Abstract
The dictyostatins are a promising class of potential anticancer drugs because they are powerful microtubule stabilizing agents, but the complexity of their chemical structures is a severe impediment to their further development. Based on both synthetic and medicinal chemistry analyses, 25,26-dihydro-16-desmethyldictyostatin and its C6 epimer were chosen as potentially potent yet accessible dictyostatin analogs, and three new syntheses were developed. A relatively classical synthesis involving vinyllithium addition and macrocyclization gave way to a newer and more practical approach based on esterification and ring-closing metathesis reaction. Finally, aspects of these two approaches were combined to provide a third new synthesis based on esterification and Nozaki-Hiyama-Kishi reaction. This was used to prepare the target dihydro analogs and the natural product. All of the syntheses are streamlined because of their high convergence. The work provided several new analogs of dictyostatin including a truncated macrolactone and a C10 E-alkene, which were 400-fold and 50-fold less active than (−)-dictyostatin. In contrast, the targeted 25,26-dihydro-16-desmethyldictyostatin analogs retained almost complete activity in preliminary biological assays.
Introduction
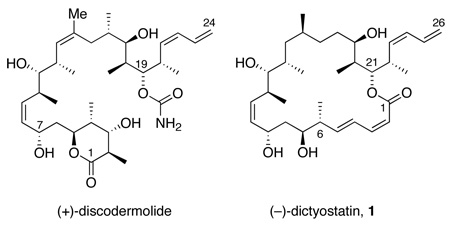
After resting in the shadow of the important natural product (+)-discodermolide for about a decade,1 (−)-dictyostatin 1 has recently emerged as a potentially useful anti-cancer agent in its own right. Pettit isolated dictyostatin in tiny quantities from a marine sponge in 1994, showed its potent anti-cancer activity, and provided its constitution.2 Several years later, Wright isolated enough sample to garner information about the mechanism of action3 and to collect a full battery of NMR spectra in collaboration with Paterson. These spectra were the basis for a complete assignment of configuration in 20044 that was confirmed almost immediately by simultaneous reports of total syntheses by Paterson5 and our group.6
These total syntheses provided precious samples and thereby paved the way for a detailed biological characterization of dictyostatin.7 It is one of the most potent microtubule stabilizing agents known, inducing the assembly of purified tubulin even more rapidly than paclitaxel. It inhibits the binding of paclitaxel, discodermolide and epothilone B to microtubules. It is a powerful anti-proliferative agent in several standard and paclitaxel-resistant cancer cell lines.
There has also been a lively interplay between synthesis and structure-activity relationship (SAR) studies in the dictyostatin class. The original syntheses have been refined and improved,8 new total syntheses by Phillips9 and Ramachandran10 have appeared, and partial syntheses of key fragments have provided additional new options.11 Building on these foundations, our group12 and Paterson’s13 have made an assortment of analogs and stereoisomers of dictyostatin either by modifications of existing synthetic routes or by introducing new routes. A clear outline of the dictyostatin SAR has emerged,14 and several potent analogs with potential advantages over dictyostatin itself have been identified. Most importantly, in the first in vivo studies of any dictyostatin, synthetic analog 6-epi-dictostatin was more effective than paclitaxel in treating mice bearing human breast cancer xenografts.15
An important next step in the field would be to advance dictyostatin, 6-epi-dictyostatin or another analog further down the road of preclinical development towards possible clinical trials. The current syntheses of dictyostatin are, however, at the outer limit of today’s technology for scale up, and the active analogs of dictyostatin are not that much simpler to make than is the parent. So, there is a need for streamlined syntheses of the class and for discovery of analogs with biological profiles that are comparable to dictyostatin’s but that are easier to make. Here we report advances towards both of these objectives.
Based on SAR and metabolic lability analyses,12f,16 we identified 25,26-dihydro-16-desmethyldictyostatin and its 6-epimer as potential analogs of dictyostatin that might be active yet considerably easier to make. We have developed three new, streamlined routes to this class of compounds that are based on vinyllithium addition, ring-closing metathesis (RCM), and Nozaki-Hiyama-Kishi (NHK) coupling. Along the way, we implemented a straightforward esterification method to make the problematic O22-C1 bond. In contrast to related macrolactonization reactions, no isomerization of the adjacent C2–C3 alkene was observed. To culminate the synthesis studies, the NHK approach was used to make (−)-dictyostatin itself. This is the first synthesis in which all of the carbon atoms of the molecule are built into the three main fragments so that none are introduced after fragment couplings begin. The increased convergency makes this the shortest current synthesis of dictyostatin. Finally, both of the new analogs exhibit interesting results in preliminary biological assays.
Results and Discussion
Analog Design and Vinyllithium Addition Route
The new analogs targeted in this work are 25,26-dihydro-16-desmethyldictyostatin 2b and its 6-epimer 2a (see Figure 1). These were selected for several reasons. First, the removal of the C16 methyl group deletes an isolated stereocenter, thereby simplifying the synthesis. 16-Desmethyldictyostatin is a known compound with an interesting biological profile.12b,13c Second, metabolism16 and SAR work in the discodermolide area17 suggested that modification of the terminal C25–C26 alkene of dictyostatin while retaining good activity might be possible. Reduction of this alkene provides a dihdyro analog that is not much simpler than the parent. We felt, however, that synthetic opportunities to make such molecules could be greatly expanded because dictyostatin has five alkenes and a sixth is present during the synthesis. Due to its exposure and therefore high reactivity, the C25–C26 mono-substituted alkene of the terminal diene complicates reactions such as metathesis or hydrogenation that are used to make or manipulate other alkenes. Such complications have been avoided by late introduction of one (or both) of the two dienes, but this sacrifices convergency. Finally, the terminal double bond of discodermolide is a metabolic soft spot16 and other dihydro analogs of dictyostatin at C10,C11 and C2,C3 are biologically active.13d,g
Figure 1.
High level plan of the vinyllithium approach
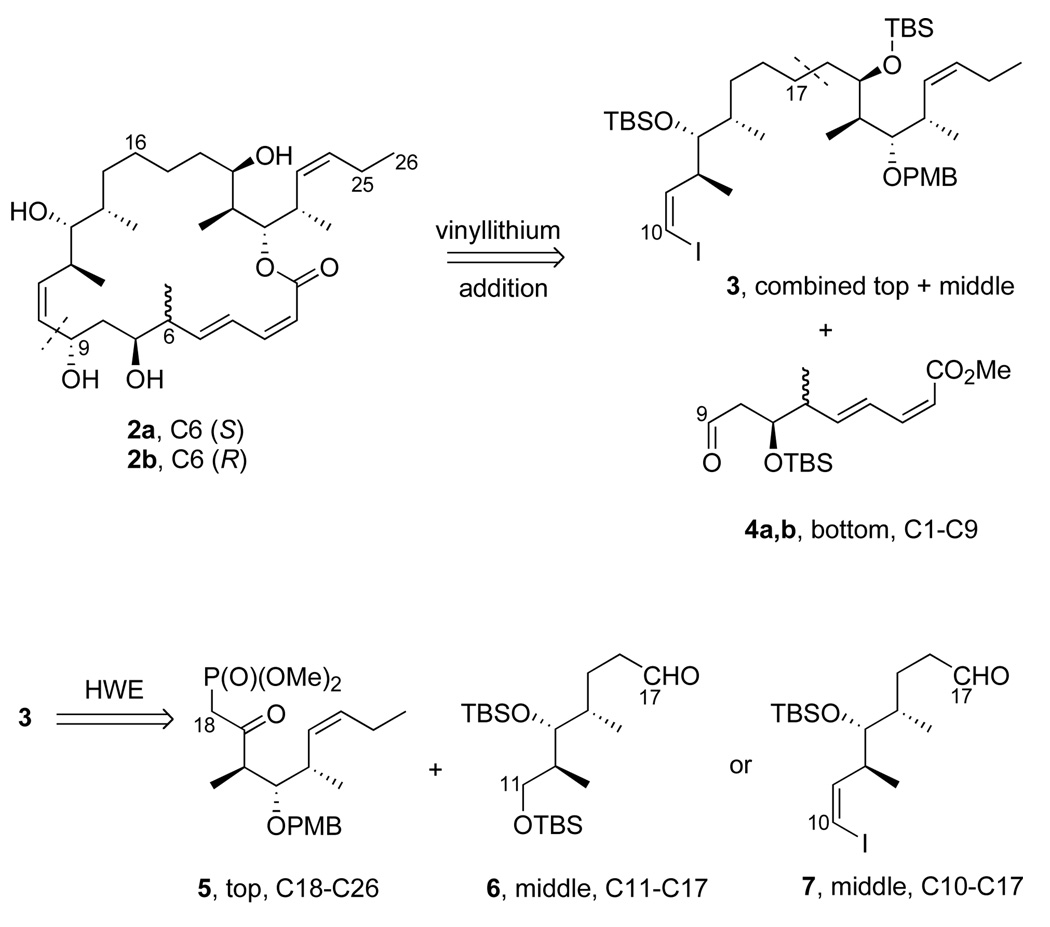
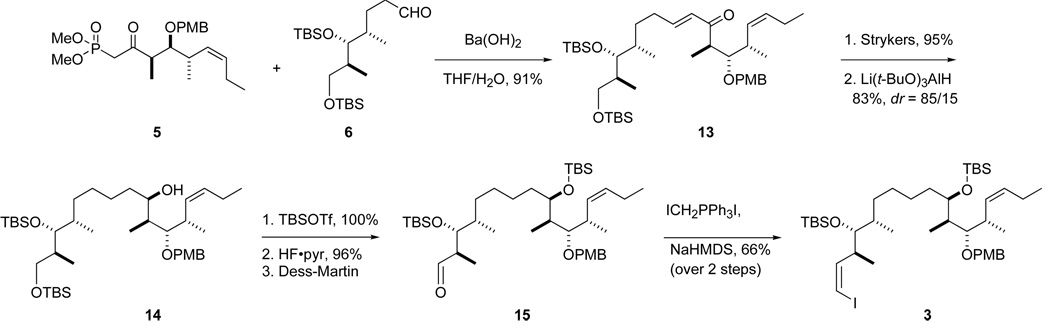
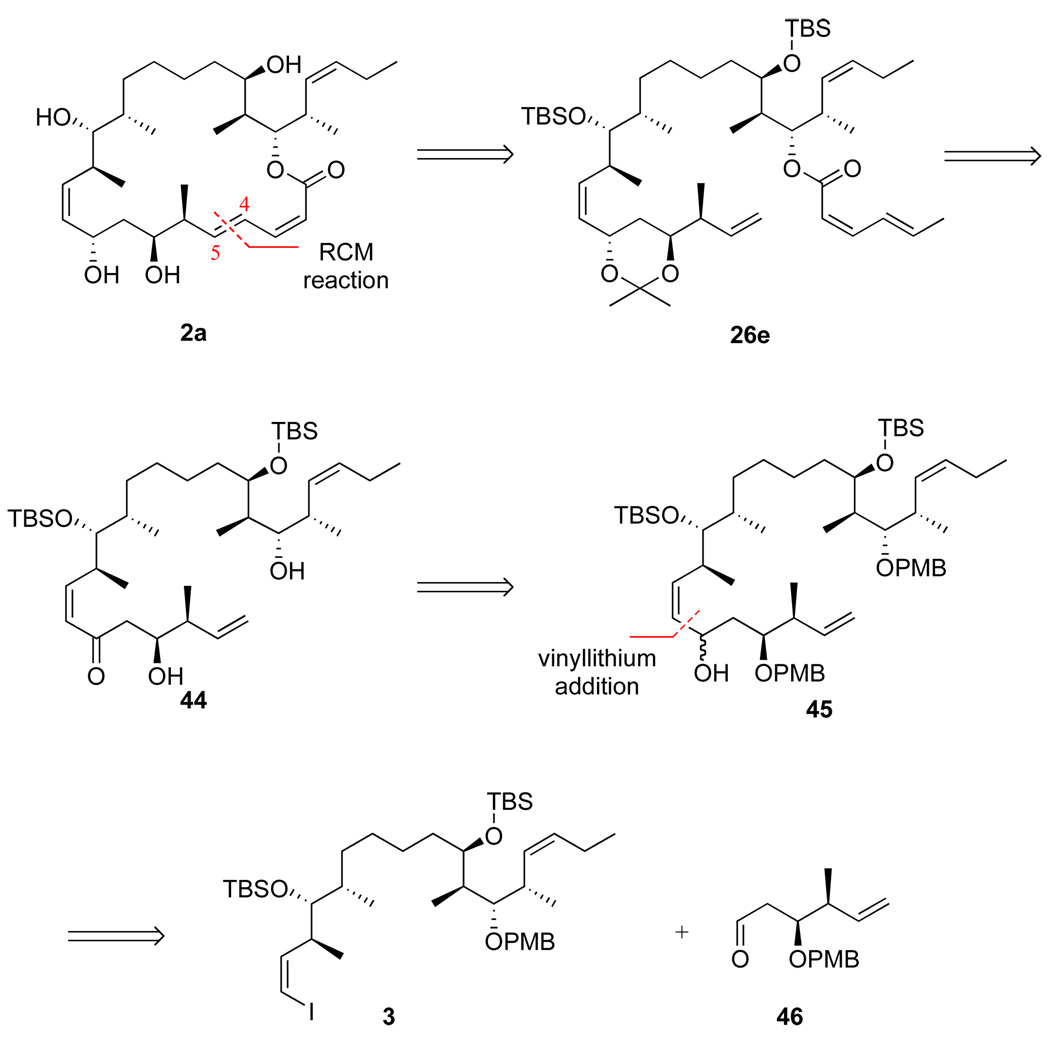
We adopted the strategy shown in Figure 1 to make the new analogs 2a,b. Three main fragments, top (C18–C26, 5), middle (C10–C17, 6 or C11–C17, 7) and bottom (C1–C9, 4) were targeted. The commonly used Horner-Wadsworth-Emmons (HWE) reaction18 first joins the top and middle fragments to give 3, while the vinyllithium addition later appends the bottom fragment. We have studied related vinyllithium approaches,12d and Ramachandran has used a vinylzinc addition as a key step in his synthesis.10 We initially used known middle fragment 6 because it was available from prior work; however, it lacks a carbon atom of the target (C10) and therefore does not meet our target of maximum convergency. We therefore later directly incorporated vinyl iodide 7 as the middle fragment to increase the convergency.
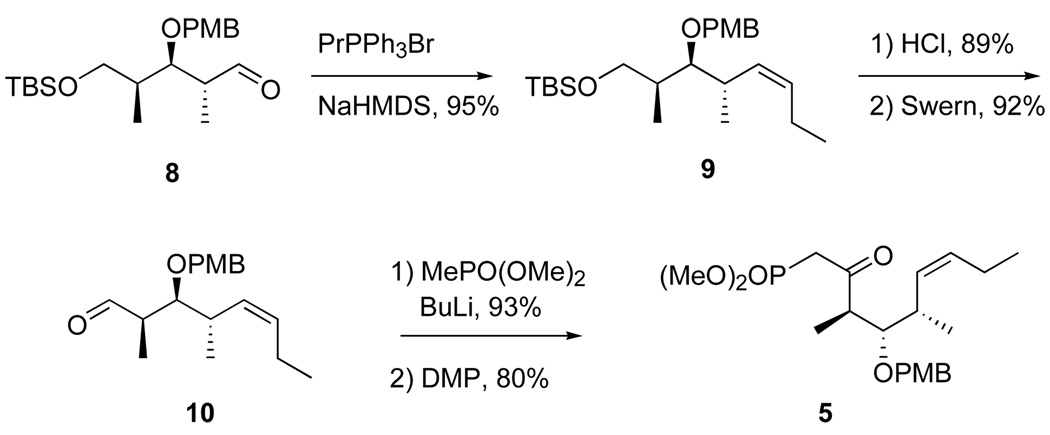
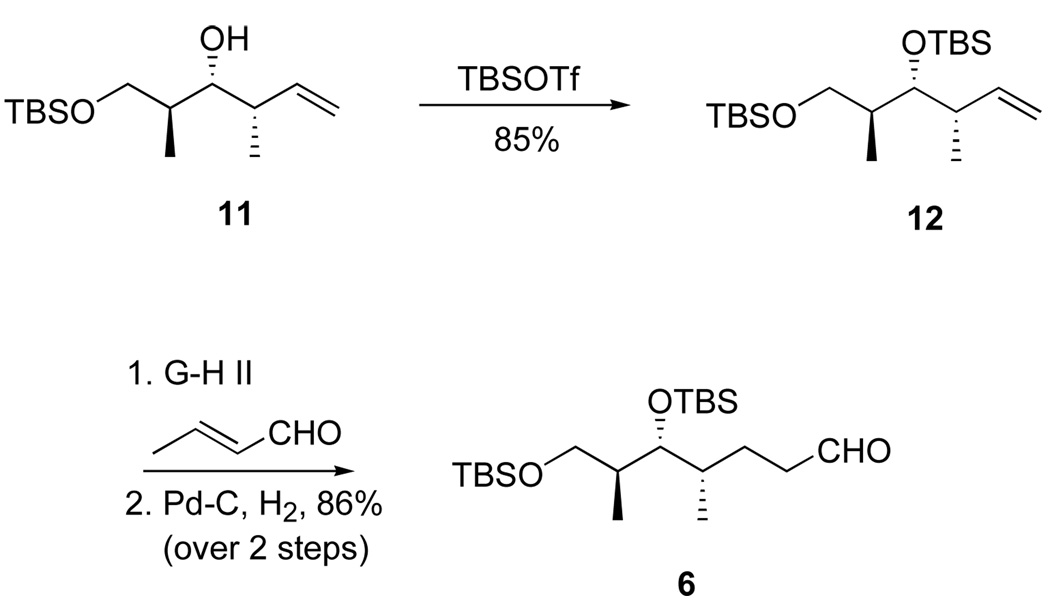
The top (C18–C26) fragment 5 was readily made in five steps from the well known intermediate 819 as summarized in Scheme 1. Briefly, Z-selective Wittig reaction to give 9, then desilylation and Swern oxidation provided an aldehyde 10 that was converted to the ketophosphonate 5 in the usual way. Full details of these and all reactions to make new intermediates and products are provided in the Supporting Information. Similarly, initial middle fragment 6 (Scheme 2) was made from homoallylic alcohol 1120 by cross metathesis with crotonaldehyde21 followed by reduction. Both epimers of the bottom fragment 4 were already in hand through an efficient six-step route based on cross metathesis.11i All three fragments were readily made on multi-gram scale.
Scheme 1.
Synthesis of top fragment 5, C18–C26
Scheme 2.
Synthesis of middle fragment 6, C11–C17
The initial synthesis of combined top-middle fragment 3 (C10–C26) is summarized in Scheme 3. HWE coupling of fragments 5 and 6 gave enone 13 in 91% yield. The α,β-alkene of enone 13 was selectively reduced with Stryker’s reagent in 95% yield.22 Stereoselective reduction of the ketone with Li(t-BuO)3AlH provided the alcohol as an 85:15 mixture of C19 epimers in 83% yield. After column chromatography to remove the minor epimer, alcohol 14 was then protected as the TBS ether in quantitative yield, and the primary TBS group was removed with a solution of HF•pyridine in pyridine/THF. The resulting alcohol was subsequently treated with DMP to give aldehyde 15, which was immediately subjected to Wittig reaction23 to install the vinyl iodide functionality in top-middle fragment 3 in 66% yield over 2 steps.
Scheme 3.
Initial synthesis of top-middle fragment 3, (C10–C26)
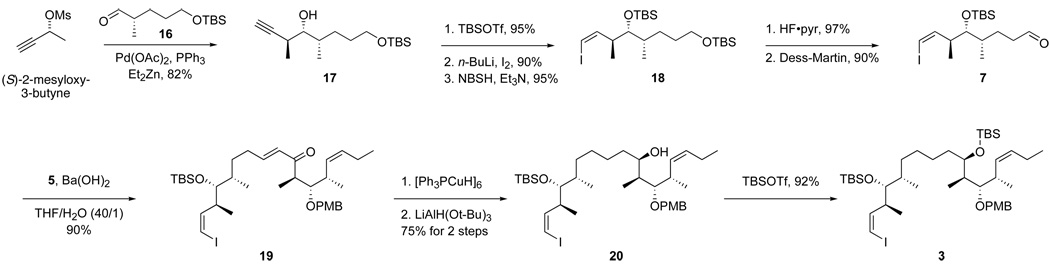
The more convergent route to top-middle fragment 3 featuring the complete middle fragment 7 is summarized in Scheme 4. First, Marshall’s palladium-catalyzed addition reaction of the allenyl zinc reagent derived from (S)-2-mesyloxy-3-butyne to aldehyde 1624 gave alcohol 17 (82%, dr >95:5).25 After protection of alcohol 17 as TBS ether, the acetylene was deprotonated, iodinated, and reduced with o-nitrobenzenesulfonyl hydrazide26 (NBSH) to provide Z-alkenyl iodide 18 (95%). Deprotection of primary TBS ether (97%) followed by Dess-Martin oxidation furnished the new middle fragment 7 (90%). This was then coupled to fragment 5 to provide top-middle fragment 3 (via enone 19 and alcohol 20) in good overall yield by using the same sequence of steps as in Scheme 3. Note that only four steps are required to produce top-middle fragment 3 from the start of the fragment coupling (fragments 5 + 7) in Scheme 4, whereas seven steps are needed to produce it from the same point (fragments 5 + 6) in Scheme 3.
Scheme 4.
Improved synthesis of top-middle fragment 3, (C10–C26)
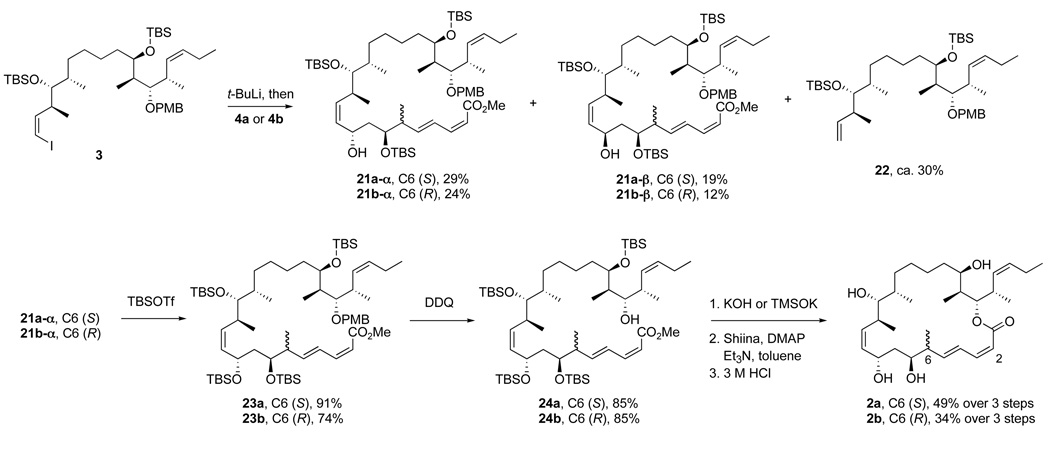
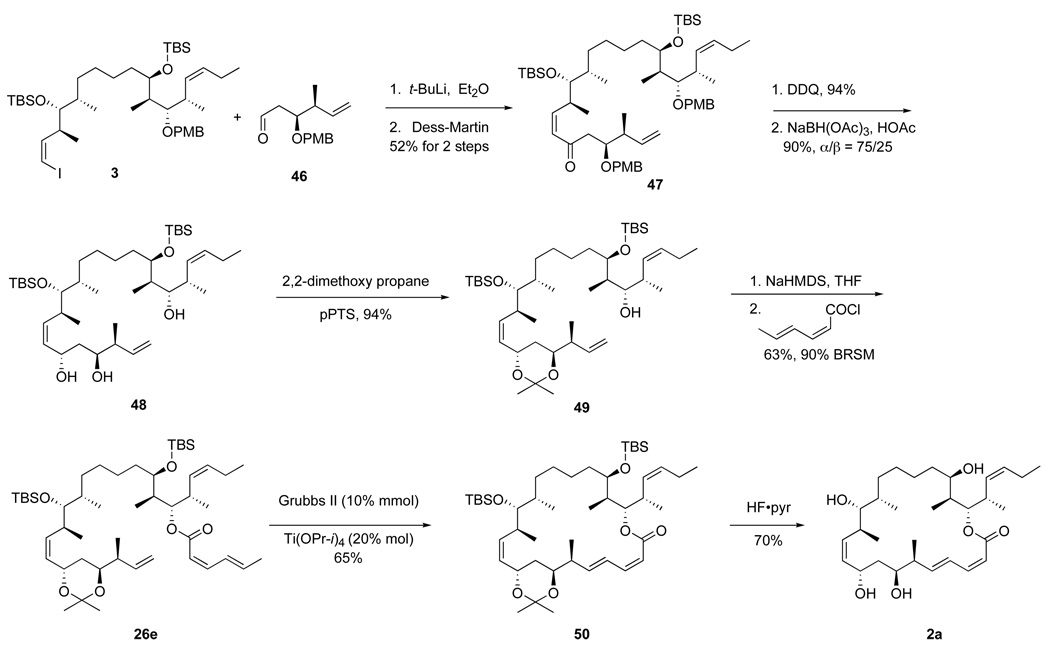
Coupling of the bottom fragments to top-middle fragment 3 and completion of the syntheses of 25,26-dihydro-16-desmethyldictyostatin 2b and its 6-epimer 2a are summarized in Scheme 5. Yields and selectivities for both epimers were comparable (see data in the Scheme), so we focus here only on the (6S)-epimer (“a” series). Lithium-iodine exchange with top-middle fragment 3 followed by addition of aldehyde 4a provided a mixture of three compounds that were separated by flash chromatography to provide C-9 epimers 21a-α (29%) and 21a-β (19%), together with the deiodinated byproduct 22 (30%).
Scheme 5.
End-game of the vinyllithium route
The established end game was then followed with C9 α-epimer 21a-α to complete the synthesis. Protection of the alcohol with TBSOTf (23a, 84%) followed by deprotection of PMB group with DDQ provided C21 alcohol 24a in 91% yield. The C1 methyl ester was then hydrolyzed by using KOH or TMSOK27 (which gave a better yield) to produce a seco-acid. The macrolactonization of the seco-acid using the Shiina reagent (2,6-methylnitrobenzoylanhydride)28 in toluene gave the crude protected macrolactone in 93% yield as a 60:40 mixture of the target (2Z,4E)-dienyl lactone and its (2E,4E)-isomer (not shown). Global deprotection of the isomer mixture with HCl followed by isomer separation provided the target analog 2a (6 mg, 49% over three steps). Likewise, the (6R)-epimer 2b was prepared on 3 mg scale by a similar sequence of reactions starting from top-middle fragment 3 and bottom fragment 4b. Again, (6R)-epimer 2b had to be separated from its (2E,4E)-isomer.
This route is considerably shorter than other syntheses of dictyostatin, needing only ten steps from the start of the fragment coupling to the end. The three fragments are also made in ten steps or fewer, for a longest linear sequence of about 20 steps. On the down side, the key vinyllithium addition was low yielding and did not scale well,29 and the formation of substantial amounts of (2E,4E)-isomer of the final macrolactonization was a persistent problem. (The E,E-isomers are not very active.12d–f) Because the final products 2a,b showed promising preliminary biological activity (see below), we decided to pursue further improvements to the synthesis.
Esterification/Ring Closing Metathesis Routes
Previous routes to dictyostatin and analogs almost all used macrolactonization as a penultimate step to close the ring. In the lone exception to date, O’Niel and Phillips closed the ring at the C2–C3 alkene by an intramolecular Still-Gennari reaction.9 In recent years, the traditional late-stage lactonization strategy for making macrolactones has received competition from a strategy involving esterification followed by late-stage ring-closing metathesis.30 This strategy is challenging for dictyostatin itself because chemoselectivity issues with the C10–C11 alkene and the two dienes arise. We11b and Phillips introduced RCM strategies to join bottom and middle fragments at the C10–C11 alkene, and Phillips carried his intermediate through to make dictyostatin.9 In such approaches, however, both dienes have to be constructed after the initial fragment couplings.
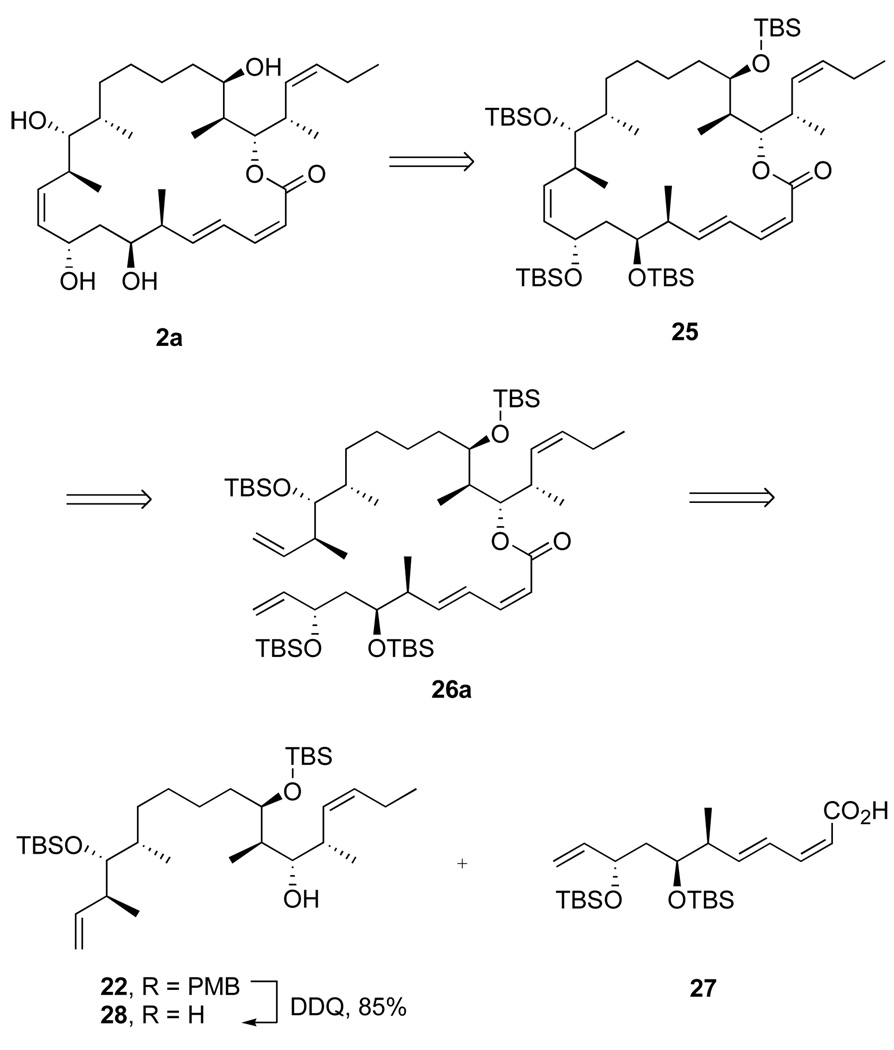
Based on an understanding of cross metathesis chemistry of dienyl esters,11i,31 we hypothesized that the simple tactic of saturating the C25–C26 terminal alkene would enable late-stage RCM reactions to make molecules like targets 2a,b. Accordingly, we drew up the strategy shown in Figure 2 involving fragments 27 and 28. The top-middle fragment 28 (C11–C26) comes from deprotection of the PMB group of alkene 22 and is coupled with fragment 27 (C1–C10) to assemble the complete carbon skeleton of diene 26a. Finally, RCM builds the C10–C11 bond and completes the macrocycle 25. The strategy is attractive because it could require as few as eight steps from the beginning of the fragment couplings to the target. Four of those steps (the top/middle coupling sequence, Scheme 4) were already well established and reliable. But, in addition to the chemoselectivity risk, there is also a risk regarding stereoselectivity. The previous RCM approaches to the C10–C11 Z alkene used a temporary 10-membered lactone to ensure Z-selectivity in the RCM.9,11b Here, there is no such insurance. In part because we already had available both fragments 22 and 27, we decided to take on the RCM challenge uninsured.
Figure 2.
RCM route to the C10,C11 alkene
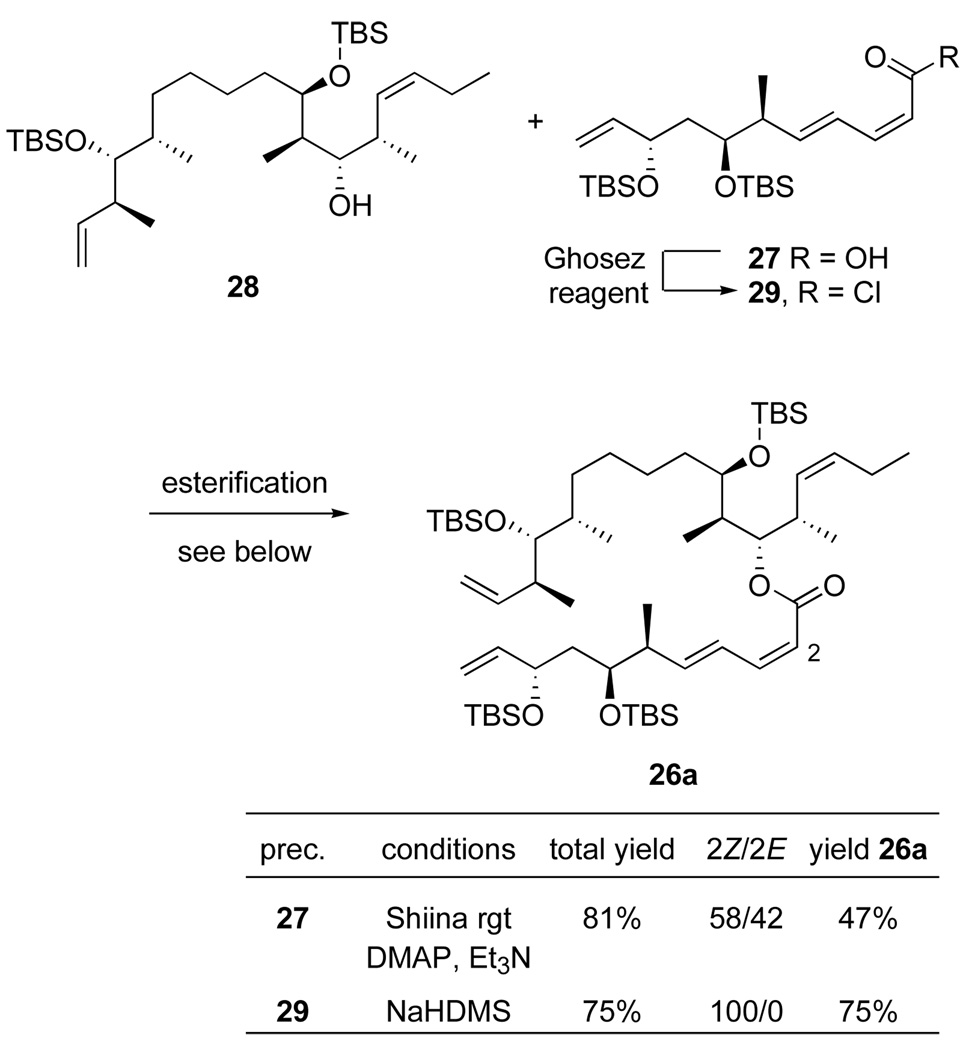
E/Z-Isomerization during macrolactonization has been a persistent problem in our and other syntheses of dictyostatins.6,12 The use of the Shiina reagent23 can sometimes suppress this isomerization, so we first tried esterification under the preferred conditions for macrolactonization. Alcohol 28 (1 equiv) and acid 27 (2 equiv) were reacted with the Shiina reagent (4 equiv) in the presence of excess Et3N and DMAP (Scheme 6). An 81% yield of ester 26a was obtained, but this was a 58:42 mixture of target (2Z,4E)- and isomerized (2E,4E)-dienyl esters. Apparently, the alkene isomerization is not associated with macrolactonization per se.
Scheme 6.
Bimolecular esterification by Shiina coupling and through an acid chloride
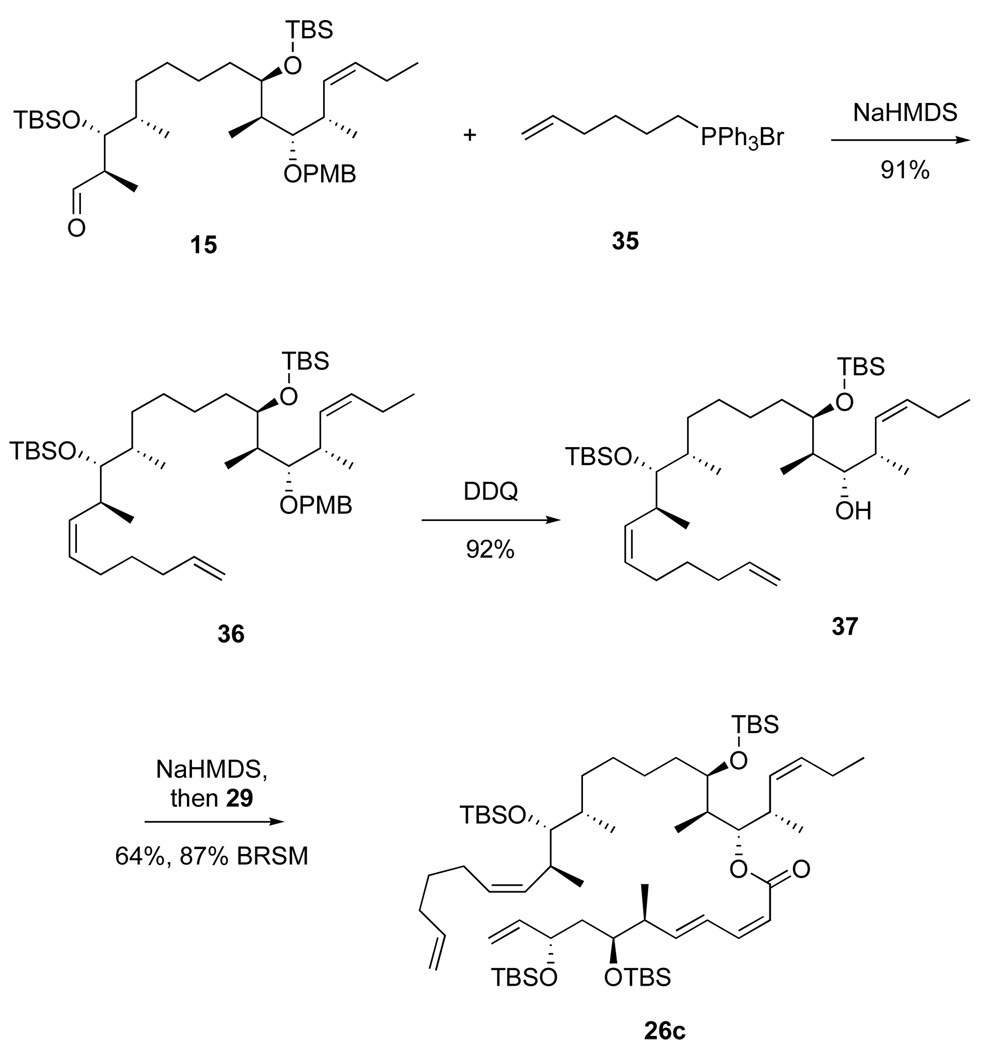
Roush has suggested that related isomerizations are induced by the acylation catalyst,32 here DMAP, but Yamaguchi and Shiina reactions in this series do not work if the DMAP is omitted. Accordingly, we transformed acid 27 to the corresponding acid chloride 29 with the Ghosez reagent (1-choro-1-(dimethylamino)-2-methyl-2-propene).33 Alcohol 28 (1 equiv) was deprotonated with NaHMDS, then crude acid chloride 29 (1 equiv) was added. After standard workup and chromatography, the Z,E isomer 26a was obtained in 75% isolated yield along with 5% of recovered 28. There was no evidence for formation of the E,E-isomer in the 1H NMR spectrum of the crude product, 26a.
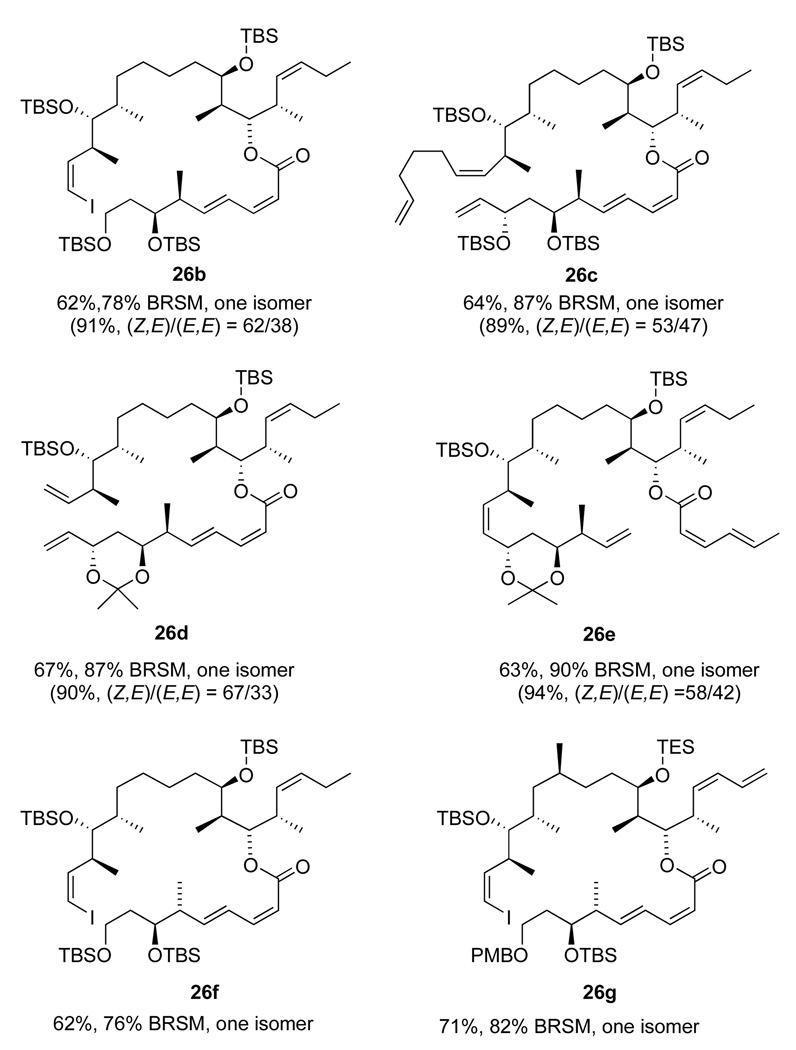
The acid chloride esterification method provided general access to the late-stage compounds needed for both this RCM approach and the subsequent Nozaki-Kishi approach. Six additional esters were made by this method, as summarized in Figure 3. In four more cases (compounds 26b–26e), the acid chloride method was compared with the Shiina method for yields and stereoselectivity. The Shiina method uniformly provided excellent yields (89–94%) but poor stereoselectivities (53:47 to 67:33). Furthermore, the alkene isomers were not separable at this point. In contrast, the acid chloride provided lower total yields of ester (62–71%), but there was no alkene isomerization in any case. The moderate yields were offset by recovery of some amounts of the more complex starting alcohol 28. (We did not attempt to recover the acid 27.) The acid chloride method was clearly superior because it provided a higher absolute yield of the Z,E isomer and because it was much easier to separate the Z,E isomer of 26 from the starting alcohol than from its E,E-isomer produced in the Shiina coupling. Furthermore, several of the acid chloride couplings in Figure 3 were only conducted once or twice on small scale, so prospects for optimization to increase conversion and yield remain.
Figure 3.
Esters made by the acid chloride route (with comparison data for the Shiina route in parentheses)
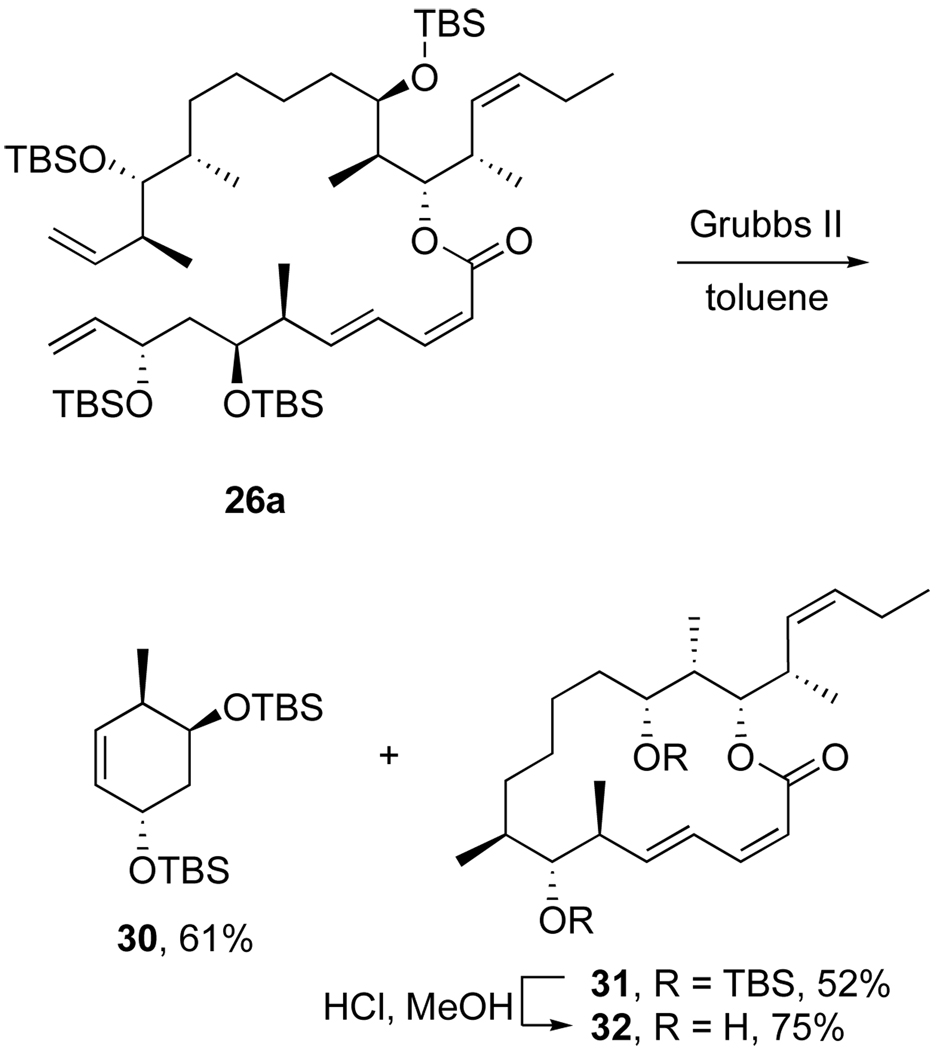
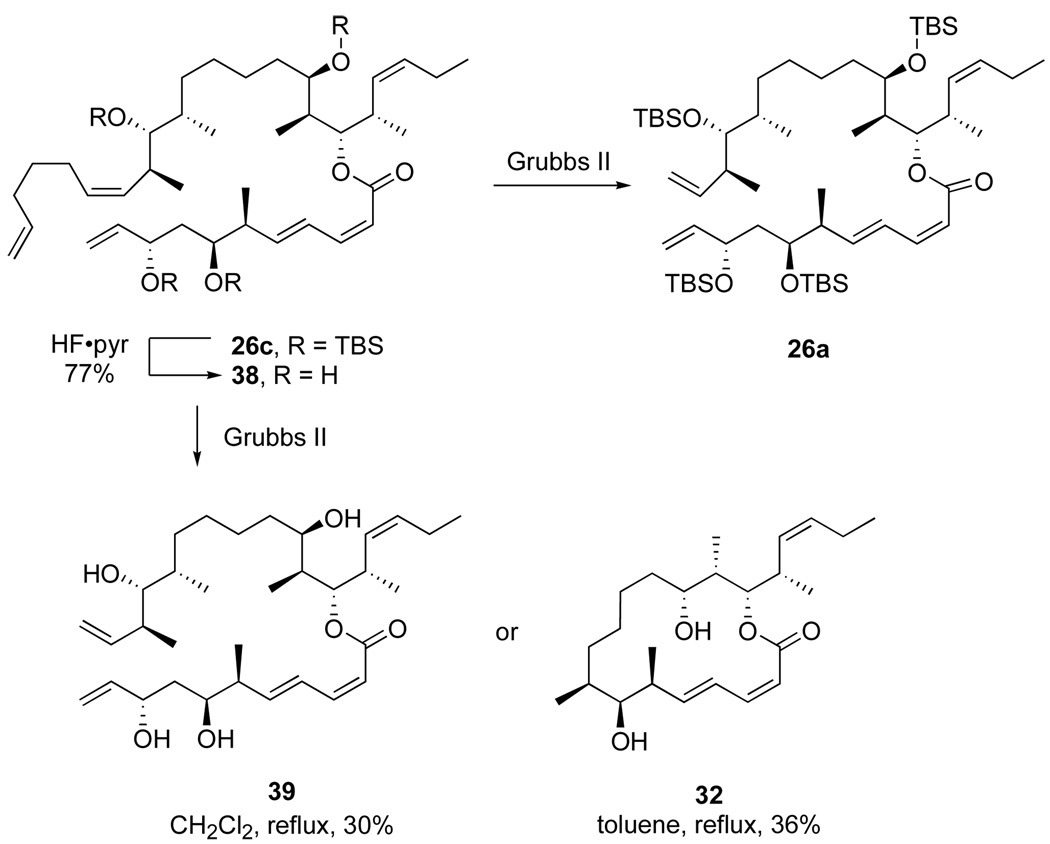
We next examined the RCM reaction of diene 26a under several conditions by varying the solvent, catalyst and temperature. The target 22-membered ring product was not, however, detected in any case. In a typical experiment (Scheme 7), treatment of diene 26a with 10% Grubbs II catalyst in refluxing toluene followed by cooling, concentration and chromatography provided cyclohexene 30 and 16-membered ring product 31 in comparable yields (61% and 52%). The silyl groups of macrolactone 31 were removed with HCl/MeOH to provide the ring-contracted dictyostatin analog 32 that was used for structure elucidation (see data in Supporting Information) and biological testing (see below).
Scheme 7.
RCM reaction of diene 26a provides truncated products by RRCM
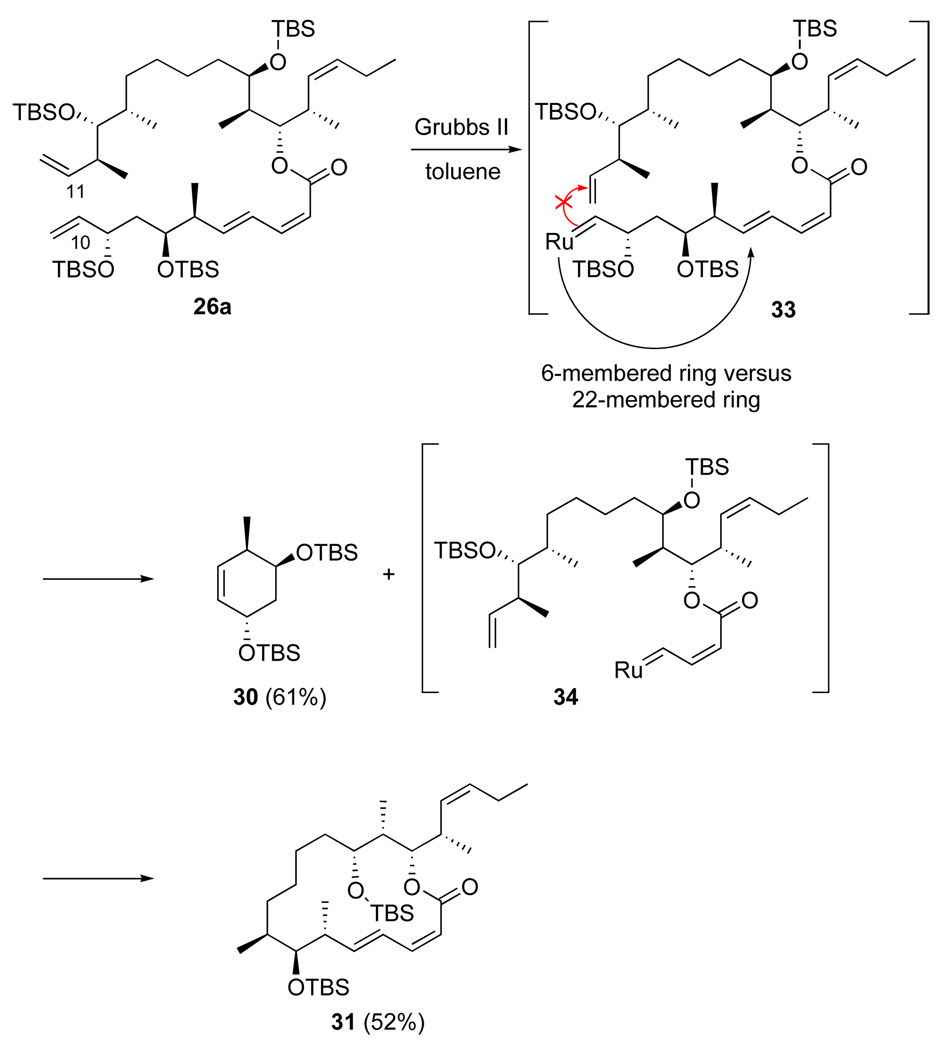
We ascribe the outcome of this RCM reaction to the initial formation of ruthenium carbene on C10 rather than C11 as shown in Figure 4. Instead of reacting with the terminal alkene at C11 to afford the 22-membered ring product, the carbene intermediate 33 reacted with the internal alkene at C5 to give a new ruthenium carbene 34 with extrusion of cyclohexene 30. Carbene 34 then reacted with the terminal alkene to furnish the 16-membered ring product 31. This failure nonetheless encouraged us because the C23–C24 alkene was a passive spectator in the process and because the structure of the side products suggested that both terminal alkenes could participate in RCM reactions.
Figure 4.
Suggested pathway for formation of truncated RCM products
In a sense, the ring-contracted product 31 was formed by a so-called “relay ring closing metathesis” (RRCM) reaction.34 We therefore first attempted to derail this RRCM reaction with a better RRCM option by designing substrate 26c, which was readily prepared as shown in Scheme 8. Wittig reaction of aldehyde 15 and the ylide derived from 35 provided alkene 36 in 91% yield. Deprotection with DDQ gave alcohol 37 (92%), which was esterified as above to give diene 26c. Assuming that the initial carbene is generated at the new terminal alkene of diene 26c, this should be relayed in turn to a carbene at C11 (not C10), so the extrusion of cyclohexene 30 should be suppressed. It was not, however.
Scheme 8.
Synthesis of RRCM precursor 26c
Again, several conditions were explored, but the RCM reactions of diene 26c produced no major new products, giving instead two products that we already had in hand (Scheme 9). Starting from diene 26c, we observed mostly product 26a in which the newly introduced side chain was simply clipped off. We also desilylated 26c to tetraol 38, but this gave similar results. Truncated product 39 was formed at short reaction times, followed by growth of its derived (doubly truncated) product 32. In a typical experiment with diene 38 (Grubbs II, dichloromethane, reflux, 14 h), we isolated 3935 and the 16-membered macrolactone 32 in 30% and 36% yields, respectively. Based on these results, we suppose that the initial metathesis was initiated at the terminal alkene, then relayed to C11 as expected. This carbene (not shown) then suffered cross metathesis to give 39 rather than RCM to give the target macrolactone. In turn, 32 is a secondary reaction product derived from 39.
Scheme 9.
Typical RCM reaction of 26c
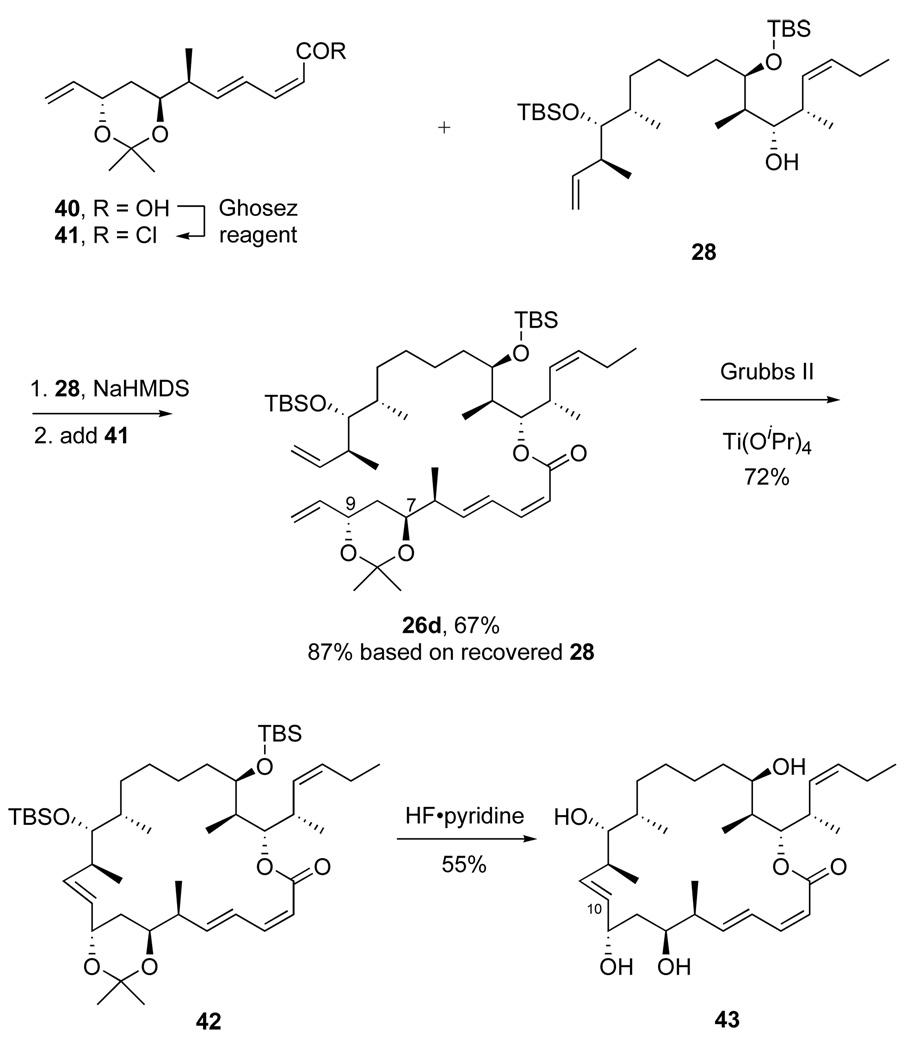
Noting the demonstration that RCM reactions could be initiated at C10, we prepared precursor 26d (Scheme 10) with a dioxane acetal protecting group on O7 and O9 in place of the two TBS groups. The RRCM contraction reaction that took place with 26a should now be prevented (because the extruded product would be a trans-bicyclo[3.3.0] ring). The acetal 40 was easily made from the bis-silyl ether by desilylation and acetonide formation (see Supporting Information). The esterification reaction to make 26d was already shown in Figure 3.
Scheme 10.
Synthesis and RCM reaction of 26d
Initial reactions of 26d with Grubbs II catalyst provided little or no conversion. Speculating that this might be caused by the increased Lewis basicity of the acetal oxygens compared to the prior silyl ethers,36 we added Lewis acids to interfere with potential chelate formation of ruthenium carbene intermediates. Indeed, treatment of 26d with 10% Grubbs II catalyst and 25% Ti(OPr-i)4 cleanly produced a new 22-membered ring macrocycle 42 in 72% isolated yield. Analysis of a set of 2D NMR spectra of 42 provided assignments for all of the protons, and the coupling constant J10,11 was extracted. Its 15 Hz magnitude showed that the RCM reaction produced exclusively the 10E isomer. Indeed, desilylation of 42 did not produce 2a, but gave instead its stereoisomer 43. Paterson has recently reported 10,11-dihydrodictyostatin,13d and 43 is the first 10,11-E isomer of the dictyostatin family. The dihdyro analog is active, but the E-isomer is not (see below).
Based on what we learned from this series of RCM reactions, we redesigned the RCM approach to form the C4–C5 E-alkene via 26e, as shown in Figure 5. In the syntheses of the bottom fragments above, we routinely used a related cross metathesis reaction. This early-stage precedent suggests that a late-stage application could work if other pathways for carbene reaction are not available. On the surface, substrate 26e has major design problems since initiation of the RCM reaction at four different places (C2, C10, C16, and C24) could be followed by rapid extrusion of a cyclohexene and a truncated carbene. We expected the reaction of 26e to initiate at C5, however, so the lone extrusion reaction to derail is the RRCM reaction starting there (which would form the same product 30 as in Scheme 7, though in the reverse direction). We again initiated this path out by using the trans-acetal protecting group.
Figure 5.
RCM route to the C4–C5 alkene
The synthesis and RCM reaction of 26e are shown in Scheme 11. Generation of the vinyllithium reagent from iodide 3 (C10–C26) and addition of aldehyde 46 (C5–C9) provided a mixture of epimeric alcohols at C9 that was not easily separated. This product was subjected to Dess-Martin oxidation to afford the enone 47 in 52% yield for 2 steps. After DDQ deprotection of the bis-PMB ether, the enone was diastereoselectively reduced with Evans-Saksena conditions37 to give the 1,3-anti triol 48 (90% yield, dr 75:25). Then, triol 48 was protected with 2,2-dimethoxypropane to give the acetonide 49 in 94% yield. The esterification reaction between acetonide 49 and (2Z,4E)-hexa-2,4-dienoyl chloride38 gave RCM precursor 26e in 63% yield (90% BRSM). The RCM reaction was run with Ti(OPr-i)4 as additive, and product 50 was isolated in 65% yield. Global deprotection of macrolactone 50 with HF•pyridine gave 6-epi-16-desmethyl-25,26-dihydrodictyostatin 2a (70%), whose analytical and spectroscopic data (Rf, 1H NMR spectra and co-injection on chiral HPLC) matched the previous synthetic sample, thus confirming the structure and stereochemical outcome of this RCM reaction.
Scheme 11.
Successful RCM route to 2a
Because top-middle fragment 3 is an intermediate in both the RCM and vinyllithium synthesis of 2, it is instructive to compare and contrast the approaches. Recall that 3 is already four steps in from the start of the fragment couplings. The vinyllithium route requires six more steps to provide 2, whereas the RCM route requires eight. The yields are about the same (8 and 9%, respectively). The RCM route is apparently less convergent because what was the “bottom fragment” in prior approaches (C1–C10) is now introduced in two pieces rather than one. The two “extra” steps in RCM route are, however, misleading because we separated the epimeric C9 alcohols directly in Scheme 1 but added the steps of oxidation and reduction to get the target epimer in Scheme 11. Therefore, the step count of the two routes is effectively the same.
Furthermore, while the vinyllithium route is already maximally convergent (assuming no changes to the basic fragment assembly strategy), the successful RCM route is not. The convergency and step count of this synthesis could be considerably improved by appending the fragment 46 (C6–C10) to the middle fragment (see 7) and dienyl ester fragment (C1–C5) to the top fragment (see 5) prior to the fragment couplings. This could provide a very efficient synthesis based on two (rather than three) main fragments with as few as six steps for the end game.
Nozaki-Hiyama-Kishi Route
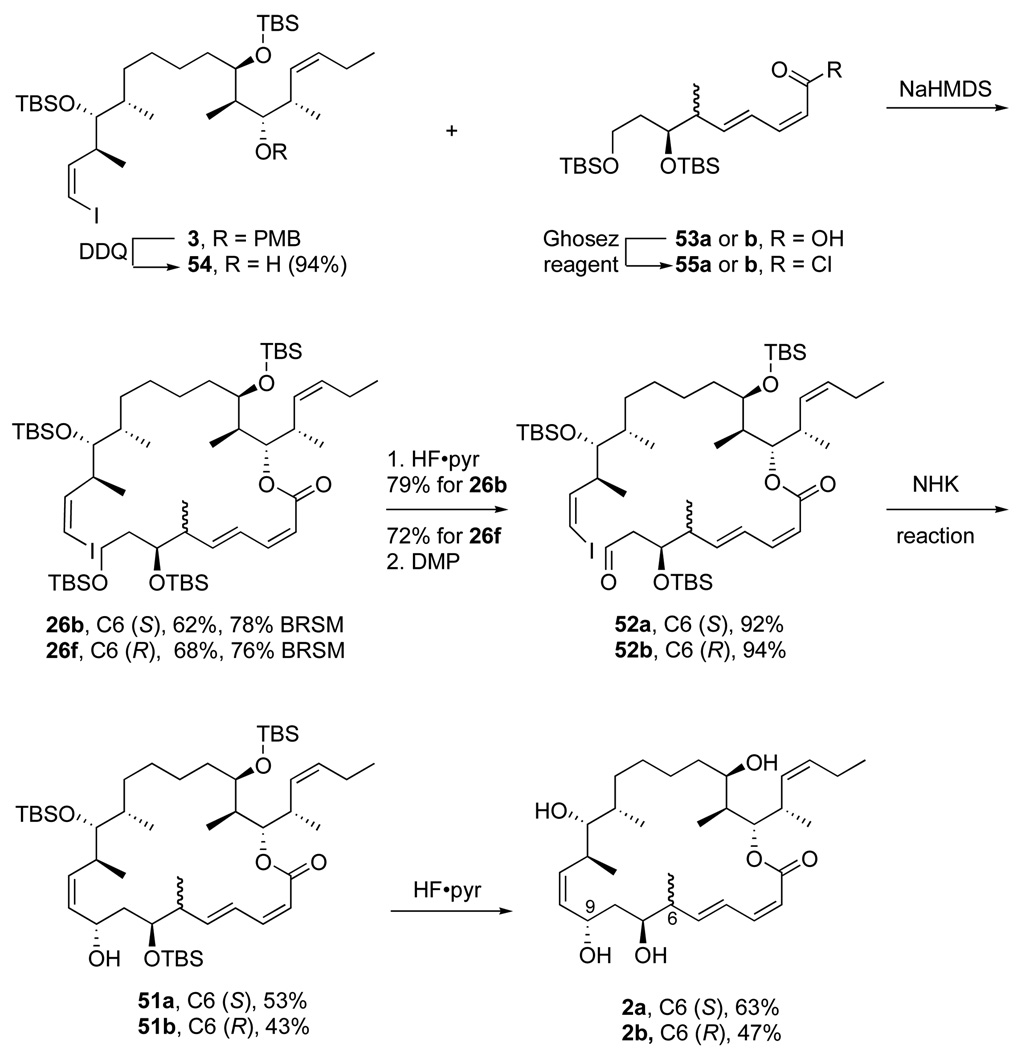
Although the RCM route has many attractive features coupled with potential for further improvement, it still probably cannot directly accommodate the C25–C26 terminal alkene present in both the natural product dictyostatin and in the preclinical candidate 6-epi-dictyostatin. Accordingly, we pursued a third approach based on macrocyclization by Nozaki-Hiyama-Kishi reaction39,40 that is a hybrid of the first two approaches. Like the RCM approach, the esterification is done before making the macrocycle. Like the vinyllithium approach, the macrocycle is made from an alkenyl iodide and an aldehyde. Maier proposed a related approach and prepared key fragments, but did not yet describe the NHK reaction.11d,e
The plan for the NHK approach to dihydro analogs 2a,b is summarized in Figure 6. Cyclization of alkenyl iodide 52 should produce alcohol 51, which is very similar to the usual penultimate intermediate 25 (one fewer TBS group). In turn, 52 is made by esterification of 54 (C10–C26) and 55 (C1–C9), followed by selective desilylation and oxidation. An attractive feature of the plan is the small number of steps needed from the beginning of the fragment coupling to the end of the synthesis (only five). The key concern is the viability and stereoselectivity of the proposed NHK reaction. Myles and coworkers used a bimolecular NHK reaction in their synthesis of discodermolide.41 We had previously explored a bimolecular NHK approach to dictyostatin with limited success.42 The model addition reactions succeeded, but the stereoselectivity at C9 was very low.
Figure 6.
Plan for the NHK route to 2
The fragment coupling and execution of the NHK reaction are shown in Scheme 12. Common intermediate 3 was first deprotected with DDQ to give alcohol 54 in 94% yield. Then 54 was coupled with the acid chloride 55a (prepared from the corresponding acid 53a and the Ghosez reagent) to afford the ester 26b (62%, 78% BRSM). The primary TBS ether was removed with HF•pyridine and the resulting alcohol was oxidized to 52a (92%) with the Dess-Martin reagent. Epimer 52b was prepared by a similar sequence of reactions in comparable yields.
Scheme 12.
Synthesis of 2a,b by the Nozaki-Hiyama-Kishi route
The key intramolecular Nozaki-Hiyama-Kishi reaction40 of 52a was then examined under various conditions, and details of this survey are contained in the Supporting Information. Under the best conditions identified, 52a was reacted with excess chromium chloride and 4,4'-di-tert-butyl-2,2'-dipyridyl (15 equiv each) and NiCl2(dppf) (0.2 equiv) in THF at room temperature. After workup and flash chromatography, macrocycle 51a was isolated in 53% yield as a single stereoisomer. The configuration of NHK product 51a was confirmed by global deprotection as usual to provide 2a, which was identical to the previous two samples.
A similar sequence of reactions in the C6-(R) series starting from 53b provided NHK precursor 52b in comparable yields. This time, however, the NHK reaction was not as stereoselective, and a 75:25 mixture of 51b and its C9 epimer (not shown) was produced. The mixture was desilylated and the target C9 epimer 2b was isolated in 43% yield after careful purification. Apparently the stereoselectivity of the NHK reaction is modulated by the configuration at C6; however, even in the less selective (R) series, the stereoselectivity is still better than related bimolecular model reactions.42
The NHK route is both convergent and efficient compared to the previous two routes. Starting from the same intermediate 3, the NHK approach provides 2a in six steps in 15.7% overall yield (6 steps, 8.3% yield for vinyllithium addition approach; 8 steps, 9% yield for RCM approach, respectively). In addition, we expected that dictyostatin analogs with the C25–C26 diene could be made by this route. To demonstrate this, we synthesized dictyostatin itself to culminate the work.
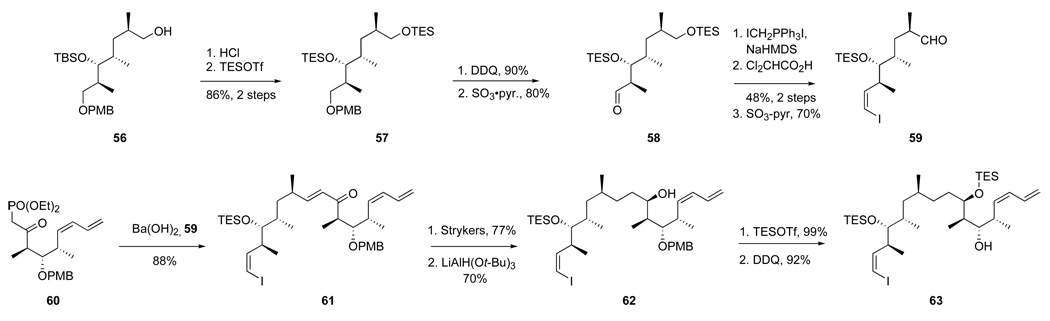
The new synthesis of dictyostatin is summarized in Schemes 13 and 14. The combined top-middle fragment 63 (C10–C26) was prepared from alcohol 56, a readily available intermediate from our first synthesis of dictyostatin (Scheme 13).6 The TBS group in 56 was removed with HCl to give a diol that was protected as bis-TES ether 57 (86% yield, two steps). Removal of the PMB group with DDQ gave the corresponding alcohol (90% yield), which was oxidized with SO3•pyridine, DMSO and TEA to provide the aldehyde 58 (80% yield). Next, Wittig olefination (ICH3PPh3, I2, BuLi and NaHMDS)43 gave a Z-vinyl iodide, which was directly treated with dichloroacetic acid to give a primary alcohol (48% yield, two steps). Oxidation under Parikh–Doering conditions provided the C10–C17 aldehyde 59 (70% yield).
Scheme 13.
NHK route to dictyostatin; synthesis of the top-middle fragment 63 (C10–C26)
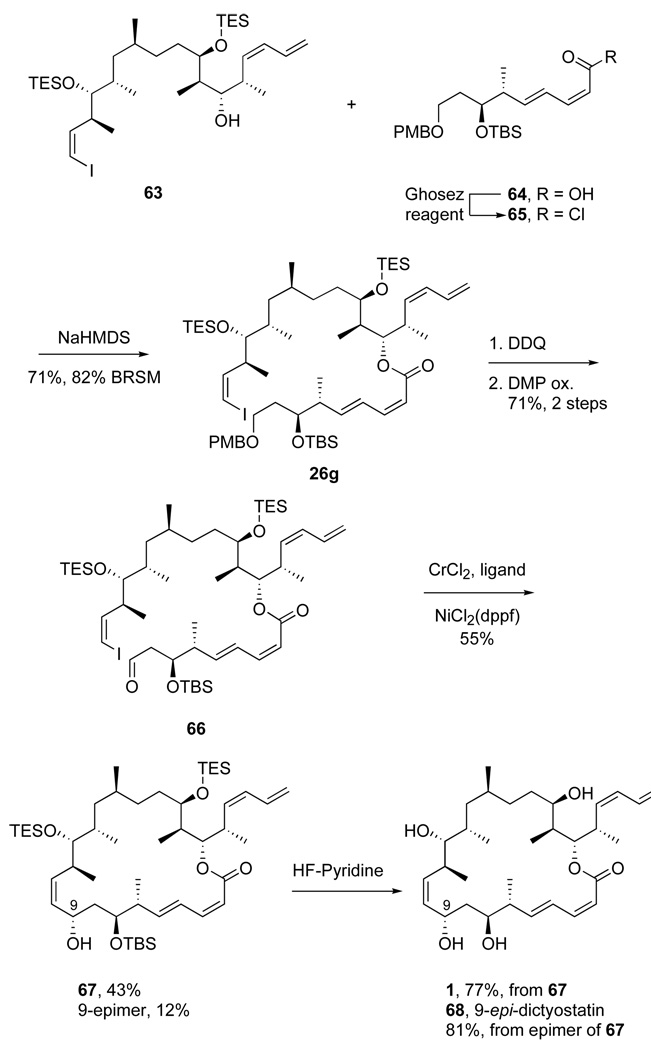
Scheme 14.
Nozaki-Hiyama-Kishi route to dictyostatin; end-game steps
The usual C18–C26 phosphonate 6012c was coupled with aldehyde 59 mediated by Ba(OH)2 to produce the enone 61 (88% yield). Again following established conditions, the C17–C18 alkene was reduced with the Stryker reagent to give a ketone (77% yield), which was reduced with LiAlH(Ot-Bu)3 to afford two C19 epimers in a 78:22 ratio favoring the C19 β-epimer 62 (70%). Alcohol 62 was protected with TESOTf to give the TES ether (99%), followed by DDQ deprotection to afford fragment 63 (92%).
To start the end game, acid 6444 was converted to the acid chloride 65 and this was coupled with alcohol 63 as usual to form the ester 26g (71%, 82% BRSM). DDQ deprotection of PMB ether 26g gave a primary alcohol, which was oxidized to aldehyde 66 (71% over two steps) by the Dess-Martin reagent. Treatment of 66 under the optimal NHK reaction conditions gave macrocyclized product 67 in 55% combined yield as two separable C9 isomers in 78:22 in favor of the alpha isomer. Global deprotection of the major isomer provided natural dictyostatin 1 in 77% yield, identical with previous natural and synthetic samples. Global deprotection of the minor C9 isomer provided 9-epi-dictyostatin 68 (81%), whose spectra matched well those reported by Paterson.13b This synthetic route provides natural dictyostatin over a 22 step linear sequence with a 3.3% overall yield.
Preliminary Biological Testing Results
The target compounds 6-epi-16-desmethyl-25,26-dihydrodictyostatin 2a and 16-desmethyl-25,26-dihydrodictyostatin 2b were tested in comparison to 16-desmethyldictyostatin and paclitaxel for antiproliferative activity against 1A9 human ovarian carcinoma cells and their paclitaxel-resistant subclones 1A9/PTX10 and 1A9/PTX22, as well as for microtubule stabilizing activity in HeLa human cervical cancer cells. The side products 9-epi-dictyostatin 68, 6-epi-10,11-E-16-desmethyl-25,26-dihydrodictyostatin 43 and the 16-member macrolactone 32 were also examined. As shown in Table 1, both 2a and 2b caused microtubule bundling in HeLa cells and retained submicromolar growth inhibitory activity against 1A9 cells. In results that parallel those with (–)-dicytostatin, the 1A9/PTX10 cells were cross-resistant to 25,26-dihydro derivatives but the 1A9/PTX22 cells were not. This suggests that this saturation of the terminal alkene does not alter the mode of binding to tubulin. Interestingly, both analogs appeared to be much less susceptible to cross-resistance in both 1A9/PTX10 and 1A9/PTX22 than was 16-desmethyldictyostatin.
Table 1.
Cell-based activities of new analogs in comparison to paclitaxel and 16-desmethyldictyostatin.
| MDECa for tubulin polymer increase nM ± SD (N) |
GI50,b nM + SD (fold resistance) (N = 4) |
|||
|---|---|---|---|---|
| Test agent | 1A9 | 1A9/PTX10 | 1A9/PTX22 | |
| 2b | 43.2 ± 10.3 (3) | 285 ± 18 | 2817 ± 37 (10) | 445 ± 27 (1.6) |
| 2a | 54.2 ± 3.1 (3) | 241 ± 7 | 4090 ± 21 (17) | 193 ± 4 (0) |
| 9-epi- dictyostatin |
74.4 ± 42.5 (4) | 79 ± 1 | 2160 ± 160 (27) | 160 ± 45 (2) |
| 32 | > 5000 (2) | 17000 ± 0.2 | 35000 ± 0.1 (2) | 23000 ± 0.2 (1.4) |
| 43 | 713.0 ± 145.3 (3) | 820 ± 10 | 2610 + 210 (3) | 1370 ± 170 (1.7) |
| 16-desmethyl- dictyostatin |
25 ± 9 (3) | 0.41 ± 0.52 | 470 ± 70 (1146) | 5.6 ± 4.7 (14) |
| (−)-dictyostatin | 12.8 ± 3.7 (4) | 0.69 ± 0.80 | 3.2 ± 2.4 (4.6) | 1.3 ±1.0 (1.9) |
| 6-epi- dictyostatin |
9.1 ± 6.3 (9) | 0.85 ± 0.03 | 4.5 ± 0.3 (5) | 0.81 ± 0.17 (0) |
| Paclitaxel | 5.2 ± 0.4 (3) | 0.71 ± 0.11 | 64 ± 8 (70) | 51 ± 9 (72) |
Minimum detectable effective concentration of the test agent in HeLa cells after 21 h of continuous exposure.
Fifty percent growth inhibitory concentration after 72 h of continuous exposure to the test agent.
Conclusions
In summary, we have developed three new routes to the dictyostatin class of natural products. A relatively classical route based on vinyllithium addition and macrocyclization was first used to make 25,26-dihydro-16-desmethyldictyostatin 2b and its 6-epimer 2a. That route is short and highly refined, but the two key reactions are problematic—the vinyllithium addition was low yielding and not stereoselective while the macrolactonization was high yielding but marred by a nagging isomerization problem at the C2–C3 alkene.
Several new approaches to the 25,26-dihydro series of analogs based on esterification and ring closing metathesis were investigated. Synthesis of a bottom fragment acid chloride and esterification occurred in good yield and without isomerization at the C2–C3 alkene. This solution to the isomerization problem proved quite general and opened the door to both RCM and NHK approaches. RCM approaches to make the C10–C11 bond were problematic, with formation of truncated products or E-isomers prevailing. In contrast, an approach to make the C4–C5 E alkene succeeded well, avoiding pitfalls like formation of truncated products and isomerization of the C2–C3 Z-alkene. This RCM approach already shows excellent potential for making diverse 25,26-dihydrodictyostatins, and room remains for improvement with increased convergency.
Finally, the discovery of practical esterification conditions allowed us to acylate intermediates from the initial vinyllithium approach and then close the macrocycle on the acylated products by an intramolecular NHK reaction. Though modest yields (up to about 50%) in the key NHK reaction have been obtained at this point, the route is attractive because it is highly convergent and because there is moderate to excellent stereoselectivity for formation of C9 secondary alcohol stereocenter. The NHK route was used to produce both C6 epimers of 25,26-dihydrodictyostatin, and dictyostatin itself.
The synthetic work marks significant advances towards the practical synthesis of dictyostatin and analogs both for drug discovery and development purposes. The “three fragment” approaches are all based on related fragments that can be made in ten steps or fewer on large scale and that can be coupled, refunctionalized and deprotected in less than 10 steps. Accordingly, the longest linear sequences of steps are 20 or less and the total numbers of steps (from three commercial starting materials) are 36–40. All the approaches maximize convergency by building the complete carbon skeleton into the fragments; no carbons are added after the fragment couplings begin. The NHK approach to dictyostatin is the first synthesis of the natural product to meet this target, and it is currently the shortest synthesis of the natural product.
Both of the new 6-epimers of 25,26-dihydrodictyostatin exhibit considerable activity in preliminary biological assays. The saturation of the potentially troublesome 25,26-terminal alkene also opens new options for synthetic manipulations. Accordingly, the further investigation of the medicinal chemistry of this series of analogs is an attractive line of research.
Supplementary Material
ACKNOWLEDGMENTS
We thank the National Institutes of Health for support through research grant CA078039 and for a grant to purchase an NMR spectrometer.
Footnotes
Supporting Information Available. Contains detailed descriptions of the synthesis and characterization of all new compounds along with copies of key NMR spectra. This material is available free of charge via the Internet at http://www.acs.org.
References and Notes
- 1.(a) Kingston DGI. J. Nat. Prod. 2009;72:507–515. doi: 10.1021/np800568j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gunasekera SP, Wright AE. In: Anticancer Agents from Natural Products. Cragg GM, Kingston DGI, Newman DJ, editors. New York, NY: CRC Taylor Francis; 2005. pp. 171–189. [Google Scholar]; (c) Paterson I, Anderson EA. Science. 2005;310:451–453. doi: 10.1126/science.1116364. [DOI] [PubMed] [Google Scholar]
- 2.(a) Pettit GR, Cichacz ZA, Gao F, Boyd MR, Schmidt JM. J. Chem. Soc., Chem. Commun. 1994:1111–1112. [Google Scholar]; (b) Pettit GR, Cichacz ZA. US Patent 5,430,053. 1995:8.
- 3.(a) Wright AE, Cummins JL, Pomponi SA, Longley RE, Isbrucker RA. PCT Int. Appl. 2001:30. WO20010162239. [Google Scholar]; (b) Isbrucker RA, Cummins J, Pomponi SA, Longley RE, Wright AE. Biochem. Pharmacol. 2003;66:75–82. doi: 10.1016/s0006-2952(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 4.Paterson I, Britton R, Delgado O, Wright AE. Chem. Commun. 2004:632–633. doi: 10.1039/b316390c. [DOI] [PubMed] [Google Scholar]
- 5.Paterson I, Britton R, Delgado O, Meyer A, Poullennec KG. Angew. Chem. Int. Ed. 2004;43:4629–4633. doi: 10.1002/anie.200460589. [DOI] [PubMed] [Google Scholar]
- 6.Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP. Angew. Chem. Int. Ed. 2004;43:4634–4637. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]
- 7.(a) Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP, Day BW. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]; (b) Buey R, Barasoain I, Jackson E, Meyer A, Giannakakou P, Paterson I, Mooberry S, Andreu JM, Diaz JF. Chem. Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 8.Review: Florence GJ, Gardner NM, Paterson I. Nat. Prod. Rep. 2008;25:342–375. doi: 10.1039/b705661n.
- 9.O'Neil GW, Phillips AJ. J. Am. Chem. Soc. 2006;128:5340–5341. doi: 10.1021/ja0609708. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran PV, Srivastava A, Hazra D. Org. Lett. 2007;9:157–160. doi: 10.1021/ol062737k. [DOI] [PubMed] [Google Scholar]
- 11.(a) O'Neil GW, Phillips AJ. Tetrahedron Lett. 2004;45:4253–4256. [Google Scholar]; (b) Kangani CO, Brückner AM, Curran DP. Org. Lett. 2005;7:379–382. doi: 10.1021/ol0478279. [DOI] [PubMed] [Google Scholar]; (c) Baba VS, Das P, Mukkanti K, Iqbal J. Tetrahedron Lett. 2006;47:7927–7930. [Google Scholar]; (d) Jagel J, Maier ME. Synlett. 2006:693–696. [Google Scholar]; (e) Prusov E, Rohm H, Maier ME. Org. Lett. 2006;8:1025–1028. doi: 10.1021/ol052917e. [DOI] [PubMed] [Google Scholar]; (f) Shaw SJ, Zhang D, Sundermann KF, Myles DC. Syn. Commun. 2006;36:1735–1743. [Google Scholar]; (g) Gennari C, Castoldi D, Sharon O. Pure Appl. Chem. 2007;79:173–180. [Google Scholar]; (h) Monti C, Sharon O, Gennari C. Chem. Commun. 2007:4271–4273. doi: 10.1039/b708820e. [DOI] [PubMed] [Google Scholar]; (i) Moura-Letts G, Curran DP. Org. Lett. 2007;9:5–8. doi: 10.1021/ol062017d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Saibaba V, Sampath A, Mukkanti K, Iqbal J, Das P. Synthesis. 2007:2797–2802. [Google Scholar]; (k) Sharon O, Monti C, Gennari C. Tetrahedron. 2007;63:5873–5878. [Google Scholar]; (l) Zanato C, Pignataro L, Hao Z, Gennari C. Synthesis. 2008:2158–2162. [Google Scholar]; (m) Dias LC, Lima DJP, Gonçalves CCS, Andricopulo AD. Eur. J. Org. Chem. 2009;10:1491–1494. [Google Scholar]; (n) Dilger AK, Gopalsamuthiram V, Burke SD. J. Am. Chem. Soc. 2007;129:16273–16277. doi: 10.1021/ja077336u. [DOI] [PubMed] [Google Scholar]
- 12.(a) Shin Y, Choy N, Turner TR, Balachandran R, Madiraju C, Day BW, Curran DP. Org. Lett. 2002;4:4443–4446. doi: 10.1021/ol026942l. [DOI] [PubMed] [Google Scholar]; (b) Shin Y, Fournier JH, Balachandran R, Madiraju C, Raccor BS, Zhu G, Edler MC, Hamel E, Day BW, Curran DP. Org. Lett. 2005;7:2873–2876. doi: 10.1021/ol050808u. [DOI] [PubMed] [Google Scholar]; (c) Fukui Y, Brückner AM, Shin Y, Balachandran R, Day BW, Curran DP. Org. Lett. 2006;8:301–304. doi: 10.1021/ol0526827. [DOI] [PubMed] [Google Scholar]; (d) Jung WH, Harrison C, Shin Y, Fournier JH, Balachandran R, Raccor BS, Sikorski RP, Vogt A, Curran DP, Day BW. J. Med. Chem. 2007;50:2951–2966. doi: 10.1021/jm061385k. [DOI] [PubMed] [Google Scholar]; (e) Shin Y, Fournier J-H, Brückner A, Madiraju C, Balachandran R, Raccor BS, Edler MC, Hamel E, Sikorski RP, Vogt A, Day BW, Curran DP. Tetrahedron. 2007;63:8537–8562. doi: 10.1016/j.tet.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Raccor BS, Vogt A, Sikorski RP, Madiraju C, Balachandran R, Montgomery K, Shin Y, Fukui Y, Jung W-H, Curran DP, Day BW. Mol. Pharmacol. 2008;73:718–726. doi: 10.1124/mol.107.042598. [DOI] [PubMed] [Google Scholar]
- 13.(a) Paterson I, Gardner NM. Chem. Commun. 2007:49–51. doi: 10.1039/b615122a. [DOI] [PubMed] [Google Scholar]; (b) Paterson I, Gardner NM, Poullenneca KG, Wright AE. Bioorg. Med. Chem. Lett. 2007;17:2443–2447. doi: 10.1016/j.bmcl.2007.02.031. [DOI] [PubMed] [Google Scholar]; (c) Paterson I, Gardner NM, Guzmán E, Wright AE. Bioorg. Med. Chem. Lett. 2008;18:6268–6272. doi: 10.1016/j.bmcl.2008.09.109. [DOI] [PubMed] [Google Scholar]; (d) Paterson I, Gardner NM, Poullennec KG, Wright AE. J. Nat. Prod. 2008;71:364–369. doi: 10.1021/np070547s. [DOI] [PubMed] [Google Scholar]; (e) Paterson I, Naylor GJ, Wright AE. Chem. Commun. 2008:4628–4630. doi: 10.1039/b811575c. [DOI] [PubMed] [Google Scholar]; (f) Paterson I, Gardner NM, Guzmán E, Wright AE. Bioorg. Med. Chem. 2009;17:2282–2289. doi: 10.1016/j.bmc.2008.10.084. [DOI] [PubMed] [Google Scholar]; (g) Paterson I, Gardner NM, Naylor GJ. Pure Appl. Chem. 2009;81:169–180. [Google Scholar]; (h) Paterson I, Naylor GJ, Fujita T, Guzmán E, Wright AE. Chem. Commun. 2010:261–263. doi: 10.1039/b921237j. [DOI] [PubMed] [Google Scholar]
- 14.Canales A, Matesanz R, Gardner NM, Andreu JM, Paterson I, Díaz JF, Jiménez-Barbero J. Chem. Eur. J. 2008;14:7557–7569. doi: 10.1002/chem.200800039. [DOI] [PubMed] [Google Scholar]
- 15.Eiseman JL, Bai L, Jung W-H, Moura-Letts G, Day BW, Curran DP. J. Med. Chem. 2008;51:6650–6653. doi: 10.1021/jm800979v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan Y, Schreiber EM, Day BW. J. Nat. Prod. 2009;72:1748–1754. doi: 10.1021/np900245k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Choy N, Shin Y, Nguyen PQ, Curran DP, Balachandran R, Madiraju C, Day BW. J. Med. Chem. 2003;46:2846–2864. doi: 10.1021/jm0204136. [DOI] [PubMed] [Google Scholar]; (b) Minguez JM, Kim S-Y, Giuliano KA, Balachandran R, Madiraju C, Day BW, Curran DP. Bioorg. Med. Chem. 2003;11:3335–3357. doi: 10.1016/s0968-0896(03)00186-x. [DOI] [PubMed] [Google Scholar]; (c) Smith AB, III, Xian M. Org. Lett. 2005;7:5229–5232. doi: 10.1021/ol052070m. [DOI] [PubMed] [Google Scholar]
- 18.Paterson I, Yeung K-P, Sunil JB. Synlett. 1993:774–776. [Google Scholar]
- 19.Curran DP, Furukawa T. Org. Lett. 2002;4:2233–2235. doi: 10.1021/ol026084t. [DOI] [PubMed] [Google Scholar]
- 20.Roush WR, Palkowitz AD, Palmer MJ. J. Org. Chem. 1987;52:316–318. [Google Scholar]
- 21.Chatterjee AK, Choi T-L, Sanders DP, Grubbs RH. J. Am. Chem. Soc. 2003;125:11360–11370. doi: 10.1021/ja0214882. [DOI] [PubMed] [Google Scholar]
- 22. Mahoney WS, Brestensky DM, Stryker JM. J. Am. Chem. Soc. 1988;110:291–293.. (b) The alkene was also reduced under nickel boride conditions (NiCl2, NaBH4) but the target product was mixed with a by-product tentatively assigned as the C-20 epimer. These were not separable by flash chromatography, even in subsequent steps.
- 23.White JD, Jensen MS. J. Am. Chem. Soc. 1995;117:6224–6233. [Google Scholar]
- 24.Paterson I, Tudge M. Tetrahedron. 2003;59:6833–6850. [Google Scholar]
- 25.Marshall JA, Yanik MM, Adams ND, Ellis KC, Chobanian HR. Org. Synth. 2005;81:157–170. [Google Scholar]
- 26.(a) Myers AG, Zheng B, Movassaghi M. J. Org. Chem. 1997;62:7507. doi: 10.1021/jo9710137. [DOI] [PubMed] [Google Scholar]; (b) Fuwa H, Goto T, Sasaki M. Org. Lett. 2008;10:2211–2214. doi: 10.1021/ol800642t. [DOI] [PubMed] [Google Scholar]
- 27.Denmark SE, Regens CS, Kobayashi T. J. Am. Chem. Soc. 2007;129:2774–2776. doi: 10.1021/ja070071z. [DOI] [PubMed] [Google Scholar]
- 28.(a) Shiina I, Ibuka R, Kubota M. Chem. Lett. 2002:286–287. [Google Scholar]; (b) Shiina I, Kubota M, Ibuka R. Tetrahedron Lett. 2002;43:7535–7359. [Google Scholar]; (c) Shiina I, Oshiumi H, Hashizume M, Yamai YS, Ibuka R. Tetrahedron Lett. 2004;45:543–547. [Google Scholar]; (d) Shiina I, Kubota M, Oshiumi H, Hashizume M. J. Org. Chem. 2004;69:1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- 29.Jung W-H. PhD Thesis. University of Pittsburgh; 2007. This document can be downloaded at: http://etd.library.pitt.edu/ETD/available/etd-03252008-125826/ [Google Scholar]
- 30.Fürstner A. Angew. Chem., Int. Ed. Eng. 2000;39:3012–3043. [PubMed] [Google Scholar]
- 31.Funk TW, Efskind J, Grubbs RH. Org. Lett. 2005;7:187–190. doi: 10.1021/ol047929z. [DOI] [PubMed] [Google Scholar]
- 32.Roush WR, Blizzard TA. J. Org. Chem. 1984;49:1772–1783. [Google Scholar]
- 33.Devos A, Remion J, Frisque-Hesbain AM, Colens A, Ghosez L. J. Chem. Soc. Chem. Commun. 1979:1180–1181. [Google Scholar]
- 34.Hoye TR, Jeffrey CS, Tennakoon MA, Wang J, Zhao H. J. Am. Chem. Soc. 2004;126:10210–10211. doi: 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]
- 35.The sample of 39 isolated from this reaction still contained some impurities, but the structure assignment of 39 as the main component was clear from 1H NMR spectroscopy and MS analysis (ESI m/z 548, [M + Na]+).
- 36.Fürstner A, Langemann K. J. Am. Chem. Soc. 1997;119:9130–9136. [Google Scholar]
- 37.(a) Evans DA, Chapman KT, Carreira EM. J. Am. Chem. Soc. 1988;110:3560–3578. [Google Scholar]; (b) Saksena AK, Magiaracina P. Tetrahedron Lett. 1983;24:273–276. [Google Scholar]
- 38.For the synthesis of the corresponding acid, see Smith AB, III, Safonov IG, Corbett RM. J. Am. Chem. Soc. 2002;124:11102–11113. doi: 10.1021/ja020635t.
- 39.(a) Okude Y, Hirano S, Hiyama T, Nozaki H. J. Am. Chem. Soc. 1977;99:3179–3181. [Google Scholar]; (b) Takai K, Tagashira M, Kuroda T, Oshima K, Utimoto K, Nozaki H. J. Am. Chem. Soc. 1986;108:6048–6050. doi: 10.1021/ja00279a068. [DOI] [PubMed] [Google Scholar]; (c) Fürstner A. Chem. Rev. 1999;99:991–1046. doi: 10.1021/cr9703360. [DOI] [PubMed] [Google Scholar]
- 40.For recent examples of intramolecular Nozaki-Hiyama-Kishi reactions, see: Takao K-I, Hayakawa N, Yamada R, Yamaguchi T, Morita U, Kawasaki S, Tadano K-I. Angew. Chem. Int. Ed. 2008;47:3426–3429. doi: 10.1002/anie.200800253. Marshall JA, Eidam PM. Org. Lett. 2008;10:93–96. doi: 10.1021/ol702024b. Pelphrey PM, Bolstad DB, Wright DL. Synlett. 2007;17:2647–2650. Matsuda M, Yamazaki T, Fuhshuku K-I, Sugai T. Tetrahedron. 2007;63:8752–8760. Venkatraman L, Salomon CE, Sherman DH, Fecik RA. J. Org. Chem. 2006;71:9853–9856. doi: 10.1021/jo062047u. Suzuki K, Takayama H. Org Lett. 2006;8:4605–4608. doi: 10.1021/ol061908i. Bian J, Van Wingerden M, Ready JM. J. Am. Chem. Soc. 2006;128:7428–7429. doi: 10.1021/ja061559n. Namba K, Kishi Y. J. Am. Chem. Soc. 2005;127:15382–15383. doi: 10.1021/ja055966v. Araki K, Saito K, Arimoto H, Uemura D. Angew. Chem. Int. Ed. 2004;43:81–84. doi: 10.1002/anie.200351750. Njardarson JT, Biswas K, Danishefsky SJ. Chem. Commun. 2002;23:2759–2761. doi: 10.1039/b209941a. Pilli RA, Victor MM. Tetrahedron Lett. 1998;39:4421–4424.
- 41.(a) Harried SS, Yang G, Strawn MA, Myles DC. J. Org. Chem. 1997;62:6098–6099. [Google Scholar]; (b) Harried SS, Lee CP, Yang G, Lee TIH, Myles DC. J. Org. Chem. 2003;68:6646–6660. doi: 10.1021/jo034521r. [DOI] [PubMed] [Google Scholar]
- 42.Moura-Letts G. Ph.D. Thesis. University of Pittsburgh; 2007. This document can be downloaded at: http://etd.library.pitt.edu/ETD/available/etd-11162007-001904/ [Google Scholar]
- 43.Stork G, Zhao K. Tetrahedron Lett. 1989;30:2173–2174. [Google Scholar]
- 44.The synthesis of acid 64 is described in the Supporting Information of reference 12d
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.