Abstract
One of the clinical hallmarks of hereditary cancer susceptibility disorders is a younger-than-usual age at diagnosis. Familial aggregation of testicular germ cell tumor (TGCT) has been reported, but data on whether familial TGCT cases are diagnosed at an earlier age are inconclusive. Here we compared the age at diagnosis of familial TGCT cases with that of population cases in several countries. Familial TGCT is defined as affected individuals from families with ≥2 cases of TGCT. Age at diagnosis of familial cases from the United States, Canada, United Kingdom, Australia and New Zealand, Norway, and Hungary was compared to cases identified in population-based cancer registries from the respective country, using the Generalized Estimation Equation (GEE) method. Age at diagnosis was statistically significantly younger for familial TGCT cases from North America (p=0.024), the United Kingdom (p<0.0001), and Australia and New Zealand (p=0.0033) compared with population cases. When stratified by histology, the difference in age at diagnosis distribution between familial and population cases was observed for seminoma cases from North America (p=0.002) and the United Kingdom (p<0.0001) and nonseminoma cases from the United Kingdom (p=0.029) and Australia and New Zealand (p=0.0023). In summary, we found that the age at diagnosis for familial TGCT cases is, on the average, 2–3 years younger than that for the population cases in North America, United Kingdom, and Australia and New Zealand. The younger age at diagnosis might be suggestive of a genetic basis for familial TGCT.
Keywords: age at diagnosis, familial, non-seminoma, population-based testicular cancer, seminoma, testicular germ cell tumor
Introduction
Testicular germ cell tumor (TGCT) is the most common malignancy in males ages 15–45. The age-standardized annual incidence rate worldwide is approximately 1.5/100,000, with substantial variations between countries [1]. Family history is among the few established risk factors for TGCT [2, 3]. Although genetic susceptibility has been implicated in familial TGCT [4–6], no high-penetrance susceptibility locus has been identified. Recent genome-wide linkage studies suggested that multiple genes, each with modest effects, contribute to TGCT susceptibility [7, 8].
Based on Knudson’s two-hit theory of carcinogenesis [9], one of the main features of single-trait gene hereditary cancers is an earlier age-at-diagnosis [10]; however, it is less clear if familial cancers not associated with a single-gene trait are also diagnosed at an earlier age. Reports on familial TGCT age-at-diagnosis have been conflicting [4, 11–14]; however, most of the data were from small series. Moreover, the clinicopathologic characteristics of familial TGCT have not been shown to be different from sporadic cases [13–15].
Histologically, TGCT is classified into seminoma and non-seminoma. The average age-at-diagnosis for seminoma is in the fourth decade of life, about 7–8 years older than non-seminoma [3, 14, 16, 17]. To investigate whether familial TGCT cases are diagnosed at a younger-than-usual age, we compared them with population cases in North America (United States and Canada), United Kingdom, Australia and New Zealand, Norway, and Hungary.
Materials and Methods
Familial Cases
Familial TGCT cases were previously ascertained through the International Testicular Cancer Linkage Consortium (ITCLC) [7, 18]. Briefly, participants were from families with ≥2 confirmed cases of invasive TGCT or a combination of TGCT and extragonadal germ cell tumor, and with DNA available from at least one case. Participants were enrolled in protocols approved by the participating centers’ Institutional Review Boards and provided informed consent for use of their genetic, demographic, and family history data. Information on deceased family members was provided by their next-of-kin or study participants. Eligibility and clinical data were ascertained by enrolling centers using pathology reports, medical records, death certificate, or participant’s report. For this analysis, we included countries contributing ≥25 cases with non-missing histology and age-at-diagnosis to the ITCLC. Cases from the United States and Canada were combined to form the “North America” group. Cases from England and Ireland were combined, as were cases from Australia and New Zealand. We analyzed all cases together and by tumor histology (seminoma and non-seminoma). Cases with missing histology were included in the overall analysis, but excluded from the analyses by histology.
Population Cases
Population cases were identified from population-based cancer registries for the respective countries. Using the International Classification of Diseases for Oncology, ICD-O-3, coding scheme, we included cases with topography code of C62.0–C62.9 and the following morphology codes: 9061, 9062, and 9063 (seminoma); 9065, 9070, 9071, 9080, 9081, 9082, 9102, 9084, 9085, 9100, 9101, 9083 (non-seminoma); cases with a morphology code of 9064 (germinoma) were included in the overall analysis, but excluded in the analyses by histology.
Statistical Analysis
The age-at-diagnosis distributions for familial and population TGCT by countries/regions were estimated using the kernel density method [19]. Differences in age-at-diagnosis for familial and population TGCT, overall and stratified by histology, were evaluated using the Generalized Estimation Equation [20], which takes the within family correlation of age-at-onset into account. Interactions between the difference in age-at-diagnosis between familiar and population TGCT and geographical location were tested using the Generalized Linear Model [21].
Results
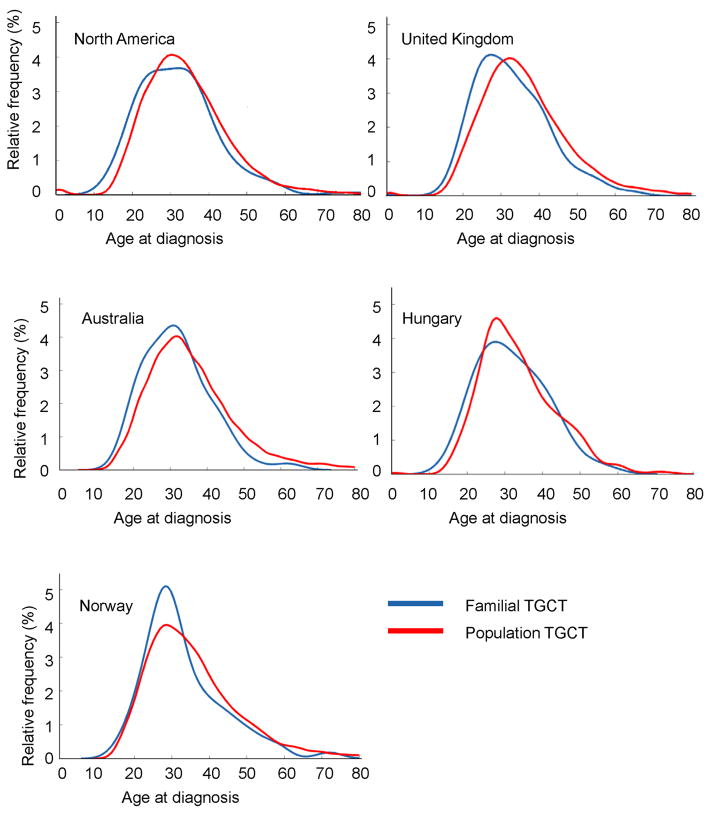
A total of 817 familial TGCT cases and 62,817 population cases from North America, the United Kingdom, Norway, Australia and New Zealand, and Hungary were included (Table). Familial and population TGCT age-at-diagnosis distribution curves for each country/region are shown in the Figure.
Table.
Number of familial and population testicular germ cell tumor cases by regions/countries
| Familial | Population† | Mean Age Differences (95%CI) p-value | ||||
|---|---|---|---|---|---|---|
| Regions/Countries | Number | Age at Diagnosis Median (range) | Number | Age at Diagnosis Median(range) | ||
| North America | All | 188 | 31 (14–82) | 24446 | 33 (0–92) | −2.0 (−3.7, −0.3), p=0.024 |
| Seminoma | 59 | 34 (20–58) | 13591 | 36 (12–92) | −3.2 (−5.2, −1.2), p=0.0017 | |
| Non-seminoma | 94 | 26 (14–56) | 10550 | 28 (0–90) | −0.9 (−2.8, 0.9), p=0.33 | |
| United Kingdom | All | 480 | 32 (2–87) | 20211 | 35 (0–95) | −3.1 (−4.0, −2.1), p<0.0001 |
| Seminoma | 212 | 35 (20–65) | 12450 | 37 (12–93) | −3.0 (−4.3, −1.8), p<0.0001 | |
| Non-seminoma | 188 | 27 (2–66) | 7517 | 30 (0–95) | −1.5 (−2.9, −0.2), p=0.029 | |
| Norway | All | 65 | 30 (16–72) | 6541 | 33 (15–95) | −2.4 (−5.3, 0.3), p=0.10 |
| Seminoma | 36 | 33 (24–72) | 3611 | 37 (15–95) | −2.8 (−6.6, 0.9), p=0.14 | |
| Non-seminoma | 27 | 28 (16–45) | 2930 | 28 (15–88) | −2.3 (−5.2, 0.6), p=0.119 | |
| Australia and New Zealand | All | 54 | 31 (19–62) | 10374 | 34 (0–90)* | −3.5(−5.9, −1.2) p=0.0033 |
| Seminoma | 31 | 31 (20–62) | 6690 | 35 (10–90) | −3.2(−6.5, 0.1) p=0.059 | |
| Non-seminoma | 22 | 30 (19–43) | 3684 | 29 (0–90) | −2.8(−5.2, −0.4) p=0.023 | |
| Hungary | All | 30 | 31 (20–55) | 1245 | 32 (0–87) | −1.69 (−4.9, 1.6), p=0.31 |
| Seminoma | 9 | 36 (20–55) | 693 | 35 (17–87) | −1.7 (−8.4, 5.1), p = 0.63 | |
| Non-seminoma | 10 | 28.5 (21–42) | 552 | 28 (0–75) | 1.1 (−3.1, 5.3), p=0.61 | |
Population cases for North America: SEER-17, 1973–2003; United Kingdom: Office of National Statistics, 1990–2004; Norway: Cancer Registry of Norway, 1953–2005; Australia and New Zealand: Australian Tumor Registry, 1982–2003; and Hungary: Hungarian Cancer Registry, 2001–2006.
Age at diagnosis for population cases were reported in 5-year age groups. Individual age at diagnosis is obtained using imputation method
Figure.
Kernel density curves for familial and population testicular germ cell tumor cases in North America, United Kingdom, Australia and New Zealand, Norway, and Hungary
North America
The mean age-at-diagnosis was 2 years younger for familial TGCT compared with population cases (95% CI = −3.7, −0.3, p=0.024). When examined by histology, familial seminoma cases were diagnosed 3.2 years younger than population seminoma cases (95% CI = −5.2, −1.2, p=0.002). Familial non-seminoma cases were also younger at diagnosis than population cases, but the difference was not statistically significant (−0.9, 95% CI = −2.8, 0.9, p=0.33).
United Kingdom
Compared with population cases, the age-at-diagnosis was 3.1 years younger (95% CI = −4.0, −2.1, p<0.0001) for all familial TGCT cases, 3.0 years younger (95% CI = −4.3, −1.8, p<0.0001) for familial seminoma cases, and 1.5 years younger (95% CI = −2.9, −0.2, p=0.029) for familial non-seminoma cases.
Australia/New Zealand
Age-at-diagnosis was reported in 5-year age groups in the Australian Tumor Registry; consequently, the median age-at-diagnosis for population cases was imputed, assuming equal distribution of cases for each age within an age group. Overall, familial TGCT cases were diagnosed 3.5 years younger than population cases (95% CI = −5.9, −1.2, p=0.0033). Familial non-seminoma cases were diagnosed at a younger age than population cases (−2.8, 95% CI= −5.2, −0.4, p=0.0023). No difference in age-at-diagnosis was observed for seminoma.
Norway
The age-at-diagnosis for familial TGCT cases was younger than that of population cases; however, the difference was not statistically significant, either for all cases (−2.4, 95% CI = −5.3, 0.3, p=0.10) or when stratified by histology (−2.8, 95% CI = −6.6, 0.9, p=0.14 for seminoma; −2.3, 95% CI = −5.2, 0.6, p=0.12 for non-seminoma).
Hungary
Familial TGCT cases were not statistically significantly younger than population cases (−1.7, 95% CI = −4.9, 1.6, p=0.31). When examined by histology, familial seminoma cases had a statistically non-significant 1.7 years younger age-at-diagnosis compared with population cases (95% CI = −8.4, 5.1, p = 0.63). There was no evidence of a younger age-at-diagnosis for familial non-seminoma (1.1, 95% CI = −3.1, 5.3, p=0.61).
There was no evidence of interaction between regions/countries and the difference in age-at-diagnosis for familial cases and population cases, either overall (p=.78), or when stratified by histology (p= 0.99 for seminoma and p=0.82 for nonseminoma).
Discussion
From this large international collection of familial TGCT cases, we observed that familial cases, on average, were diagnosed 2–3 years younger than population cases, with seminoma demonstrating a larger difference. Moreover, the younger age-at-diagnosis among familial cases is observed in the U.S. and Canada, the United Kingdom, and Australia and New Zealand, with no evidence of a differential effect by geographical locations. Although familial TGCT cases were diagnosed at a younger age than population cases in Norway and Hungary, these differences did not reach statistical significant levels, likely because of the small number of familial cases enrolled.
Previously reported data on age-at-diagnosis of familial TGCT are inconsistent; while some studies suggested that familial TGCT was diagnosed about 3 to 5 years earlier than sporadic cases [4, 11], which is similar to our findings, others did not show any differences [12–14]. However, most of these studies were small series with limited numbers of familial cases. Our study compared a large number of familial TGCT cases to unselected population cases from several countries. Because the mean age-at-diagnosis for TGCT varies by country/geographical region, we compared familial cases from each region to the corresponding population-based cancer registry.
Earlier screening for breast, colorectal, and prostate cancer may be recommended for those with a family history of early diagnosis of the same cancer [22]. Although we observed a statistically significant younger age-at-diagnosis for familial TGCT, it is unclear if this difference is clinically meaningful. Currently, there are no standard screening recommendations for TGCT, either in the general population or among individuals at increased risk. Thus, our finding, though may be suggestive of a genetic basis for familial TGCT, is unlikely to alter health care recommendations for individuals with a family history of TGCT.
This study has several strengths. First, this is the largest group of familial TGCT cases with age-at-diagnosis reported to date. Second, we compared familial TGCT from each region/country with cases from the respective population-based cancer registries, thus eliminating the effect of differences in TGCT age-at-diagnosis by geographical locations. Furthermore, despite the variations in mean age-at-diagnosis of TGCT by country, the earlier age-at-diagnosis for familial TGCT was consistently observed across all regions/countries examined. Lastly, we were able to examine seminoma and non-seminoma separately, which is important given known differences in average age-at-diagnoses by histology.
This report also has some potential limitations. Clinicopathologic data for familial cases was obtained from multiple sources; thus, the age-at-diagnosis might not have been accurate in cases where pathology reports were not available. Additionally, study participants were either referred by their healthcare providers or self-referred to one of the study centers in response to advertisement. It is possible that families/cases with earlier age-at-diagnosis were more likely to participate in the ITCLC.
In summary, in this international, multicenter study, we observed a younger age-at-diagnosis for familial TGCT cases compared with population cases obtained from cancer registries across all regions/countries examined. This finding is suggestive of a genetic basis for familial TGCT, though further studies are needed.
Acknowledgments
We would like to thank Dr. Parry Guilford for his contribution to the familial TGCT cases. We also thank Istvan Gaudi (Cancer Registry of National Institute of Oncology, Budapest, Hungary) and Sue Westlake (Social & Health Analysis & Reporting Division, Office for National Statistics, UK) for their contribution from the cancer registries. This research was funded in part by the Intramural Research Program of the National Cancer Institute, National Institutes of Health, and supported by contracts N02-CP-11019 and N02-CP-65504 with Westat, Incorporated.
Abbreviations
- ITCLC
International Testicular Cancer Linkage Consortium
- TGCT
testicular germ cell tumor
Footnotes
The authors have no conflict of interest or financial disclosures to report.
References
- 1.Ferlay J, Bray F, Pisani P, et al. GLOBOCAN 2002: Cancer incidence, mortality and prevalence worldwide. Lyon: IARC ; 2004. Press. [Google Scholar]
- 2.Hemminki K, Sundquist J, Bermejo JL. Familial risks for cancer as the basis for evidence-based clinical referral and counseling. Oncologist. 2008;13:239–247. doi: 10.1634/theoncologist.2007-0242. [DOI] [PubMed] [Google Scholar]
- 3.Holzik MFL, Rapley EA, Hoekstra HJ, et al. Genetic predisposition to testicular germ-cell tumours. Lancet Oncol. 2004;5:363–371. doi: 10.1016/S1470-2045(04)01493-7. [DOI] [PubMed] [Google Scholar]
- 4.Nicholson PW, Harland SJ. Inheritance and testicular cancer. Br J Cancer. 1995;71:421–426. doi: 10.1038/bjc.1995.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimdal K, Olsson H, Tretli S, et al. A segregation analysis of testicular cancer based on Norwegian and Swedish families. Br J Cancer. 1997;75:1084–1087. doi: 10.1038/bjc.1997.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harland SJ, Daugaard G, Horwich A, et al. The familial influence on bilateral testicular germ cell cancer: Medical Research Council study TER2. Annual Meeting Proceedings. J Clin Oncol. 2006;24 Abstract no. 4590. [Google Scholar]
- 7.Crockford GP, Linger R, Hockley S, et al. Genome-wide linkage screen for testicular germ cell tumour susceptibility loci. Hum Mol Genet. 2006;15:443–451. doi: 10.1093/hmg/ddi459. [DOI] [PubMed] [Google Scholar]
- 8.Rapley EA, Crockford GP, Easton DF, et al. Localisation of susceptibility genes for familial testicular germ cell tumour. APMIS. 2003;111:128–135. doi: 10.1034/j.1600-0463.2003.11101171.x. [DOI] [PubMed] [Google Scholar]
- 9.Knudson AG., Jr Hereditary cancer, oncogenes, and antioncogenes. Cancer Res. 1985;45:1437–1443. [PubMed] [Google Scholar]
- 10.Lindor NM, McMaster ML, Lindor CJ, et al. Concise handbook of familial cancer susceptibility syndromes - Second edition. J Natl Cancer Inst Monogr. 2008;2008:3–93. doi: 10.1093/jncimonographs/lgn001. [DOI] [PubMed] [Google Scholar]
- 11.Forman D, Oliver RT, Brett AR, et al. Familial testicular cancer: a report of the UK family register, estimation of risk and an HLA class 1 sib-pair analysis. Br J Cancer. 1992;65:255–262. doi: 10.1038/bjc.1992.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieckmann K-P, Pichlmeier U. The prevalence of familial testicular cancer. Cancer. 1997;80:1954–1960. [PubMed] [Google Scholar]
- 13.Sonneveld DJA, Sleijfer DT, Schraffordt Koops H, et al. Familial testicular cancer in a single-centre population. Eur J Cancer. 1999;35:1368–1373. doi: 10.1016/s0959-8049(99)00140-9. [DOI] [PubMed] [Google Scholar]
- 14.Dong C, Lonnstedt I, Hemminki K. Familial testicular cancer and second primary cancers in testicular cancer patients by histological type. Eur J Cancer. 2001;37:1878–1885. doi: 10.1016/s0959-8049(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 15.Mai PL, Friedlander M, Tucker K, et al. The International Testicular Cancer Linkage Consortium: A clinicopathologic descriptive analysis of 461 familial malignant testicular germ cell tumor kindred. Urol Oncol. 2009 doi: 10.1016/j.urolonc.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokoloff MH, Joyce GF, Wise M. Testis cancer. J Urol. 2007;177:2030–2041. doi: 10.1016/j.juro.2007.01.127. [DOI] [PubMed] [Google Scholar]
- 17.Fossa SD, Chen J, Schonfeld SJ, et al. Risk of contralateral testicular cancer: A population-based study of 29 515 U.S. men. J Natl Cancer Inst. 2005;97:1056–1066. doi: 10.1093/jnci/dji185. [DOI] [PubMed] [Google Scholar]
- 18.The International Testicular Cancer Linkage Consortium. Candidate regions for testicular cancer susceptibility genes. APMIS. 1998;106:64–72. [PubMed] [Google Scholar]
- 19.Parzen E. On estimation of a probability density function and mode. Ann Math Stat. 1962;33:1065–1076. [Google Scholar]
- 20.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 21.McCullagh P, Nelder JA. Generalized Linear Models. 2. London: Chapman and Hall/CRC; 1989. [Google Scholar]
- 22.Smith RA, Cokkinides V, Brawley OW. Cancer Screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin. 2008;58:161–179. doi: 10.3322/CA.2007.0017. [DOI] [PubMed] [Google Scholar]