Abstract
The clinical course of prostate cancer is grouped into two broad phases. The first phase, which is the growth of the androgen-dependent cancer (AD-Ca), responds well to androgen depletion treatment while the second phase that could be termed as androgen-depletion-independent cancer (ADI-Ca) does not. We used two separate prostate tumors, one AD-Ca and one ADI-Ca from the conditional Pten deletion mouse model, to generate from each a pair of cell lines. The AD-Ca cell lines (E2 and E4) and the ADI-Ca cell lines (cE1 and cE2) display biallelic deletion at the Pten gene locus, an event which is specific for the prostate epithelium for this mouse model and a fairly similar level of expression of the androgen receptor (AR). The ADI-Ca cell lines (cE series) grow well in the absence of androgen, display increased AR transcription under androgen-deprived environment, and retain the sensitivity to increased proliferation when androgen is supplemented. The AD-Ca cell lines (E series) grow slowly in the absence of androgen and, unlike cE cells, do not show increased AR expression when maintained in the absence of androgen. The detection of epithelial cell markers, such as CK8, CK14, CK18, and E-cadherin in the cE series is conforming with the polygonal epithelial morphology of these cells in culture. The E cells also present mostly polygonal-shaped morphology with a small percent of cells with fibroblastoid morphology and produce little or very low levels of cytokeratins but increased levels of vimentin, Twist, and Slug, the markers known to be associated with epithelial–mesenchymal transition. Each of the cell lines, when inoculated subcutaneously into male or female NOD.SCID mice induced tumors within 8 weeks with 100% incidence. Histopathological examinations of the tumor sections, however, led to noticeable biological differences. The cE series engenders adenocarcinomas, particularly in male hosts, and the E series induces sarcomatoid carcinomas (positively stained for CK8 and AR as well as vimentin expression) in either male or female hosts. These new cell lines are promising models for the elucidation of the androgen metabolism and their role in prostate cancer.
Keywords: Conditional Pten deletion mouse model of prostate adenocarcinoma, Mouse prostate cancer cell lines, Androgen-dependent and androgen-depletion-independent prostate cancer, Prostate sarcomatoid carcinoma
Introduction
Androgen depletion has been a gold standard therapy in the clinical management of prostate cancer with localized or metastasized disease [1, 2]. Because androgen is necessary to maintain the function of the prostate gland as well as for the initial growth of the prostate tumors, this therapy of the androgen-dependent cancer (AD-Ca) rapidly leads to the shrinkage of the tumors. Unfortunately, within a period of 2 years, most patients do, however, manifest a relapse resulting in recurrent tumor, which may be best described [3] as androgen-depletion-independent cancer (ADI-Ca). How prostate cancer cells survive and proliferate under the low-androgen environment remains unclear, although multiple mechanisms, such as increased level of expression of androgen receptor (AR) in the cancer cells, mutations or posttranslational modifications of AR that broaden its ligand specificity, bypassing of the androgen–AR signaling to an extent through utilization of alternative pathways, etc., have been implicated [4–7].
There is currently a strong interest in the application of the knowledge gained from the analyses of human prostate cancer in modeling this disease in mice. Several mouse models of prostate cancer have been developed based on genetic alternations which are found frequently in human prostate cancer in order to recapitulate its natural course of initiation and progression [8–13]. Among these models, the conditional Pten deletion mouse model (cPten −/−) demonstrates a short latency for the development of adenocarcinoma [14]. A refined (cPten −/− L) model further increases the utility of the conditional Pten deletion model by combining it with a conditional luciferase expression, which is mediated by the same Cre/LoxP regulation system that is activated in the prostate epithelial cells [15]. In this model, the progression of tumor development and growth can be longitudinally monitored in living animals using bioluminescence imaging (BLI) technology. Importantly, the tumor regression after castration and then relapse can also be similarly monitored in these animals.
For studies of the mechanisms of cancer, cancer cell lines are powerful tools. A limited number of human prostate cancer lines have been established from primary or metastatic cancers [16–19], and there is indeed a dearth of parallel cell lines developed from both AD-Ca and ADI-Ca phases. Mouse models of prostate cancer can readily provide tissue materials for establishing cell lines from different phases of the disease progression. However, only a few murine prostate cancer cell lines have been derived from the primary tumors to date [20–22], and none from the ADI-Ca phase. Here, we report establishment and characterization of four prostate cancer cell lines from the cPten −/− L mouse model, two derived from an AD-Ca and the other two from a separate ADI-Ca.
Material and Methods
Animals
Mice with prostate epithelium-specific inactivation of Pten coincidental with luciferase reporter gene activation, as well as castration experiments after tumor growth, were as described before [15]. All mice were maintained under identical conditions, and animal experimentation was conducted using the standards for humane care in accordance with the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
Generation of Cell Lines from cPten−/−L Prostate Cancer Model
Freshly collected prostate tumor tissues were minced with crossed scalpels (size 11 blades), transferred to a 50-ml tube, and incubated in 5 ml of a digestion medium at 37°C overnight on a rotator (37°C, 140 rpm). The digestion medium contained Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Invitrogen), 10% fetal bovine serum (FBS; Gemini), collagenase (1 mg/ml), hyaluronidase (1 mg/ml), and DNase I (1 μg/ml). Collagenase, hyaluronidase, and DNase I were purchased from Sigma-Aldrich, St. Louis, MO, USA. After low-speed centrifugation, single cells and cell clumps were collected and subjected to treatment with 0.05% trypsin–EDTA for 10 min at 37°C and sequentially passed through 100- and 40-μm cell strainers (BD Bioscience). The preparation was then cultured in a maintenance medium that contained DMEM, 10% FBS, 25 µg/ml bovine pituitary extract (Invitrogen), 5 µg/ml insulin (Sigma-Aldrich), and 6 ng/ml recombinant human epidermal growth factor (rhEGF; Invitrogen). When cell colonies with epithelial cell morphology were observed, cells were trypsinized, diluted with culture medium to obtain a concentration of approximately one cell per 100 µl, and seeded in 96-well plates (100 µl per well). After culturing overnight at 37°C, each well was carefully examined under the bright field in microscopy. Wells containing single cells with the epithelial morphology were marked, and the culture medium in such wells was changed every 3 days. Expanded cell populations derived from these colonies were subjected to further characterization.
Quantitative RT- PCR
Total cellular RNA (1 µg), extracted by RNAqueous-4PCR Kit (Ambion) was reverse-transcribed by random hexamers using qScript™ cDNA Synthesis Kit (Quanta), and the reverse transcription reaction (1 µl) was then subjected to polymerase chain reaction (PCR) amplification using FastStart Universal SYBR Green Master (Roche). PCR signals were recorded and analyzed in Stratagene MX3000P qPCR system with MxPro software (Stratagene; v4.01). Primer sets are listed in Table 1.
Table 1.
Primer sets used in real-time PCR and genotyping
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| CK5 | ACCTTCGAAACACCAACGAC | TTGGCACACTGCTTCTTGAC |
| p63 | GAAGGCAGATGAAGACAGCA | GGAAGTCATCTGGATTCCGT |
| AR | AACCAACCAGATTCCTTTGC | ATTAGTGAAGGACCGCCAAC |
| CK14 | GACTTCCGGACCAAGTTTGA | CCTTGAGGCTCTCAATCTGC |
| CK8 | ATCGAGATCACCACCTACCG | TGAAGCCAGGGCTAGTGAGT |
| CK18 | ACTCCGCAAGGTGGTAGATG | GCCTCGATTTCTGTCTCCAG |
| PSCA | GCTGCTACTCTGACCTGTGC | TTCACAATCGGGCTATGGTA |
| CgA | GGGAGCTGGAACATAAGCAG | TGTCCTCCCATTCTCTGGAC |
| Syph | CTTTGTGAAGGTGCTGCAAT | GTCTTGTTGGCACAATCCAC |
| E-cadherin | TCCAGGAACCTCCGTGATG | GGGTAACTCTCTCGGTCCAG |
| N-cadherin | AGCCTGGGACGTATGTGATG | ATGTTGGGTGAAGGTGTGCT |
| Vimentin | CAAGTCCAAGTTTGCTGACCT | TCTTCCATCTCACGCATCTG |
| Twist | CTCGGACAAGCTGAGCAAG | ACGGAGAAGGCGTAGCTGAG |
| Snai1 | ACCCACACTGGTGAGAAGC | GACCAAGGCTGGAAGGAGTC |
| Slug | CACAGTTATTATTTCCCCATATCT | GCAGTCTCTCCTCTTCGTCA |
| β-actina | AGTGTGACGTTGACATCCGT | CTTGCTGATCCACATCTGCT |
| Pten | AAGCAAGCACTCTGCGAAACTG | GATTGTCATCTTCACTTAGCCATTGGT |
| Cre | GATCCTGGCAATTTCGGCTAT | TTGCCTGCATTACCGGTCGAT |
PCR Genotyping
DNA extracted from cell lines were used as templates for PCR reactions using specific primer sets for Pten and Cre. These primer sets are also listed in Table 1.
Western Blot Analysis
The whole-cell lysates were prepared as described [23]. The antibodies used in this Western blots were anti-AR (1:100; Santa Cruz) and anti-actin (1:1,000; Santa Cruz).
Assays for Growth in Serum-Free Medium
For testing the efficiency of growth of these cell lines in an androgen-free environment, a modified serum-free medium (SFM) was used [24]. It was composed of DMEM/F-12 supplemented with 0.5% glucose (Sigma) and 2 mM glutamine, 10 g/ml insulin, 5.5 g/ml transferrin, 6.5 ng/ml selenium, and 6 ng/ml rhEGF (all from Invitrogen). Initially, cells (5 × 104) were plated in six-well plates in the maintenance medium overnight, washed two times with 1× PBS, and then cultured in SFM for 5 days. The culture medium was changed every 2 days, and the cell proliferation rate was determined at those time points by cell counting. SFM containing 1 or 5 nM of methyltrienolone (R1881; Perkin Elmer), a synthetic androgen agonist, was utilized to determine the effect of androgen on cell proliferation.
Tumorigenicity Assays
Cells (1 × 106) were mixed with 100 µl Matrigel (BD bioscience)/maintenance medium at a ratio of 1:1 and then inoculated subcutaneously into NOD.SCID mice of 8–12 weeks of age. Grafts were collected surgically at 8 weeks postinoculation from the euthanized animals.
Immunostainings
Paraffin sections (5 µm) of tissues fixed in 4% PFA were stained for either H&E or immunohistochemistry (IHC) or immunofluorescence (IF), following the published protocol [15]. Antigen retrieval for IHC was conducted by boiling the slides in 10 mM citrate buffer, pH 6.0, for 15 min and then cooling down to room temperature. The sections were allowed to react with primary antibodies against AR (1:200; Santa Cruz), CK8 (1:100; TROMA-1 antibody; Developmental Studies Hybridoma Bank, University of Iowa), vimentin (1:50; Cell Signaling), or Ki67 (1:200; Vector Laboratories) overnight at 4°C, followed by incubation with biotinylated secondary antibodies (1:200) against rabbit or rat IgG (Vector Laboratories) for 30 min, and then stained using the ABC Elite Kit (Vector Laboratories) and 3,3′-diaminobenzidine (Dako) as substrate. IF analysis of the parallel sections was done by using primary antibodies against CK8, E-cadherin (1:100; Cell Signaling), N-cadherin (1:200; Abcam), and α-smooth muscle actin (SMA; Cy3-conjugated; 1:200; Sigma-Aldrich). FITC-conjugated secondary antibodies against rabbit or rat IgG (1:80; Sigma-Aldrich) were then applied. After staining, sections were mounted with medium containing DAPI for the labeling the nuclei.
Karyotyping
Analysis of karyotypes was performed essentially as described in [25]. Briefly, actively growing cells were cultured in the presence of 0.05 µg/ml colcemid (Invitrogen) for 4 h, harvested after trypsin dissociation, and collected in 15-ml conical centrifuge tubes. Cell pellets were suspended in 1.5 ml of fresh growth medium; 10 ml of a 37°C potassium chloride solution (0.075 M) was slowly added followed by 17-min incubation at 37°C. Then, two to three drops of ice-cold fixative (methanol/glacial acetic acid = 3:1) were mixed into the cell suspension. After immediate centrifugation, the fixation step with 10-ml ice-cold fresh fixative was repeated three times. Finally, the cell pellets were suspended in 1 to 0.5 ml fixative, and chromosome spreads were made by dropping 70 µl onto glass slides. Slides stained with Giemsa dye were counted using a ×100 or a ×40 oil immersion objective. At least 25 spreads were counted for each cell line. A statistical analysis was performed with InStat 3.05 (GraphPad).
Statistical Analysis
The results of cell growth and marker analyses were evaluated as the mean ± SE of at least two different experiments performed in triplicate. Statistical calculations were done with Microsoft Excel analysis tools. Differences between individual groups were analyzed by independent t test. P values of <0.05 were considered statistically significant.
Results
Characterization of the New Murine Prostate Tumor Cell Lines
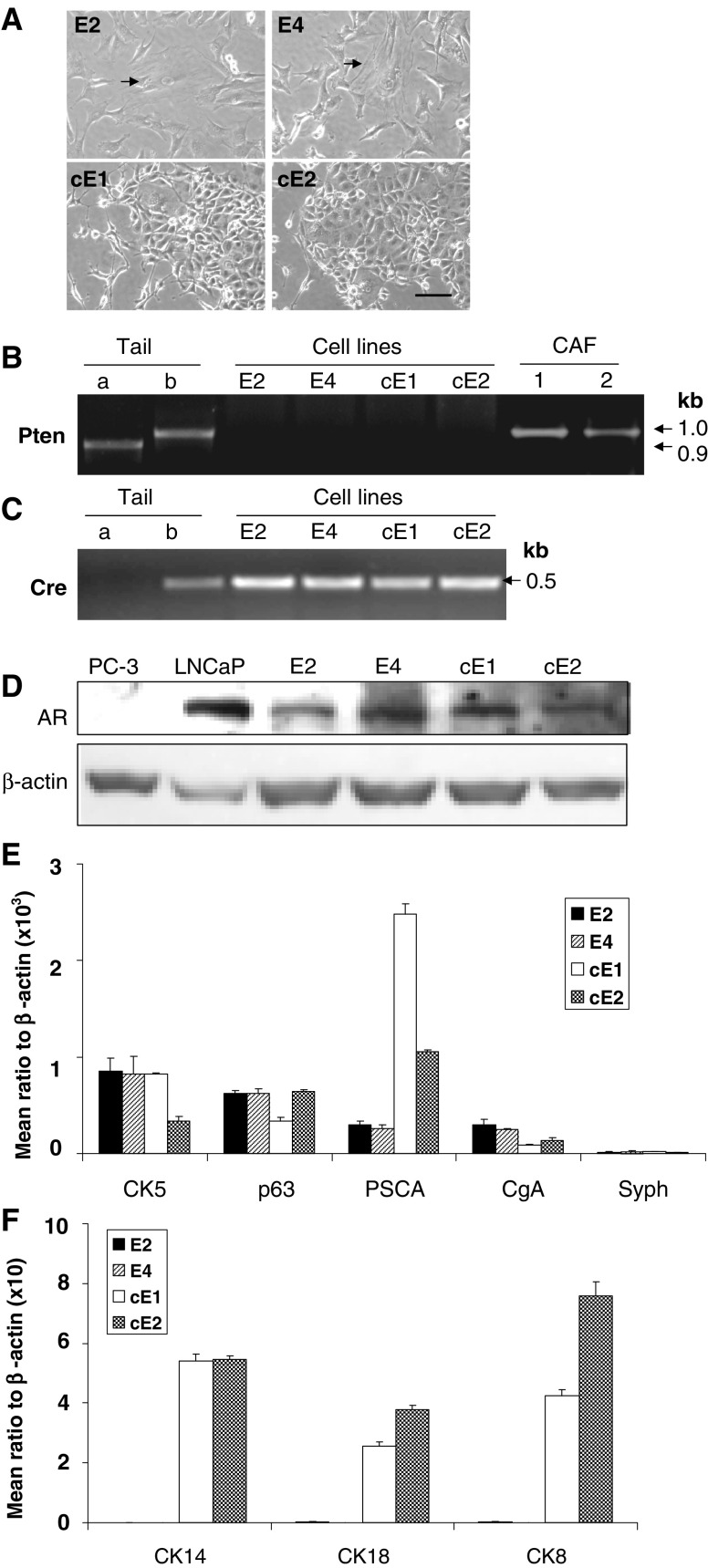
We first isolated cells from an AD-Ca tissue from an intact mouse of 8 months of age. After culturing of single cells in 96-well plates, five colonies from different wells were obtained and cultured to enlarge the population, two from which, named E2 and E4, were selected for further characterization. By using the same method, cell lines were derived from an ADI-Ca tumor collected from a 13-month-old male mouse, which was castrated at the age of 8 months. Initial decrease followed by increase in BLI signals was monitored in this mouse for the growth of the recurrent tumor during the 5 months of observation after castration. From this recurrent tumor at the primary site, three single-cell colonies were produced, two of which were named cE1and cE2 and subjected to further characterization. As illustrated in Fig. 1a, E2 and E4 cells appeared to display generally a polygonal-shaped morphology along with the presence of a small fraction of cells (<5%) that assumed a flattened and elongated shape (indicated by arrows). The cE1 and cE2 cells mostly exhibited polygonal shapes but apparently with increased intracellular adhesion as these cells tend to grow in clumps in the culture.
Fig. 1.
Characteristics of the E2 and E4 and cE1 and cE2 cell lines. a Comparison of the morphology of cells under the bright field in microscopy. While all cell lines appear to display polygonal-shaped cells that are distinctive of epithelial cells, E2 or E4 cells also manifest a small amount of cells with elongated morphology, as indicated by arrows. Bar, 100 μm. b PCR genotyping of the cell lines using Pten-specific primers. (a normal mouse tail tissue; b cPten −/− L mouse tail tissue). c PCR detection of the Cre gene in the cell lines. d Analysis of AR expression in the mouse cell lines by Western blots; human prostate cancer cell lines used as controls were: PC3, negative; LNCaP, positive. e, f Comparative real-time PCR analysis of marker gene expression including basal (CK5, p63, CK14), luminal (CK18, CK8), neuroendocrine cell markers (CgA, Syph), and PSCA.
To determine the Pten gene status in these four cell lines, a genotyping analysis was performed. Genomic DNA extracted from these cell line cells were subjected to PCR reactions with a primer set specific for the exon 5 region of the Pten floxed allele. This primer set was able to amplify a 1-kb-long DNA fragment in the absence of DNA recombination. However, in the event of Cre-mediated recombination at the floxed site, there should not be any amplified product. Genomic DNA extracted from the tail tissue of cPten −/− L mice and their normal counterparts were used as controls for the PCR products. The sizes were expected to be 1.0 and 0.9 kb for the floxed allele and the wild-type allele, respectively. As illustrated in Fig. 1b, such appropriate bands were detected in the tail DNA collected from the cPten −/− L and normal mice, with no evidence for such products from any of the DNAs isolated from the cell lines. The results implied homozygous deletion of the floxed Pten allele in each of the epithelial cell lines. A similar analysis with the DNA isolated from the cancer-associated fibroblasts from the same mouse model showed the presence of only unrecombined floxed allele, reconfirming the prostate epithelium specificity of the Cre/loxP system in the model [23]. The results supported an epithelial origin for each of the four cell lines established. The retention of the Cre gene in the cell lines was also confirmed by PCR analysis (Fig.1c). When the cell lines were subjected to karyotyping analysis, some individual differences became apparent. As shown in Table 2, E2, E4, and cE2 appeared near tetraploid, whereas cE1 was clearly aneuploid.
Table 2.
Representative karyotypes of the cell lines
| Cell line | Chromosome number | Sample number |
|---|---|---|
| E2 | Near 4N (79.3 ± 14.9) | 40 |
| E4 | Near 4N (76.1 ± 23) | 31 |
| cE1 | Near 3N (63.8 ± 15.1) | 26 |
| cE2 | Near 4N (78.6 ± 29.5) | 25 |
For determining the characteristics of molecular expressions, we used Western blot to analyze AR and real-time PCR technique for mRNAs for basal cell markers (CK5, p63, CK14), luminal cell markers (CK18, CK8), neuroendocrine phenotype markers [26–28], namely chromogranin A (CgA) and synaptophysin (Syph), and the so-called prostate stem cell antigen (PSCA) [29], a marker for intermediate epithelial cell differentiation. The values obtained from each PCR reaction was normalized to that of β-actin. As shown in Fig. 1d, the level of AR expression was fairly similar in all of these cell lines. Each of the cell lines expressed only very low levels of CK5, p63, CgA, and PSCA and a barely detectable level of Syph (Fig. 1e). The cE1 and cE2 cells, but not E2 and E4 cells, displayed significant levels of CK8, CK14, and CK18 (Fig. 1f).
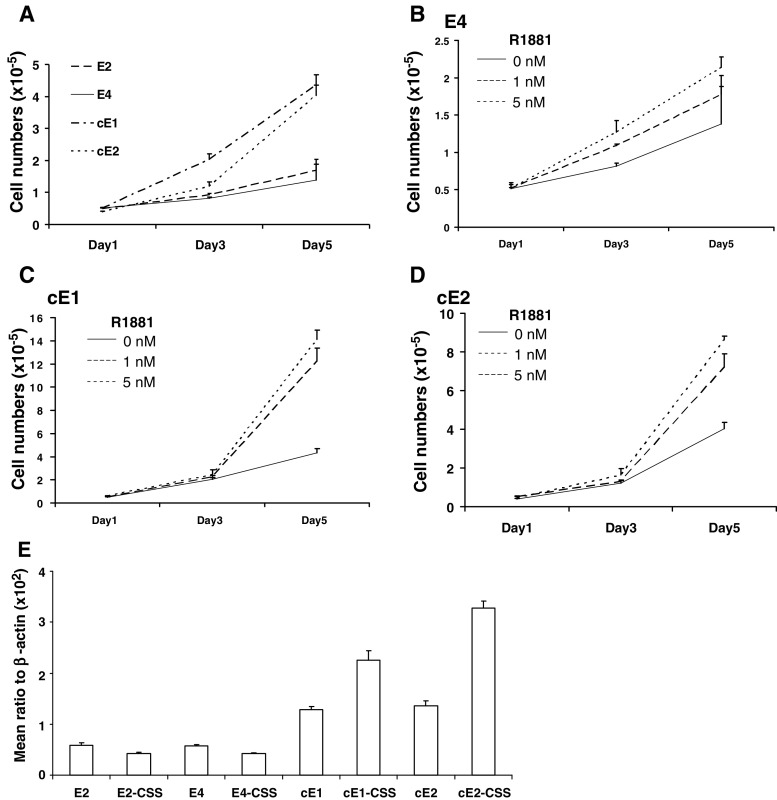
We next examined these cell lines for the effect of androgen on the proliferation rate. In SFM, E2 and E4 cells grew slowly. However, the proliferation rate for cE1 and cE2 cells under identical conditions was higher, displaying an approximately fivefold increase in the cell number after 5-day culture (Fig. 2a). The cell proliferation rate of E2 and E4 cells in SFM could be increased by about 1.2- to 1.7-fold by the addition of 1- to 5-nM concentration of androgen (R1881), as illustrated for the E4 cells in Fig. 2b. The response of cE1 and cE2 cells to the same levels of androgen was higher, displaying a 3.0- and 3.5-fold increase in the proliferation rate (Fig. 2c, d). To determine the effect of androgen on the AR expression in these cell lines, we cultured the cells in a medium composed of DMEM and 10% charcoal-stripped serum (CSS) up to 18 days. RNA extracted from the cells cultured either in the maintenance medium or CSS medium were examined using real-time PCR with primers specific to AR. As illustrated in Fig. 2e, a significant (2.0- to 2.5-fold; P < 0.01) increase of AR transcripts was detected in cE series cell lines cultured in CSS medium relative to cells in maintenance medium. However, in contrast to cE cells, there was no striking increase in the AR expression level between E cells cultured in the maintenance and the CSS medium; rather a slight decrease was observed.
Fig. 2.
The comparison of proliferation rates of the cell lines in cultures. a Growth rates in SFM. The differences in the number of cells counted at day 5 were significant between the E and cE series (P < 0.01). b Illustration of the effect of low concentrations of androgen (R1881) on the growth of E4 cells in SFM (similar results were also obtained with E2 cells). The growth rate was significantly increased (P < 0.05) in the presence of 5 nM, but not 1 nM R1881. c Effect of similar concentrations of androgen on the growth of cE1 and cE2 cells (d). At day 5, the stimulation by androgen was significant (P < 0.01) with both cell lines. e Analyses of the AR expression levels in E and cE series cultured in the maintenance or an androgen-free medium derived by using charcoal-stripped serum in the place of normal serum (CSS). The differences between cE1 and cE1-CSS and between cE2 and cE2-CSS were significant (P < 0.01).
Tumorigenicity of the Cell Lines
For testing the tumorigenicity of the cell lines, we used grafting on both male and female NOD.SCID recipients to examine the influence of gender on the intake of grafts and tumor histology. Mice were euthanized after 8 weeks, and grafts were collected and weighed, and tissue sections prepared. Our data demonstrated that the tumor incidence for all cell lines was 100% (Table 3), and, in general, grafts formed in male were larger than those formed in female. When the tumors formed in the males from different cell lines were compared, the tumor weights appeared to be similar for the E2, cE1, and cE2 cells, while the grafts from E4 weighed threefold higher; in females, E series grafts weighed threefold to 18-fold more than the cE series grafts.
Table 3.
The comparison of the incidence and weights of the tumors formed
| Host | Cell line | Incidence (%) | Tumor weight (g) |
|---|---|---|---|
| Male | E2 | 4/4 (100) | 0.25 ± 0.24 |
| Male | E4 | 4/4 (100) | 0.80 ± 0.54 |
| Male | cE1 | 4/4 (100) | 0.25 ± 0.08 |
| Male | cE2 | 4/4 (100) | 0.26 ± 0.07 |
| Female | E2 | 4/4 (100) | 0.17 ± 0.03 |
| Female | E4 | 8/8 (100) | 0.56 ± 0.15 |
| Female | cE1 | 8/8 (100) | 0.05 ± 0.01 |
| Female | cE2 | 4/4 (100) | 0.03 ± 0.02 |
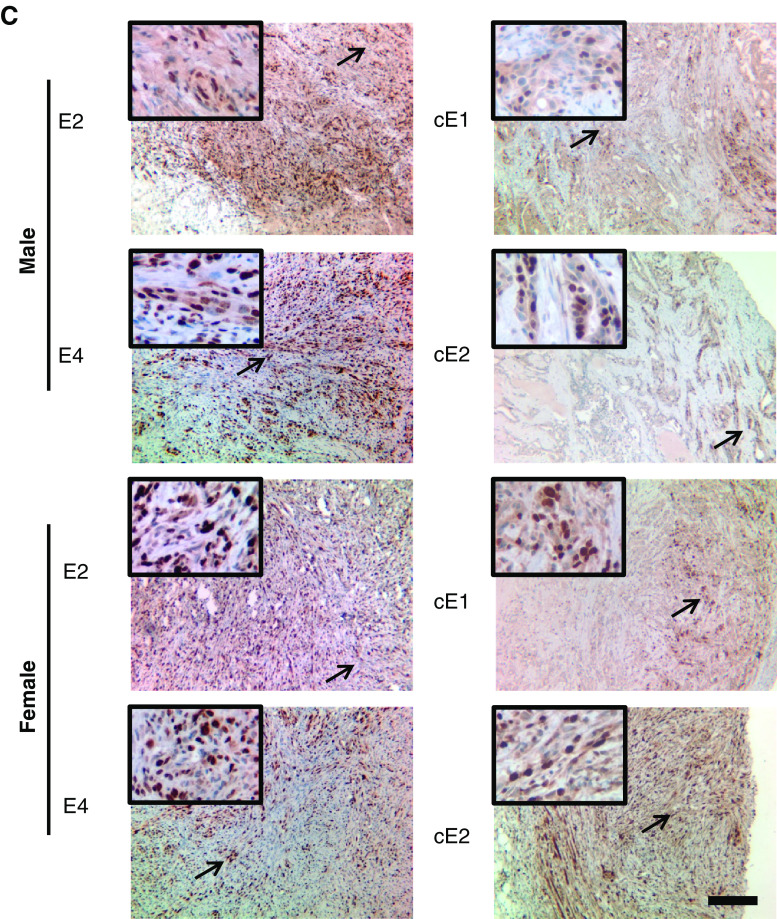
When the grafts from the male mice were examined for pathology, a major difference between the cell lines from AD-Ca and ADI-Ca series was noted. As illustrated in Fig. 3a, H&E staining of E2 and E4 grafts demonstrated spindle cell neoplasms. Since neoplastic cells expressed not only AR but also vimentin and CK8, these neoplasms were diagnosed as sarcomatoid carcinoma [30] as per the “Pathological Classification of Prostate Lesions in Genetically Engineered Mice” [31]. Distinct from the E2 and E4 grafts (Fig. 3a, upper part), cE1 and cE2 grafts demonstrated the histology of adenocarcinoma with presentation of glandular structures composed of round-shaped neoplastic epithelial cells expressing AR and CK8 (Fig. 3b) and with such epithelial cells that had penetrated into the stromal layers. Vimentin-positive cells could be detected in the stromal regions, but not inside the epithelial layers. Sections from E2 and E4 grafts from the female mice also demonstrated histopathology of sarcomatoid carcinoma, except that the percentage of CK8+ cells was higher than that in the E2 and E4 grafts in male mice (Fig. 3a). While cE1 and cE2 cells were also able to form tumors in the female mice, the morphology of glandular structures in these grafts was not as clear as those observed in the male. The IHC staining demonstrated that cE1 and cE2 grafts in male had positive nuclear staining for AR. In contrast, their counterparts in the female displayed mostly a diffuse cytoplasmic AR staining. Ki67-positive, the cell proliferation marker, cells were detected in all grafts (Fig. 3c).
Fig. 3.
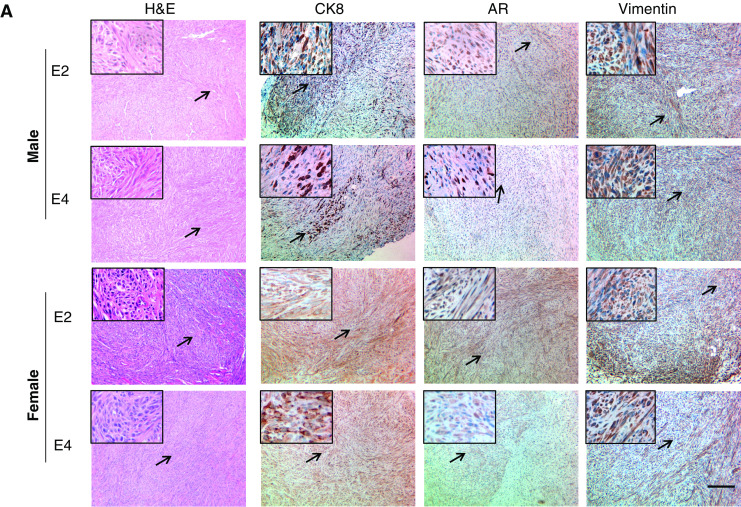
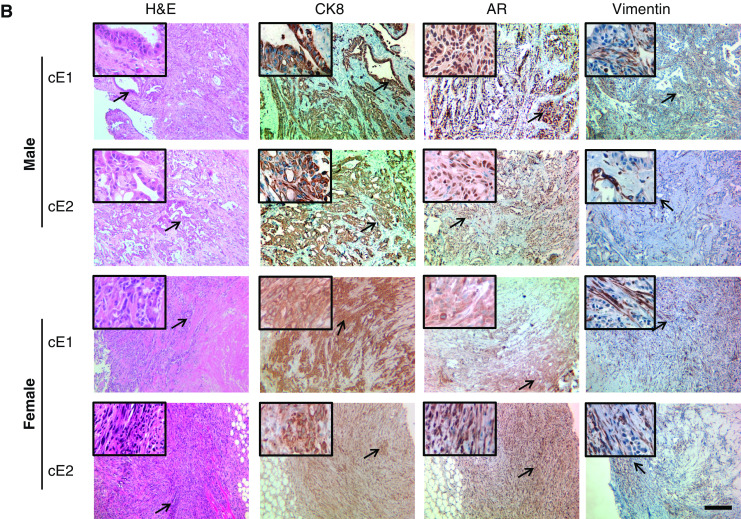
Microanatomic analyses of tumors induced by the cell lines in male or female in NOD.SCID mice. a H&E and IHC staining of sections of E2 and E4 grafts for AR, CK8, and vimentin. A higher magnification of an area marked by the arrow is shown in the upper-left corner of each section. Bar, 100 μm. b Similar staining experiments with sections from cE1 and cE2 grafts. Bar, 100 μm. c IHC staining of the grafts for Ki67. Bar, 100 μm.
Analysis for EMT Markers
Suspecting that E2 and E4 cells might have undergone some degree of epithelial–mesenchymal transition (EMT) conversion in cultures, we further tested for the expression levels of certain EMT markers in all cell lines by using real-time PCR. As illustrated in Fig. 4a, while E-cadherin expression was present in cE1 and cE2 cells, it was practically absent in E2 and E4 cells; N-cadherin levels were very low in all cell lines. E2 and E4 cells appeared to produce significantly more vimentin than the cE1 and cE2 cells. Expression of Twist, Slug, and Snail, the known transcriptional repressors for EMT [28–31], was also increased in the E2 and E4 cells relative to the cE1 and cE2 cells (Fig. 4b). A few markers were also examined at the protein level using the grafts formed in vivo. Tissue sections adjacent to those used for H&E and IHC staining were analyzed by IF using antibodies against CK8, E-cadherin, N-cadherin, and α-SMA. As illustrated in Fig. 4c–e, cells that stained for α-SMA did not appear to simultaneously express CK8, E-cadherin, or N-cadherin on sections from cE1 and cE2 grafts grown either in male or female mice. However, many such cells (arrows) were detected in the sections from E2 and E4 grafts.
Fig. 4.
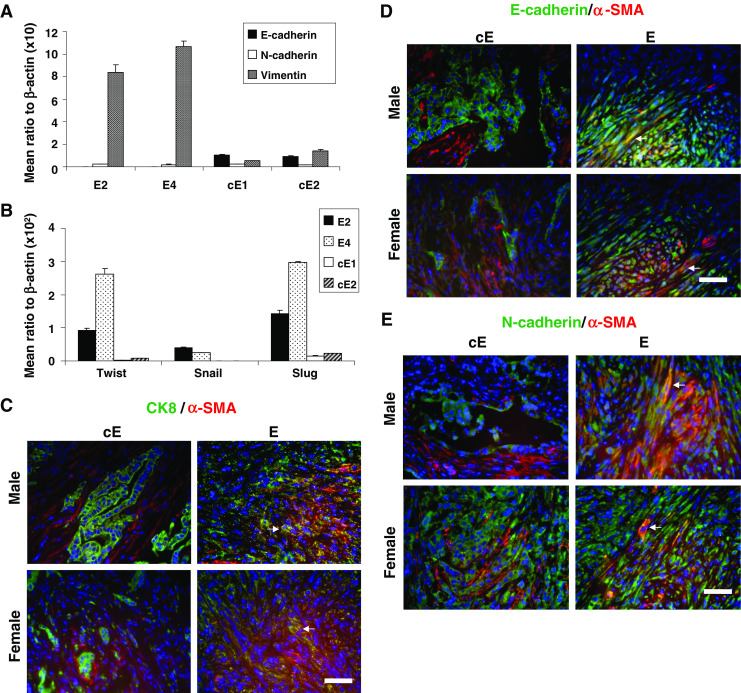
Analysis for the expression of EMT-related markers in the cell lines. a Relative expression of E-cadherin, N-cadherin, and vimentin and b that of Twist, Snail, and Slug as determined by real-time PCR. The difference in the level of expression of each of vimentin, Twist, Snail, or Slug in the E series relative to the cE series was significant (P < 0.01). c–e Illustrations of coimmunofluorescence results when tumor grafts from the E and cE cell lines were stained red for α-SMA and green for either CK8 (c), E-cadherin (d), or N-cadherin (e). White arrows indicate cells with coexpression (yellow). Bar, 50 μm.
Discussion
The importance of this report is that it describes isolation and properties of new tumorigenic cell lines derived from two different tumors developed in the cPten −/− L mouse model. These cell lines add to the limited repertoire of cell lines currently available for the study of prostate cancer. Of the four cell lines we describe here, E2 and E4 were derived from an AD-Ca and cE1 and cE2 from ADI-Ca of a separate mouse. While each of the cell lines is strong in AR production, the two series vary in their growth rates, levels of expression of certain cell markers, and sensitivity to androgen exposure. In general, E2 and E4 cells, expressing low levels of basal cell markers (CK5 and p63) but practically no luminal cell markers, show a requirement for androgen for optimal growth in cell cultures. E2 and E4 cells appear to express a few EMT markers, namely vimentin, Twist, Slug, and Snail, at levels significantly higher than the cE series. The cE1 and cE2 cells, which express significantly higher levels of two luminal cell markers (CK8 and CK18) as well as a basal cell marker (CK14) than the E2 and E4 cells, can grow well in the absence of androgen and at the same time retain the capacity to produce increased AR transcripts when deprived of androgen and to increase proliferation when androgen is supplemented. Since AR expression is similar in both series, the difference in the androgen sensitivity is likely to be regulated by mechanisms much more complex than simply by the levels of AR. It has been reported that AR remains critical for cell cycle progression of human prostate cancer cell lines that are not dependent on androgen for growth [32, 33], and the loss of PTEN function in a set of mouse neoplastic epithelial cell lines enhances androgen independence [21]. In the Pten-deleted cell lines we have generated, a clear picture of the effect of androgen on cell growth is also not derived. We find that the two cell lines obtained from the androgen-dependent phase of the mouse prostate tumor can slowly proliferate for a limited time under androgen deprivation, and the cell lines from the ADI phase of the tumor show the ability to grow significantly more efficiently under such an environment while retaining the potential of increased proliferation when exposed to androgen. Since the study was performed using the E and cE lines from two different mice, it is unclear whether the cE cells represent an evolution from an androgen-dependent state correlating with a linear progression of the disease or an entirely independent phenomenon. To address this issue, it would be necessary to analyze samples of the same tumor and derived cell lines from a single animal at successive stages of progression from AD-Ca to ADI-Ca.
Another interesting observation we made is that these two series of cell lines drastically differ in their ability to induce tumor types in the in vivo assays. E series cell lines formed sarcomatoid carcinoma in both male and female mice, whereas cE series cell lines formed adenocarcinoma in males but undifferentiated tumors in the female (Fig. 3b). Consistent with the detection of reduced cell–cell adhesion properties as well the presence of a small fraction of cells with elongated and flattened shapes in the in vitro cultures of the E series, the real-time PCR results from these cells also indicate increased levels of some EMT markers, as stated above. The possibility that the E series cells might have acquired a degree of EMT is supported by the demonstration that spindle-shaped cells coexpressing both epithelial and mesenchymal markers could be readily detected in the tumors formed in vivo from these cells. No such evidence was obtained with the cE cell lines (Fig. 4c–e). In the tumors induced by the cE series, however, a major influence of the gender of the host animal was clearly evident. Tumors induced in the males by the cE cells present distinct glandular structures with the presence of CK8-positive cells both inside and outside the lumen, whereas, there is a dense distribution of CK8-positive cells in the undifferentiated tumors formed in the females, especially in cE2 grafts (Fig. 3b). These results suggest that the cE cells retain the capacity to generate lesions akin to prostatic adenocarcinoma, and this property appears to be dependent on the levels of male hormones. In this connection, the AR staining in the tumor sections of both E and cE cell lines that are formed in the male mice is found to be located mostly in the nuclei but is detected primarily in the cytoplasm of the tumor cells in the grafts formed in the female mice, where the circulating androgen levels are expected to be low. Thus, a regulation by androgen appears to be more prominent in the formation of adenocarcinomas from the cE1 and cE2 cells and less for the histopathological phenotype of the tumors induced by E2 and E4 cells. Induction of sarcomatoid carcinoma by the E series, however, remains quite intriguing. First of all, sarcomatoid carcinomas of the prostate are rare both in humans and mice [31]. Second, contrary to our expectation, these cell lines from the relatively early phase of the prostate tumorigenesis appear to be less differentiated than those from the late ADI-Ca phase. It is, thus, unclear whether the isolation of E2 and E4 cells is related to clonal variations that may not be representative of the majority of the cancer cells but are coincidentally selected in the cultures, or their presence in the tumors may have a hitherto unknown biological implication in tumorigenesis induced by Pten gene activation in the prostate epithelium. Isolation and characterization of additional neoplastic cell clones from the AD-Ca phase will be necessary for further evaluation of these issues.
In summary, we have generated two pairs of new mouse prostate cancer cell lines with homozygous deletion of the Pten tumor suppressor gene from two different prostate tumors: one pair of cell lines from an androgen-dependent tumor and the other pair from an androgen-depletion-independent (recurrent) tumor. The particular cell lines, derived from spontaneous tumor models of a similar genetic background, display properties that are distinctive. Each of two cell lines derived from the recurrent tumor grows well in the absence of androgen, displays the ability to increase AR transcription when deprived of androgen, maintains sensitivity to androgen levels for increased proliferation, and can produce adenocarcinomas in male NOD.SCID mice. The pair of cell lines obtained from the androgen-dependent phase requires androgen for optimal growth, exhibits certain EMT-like properties in vitro, and can induce sarcomatoid carcinomas displaying a hybrid epithelial–mesenchymal cell phenotype in vivo when transplanted in either male or female hosts. These cell lines would likely be important resources for exploring the mechanisms in prostate cancer progression and manifestation of the diverse histopathological phenotypes.
Acknowledgement
Grant support: NIH RO1 CA113392 and NIH RO1 CA59705 (P. Roy-Burman) and NIH RO1 CA96823 (A. Nikitin).
We thank all members of the Roy-Burman Laboratory for assistance in various aspects of this work, especially Lauren Geary for the design of primary sets of Snail and Slug and Erik Haw for genotyping. We also thank Dr. Zongxiang Zhou for his initial contributions to karyotyping analyses.
Footnotes
Chun-Peng Liao and Mengmeng Liang contributed equally to this work.
References
- 1.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 2.Bonkhoff H, Berges R. From pathogenesis to prevention of castration resistant prostate cancer. Prostate. 2009;70:100–112. doi: 10.1002/pros.21042. [DOI] [PubMed] [Google Scholar]
- 3.Roy-Burman P, Tindall DJ, Robins DM, et al. Androgens and prostate cancer: are the descriptors valid? Cancer Biol Ther. 2005;4:4–5. doi: 10.4161/cbt.4.1.1563. [DOI] [PubMed] [Google Scholar]
- 4.Brooke GN, Bevan CL. The role of androgen receptor mutations in prostate cancer progression. Curr Genomics. 2009;10:18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61:3550–3555. [PubMed] [Google Scholar]
- 6.Waltering KK, Helenius MA, Sahu B, et al. Increased expression of androgen receptor sensitizes prostate cancer cells to low levels of androgens. Cancer Res. 2009;69:8141–8149. doi: 10.1158/0008-5472.CAN-09-0919. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Clegg NJ, Scher HI. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 2009;10:981–991. doi: 10.1016/S1470-2045(09)70229-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roy-Burman P, Wu H, Powell WC, Hagenkord J, Cohen MB. Genetically defined mouse models that mimic natural aspects of human prostate cancer development. Endocr Relat Cancer. 2004;11:225–254. doi: 10.1677/erc.0.0110225. [DOI] [PubMed] [Google Scholar]
- 9.Abate-Shen C, Brown PH, Colburn NH, et al. The untapped potential of genetically engineered mouse models in chemoprevention research: opportunities and challenges. Cancer Prev Res (Phila Pa) 2008;1:161–166. doi: 10.1158/1940-6207.CAPR-08-0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trotman LC, Niki M, Dotan ZA, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003;1:385–396. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma X, Ziel-van der Made AC, Autar B, et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res. 2005;65:5730–5739. doi: 10.1158/0008-5472.CAN-04-4519. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Z, Flesken-Nikitin A, Corney DC, et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 2006;66:7889–7898. doi: 10.1158/0008-5472.CAN-06-0486. [DOI] [PubMed] [Google Scholar]
- 13.Bruxvoort KJ, Charbonneau HM, Giambernardi TA, et al. Inactivation of Apc in the mouse prostate causes prostate carcinoma. Cancer Res. 2007;67:2490–2496. doi: 10.1158/0008-5472.CAN-06-3028. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Gao J, Lei Q, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/S1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 15.Liao CP, Zhong C, Saribekyan G, et al. Mouse models of prostate adenocarcinoma with the capacity to monitor spontaneous carcinogenesis by bioluminescence or fluorescence. Cancer Res. 2007;67:7525–7533. doi: 10.1158/0008-5472.CAN-07-0668. [DOI] [PubMed] [Google Scholar]
- 16.van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 17.Navone NM, Olive M, Ozen M, et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res. 1997;3:2493–2500. [PubMed] [Google Scholar]
- 18.Chen ME, Lin SH, Chung LW, Sikes RA. Isolation and characterization of PAGE-1 and GAGE-7. New genes expressed in the LNCaP prostate cancer progression model that share homology with melanoma-associated antigens. J Biol Chem. 1998;273:17618–17625. doi: 10.1074/jbc.273.28.17618. [DOI] [PubMed] [Google Scholar]
- 19.Ishikura N, Kawata H, Nishimoto A, Nakamura R, Ishii N, Aoki Y (2009) Establishment and characterization of an androgen receptor-dependent, androgen-independent human prostate cancer cell line, LNCaP-CS10. Prostate (in press) [DOI] [PubMed]
- 20.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–3330. [PubMed] [Google Scholar]
- 21.Jiao J, Wang S, Qiao R, et al. Murine cell lines derived from Pten null prostate cancer show the critical role of PTEN in hormone refractory prostate cancer development. Cancer Res. 2007;67:6083–6091. doi: 10.1158/0008-5472.CAN-06-4202. [DOI] [PubMed] [Google Scholar]
- 22.Choi J, Burns AA, Williams RM, et al. Core–shell silica nanoparticles as fluorescent labels for nanomedicine. J Biomed Opt. 2007;12:1–12. doi: 10.1117/1.2823149. [DOI] [PubMed] [Google Scholar]
- 23.Wu X, Wu J, Huang J, et al. Generation of a prostate epithelial cell specific Cre transgenic mouse model for tissue specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/S0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 24.Leong KG, Wang BE, Johnson L, Gao WQ. Generation of a prostate from a single adult stem cell. Nature. 2008;456:804–808. doi: 10.1038/nature07427. [DOI] [PubMed] [Google Scholar]
- 25.Nagy A, Gertsenstein M, Vintersten K, Behringer R. Manipulating the mouse embryo. A laboratory manual. 3. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2003. p. 389. [Google Scholar]
- 26.Leiblich A, Cross SS, Catto JW, Pesce G, Hamdy FC, Rehman I. Human prostate cancer cells express neuroendocrine cell markers PGP 9.5 and chromogranin A. Prostate. 2007;67:1761–1769. doi: 10.1002/pros.20654. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Z, Flesken-Nikitin A, Nikitin AY. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 2007;67:5683–5690. doi: 10.1158/0008-5472.CAN-07-0768. [DOI] [PubMed] [Google Scholar]
- 28.Isayeva T, Chanda D, Kallman L, Eltoum IE, Ponnazhagan S. Effects of sustained antiangiogenic therapy in multistage prostate cancer in TRAMP model. Cancer Res. 2007;67:5789–5797. doi: 10.1158/0008-5472.CAN-06-3637. [DOI] [PubMed] [Google Scholar]
- 29.Tran CP, Lin C, Yamashiro J, Reiter RE. Prostate stem cell antigen is a marker of late intermediate prostate epithelial cells. Mol Cancer Res. 2002;1:113–121. [PubMed] [Google Scholar]
- 30.Hansel DE, Herawi M, Montgomery E, Epstein JI. Spindle cell lesions of the adult prostate. Mod Pathol. 2007;20:148–158. doi: 10.1038/modpathol.3800676. [DOI] [PubMed] [Google Scholar]
- 31.Shappell SB, Thomas GV, Roberts RL, et al. Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res. 2004;64:2270–2305. doi: 10.1158/0008-5472.CAN-03-0946. [DOI] [PubMed] [Google Scholar]
- 32.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 33.Yuan X, Li T, Wang H, et al. Androgen receptor remains critical for cell-cycle progression in androgen-independent CWR22 prostate cancer cells. Am J Pathol. 2006;169:682–696. doi: 10.2353/ajpath.2006.051047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]