Abstract
Adult-onset stuttering (AS) typically occurs following neurological and/or psychological trauma, considered different from developmental stuttering (DS), which starts during early childhood with few if any new cases reported after adolescence. Here we report four cases of AS, two with apparent psychological trigger and two without, none with evidence of neurological injury, and none conforming to previously reported characteristics of psychogenic stuttering. We asked whether this group of AS would have similar speech and neuroanatomical characteristics to those with DS. We conducted blinded analyses of speech samples in both AS cases and 14 cases of DS on type, frequency, and loci of disfluencies. Diffusion tensor imaging (DTI) was conducted to compare white matter tracts using fractional anisotropy (FA). We found that AS did not differ significantly from DS in any of the speech characteristics measured. On DTI, DS had significantly increased FA relative to controls in the right superior longitudinal tract. AS cases showed a similar trend for increases in these regions when compared to controls. The results of this study suggest that symptoms of idiopathic stuttering can begin during adulthood, and that similar neuroanatomical differences from controls may be associated with both developmental and adult onset idiopathic stuttering.
Keywords: Adult-onset stuttering, developmental stuttering, DTI, white matter, speech
1. Introduction
Adult-onset stuttering usually occurs following a neurological insult such as missile wounds (Ludlow, Rosenberg, Salazar, Grafman, & Smutok, 1987), stroke (Ardila & Lopez, 1986; Ciabarra, Elkind, Roberts, & Marshall, 2000; Doi et al., 2003; Fawcett, 2005; Fleet & Heilman, 1985; Grant, Biousse, Cook, & Newman, 1999; Hamano et al., 2005; Mouradian, Paslawski, & Shuaib, 2000; Sahin, Krespi, Yilmaz, & Coban, 2005; Turgut, Utku, & Balci, 2002), or neurodegenerative diseases (Lim, Wilder-Smith, Ong, & Seet, 2005; K. Sakai, Furui, Komai, Notoya, & Yamada, 2002; T. Sakai, Miyamura, & Kuzuhara, 1992). In a small number of cases, however, psychogenic stuttering has been identified when the onset of stuttering could be traced to emotional/psychological trauma. This type of stuttering has been considered distinct from both adult onset neurogenic stuttering following brain lesions/disorders, and developmental stuttering, the typical form of stuttering that starts during early in childhood that can continue throughout adulthood.
Several case studies of psychogenic stuttering have been reported. Symptom characteristics include indifference to stuttering (Deal, 1982; Mahr & Leith, 1992), lack of adaptation (Baumgartner & Duffy, 1997; Freund, 1966), rapid recovery with speech therapy (Baumgartner & Duffy, 1997; Duffy, 1989; Dworkin, Culatta, Abkarian, & Meleca, 2002; Roth, Aronson, & Davis, 1989), and no effect of fluency inducing conditions on stuttering (Deal, 1982; Dworkin et al., 2002). Some reports, however, include characteristics similar to developmental stuttering (DS) such as struggle behaviors associated with moments of stuttering (Baumgartner & Duffy, 1997; Duffy, 1989), while some do not (Attanasio, 1987; Deal, 1982; Mahr & Leith, 1992; Weiner, 1981). Further, similar to DS, some researchers have reported a speech pattern that is worse in certain situations or speech tasks (Attanasio, 1987; Baumgartner & Duffy, 1997; Deal & Doro, 1987; Duffy, 1989; Weiner, 1981), whereas others have reported little variations in different settings with no “islands of fluency” (Deal, 1982; Freund, 1966; Mahr & Leith, 1992). (Table 1)
Table 1.
Previously reported cases of psychogenic stuttering.
| Author | Year | # of Cases | Psychological Trigger | Methods Used | Duration/Therapy/Recovery | Behavioral (Speech) Characteristics |
|---|---|---|---|---|---|---|
| Wallen | 1961 | 1 age: 28 male (M) |
“Overworked” for several months. | Speech-language Pathology (SLP) and psychology consults. | Recovered to fluent speech spontaneously after three weeks. | Initial syllable repetitions, vowel prolongations. No evidence of anxiety when stuttering. |
| Weiner | 1981 | 1 age: 36 onset:33 M |
Forced to take over partner’s role in company. | Neurology, psychiatry, behavior therapy, and SLP consults. | Improved from 50% to 2–5% stuttering at work over 6 month period of speech therapy. | “Mild” on Stuttering Severity Instrument. Sound and syllable repetitions, fleeting prolongations. No stuttering while reading. No struggle behaviors. Worse in certain situations or with certain people. |
| Deal | 1982 | 1 age: 28 M |
Attempted suicide (not specified). | Psychiatric evaluation, SLP referral. | Recovery: 2 months psychiatric group therapy, and speech therapy using delayed auditory feedback (DAF). | Sound and syllable repetitions, prolongations of single syllable words. No struggle behaviors; no concern about stuttering; no islands of fluency. No improvement with DAF (initially), white noise, choral reading, singing, miming. |
| Attanasio | 1987 | 1 age: 36 onset: 29 M |
Marriage difficulty and divorce. | SLP consult. | Improved with speech therapy but relapsed following therapy. | Multiple sound and syllable repetitions, blocks. Episodic in nature. Few “accessory features.” |
| Roth, et al. | 1989 | 12 ages: 21–79 6 M, 6 female (F) |
10 of 12 patients: environmental stress or interpersonal conflict. | Neurology consult, SLP consult, Minnesota Multiphasic Personality Inventory or psychiatric evaluation. | Recovery: speech therapy, psychotherapy or spontaneously Duration between 4hr to 1.5yr. | Repetitions, blocking, prolongations. 12 with somatic complaints, 6 with head movements. |
| Duffy | 1989 | 1 age: 50 M |
Stress over whether or not to quit job. | Neurology, SLP, psychiatry exams. | Intermittent over 18 months, periods of disfluency and remittance. Dramatic improvement after 2 minutes of symptomatic therapy; complete recovery after second session. | Sound/syllable prolongations, repetitions and hesitations. Easily observable secondary movements including neck hyper-extension, lip pursing, etc. Frequency of disfluencies fluctuated. Felt he could anticipate the onset of the next recurrence 2 days ahead. |
| Mahr & Leith | 1992 | 4 ages: 32–44 1 M, 3 F |
Physical trauma, traumatic relationship, or childhood abuse. | Neurology consult for 1 patient, psychological or psychiatric evaluation for 3 patients, neuro- psychological testing for 1 patient, SLP referral for 3 patients. | 3 recovered due to psychotherapy or speech therapy. | Stereotypical repetitions, prolongations, blocking. Head/body movements in 2; none in 1; unknown in 1. |
| Baumgartner & Duffy | 1997 | 49 20 with neurologic disease (ND) and 49 without ND ages: 19–79 35 M, 34 F |
Mixed – some with psychiatric history, some without, some unknown. | Neurology consult, SLP consult, half had psychiatric evaluation. | 32 treated by SLP: 70% recovered within 2 sessions. 2 recovered spontaneously. | Sound and syllable repetitions, prolongations, blocking. Many with struggle behavior, most with other somatic complaints. |
| Dworkin, et al. | 2002 | 1 age: 39 M |
Motor vehicle accident/whiplash. | Neurology, psychiatry and psychology consults. | Immediate improvement following topical laryngeal anesthetic and 15 minutes of behavioral speech therapy. Normal speech by 5 months post-treatment. | Multiple repetitions and brief blocks. No improvement in song, memorized passages or whispering. No secondary characteristics. |
Most case studies of psychogenic stuttering have reported that stuttering in this group involves sound/syllable repetitions on initial syllables, prolongations, and blocks. None, however, have systematically compared the speech characteristics of adult-onset stuttering (without neurogenic deficits) with developmental stuttering in terms of stuttering severity, disfluency type, and stuttering loci in relation to word type (content versus function), and place within syntactical structure. We hypothesized that adults with idiopathic adult-onset stuttering without neurological insults would have unique speech characteristics compared to individuals with developmental stuttering.
Recently it has been shown that children and adults with persistent developmental stuttering have decreased fractional anisotropy (FA; a measure derived from diffusion tensor imaging that reflects white matter organization) in the superior longitudinal/arcuate fasciculus, a major white matter tract that interconnects frontal and temporoparietal regions in the left hemisphere. We wanted to consider three alternatives with regard to neuroanatomical differences in adult onset stuttering relative to controls. First, if adult-onset stuttering is distinct from developmental stuttering, groups may have differences in neuroanatomy. Second, if decreased white matter organization previously found in developmental stutterers (Chang, Erickson, Ambrose, Hasegawa-Johnson, & Ludlow, 2008; Sommer, Koch, Paulus, Weiller, & Buchel, 2002) underlies stuttering behavior in general, such characteristics may also be present in both adult-onset and developmental stutterers. Alternatively, because both groups include adults who have stuttered for some time, if neuroanatomical differences from controls are secondary to attempts to overcome disfluency, similar neuroanatomy may be found in both groups of stuttering individuals.
Here we report on four cases of long-standing adult-onset idiopathic stuttering not associated with either neurological or other physical injuries. One reported stuttering onset unrelated to psychological/emotional trauma and denied any family or personal history of stuttering. Two cases reported psychological/emotional trauma associated with onset of stuttering, without physical insult. The final case had a family history of stuttering without a personal history of developmental stuttering. In all of the cases, stuttering onset was at 17 years or older, and no history of childhood stuttering. In each of these cases of late onset, stuttering was relatively long-standing and none had remitted with treatment.
We asked if these stuttering individuals would exhibit different stuttering characteristics, physical concomitants, loci of disfluencies, and brain structure (white matter), from persons with persistent developmental stuttering. Our hypothesis was that if there is a common brain basis for both developmental and adult-onset idiopathic stuttering, these adult-onset cases would show similar white matter differences from controls as have been reported in developmental stuttering (Chang et al., 2008; Sommer et al., 2002), and similar speech characteristics.
2. Methods
2.1. Participants
14 (6 females) subjects with developmental stuttering (DS), 14 controls (7 females), and 4 subjects (2 females) with adult-onset stuttering (AS) participated in this study. The first two groups were recruited as part of a larger study examining functional and structural connectivity. The DS participants exhibited typical history and speech disfluencies that are characteristic of developmental stuttering. Ten of the 14 DS participants had a positive family history of stuttering. All subjects were strongly right handed on the Edinburgh handedness inventory (Oldfield, 1971), native North American English speakers, and were within 2 standard deviations of the age-adjusted mean on speech, language, and cognitive testing. Stuttering severity in both stuttering groups was assessed using the Stuttering Severity Instrument (SSI-3) (Riley, 1972), while they engaged in conversation, monologue, and reading tasks in front of a small audience of strangers. All subjects were required to be free of neurological or medical disorders, passed audiometric screening, and had normal structural MRI scans as confirmed by a radiologist. The AS group participants were additionally seen by a neuropsychologist for a comprehensive assessment of motor, cognitive, language, and visuospatial skills. All subjects signed an informed consent form approved by the Internal Review Board of the National Institutes of Neurological Disorders and Stroke. All were paid for their participation.
2.2 Case histories of adult-onset stuttering participants
2.2.1
Case 1, a 51 year old male, reported stuttering onset at age 17 unrelated to any obvious emotional/physical trauma. He denied prior developmental stuttering as a child or a history of stuttering in the family. He noted that he had always spoken at a rapid rate, and considered this may have contributed to developing stuttering. He reported receiving speech therapy for approximately two and a half years in his early twenties, with some benefit. He has experienced an overall improvement in his speech over the past 15 years, due to improved mental and physical well-being and an accepting attitude toward his stuttering. He is an active member of stuttering support groups.
2.2.2
Case 2, a 30 year-old female, reported stuttering onset at 26, following a painful breakup with an abusive boyfriend. She later developed depression and anxiety, and was prescribed Celexa for up to one year. She was not taking any medications at the time of testing. She described her stuttering onset as “gradual”, with stuttering occurring initially on one to two words only, and then spreading to more words. She noted that her stuttering gradually worsened over several months and then stabilized. She noted that her symptoms were first syllable repetitions. Exacerbating conditions include talking on the phone, and speaking to colleagues at work. She reported she stuttered less with her family and did not stutter during singing or whispering. She had no significant medical history, denied any personal history of developmental stuttering as a child, or a family history of stuttering. She received speech therapy from two different speech therapists for short periods of time, with little benefit.
2.2.3
Case 3, a 43 year-old male, a part-time book keeper, was a University student counselor for developmental testing when his stuttering started. At age 38, he began feeling dizzy and started to stutter while interviewing a student. The stuttering lasted for a few days and subsided, but then came back some time later. He subsequently quit his job due to his communication difficulty. He reported that the stuttering onset was preceded by a painful divorce that took place one year before. Following the divorce, he was diagnosed with anxiety disorder, depression, and acid reflux. He was prescribed Paxil, but finding that this made his stuttering worse, he stopped taking it after two months. He was not on any medication during the testing, and visibly emotional while talking about the time of his divorce five years earlier. The situations that exacerbated his stuttering were stress and speaking in front of authority figures. He denied any personal history of developmental stuttering or receiving conventional speech therapy to treat his stuttering, although he had tried SpeechEasy, a delayed auditory feedback device, without any benefit and discontinued use after one day. He reported that an aunt on his mother’s side had a “trembling voice”, and his mother’s paternal uncle had a noticeable voice disorder during conversation. He also reported that his paternal grandmother had a tic disorder involving the right arm and facial muscles.
2.2.4
Case 4 is a 36 year-old female who started stuttering at 17, without a history of childhood stuttering. She reported that her father had stuttered (onset uncertain) and still has a mild stutter. Of her three sons, the eldest one stuttered transiently for six months at age three. No other relatives were known to stutter. The onset of her stuttering was not associated with any apparent emotional/physical trauma, although she did note that the severity of stuttering seemed to increase with each childbirth. She denied having any post-partum depression associated with her pregnancies. She was more disfluent when speaking with family and friends, and her husband in particular. She sought speech therapy, but was not able to find a therapist that would treat adults who stutter.
2.3 Procedure
2.3.1. Speech-Language-Hearing evaluation
All subjects underwent testing with a battery of standardized speech, language, and cognitive tests, audiometric hearing screening, oral-motor screening, and cognitive evaluations. The tests included the Peabody Picture Vocabulary Test (PPVT-3) (Dunn, 1959), Expressive vocabulary test (EVT-3) (Williams, 1999), Test of Nonverbal Intelligence (TONI-3) (Brown, Sherbenou, & Johnsen, 1997), Revised token test (McNeil & Prescott, 1978), Wechsler digit span test (Wechsler, 1997), Goldman-Fristoe Test of articulation (Goldman & Fristoe, 2006), Kahn-Lewis Phonological Analysis (Kahn & Lewis, 1986), and Test of Auditory Comprehension of Language (TACL) (Carrow-Woolfolk, 1999).
2.3.2. Speech sample
A 20–30 minute speech sample was audio and videotaped while the subject engaged in monologue, conversation, and reading all in front of three to six strangers. The speech severity was assessed with SSI-3 (Riley, 1972), and a sample of approximately 200 words of conversational speech was transcribed off-line by a speech-language pathology student with an undergraduate degree in linguistics(AS). Speech samples were transcribed for a total of 14 DS, 4 AS, and 4 controls. Numbers of stuttering-like disfluencies (part-word repetitions, whole-word repetitions, dysrhythmic phonations, blocks), normal disfluencies (interjections, revisions, phrase repetitions, abandoned phrases), and their loci of occurrence were analyzed in relation to semantic (content versus function words) and syntactic (noun, verb phrase, initial, and within phrase locations, and prepositional phrase, initial) loci. Intra-rater reliability, and inter-rater reliability with an experienced speech-pathologist specializing in stuttering (SC) was assessed for all speech measures. To measure reliability, intra-class correlations (ICC) between intra-judgments and between judges (AS, SC) were calculated separately for each of the speech measure categories (stuttering-like disfluencies, normal disfluencies, word loci, sentence loci). The ICC coefficients were derived using the following equation: r= (Between group mean squares−Within group mean squares) / Between group mean squares + (n-1)*Within group squares), where n was the number of samples taken to compare within and between judges. In this case, we took 7 random speech samples to measure intra- and inter-rater reliability, hence n equaled 7.
2.3.3. Neuropsychology testing
All participants underwent neuropsychology testing with a licensed neuropsychologist. The following tests were administered to test the neurocognitive functioning of attention (Neuropsychological Assessment Battery (NAB): Attention module), processing speed (Symbol Digit Modalities Test, Trail Making Test), motor speed (Grooved Pegboard Test), memory (Neuropsychological Assessment Battery (NAB): Memory module, Symbol Digit Modalities Test, Trail Making Test), language (Boston Naming Test, Controlled Oral Word Association Test), visuospatial skills (Judgment of Line Orientation Drawings), executive function (Frontal Systems Behavioral Inventory (FrSBe)), intelligence (Wechsler Abbreviated Scale of Intelligence (WASI)). The neuropsychological evaluation typically took 2–3 hours, which took place on a separate day from other testing.
2.3.4. MRI procedure
All participants were scanned on a 3.0 Tesla GE Signa scanner equipped with an eight-channel receive-only coil (General Electric Medical System, Milwaukee, WI, USA). Diffusion tensor imaging (DTI) scans were acquired with whole-brain coverage using a single-shot spin-echo echo-planar imaging sequence with paired gradient pulses. Imaging parameters for the diffusion-weighted sequence were: TE/TR=73.4/13,000, FOV=2.4 × 2.4 cm2; matrix=96 × 96 mm2 zero-filled to 256 × 256 mm2; 51 contiguous slices with slice thickness of 2.6 mm. Diffusion was measured along 33 non-collinear directions with a b factor of 1000s/mm2. Three reference images were acquired with no diffusion gradients applied (b0 scans). Three anatomical scans were additionally acquired in all participants and were sent to a staff radiologist for clinical evaluation, to rule out gross abnormalities.
2.3.5. MRI data analysis
Diffusion Tensor Imaging (DTI) allows quantification of random movement of water molecules in the brain. Since water molecules are bound by fatty structures such as myelin and axonal membranes, tracking the directionality and magnitude of its movement provides measures of axonal organization. Fractional anisotropy (FA) quantifies the degree of water diffusion in a preferential direction. An FA measure of 1 represents perfect anisotropic diffusion, while an FA measure of 0 represents perfect isotropic diffusion. Faster and more efficient information transfer achieved through white matter growth and myelination may be critical in regions such as the left arcuate fasciculus, the major white matter bundle interconnecting the frontal-temporal regions. White matter density in the left arcuate fasciculus and age are positively correlated (Paus et al., 2001), and FA (Buchel et al., 2004; Parker et al., 2005) as well as fiber density (Nucifora, Verma, Melhem, Gur, & Gur, 2005; Vernooij et al., 2007) is highly asymmetric and greater on the left in adults. DTI has been used to examine stuttering in children (Chang et al., 2008), adolescents (Watkins et al., 2008), and adults (Sommer et al., 2002), which revealed decreased FA in similar regions in the left arcuate fasciculus in the stuttering participants. Here we examined FA in the right and left arcuate fasciculus in developmental, adult-onset stuttering, and controls for group differences in FA.
For each subject, we calculated FA images using FMRIB’s Diffusion Toolbox (FDT), part of the FMRIB Software Library (FSL, www.fmrib.ox.ac.uk/fsl). We then used tract-based spatial statistics (TBSS) (S. M. Smith et al., 2006) to nonlinearly align individual FA maps onto a common registration target in standard space. The method involves non-linear registration of all subjects’ FA data onto a common registration target, and creating a mean white matter “skeleton” that is comprised of tracts common to all subjects. TBSS projects all subjects’ FA data onto this mean skeleton before applying voxel-wise across-subject statistics, resulting in robust and sensitive analysis of multiple subject diffusion imaging data (for detailed steps, see Smith et al., 2006).
Due to the small sample size in the AS group, we did not conduct voxel-wise whole brain analyses for group comparisons, but conducted a-priori defined ROI analyses focused on the left and right superior longitudinal fasciculi underlying the rolandic operculi and inferior frontal regions, which were placed on each subject’s nonlinearly aligned FA maps. The choice of these regions were based on previous studies that have shown FA decreases in the left superior longitudinal fasciculus near the rolandic operculum (RO) in adults (Sommer et al., 2002) and children (Chang et al., 2008) and in a more frontal region in adolescents/young adults (Watkins, Smith, Davis, & Howell, 2008). A 5×5×5 cube was placed in the left (center voxel coordinate: −42, −9, 25) and right (center voxel coordinate: 40, −9, 29) superior longitudinal fasciculi underlying bilateral rolandic opercular regions, and in the frontal regions lying on the same tract (left center coordinate: −37, 24, 16; right center coordinate: 36, 24, 21) in each subject’s non-linearly aligned FA images. Mean FA was calculated across all voxels within these ROIs in each subject, and the effects of group (DS, AS, Control) and ROI (left RO, Right RO, left Frontal, Right Frontal) on FA was examined using box plot distributions. The mean FA from each subject from all ROIs were also plotted against SSI scores within the stuttering groups to examine the spread of values in the two groups across stuttering severity levels.
3. Results
Speech testing
All subjects tested within 1 SD of the norm on the standardized language tests. Case 2 exhibited a wide discrepancy in her performance between her expressive (14th percentile) and receptive (84th) vocabulary scores (Table 3). All passed hearing screening, and oral-motor function as judged to be normal in all subjects. The SSI scores ranged from 16 (very mild) to 22 (moderate) in AS participants (mean: 18.25, SD: 2.87) and from 13 (very mild) to 39 (very severe) (mean: 23.36 SD: 6.46) in our DS participants (Figure 1).
Table 3.
Speech test results of the adult-onset stuttering group
| Case number | SSIa Total | Edinburgh score | PPVTb %ile | EVTc %ile | TONId- 2 %ile | RTTe %ile | WISC DSf | GFTAg | Kahn-Lewis | TACLh |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 19 | 100 | 23rd | 27th | 53rd | 52nd | 15 | 100% | 100% | 25th |
| 2 | 16 | 80 | 84th | 14th | 99th | 79th | 12 | 100% | 100% | 84th |
| 3 | 22 | 90 | 21st | 25th | 79th | 66th | 11 | 100% | 100% | 91st |
| 4 | 16 | 79 | 55th | 58th | 37th | 10th | 16 | 100% | 100% | 75th |
Stuttering Severity Instrument
Peabody Picture Vocabulary Test
Expressive Vocabulary Test
Test of Nonverbal Intelligence
Revised Token Test
Wechsler Digit Span test
Goldman-Fristoe Test of Articulation
Test of Auditory Comprehension of Language
Figure 1.
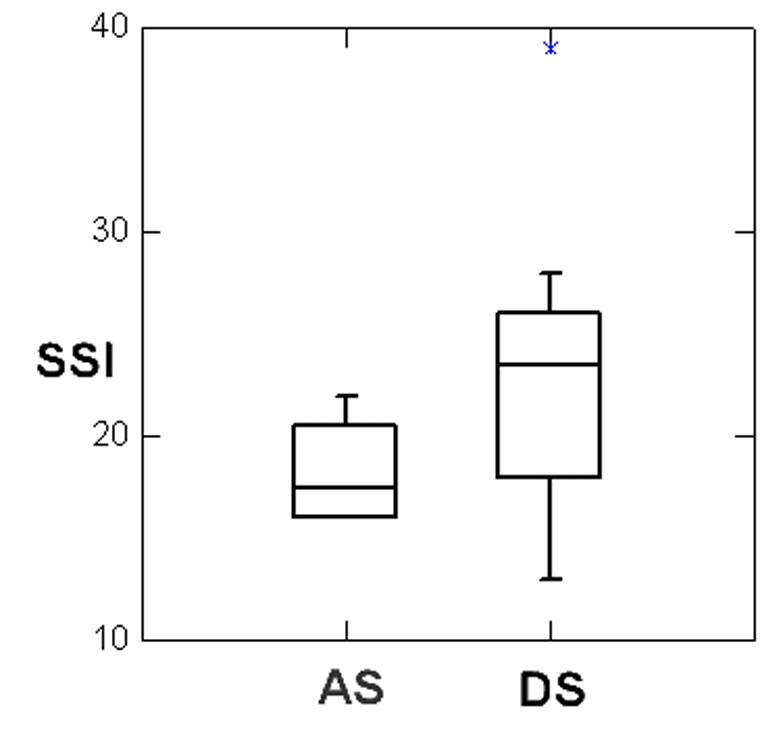
Boxplot comparing the Stuttering Severity Index (SSI) ratings of Adult-onset stuttering (AS) and Developmental stuttering (DS) groups.
3.2. Speech analysis
The intra-rater reliability assessed through intra-class correlation (ICC) between two time points for judge AC was as follows: for frequency of stuttering-like disfluency (SLD) types, 0.89, for frequency of normal disfluencies (ND), 0.96, for judgment of semantic loci of stuttering, 0.97, and for judgment of syntactic loci of stuttering, 0.91. The inter-rater reliability calculated with ICC for each of these categories was: 0.92, 0.97, 0.92, and 0.74, respectively. Pie charts demonstrate similar distributions between the AS and DS groups in dysfluency type, frequency, and loci of disfluencies (Figure 2 and Figure3).
Figure 2.
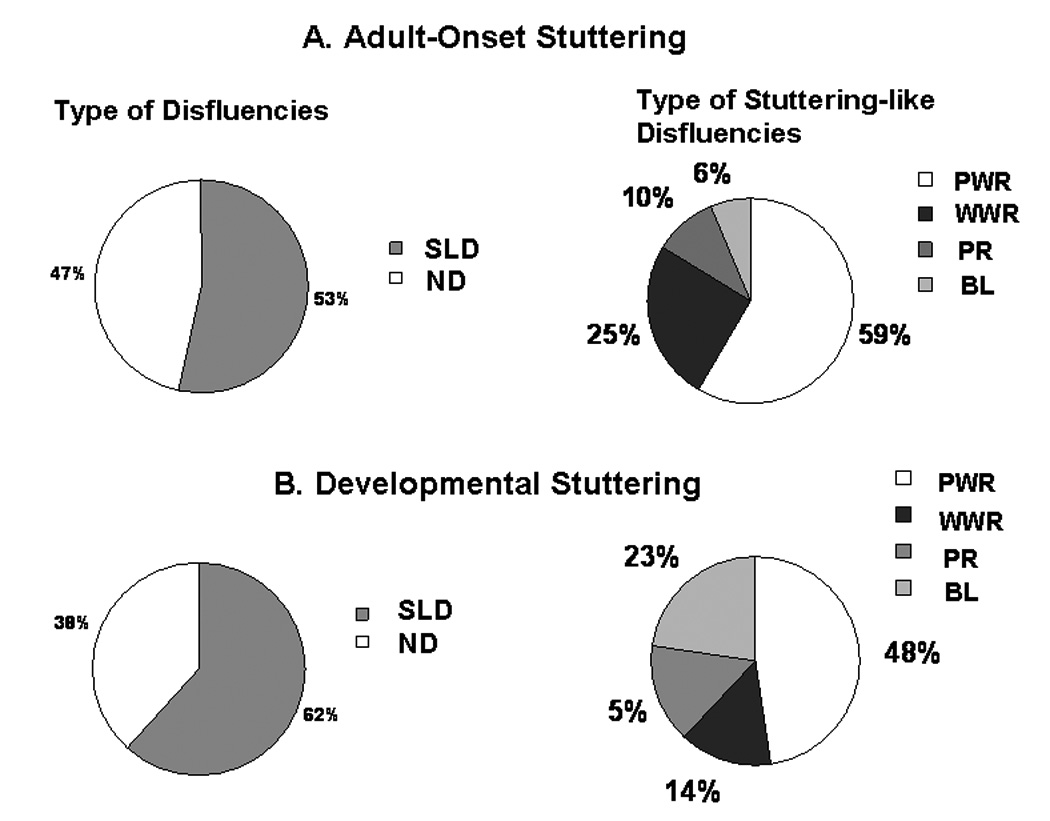
Relative frequencies of type of disfluencies and frequencies within the stuttering-like disfluencies (SLD) in (1) AS and (2) DS groups. SLD: stuttering-like disfluencies; ND: normal disfluencies; PWS: part-word repetitions; WWR: whole-word repetitions; PR: prolongations; BL: blocks.
Figure 3.
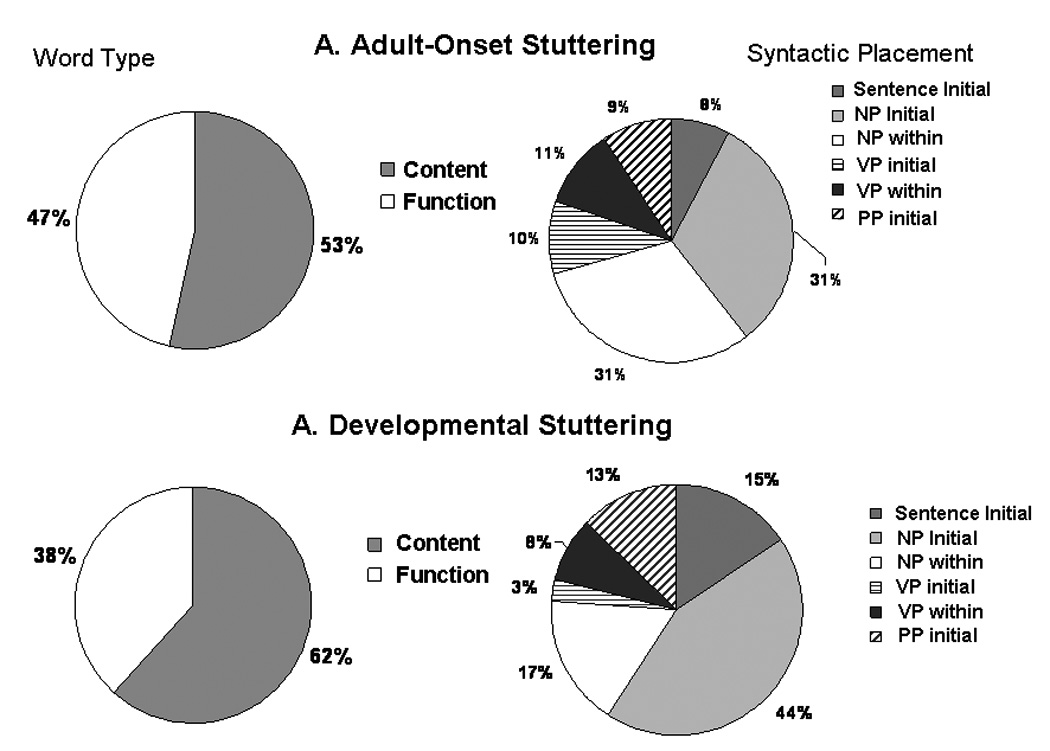
Relative frequencies of content versus function word loci and syntactic loci of disfluencies in (1) AS and (2) DS groups. NP: noun phrase; VP: verb phrase; PP: prepositional phrase.
A somewhat greater proportion of stuttering was in the initial part of the sentence or the initial noun phrase in the DS adults who had stuttered since childhood (total=59%) while AS who developed stuttering as adults had less stuttering at the beginning of the sentence and in the initial noun phrase (39%). On the other hand, less stuttering occurred in the initial verb phrase or within the verb phrase in the DS group (11%) and somewhat more in the AS group (21%) (Figure 3). The only difference in the distribution in the types of disfluencies between the two groups was the tendency for more blocks to occur in the DS group (23%), with blocks only being 6% of stuttering in the AS group. This suggests a greater struggle involved in stuttering in the DS group who had stuttered since childhood while the AS only reported stuttering a mean of 15 years (between 4 and 34 years) (Figure 2).
3.3. Neuropsychology test results
All AS subjects tested within the normal range on intelligence tests. The AS subjects’ performance were mixed on all other tests; two (cases 1 and 2) of the four scored less than 1 standard deviation (SD) below the norm or lower on tests assessing processing speed (Symbol digit manipulation, trails test), and two (cases 2 and 3) were below 1 SD from the normal mean on attention/working memory testing (digit forward, dots), with case 2 more than 2 SDs below the normal mean on digit forward testing. All four cases had low average performance on the learning/recall tests and had attenuated (low average) performance on motor speed testing. Two (cases 1 and 3) were between 2SD and 1SD below the normal mean on the Boston naming test, while cases 1 and 2 had low average performance on the phonemic generation test. The performance scores on all of these tests, with the exception of case 2’s score on the digit forward test and case 3’s score on the Boston naming test, were within 2 SD of the norm and could be considered within normal range. Hence, although many of the scores assessed with these tests were in the low average range, these were not considered clinical deficits.
3.4. ROI analyses of FA
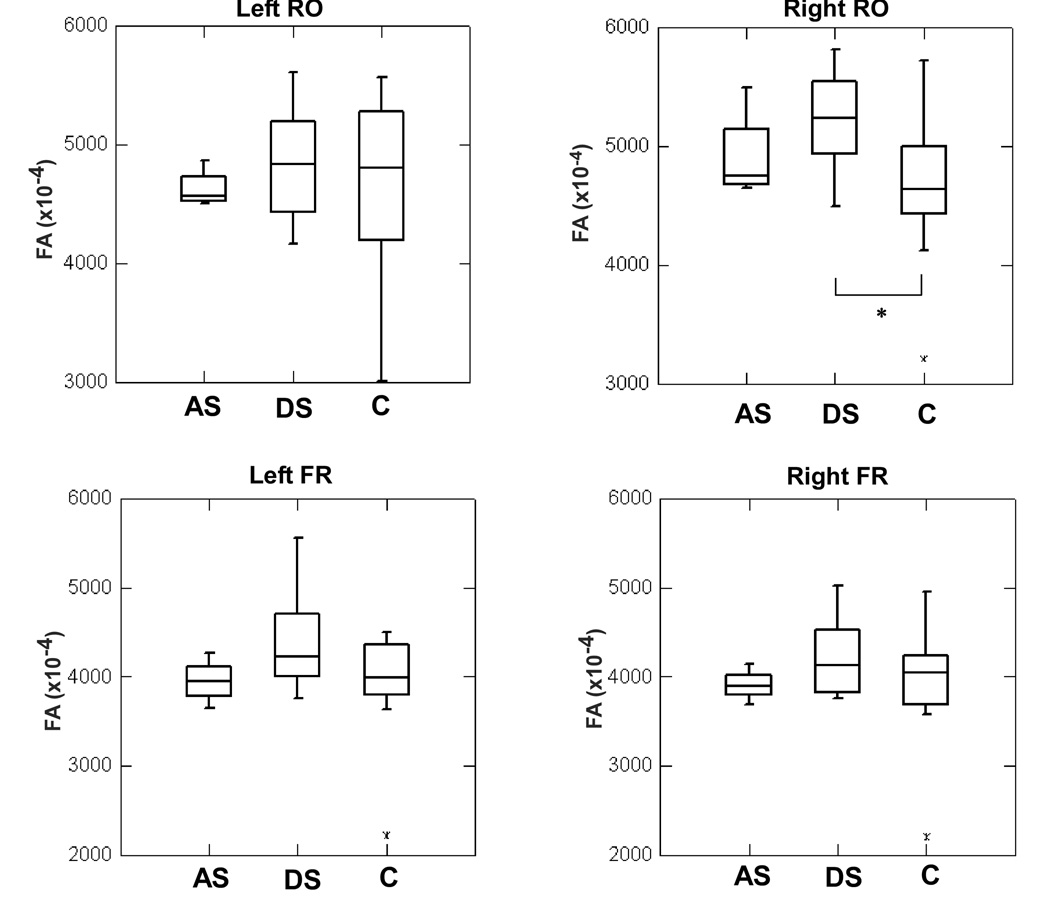
FA was examined in four ROIs placed along the bilateral arcuate fasciculus, underlying the left and right rolandic operculum (RO), and in the left and right inferior frontal regions. Differences between the DS group and the controls was significant only in the right RO (F(2,29)=3.685, p= 0.037). No significant group differences were found in the left RO (F(2,29)=0.378, p= 0.689), right inferior frontal (F(2,29)=0.854, p= 0.436) or in the left inferior frontal regions (F(2,29)=2.325, p= 0.116) (Figure 4).
Figure 4.
Box-plot graph of FA values in the right rolandic opercular (RO) region plotted against groups (AS: adult-onset stuttering, DS: developmental stuttering, C: controls). There was a significant difference between DS and controls (p < 0.05). See text for more details.
The DS group had higher FA values only in the right rolandic operculum and the AS group FA values were in the range between the FA values of the DS group and the controls. The distribution of FA values were very similar in the three groups in the left RO and the left and right inferior frontal regions (Figure 5).
Figure 5.
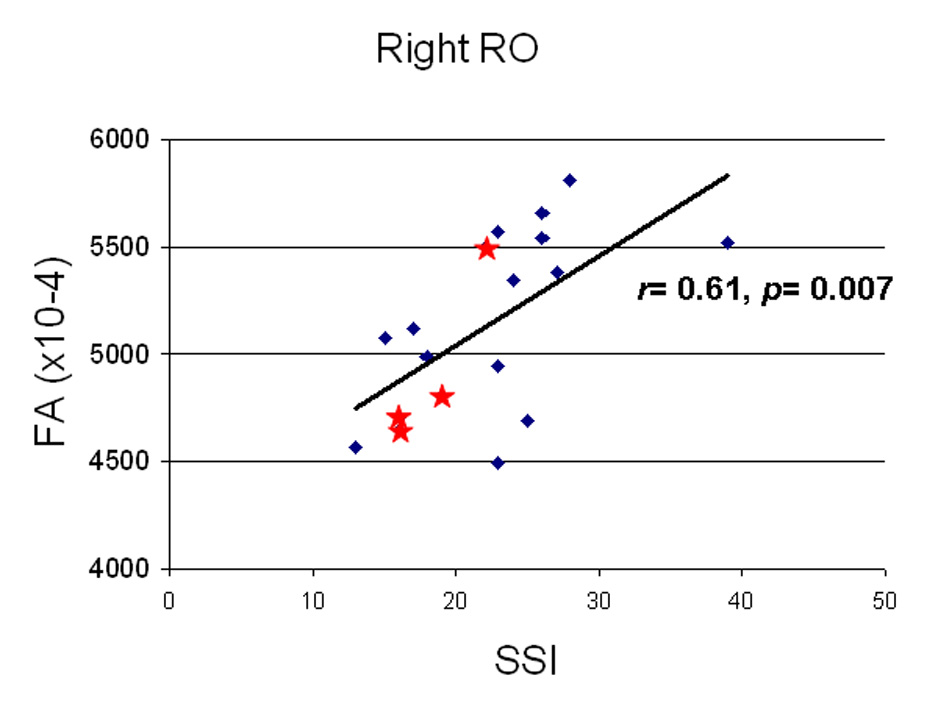
Correlation of stuttering severity measured through the stuttering severity instrument (SSI) and mean FA in the right rolandic operculum (RO) ROI. There was a significant positive correlation only in the right RO and SSI scores in stuttering individuals. The spread of values in AS appeared to be within the range of DS (Blue diamonds are DS, red stars are AS).
The range of mean FA values from each ROI were examined in relation to stuttering severity in the two stuttering groups (AS, DS). Pearson correlation coefficient was significant for Right RO (r= 0.61, p= 0.007), indicating that there was a positive correlation between stuttering severity and increases in FA in this region. The spread of values in AS were within the range of that of DS (Figure 5).
4. Discussion
The idiopathic adult-onset stuttering cases in this study were unique in that they did not conform to previous reports of psychogenic cases of stuttering: two of the four did not report emotional trauma associated with their stuttering onset. All four cases presented with stuttering behavior similar to that of individuals with developmental stuttering, in disfluency type, frequency, and loci of occurrence. They were normal on speech and language testing, exhibited normal speech motor function, were within normal limits on neuropsychology assessments, and were without clinical neurological deficits. Three of the four participants had been in speech therapy for some length of time, without elimination of their stuttering. Rapid recovery from stuttering with therapy has been the most consistently reported characteristic of psychogenic stuttering and did not occur in these cases. Hence the cases reported here seem to more closely fit the definition of developmental stuttering, although the onset of stuttering was in late adolescence or adulthood.
The onset of the vast majority of cases of stuttering are developmental often between two and four years of age (Yairi & Ambrose, 1999), with very few cases reported after 8 years. Most stuttering cases occurring after adolescence have been considered neurogenic, psychogenic, or a mixture of the two in origin. The cases reported here, however, suggest that stuttering characteristics considered typical for developmental stuttering can indeed first become evident during adulthood. Only one of our cases had a family history of developmental stuttering, although she did not stutter in childhood. Possibly these late onset cases represent persons at risk for stuttering, whose speech difficulties were not triggered until later in life.
In our white matter analysis, the most striking finding was that DS, and to a lesser extent AS, had increased FA in the right RO region relative to the control group. Others have reported white matter increases in the right hemisphere homologues to the speech areas in adults who stutter (Jancke, Hanggi, & Steinmetz, 2004), and past studies have shown that DS have relatively greater right side volume and decreased asymmetry in the planum temporale relative to controls (Foundas, Bollich, Corey, Hurley, & Heilman, 2001), increases in cortical folding in the right perisylvian region (Cykowski et al., 2008), and right cerebral over-activity during speech-language tasks (De Nil, Kroll, Kapur, & Houle, 2000; Fox et al., 1996; Neumann et al., 2003).
Increased FA on the right in the DS group may reflect compensatory neuroplasticity in response to impairments in the left hemisphere. Decreased white matter integrity in the left hemisphere has been suggested as a possible basis for developing stuttering (Chang et al., 2008; Sommer et al., 2002). Given this, the right homologues may have increased in white matter as a result of compensation for this deficit in the left regions in stuttering individuals.
Between the two stuttering groups, FA tended to be greater in DS compared to AS in the right RO regions, which may be related to differences in the duration of stuttering between the groups. Namely, coping with stuttering since early childhood may have resulted in more right-sided compensation by the DS, relative to AS who have been stuttering only since adolescence or adulthood. Several different mechanisms can contribute to the FA value, such as myelination, alignment of axons, axon diameter, and density (Beaulieu, 2002), but also the presence of crossing fiber pathways. White matter development continues throughout adolescence in the form of continuing myelination (Benes, 1989), increased density and organization (Schmithorst, Wilke, Dardzinski, & Holland, 2002), and it has been shown that FA changes continue during adulthood in the right superior longitudinal fasciculus (Giorgio et al., 2008). This suggests that skill learning and experience that continues throughout adolescence up to adulthood could influence microstructural changes in white matter. Development of normal speech motor control has been shown to continue during adolescence (A. Smith, 2006; Walsh & Smith, 2002), which may be aberrant in stuttering (Kelly, Smith, & Goffman, 1995). The white matter differences we observed among the groups may thus represent neuroplastic changes that accompany differences in speech motor development, as well as the resultant compensation by the right homologues.
Stuttering severity was significantly correlated with FA values in the right RO in both stuttering groups. This indicates that increases in the right FA, which may indicate denser and greater interconnection between speech regions on the right, is associated with increased stuttering severity. Foundas et al. (2004) similarly reported that atypical rightward asymmetry in the planum temporale (PT) (greater volume on the right than left) was associated with greater stuttering severity, and was also associated with greater benefit from delayed auditory feedback (Foundas et al., 2004). Our results are also in line with past research that reported right-sided white matter increases relative to controls (Jancke et al., 2004). Given that such differences in asymmetry were not found in children in gray or white matter volume (Chang et al., 2008), but rather differences were found in the left arcuate fasciculus in both children and adolescents (Chang et al., 2008; Watkins et al., 2008), it is likely that right hemisphere increases occur later on as stuttering continues.
It is interesting that the present study did not replicate a previous study that found FA decrease in the left RO in DS adults compared to controls (Sommer et al., 2002). The present findings rather point to FA increase in the right RO homologue in DS compared to controls. This may represent differences in methodology, as well as possibly subject characteristics. We were able to take advantage of advanced DTI analysis methods that involved robust inter-subject registration and less possibility of partial volume effects provided by tract-based spatial statistics (S. M. Smith et al., 2007). In the Sommer et al. (2002) study, only males were included, whereas the present study included males and females in each group.
There are several caveats in this study. While AS group appeared to have similar trends as shown in DS, the white matter results are considered preliminary due to small sample size. Considering that clinical populations tend to have more variability in task performance (Hill, Hogben, & Bishop, 2005), which may be associated with more variability in brain structure (Foundas et al., 2001), it may be all the more important to increase sample size to mitigate the effects of individual variations affecting brain structure. Approaches that enable quantification of individual variability in cortical surface and sulcal structure (Sowell et al., 2002; Thompson, Schwartz, Lin, Khan, & Toga, 1996), as well as use of a disease-specific atlas (Toga & Thompson, 2002) may also enable more accurate characterization of the neuroanatomy specific to different subtypes of stuttering. Future studies may in addition attempt to conduct more detailed behavioral (including neurology, psychiatric evaluations) as well as imaging (tractography and radial diffusivity measurements) analyses to better characterize adult-onset stuttering that is unrelated to apparent psychological or neurological trauma.
In conclusion, we have shown evidence that idiopathic adult-onset stuttering can occur with similar characteristics to developmental stuttering, including speech disfluencies and neuropsychology function. Subtle evidence of neuroanatomicial differences were found in both stuttering groups relative to controls; greater FA in the right hemisphere RO was found in the DS, and a trend for increased right RO FA relative to controls in AS. We interpret this as an indication that right hemisphere anatomical differences in adults with DS is a result of compensatory mechanisms involved in stuttering people over a life time.
Table 2.
Speech history of adult-onset stuttering cases included in this study
| Case number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 51 | 30 | 43 | 36 |
| Sex | M | F | M | F |
| Age at onset | 17 | 26 | 38 | 17 |
| Manner of onset | Gradual | Gradual | Sudden | Gradual |
| Duration of stuttering (yrs.) | 34 | 4 | 5 | 19 |
| Trigger | None | Stressful breakup | Divorce | None |
| Childhood stuttering | None | None | None | None |
| Family history of DS | None | None | None | Yes, father, son |
| Neurological deficit | None | None | None | None |
| Speech Therapy | Yes | Yes | Yes--DAF | None |
| Physical concomitants | No | Yes | Yes | Yes |
Table 4.
Neuropsychology test results in adult-onset stuttering participants.
| Case number | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Age | 51 | 30 | 43 | 36 |
| IQ | ||||
| Full scale | 99 | 105 | 102 | 117 |
| Verbal | 92 | 108 | 98 | 109 |
| Performance | 104 | 101 | 106 | 123 |
| Processing Speeda | ||||
| Symbol digit manipulation | −1 | 1.35 | 0.05 | 0.05 |
| Trails A | −1.5 | −1 | 0 | missing |
| Trails B | −0.4 | −1.5 | 0 | missing |
| Trails C | −0.2 | −1.5 | 0 | missing |
| Attention/Working Memoryb | ||||
| Digit forward | 40 | 28 | 33 | 41 |
| Digit backward | 50 | 42 | 43 | 55 |
| Dots (visual attention) | 55 | 59 | 39 | 59 |
| Learning/Memory (recall) b | ||||
| words-immediate | 32 | 50 | 47 | 36 |
| words-delay | 31 | 59 | 48 | 51 |
| shape-immediate | 46 | 58 | 56 | 41 |
| shape-delay | 52 | 58 | 52 | 47 |
| story-immediate | 44 | 32 | 40 | 35 |
| story-delay | 49 | 34 | 35 | 38 |
| Daily Living-immediate | 52 | 41 | 39 | 49 |
| Daily Living-delay | 55 | 51 | 58 | 37 |
| Motor Speeda | ||||
| GPT-dominant | −0.8 | −1.18 | −1.5 | −0.84 |
| GPT-nondominant | −0.9 | −1.05 | −1.98 | −0.66 |
| Languagea | ||||
| Boston Naming Test | −1.8 | −0.32 | −2.18 | −0.6 |
| Phonemic Word Generation | −1.8 | −1.24 | 0.4 | 0.95 |
| Semantic Word Generation | −0.9 | −0.59 | missing | missing |
| Executive Function | ||||
| FrSBe | 0 | 0 | missing | 0 |
| Visuospatial | ||||
| Judgment of line orientation | n | y | y | y |
| Aphasia Screening Test | y | y | y | y |
z scores
T scores
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS). The authors wish to thank Dr. Edythe Wiggs for her help in conducting neuropsychology evaluations and Sandra Martin for her help in conducting speech-language-hearing evaluations.
REFERENCES
- Ardila A, Lopez MV. Severe stuttering associated with right hemisphere lesion. Brain and Language. 1986;27(2):239–246. doi: 10.1016/0093-934x(86)90018-0. [DOI] [PubMed] [Google Scholar]
- Attanasio JS. A case of late-onset or acquired stuttering in adult life. Journal of Fluency Disorders. 1987;12:287–290. [Google Scholar]
- Baumgartner J, Duffy JR. Psychogenic stuttering in adults with and without neurologic disease. Journal of Medical Speech-Language Pathology. 1997;5:75–95. [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - A technical review. NMR in Biomedicine. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophrenia Bulletin. 1989;15(4):585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. 3 ed. American Guidance Services; 1997. [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cerebral Cortex. 2004;14(9):945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Carrow-Woolfolk E. Test of Auditory Comprehension on Language. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- Chang SE, Erickson KI, Ambrose NG, Hasegawa-Johnson MA, Ludlow CL. Brain anatomy differences in childhood stuttering. NeuroImage. 2008;39(3):1333–1344. doi: 10.1016/j.neuroimage.2007.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabarra AM, Elkind MS, Roberts JK, Marshall RS. Subcortical infarction resulting in acquired stuttering. Journal of Neurology, Neurosurgery, and Psychiatry. 2000;69(4):546–549. doi: 10.1136/jnnp.69.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cykowski MD, Kochunov PV, Ingham RJ, Ingham JC, Mangin JF, Riviere D, et al. Perisylvian sulcal morphology and cerebral asymmetry patterns in adults who stutter. Cerebral Cortex. 2008;18(3):571–583. doi: 10.1093/cercor/bhm093. [DOI] [PubMed] [Google Scholar]
- De Nil LF, Kroll RM, Kapur S, Houle S. A positron emission tomography study of silent and oral single word reading in stuttering and nonstuttering adults. Journal of Speech, Language, and Hearing Research. 2000;43(4):1038–1053. doi: 10.1044/jslhr.4304.1038. [DOI] [PubMed] [Google Scholar]
- Deal JL. Sudden onset of stuttering: A case report. Journal of Speech and Hearing Disorders. 1982;47(3):301–304. doi: 10.1044/jshd.4703.301. [DOI] [PubMed] [Google Scholar]
- Deal JL, Doro JM. Episodic hysterical stuttering. Journal of Speech and Hearing Disorders. 1987;52(3):299–300. doi: 10.1044/jshd.5203.299. [DOI] [PubMed] [Google Scholar]
- Doi M, Nakayasu H, Soda T, Shimoda K, Ito A, Nakashima K. Brainstem infarction presenting with neurogenic stuttering. Internal Medicine. 2003;42(9):884–887. doi: 10.2169/internalmedicine.42.884. [DOI] [PubMed] [Google Scholar]
- Duffy JR. A puzzling case of adult onset stuttering. In: Helm-Estabrooks N, Aten JL, editors. Difficult diagnoses in communication disorders. Boston: College-Hill Press; 1989. pp. 13–22. [Google Scholar]
- Dunn L. Peabody picture vocabulary test. Circle Pines, MN: American Guidance Service; 1959. [Google Scholar]
- Dworkin JP, Culatta RA, Abkarian GG, Meleca RJ. Laryngeal anesthetization for the treatment of acquired disfluency: a case study. Journal of Fluency Disorders. 2002;27(3):215–225. doi: 10.1016/s0094-730x(02)00129-8. [DOI] [PubMed] [Google Scholar]
- Fawcett RG. Stroke-associated acquired stuttering. CNS Spectrum. 2005;10(2):94–95. doi: 10.1017/s1092852900019416. [DOI] [PubMed] [Google Scholar]
- Fleet WS, Heilman KM. Acquired stuttering from a right hemisphere lesion in a right-hander. Neurology. 1985;35(9):1343–1346. doi: 10.1212/wnl.35.9.1343. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Corey DM, Hurley M, Heilman KM. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001;57(2):207–215. doi: 10.1212/wnl.57.2.207. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Feldman J, Corey DM, Hurley M, Lemen LC, et al. Aberrant auditory processing and atypical planum temporale in developmental stuttering. Neurology. 2004;63(9):1640–1646. doi: 10.1212/01.wnl.0000142993.33158.2a. [DOI] [PubMed] [Google Scholar]
- Fox PT, Ingham RJ, Ingham JC, Hirsch TB, Downs JH, Martin C, et al. A PET study of the neural systems of stuttering. Nature. 1996;382(6587):158–161. doi: 10.1038/382158a0. [DOI] [PubMed] [Google Scholar]
- Freund H, editor. Psychopathology and the problems of stuttering. Springfield, IL: Charles C. Thomas; 1966. [Google Scholar]
- Giorgio A, Watkins KE, Douaud G, James AC, James S, De Stefano N, et al. Changes in white matter microstructure during adolescence. NeuroImage. 2008;39(1):52–61. doi: 10.1016/j.neuroimage.2007.07.043. [DOI] [PubMed] [Google Scholar]
- Goldman R, Fristoe M. Goldman-Fristoe Test of Articulation. American Guidance Services; 2006. [Google Scholar]
- Grant AC, Biousse V, Cook AA, Newman NJ. Stroke-associated stuttering. Archives of Neurology. 1999;56(5):624–627. doi: 10.1001/archneur.56.5.624. [DOI] [PubMed] [Google Scholar]
- Hamano T, Hiraki S, Kawamura Y, Hirayama M, Mutoh T, Kuriyama M. Acquired stuttering secondary to callosal infarction. Neurology. 2005;64(6):1092–1093. doi: 10.1212/01.WNL.0000154472.51190.FB. [DOI] [PubMed] [Google Scholar]
- Hill PR, Hogben JH, Bishop DM. Auditory frequency discrimination in children with specific language impairment: a longitudinal study. Journal of Speech, Language, and Hearing Research. 2005;48(5):1136–1146. doi: 10.1044/1092-4388(2005/080). [DOI] [PubMed] [Google Scholar]
- Jancke L, Hanggi J, Steinmetz H. Morphological brain differences between adult stutterers and non-stutterers. BMC Neurology. 2004;4(1):23. doi: 10.1186/1471-2377-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LM, Lewis NP. Kahn-Lewis Phonological Analysis (KLPA): American Guidance Services, Inc.; 1986. [Google Scholar]
- Kelly EM, Smith A, Goffman L. Orofacial muscle activity of children who stutter: a preliminary study. Journal of Speech and Hearing Research. 1995;38(5):1025–1036. doi: 10.1044/jshr.3805.1025. [DOI] [PubMed] [Google Scholar]
- Lim EC, Wilder-Smith E, Ong BK, Seet RC. Adult-onset re-emergent stuttering as a presentation of Parkinson's disease. Annals Academy of Medicine Singapore. 2005;34(9):579–581. [PubMed] [Google Scholar]
- Ludlow CL, Rosenberg J, Salazar A, Grafman J, Smutok M. Site of penetrating brain lesions causing chronic acquired stuttering. Annals of Neurology. 1987;22(1):60–66. doi: 10.1002/ana.410220114. [DOI] [PubMed] [Google Scholar]
- Mahr G, Leith W. Psychogenic stuttering of adult onset. Journal of Speech and Hearing Research. 1992;35(2):283–286. doi: 10.1044/jshr.3502.283. [DOI] [PubMed] [Google Scholar]
- McNeil MR, Prescott TE. Revised Token Test. Baltimore: University Park Press; 1978. [Google Scholar]
- Mouradian MS, Paslawski T, Shuaib A. Return of stuttering after stroke. Brain and Language. 2000;73(1):120–123. doi: 10.1006/brln.2000.2289. [DOI] [PubMed] [Google Scholar]
- Neumann K, Euler HA, von Gudenberg AW, Giraud AL, Lanfermann H, Gall V, et al. The nature and treatment of stuttering as revealed by fMRI A within-and between-group comparison. Journal of Fluency Disorders. 2003;28(4):381–409. doi: 10.1016/j.jfludis.2003.07.003. quiz 409–410. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16(8):791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. NeuroImage. 2005;24(3):656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Paus T, Collins DL, Evans AC, Leonard G, Pike B, Zijdenbos A. Maturation of white matter in the human brain: A review of magnetic resonance studies. Brain Research Bulletin. 2001;54(3):255–266. doi: 10.1016/s0361-9230(00)00434-2. [DOI] [PubMed] [Google Scholar]
- Riley GD. A stuttering severity instrument for children and adults. Journal of Speech and Hearing Disorders. 1972;37(3):314–322. doi: 10.1044/jshd.3703.314. [DOI] [PubMed] [Google Scholar]
- Roth CR, Aronson AE, Davis LJ. Clinical studies in psychogenic stuttering of adult onset. Journal of Speech and Hearing Disorders. 1989;54(4):634–646. doi: 10.1044/jshd.5404.634. [DOI] [PubMed] [Google Scholar]
- Sahin HA, Krespi Y, Yilmaz A, Coban O. Stuttering due to ischemic stroke. Behav Neurol. 2005;16(1):37–39. doi: 10.1155/2005/941926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai K, Furui E, Komai K, Notoya M, Yamada M. Acquired stuttering as an early symptom in a patient with progressive supranuclear palsy. Rinsho Shinkeigaku. 2002;42(2):178–180. [PubMed] [Google Scholar]
- Sakai T, Miyamura M, Kuzuhara S. Palilalia and acquired stuttering in a case of Parkinson's disease. Rinsho Shinkeigaku. 1992;32(8):859–863. [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: A cross-sectional diffusion-tensor MR imaging study. Radiology. 2002;222(1):212–218. doi: 10.1148/radiol.2221010626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. Speech motor development: Integrating muscles, movements, and linguistic units. Journal of Communication Disorders. 2006;39(5):331–349. doi: 10.1016/j.jcomdis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, et al. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature protocols. 2007;2(3):499–503. doi: 10.1038/nprot.2007.45. [DOI] [PubMed] [Google Scholar]
- Sommer M, Koch MA, Paulus W, Weiller C, Buchel C. Disconnection of speech-relevant brain areas in persistent developmental stuttering. Lancet. 2002;360(9330):380–383. doi: 10.1016/S0140-6736(02)09610-1. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Rex D, Kornsand D, Tessner KD, Jernigan TL, et al. Mapping sulcal pattern asymmetry and local cortical surface gray matter distribution in vivo: maturation in perisylvian cortices. Cerebral Cortex. 2002;12(1):17–26. doi: 10.1093/cercor/12.1.17. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. Journal of Neuroscience. 1996;16(13):4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. New approaches in brain morphometry. American Journal of Geriatric Psychiatry. 2002;10(1):13–23. [PubMed] [Google Scholar]
- Turgut N, Utku U, Balci K. A case of acquired stuttering resulting from left parietal infarction. Acta Neurologica Scandinavica. 2002;105(5):408–410. doi: 10.1034/j.1600-0404.2002.01126.x. [DOI] [PubMed] [Google Scholar]
- Vernooij MW, Smits M, Wielopolski PA, Houston GC, Krestin GP, van der Lugt A. Fiber density asymmetry of the arcuate fasciculus in relation to functional hemispheric language lateralization in both right- and left-handed healthy subjects: A combined fMRI and DTI study. NeuroImage. 2007;35(3):1064–1076. doi: 10.1016/j.neuroimage.2006.12.041. [DOI] [PubMed] [Google Scholar]
- Walsh B, Smith A. Articulatory movements in adolescents: evidence for protracted development of speech motor control processes. Journal of Speech, Language, and Hearing Research. 2002;45(6):1119–1133. doi: 10.1044/1092-4388(2002/090). [DOI] [PubMed] [Google Scholar]
- Watkins KE, Smith SM, Davis S, Howell P. Structural and functional abnormalities of the motor system in developmental stuttering. Brain. 2008;131(Pt 1):50–59. doi: 10.1093/brain/awm241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd ed. San Antonio: Pearson Education, Inc; 1997. [Google Scholar]
- Weiner AE. A case of adult onset stuttering. Journal of Fluency Disorders. 1981;6:181–186. [Google Scholar]
- Williams KT. Expressive Vocabulary Test. Circle Pines: American Guidance Service, Inc; 1999. [Google Scholar]
- Yairi E, Ambrose NG. Early childhood stuttering I: persistency and recovery rates. Journal of Speech, Language, and Hearing Research. 1999;42(5):1097–1112. doi: 10.1044/jslhr.4205.1097. [DOI] [PubMed] [Google Scholar]