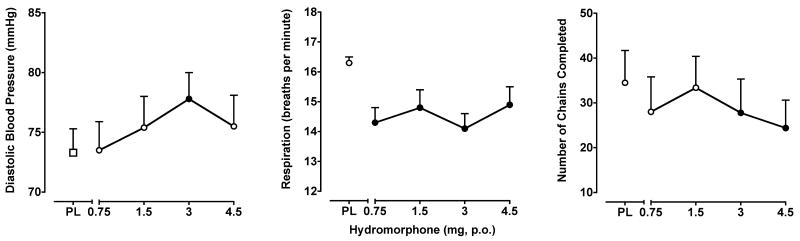
Like Δ9-THC, hydromorphone produced only modest and somewhat inconsistent impairment on the DSST and RA Task. Hydromorphone (0.75 mg only) decreased DSST correct trial (F4,28 = 3.1; p < 0.05), but not total trial rate. Hydromorphone also decreased the number of chains completed during the acquisition component of the RA task (F4,28 = 2.7; p < 0.05; Figure 4, right panel, 3 and 4.5 mg doses) with a trend noted during the performance component (p = 0.06). No significant effects on IOC were found during the performance component, and hydromorphone did not produce any effects on Time Reproduction Task performance.
Figure 4.
Left Panel: Peak (maximum value) effects of hydromorphone on diastolic blood pressure. Middle Panel: Peak (minimum value) effects of hydromorphone on respiration rate. Right Panel: Peak (minimum value) effects of hydromorphone on the number of chains completed on the RA task. All other details are as in Figure 1.
Of the cardiovascular measures, hydromorphone increased only diastolic blood pressure (F4,28 = 2.6; p < 0.05), with planned comparisons indicating that the 3 mg dose elevated diastolic pressure compared to placebo (Figure 4, left panel). Hydromorphone also decreased respiration rate (F4,28= 2.8; p < 0.05), with decreases observed at every dose. Hydromorphone had no hypothermic effects.
Discussion
The aim of the present article was to report on the reinforcing, self-reported, performance and physiological effects of psychoactive drugs from different pharmacological classes in regular cannabis users. All of the drugs tested produced significant effects on several of the experimental measures in a manner consistent with their known pharmacokinetic and pharmacodynamic profiles.
The Multiple-Choice Procedure was used as a means to assess the reinforcing effects of the various drugs tested. Only the 25 mg dose of Δ9-THC significantly increased the crossover point compared to placebo. The ability of Δ9-THC to function as a reinforcer is generally consistent with previous findings (Chait and Zacny, 1992; Hart et al., 2005), although in the study by Hart and colleagues, lower doses of Δ9-THC (i.e., 10 and 20) maintained choice compared to placebo. Potency differences in the reinforcing effects of Δ9-THC could be explained, in part, by procedural variations for the choice procedures used across studies. For example, under the conditions of the Multiple-Choice Procedure used here, only one of the choices was reinforced. As a result, there was a low likelihood that a particular choice would be reinforced, and a low probability of reinforcement has been shown to decrease the reinforcing effects of a stimulus (e.g., Woolverton and Rowlett, 1998). The reinforcing effects of a stimulus are also influenced by the time between the response and stimulus delivery (e.g., Anderson and Woolverton, 2003), and this effect has been observed using the Multiple-Choice Procedure (Benson et al., 2009). The long delay between a choice and the delivery of the reinforcer (i.e., drug or money), and the low probability of reinforcement in the present study could explain the modest reinforcing effects of Δ9-THC in the present study, and possibly the lack of reinforcing effects of the other drugs. Moreover, these subjects had some experience with other psychoactive drugs, but used only cannabis on a regular basis, indicating a preference for Δ9-THC, which is consistent with these results.
Despite the absence of reinforcing effects, triazolam, hydromorphone and methylphenidate produced “positive” subject-rated drug effects, such as Good Drug Effects (see Lile et al., 2009), Like Drug, Take Again and Rush, consistent with their potential for abuse. These data are in agreement with previous findings demonstrating the dissociation between reinforcing and subject-rated effects (e.g., Johanson and Uhlenhuth, 1982; Lamb et al., 1991; Stoops et al., 2005). With regard to hydromorphone, there are conflicting data about the self-reported effects in non-opioid using individuals, with some studies reporting no effects or simultaneous “positive” and “negative” effects (e.g., Hill and Zacny, 2000; Oliveto et al., 1994; Pickworth et al., 1997; Walker and Zacny, 1999). A unique constellation of subject-rated drug effects was found for the different compounds. For example, Δ9-THC alone produced significant effects on certain measures usually associated with cannabis, specifically Stoned and High. Although these adjectives are sometimes used to describe intoxication on other drugs, they appear to have a specific connotation in these subjects, because hydromorphone, triazolam and methylphenidate did not increase these ratings. Interestingly, compared to Δ9-THC, fewer VAS drug-effect questionnaire items were increased by the other drugs tested, and the magnitude of the positive subject-rated effects of these drugs appears less than those engendered by Δ9-THC. These findings could be a function of dose (i.e., the dose of Δ9-THC was relatively higher than the other drugs because tolerance was expected) or that cannabis users are more familiar with the positive effects of Δ9-THC, making them easier to detect or more likely to be reported.
Of the drugs tested, triazolam produced the most robust performance decrements, significantly impairing performance on both the acquisition and performance components of the Repeated Acquisition of Response Sequences Task. Because the performance component of the RA Task was also impacted here, it appears that the effects of triazolam were due to nonspecific impairment in psychomotor performance instead of selective effects on learning, memory or attention under these conditions. Triazolam also reduced both the rate and accuracy of trials on the DSST, again indicating a non-specific impairment in psychomotor performance. In contrast, Δ9-THC decreased, and methylphenidate increased, the percentage of correct DSST trials, but not total trials completed, suggestive of more selective effects of these drugs on performance, and in the case of Δ9-THC, a compensatory slowing of trial rate to maintain accurate responding.
Although certain performance task outcomes were significantly decreased by Δ9-THC, it did not impact performance in a comparable manner to triazolam. Although the majority of the subjects who participated in this study did not exhibit substantial decreases in psychomotor performance following Δ9-THC administration, there was one subject who was particularly susceptible to the performance-impairing effects. For example, following administration 25 mg Δ9-THC, this subject showed a 68% reduction in the number of correct trials completed on the DSST and a 72% reduction in the number of 10-sequence chains completed during the acquisition component of the RA task. Interestingly, this subject reported the least frequent cannabis use in the month prior to enrollment, although this apparent relationship between the effects of Δ9-THC on measures of psychomotor performance and the length of cannabis use or past month use was not observed for all subjects, as described below.
The majority of human studies testing cannabis or Δ9-THC studies have included cardiovascular measures; however, fewer studies have evaluated the hypothermic effects of cannabis or Δ9-THC in humans, although the hypothermic effects of cannabinoids are well documented in animals (e.g., Crawley et al., 1993; McMahon and Koek, 2007). The results of previous reports on the thermoregulatory effects of smoked cannabis or Δ9-THC in humans are varied (reviewed in Clark, 1987; Clark and Clark, 1981). In the present study, however, both finger skin temperature and oral temperature were reduced by Δ9-THC. The temperature variations detected by the infrared thermometer are attributed to underlying variations in perfusion (User Manual, DermaTemp 1001, Exergen Corporation), and therefore the impact of Δ9-THC on index finger skin temperature could be the result of direct effects on the vasculature and not centrally mediated changes in thermoregulation, per se. Nonetheless, the dose-dependent effects on index finger skin temperature indicate that this outcome measure is a sensitive and rapid means to assess the pharmacological activity of cannabinoids.
Hydromorphone produced minimal effects on these outcome measures. One possible explanation for these findings is that the doses of hydromorphone were relatively low. Human laboratory research that tested the abuse-related effects of hydromorphone in opioid users have tested higher dose ranges (e.g., 10-25 mg, Walsh et al., 2008), although doses comparable to those tested in the present study increased positive subject-rated effects such as Drug Liking and Good Drug Effects (4 mg, p.o., Lamb and Henningfield, 1994; 0.75-3 mg, i.m., Jones et al., 1999). Another plausible explanation for the modest effects engendered by hydromorphone is that cross-tolerance to the effects of opioids develops with regular cannabis use. In rodent models of locomotor sensitization, repeated cannabinoid agonist treatment enhanced the locomotor response to heroin (Norwood et al., 2003) and morphine (Cadoni et al., 2001; Lamarque et al., 2001). In addition, repeated Δ9-THC administration modified the place preference conditioned by morphine (Jardinaud et al., 2006) and heroin self-administration (Solinas et al., 2004) in a manner consistent with tolerance to the rewarding/reinforcing effects of those opioids. It appears that only a single human study has compared the effects of an opioid in cannabis users versus non-users (Haney, 2007); however, the use of a single dose that differed with group (10 vs. 7.5 mg) limited the conclusions that could be drawn.
The profile of the effects of Δ9-THC, triazolam, hydromorphone and methylphendiate were consistent with previous reports that have tested these drugs in individuals with varied or limited drug use histories. However, it is possible that significant differences could exist in the potency or efficacy of these drugs as a function of cannabis use history. To begin to address this possibility, an exploratory regression analysis was conducted to determine if the number of years of cannabis use or frequency of past month cannabis use was associated with drug response. Cannabis use was not significantly correlated with the peak drug effects at any dose; however, one notable exception was a trend (p < 0.1) towards a decreased response to Δ9-THC for certain subject-rated items with greater past-month use, perhaps suggesting some tolerance to Δ9-THC's effects. It is worth noting, though, that the cannabis users enrolled in this study were non-dependent and had an average use history of 5.5 years. A more prolonged or intensive use history might be required for potential differences in drug effects to emerge. Preclinical research has demonstrated that the pharmacological history of a subject is an important determinant of the behavioral effects of psychoactive drugs (e.g., Lile et al., 2000; Nader and Mach, 1996; Young et al., 1981), yet clinical research in this area is limited. Further research to evaluate the effects of other commonly abused drugs in cannabis users and comparison groups would be informative to determine the extent to which cannabis use alters the response to drugs, and to reveal potential neurobiological changes that might occur with regular cannabis use.
Acknowledgments
We are grateful to David Hudson, M.D., Rodrick Stuart, M.D. and Matthew Neltner, M.D. and the University of Kentucky General Clinical Research Center nursing staff for expert medical assistance. We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We also thank David Pinsky, Drew Lally, Lisa Purdy and David Splichal for their technical assistance. Finally, we would like to thank the Reviewers for their helpful comments on a previous version of this manuscript.
These experiments comply with the current laws of the United States.
This research and the preparation of this manuscript were supported by grants from the National Institute on Drug Abuse (K01 DA018772) and the University of Kentucky Research Foundation awarded to Dr. Joshua A. Lile. Support was also provided by a Center for Biomedical Research Excellence (P20 RR015592) awarded to Dr. Thomas Curry and a General Clinical Research Center (M01 RR002602) awarded to Dr. Jay Perman by the National Center for Research Resources. The authors do not have any financial relationship with these funding sources.
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, Fourth edition, Text revision. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Anderson KG, Woolverton WL. Effects of dose and infusion delay on cocaine self-administration choice in rhesus monkeys. Psychopharmacology. 2003;167:424–30. doi: 10.1007/s00213-003-1435-9. [DOI] [PubMed] [Google Scholar]
- Benson TA, Little CS, Hensless AM, Correia CJ. Effects of reinforcer magnitude and alternative reinforcer delay on preference for alcohol during a multiple-choice procedure. Drug and Alcohol Depend. 2009;100:161–3. doi: 10.1016/j.drugalcdep.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cadoni C, Pisanu A, Solinas M, Acquas E, Di Chiara G. Behavioural sensitization after repeated exposure to Δ9-tetrahydrocannabinol and cross-sensitization with morphine. Psychopharmacology. 2001;158:259–66. doi: 10.1007/s002130100875. [DOI] [PubMed] [Google Scholar]
- Chait LD, Zacny JP. Reinforcing and subjective effects of oral delta 9-THC and smoked marijuana in humans. Psychopharmacology. 1992;107:255–62. doi: 10.1007/BF02245145. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Corwin RL, Robinson JK, Felder CC, Devane WA, Axelrod J. Anandamide, an endogenous ligand of the cannabinoid receptor, induces hypomotility and hypothermia in vivo in rodents. Pharmacol Biochem Behav. 1993;46:967–72. doi: 10.1016/0091-3057(93)90230-q. [DOI] [PubMed] [Google Scholar]
- Clark WG. Changes in body temperature after administration of antipyretics, LSD, Δ9-THC and related agents: II. Neurosci Biobehav Rev. 1987;11:35–96. doi: 10.1016/s0149-7634(87)80003-9. [DOI] [PubMed] [Google Scholar]
- Clark WG, Clark YC. Changes in body temperature after administration of antipyretics, LSD, Δ9-THC, CNS depressants and stimulants, hormones, inorganic ions, gases, 2,4-DNP and miscellaneous agents. Neurosci Biobehav Rev. 1981;5:1–136. doi: 10.1016/0149-7634(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW, Nestadt G, Pearlson GD. Effects of desipramine maintenance on cocaine self-administration. J Pharmacol Exp Ther. 1990;253:160–70. [PubMed] [Google Scholar]
- Fry W, Kelleher RT, Cook L. A mathematical index of performance on fixed-interval schedules of reinforcement. J Exp Anal Behav. 1960;3:193–9. doi: 10.1901/jeab.1960.3-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, II, Silverman K, Mumford GK. Multiple-choice procedure: An efficient approach for investigating drug reinforcement in humans. Behav Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Griffiths RR, Rush CR, Puhala KA. Validation of the multiple-choice procedure for investigating drug reinforcement in humans. Exp Clin Psychopharmacol. 1996;4:97–106. [Google Scholar]
- Hall WD, Lynskey M. Is cannabis a gateway drug? Testing hypotheses about the relationship between cannabis use and the use of other illicit drugs. Drug Alcohol Rev. 2005;24:39–48. doi: 10.1080/09595230500126698. [DOI] [PubMed] [Google Scholar]
- Haney M. Opioid antagonism of cannabinoid effects: differences between marijuana smokers and nonmarijuana smokers. Neuropsychopharmacology. 2007;32:1391–403. doi: 10.1038/sj.npp.1301243. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–43. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Hill JL, Zacny JP. Comparing the subjective, psychomotor and physiological effects of intravenous hydromorphone and morphine in healthy volunteers. Psychopharmacology. 2000;152:31–9. doi: 10.1007/s002130000500. [DOI] [PubMed] [Google Scholar]
- Jardinaud F, Roques BP, Noble F. Tolerance to the rewarding effects of morphine in delta9-tetrahydrocannabinol treated mice. Behav Brain Res. 2006;173:255–61. doi: 10.1016/j.bbr.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Uhlenhuth EH. Drug preferences in humans. Fed Proc. 1982;41:228–33. [PubMed] [Google Scholar]
- Jones HE, Bigelow GE, Preston KL. Assessment of opioid partial agonist activity with a three-choice hydromorphone dose-discrimination procedure. J Pharmacol Exp Ther. 1999;289:1350–61. [PubMed] [Google Scholar]
- Lamarque S, Taghzouti K, Simon H. Chronic treatment with Δ9-tetrahydrocannabinol enhances the locomotor response to amphetamine and heroin. Implications for vulnerability to drug addiction. Neuropharmacology. 2001;41:118–29. doi: 10.1016/s0028-3908(01)00039-9. [DOI] [PubMed] [Google Scholar]
- Lamb RJ, Henningfield RJ. Human d-amphetamine drug discrimination: methamphetamine and hydromorphone. J Exp Anal Behav. 1994;61:169–80. doi: 10.1901/jeab.1994.61-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb RJ, Preston KL, Schindler CW, Meisch RA, Davis F, Katz JL, Henningfield JE, Goldberg SR. The reinforcing and subjective effects of morphine in post-addicts: a dose-response study. J Pharmacol Exp Ther. 1991;259:1165–73. [PubMed] [Google Scholar]
- Leggett T, United Nations Office on Drugs and Crime A review of the world cannabis situation. Bull Narc. 2006;58:1–155. [PubMed] [Google Scholar]
- Lile JA, Morgan D, Freedland CS, Sinnott RS, Davies HM, Nader MA. Self-administration of two long-acting monoamine transport blockers in rhesus monkeys. Psychopharmacology. 2000;152:414–21. doi: 10.1007/s002130000554. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:241–50. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the Digit Symbol Substitution Test (DSST) Behav Res Methods Instrument. 1982;14:463–6. [Google Scholar]
- McMahon LR, Koek W. Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol. 2007;569:70–6. doi: 10.1016/j.ejphar.2007.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppelman LF. Profile of mood states [manual] Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- Nader MA, Mach RH. Self-administration of the dopamine D3 agonist 7-OH-DPAT in rhesus monkeys is modified by prior cocaine exposure. Psychopharmacology. 1996;125:13–22. doi: 10.1007/BF02247388. [DOI] [PubMed] [Google Scholar]
- Norwood CS, Cornish JL, Mallet PE, McGregor IS. Pre-exposure to the cannabinoid receptor agonist CP 55,940 enhances morphine behavioral sensitization and alters morphine self-administration in Lewis rats. Eur J Pharmacol. 2003;465:105–14. doi: 10.1016/s0014-2999(03)01455-9. [DOI] [PubMed] [Google Scholar]
- Oliveto AH, Bickel WK, Kamien JB, Hughes JR, Higgins ST. Effects of diazepam and hydromophone in triazolam-trained humans under a novel-response drug discrimination procedure. Psychopharmacology. 1994;114:417–23. doi: 10.1007/BF02249331. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Solinas M, Matthews SA, Goldberg SR. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology. 2007;32:646–57. doi: 10.1038/sj.npp.1301109. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Rohrer MS, Fant RV. Effects of abused drugs on psychomotor performance. Exp Clin Psychopharmacol. 1997;5:235–41. doi: 10.1037//1064-1297.5.3.235. [DOI] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. Long lasting consequences of cannabis exposure to adolescence. Mol Cell Endocrinol. 2008;286(Suppl 1):S108–13. doi: 10.1016/j.mce.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Solinas M, Panlilio LV, Goldberg SR. Exposure to Δ9-tetrahydrocannabinol (THC) increases subsequent heroin taking but not heroin's reinforcing efficacy: a self-administration study in rats. Neuropsychopharmacology. 2004;32:646–57. doi: 10.1038/sj.npp.1300431. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;177:349–55. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Results from the 2007 National Survey on Drug Use and Health: National Findings (NSDUH Series H-34, DHHS Publication No SMA 08-4343) Rockville, MD: 2008. [Google Scholar]
- Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders 1. Common mechanisms and manifestations. Neurosci Biobehav Rev. 2003;27:507–15. doi: 10.1016/j.neubiorev.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Walker DJ, Zacny JP. Subjective, psychomotor, and physiological effects of cumulative doses of opioid mu agonists in healthy volunteers. J Pharmacol Exp Ther. 1999;289:1454–64. [PubMed] [Google Scholar]
- Walsh SL, Nuzzo PA, Lofwall MR, Holtman JR., Jr The relative abuse liability of oral oxycodone, hydrocodone and hydromorphone assessed in prescription opioid abusers. Drug Alcohol Depend. 2008;98:191–202. doi: 10.1016/j.drugalcdep.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Rowlett JK. Choice maintained by cocaine or food in monkeys: effects of varying probability of reinforcement. Psychopharmacology. 1998;138:102–6. doi: 10.1007/s002130050651. [DOI] [PubMed] [Google Scholar]
- Yajnik S, Thapar P, Lichtor JL, Patterson T, Zacny JP. Effects of marijuana history on the subjective, psychomotor and reinforcing effects of nitrous oxide in humans. Drug Alcohol Depend. 1994;36:227–36. doi: 10.1016/0376-8716(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Young AM, Woods JH. Maintenance of behavior by ketamine and related compounds in rhesus monkeys with different self-administration histories. J Pharmacol Exp Ther. 1981;218:720–7. [PubMed] [Google Scholar]