Abstract
The purpose of this study was to summarise the best evidence to assess radiological outcomes of highly cross-linked polyethylene compared with conventional polyethylene in total hip arthroplasty. All randomised, controlled clinical trials comparing highly cross-linked polyethylene with conventional polyethylene were sought and then analysed by two independent reviewers using the Cochrane collaboration guidelines. Eight studies in seven articles were identified as eligible for inclusion. Due to the clinical and methodological heterogeneity, data from the studies included could not be pooled. No failures related to highly cross-linked polyethylene were reported. All highly cross-linked polyethylene groups had a significantly lower wear or penetration than conventional polyethylene groups. This preliminary result suggests that highly cross-linked polyethylene has significantly less wear than conventional polyethylene.
Résumé
Le but de cette étude est de résumer les données indiscutables dans les prothèses totales de hanche concernant l’évaluation du devenir radiologique du polyéthylène hautement réticulé comparé au polyéthylène conventionnel. Tous les essais contrôlés, randomisés comparant le polyéthylène hautement réticulé au polyéthylène conventionnel ont été analysés par deux reviewers indépendants à partir de la base de Cochrane. 8 études dont 7 articles ont été identifiés et inclus dans ce travail. Du fait de l’hétérogénéicité clinique et méthodologique de ces études, toutes les données n’ont pu être regroupées. Aucun échec du polyéthylène hautement réticulé n’a été rapporté. Dans ces études, tous les groupes concernant le polyéthylène hautement réticulé et ont une usure et une pénétration plus basses que le polyéthylène conventionnel ceci de façon significative. Ces données préliminaires permettent de penser que le polyéthylène hautement réticulé a de façon significative moins d’usure que le polyéthylène conventionnel.
Introduction
The metal-on-polyethylene bearing surface remains the most common articulation in total hip arthroplasty and has provided satisfactory results [1, 2, 24]. However, polyethylene wear, leading to periprosthetic osteolysis, is one of the most important causes of aseptic loosening and revision total hip arthroplasty [11, 18, 21].
To reduce wear and improve longevity of total hip arthroplasty procedures, highly cross-linked polyethylenes have emerged as an alternative bearing. In vitro studies have shown that these materials show great improvements in resistance to wear [13, 16, 20]. Nevertheless, hip simulator data is not always consistent with clinical findings [10, 14].
Since 1998, highly cross-linked polyethylene has been introduced for clinical use in total hip arthroplasty. Although many clinical studies reported dramatic wear reduction, most of them were not randomised, controlled trials and lacked high-level clinical evidence. Moreover, no systematic review or meta-analysis has been published on this matter.
We therefore performed this systematic review to summarise the best evidence in the literature to assess wear performance of highly cross-linked polyethylene acetabular liners compared with conventional polyethylene liners. We postulated that highly cross-linked polyethylene should have a significantly lower wear than conventional polyethylene.
Materials and methods
Search strategy
We searched Medline, EMBASE, Cochrane Central Register of Controlled Trials, and ISI Web of Science, with no restrictions on date of publication or language. The search strategy employed the following terms: highly cross-linked; polyethylene; randomised. The reference list of published trials was manually examined to find additional relevant studies. We also contacted experts in the field to identify additional studies. The closing date for retrieval of studies was 13 October 2008.
Selection criteria
We included randomised total hip arthroplasty clinical trials comparing highly cross-linked polyethylene liners with conventional polyethylene liners and excluded those trials which were only reported in proceedings with the full text unavailable. When data in studies was presented repeatedly, we included the longest follow-up outcomes.
One reviewer performed an initial title and abstract screening of articles to discard those which were clearly ineligible, then two reviewers independently examined the full article to assess the trials for eligibility for inclusion, with disagreements resolved by discussion. If necessary, we attempted to contact the author of the original reports to obtain further details.
Data extraction and assessment of risk of bias
Two reviewers independently extracted data from each trial including the location of the trial, study design, participants, liner type, methods of fixation, outcomes, follow-up duration, dropout or lost to follow-up data, and conflict of interest by using standardised forms. Risk of bias in the studies was assessed independently by at least two reviewers according to the criteria [12, 25]. The criteria involved the judgement for four features of interest including sequence generation, allocation sequence concealment, blinding, and incomplete outcome data. Any disagreements between reviewers arising at any stage were resolved by discussion when necessary with the help of a third reviewer.
Data analysis
Data could not be analysed using a meta-analysis due to the clinical and methodological heterogeneity in the available studies. They were expressed as mean and standard deviation.
Results
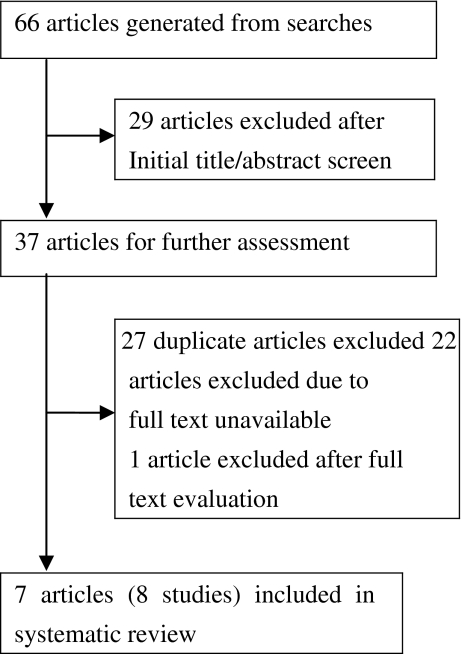
The search strategy generated 18 articles from Medline, 13 from EMBASE, 17 from Cochrane Central Register of Controlled Trials, and 18 from ISI Web of Science. Due to two studies reported by the same article [5], eight studies in seven articles [4, 5, 7–9, 15, 23] were identified as eligible for inclusion (Fig. 1). Four highly cross-linked polyethylene liners were used: Marathon in two studies [4, 7], Longevity in two [5, 9], Durasul in three [5, 8, 23], and Crossfire in one [15]. The types of hip implant and fixation method were different among studies.
Fig. 1.
Flow diagram showing selection process of studies included in systematic review
The randomisation process was described and appropriate for three studies [4, 9, 23]; the other studies mentioned randomisation allocation but lacked a description of the randomisation method [5, 7, 8, 15]. With respect to allocation concealment, four studies [4, 9, 15, 23] were adequate and four [5, 7, 8] were unclear. Regarding blinding of outcome assessment, six studies [4, 7–9, 15, 23] were adequate and two [5] were unclear. Four studies [5, 7, 15, 23] reported the rate of exclusion were greater than 15℅ and one study [4] did not describe the exact number of participants in both groups.
Characteristic details and risk of bias of seven of the studies are summarised in Tables 1 and 2, respectively. Table 3 shows the results of radiological evaluation of highly cross-linked polyethylene compared with conventional polyethylene. Due to clinical and methodological heterogeneity, data from the studies which included comparison of highly cross-linked polyethylene with conventional polyethylene could not be pooled and thus are described individually according to the polyethylene liners used.
Table 1.
Characteristics of the studies included
| Study | Design | Participants analysed | Linear | Fixation | Follow-up (y) | Notes |
|---|---|---|---|---|---|---|
| Clavert et al. [4] | RCT | Unclear | Marathon | Hybrid | 4 | Two participants died of causes unrelated to surgery. Evidence of conflict of interests |
| Enduron | ||||||
| Digas et al. [5]a | RCT | HXLPE = 28 | Durasul | Cemented | 5 | Five participants did not attend the follow-up. Evidence of conflict of intrests |
| CPE = 27 | Sulene | |||||
| Digas et al. [5]a | RCT | HXLPE = 19 | Longevity | Hybrid | 5 | 13 participants (26 hips) could not be evaluated. Evidence of conflict of intrests |
| CPE = 19 | CPE | |||||
| Engh et al. [7] | RCT | HXLPE = 76 | Marathon | Cementless | 4.3 | 226 participants included, 148 measured wear rate, 186 identified osteolysis Incidence. Evidence of conflict of intrests |
| CPE = 72 | Enduron | |||||
| Garcia-Rey et al. [8] | RCT | HXLPE = 45 | Durasul | Cementless | 5.5 | No osteolysis. No conflict of intrests |
| CPE = 45 | Sulene | |||||
| Glyn-Jones et al. [9] | RCT | HXLPE = 26 | Longevity | Hybrid | 3 | Three participants excluded. Evidence of conflict of intrests |
| CPE = 25 | CPE | |||||
| Martell et al. [15] | RCT | HXLPE = 24 | Crossfire | Cementless | 2.3 | 97 hips included, only 46 hips measured 2D wear and 29 hips 3D wear. Evidence of conflict of intrests |
| CPE = 22 | N2/Vac | |||||
| Triclot et al. [23] | RCT | HXLPE = 33 | Durasul | Hybrid | 4.9 | 35 participants excluded. Evidence of conflict of intrests |
| CPE = 34 | Sulene |
RCT randomised, controlled trial, HXLPE highly cross-linked polyethylene, CPE conventional polyethylene
aData from two studies reported in one article
Table 2.
Risk of bias of the studies included
| Study | Randomisation process | Allocation concealment | Blinding | Incomplete outcome data addressed |
|---|---|---|---|---|
| Clavert et al. [4] | Low | Low | Low | High |
| Digas et al. [5]a | Unclear | Unclear | Unclear | Low |
| Digas et al. [5]a | Unclear | Unclear | Unclear | High |
| Engh et al. [7] | Unclear | Unclear | Low | High |
| Garcia-Rey et al. [8] | Unclear | Unclear | Low | Low |
| Glyn-Jones et al. [9] | Low | Low | Low | Low |
| Martell et al. [15] | Unclear | Low | Low | High |
| Triclot et al. [23] | Low | Low | Low | High |
“High” indicates high risk of bias in the according stage; “Low” indicates low risk of bias in the according stage; “Unclear” indicates either lack of information or uncertainty over the potential for bias
aData from two studies reported in one article
Table 3.
Results of radiological evaluation of highly cross-linked polyethylene compared with conventional polyethylene
| Study | Outcomes | ||||
|---|---|---|---|---|---|
| Clavert et al. [4]a | Wear rate | Linear (mm/y) | 3D linear (mm/y) | Volume (mm3/y) | |
| p = 0.002 | p = 0.012 | p = 0.017 | |||
| HXLPE = 0.0239 | 0.0242 | 13.741 | |||
| CPE = 0.1276 | 0.1109 | 60.24 | |||
| Digas et al. [5]b | Penetration | M/L (mm) | P/D (mm) | A/P (mm) | Total (mm) |
| p = 0.3 | p < 0.001 | p = 0.5 | p < 0.001 | ||
| HXPEL = −0.019 ± 0.021 | 0.15 ± 0.030 | −0.01 ± 0.026 | 0.23 ± 0.030 | ||
| CPE = −0.05 ± 0.020 | 0.36 ± 0.046 | 0.02 ± 0.026 | 0.41 ± 0.046 | ||
| Digas et al. [5]b | Penetration | M/L (mm) | P/D (mm) | A/P (mm) | Total (mm) |
| p = 0.2 | p < 0.001 | p = 0.4 | p < 0.001 | ||
| HXLPE = 0.001 ± 0.0261 | 0.08 ± 0.020 | 0.09 ± 0.036 | 0.20 ± 0.026 | ||
| CPE = −0.04 ± 0.015 | 0.34 ± 0.067 | 0.03 ± 0.036 | 0.41 ± 0.056 | ||
| Engh et al. [7] | Wear rate Osteolysis |
Linear 2D (mm/y) | Volume(mm3/y) | Osteolysis | Osteolysis>1cm2 |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.002 | ||
| HXLPE = 0.01 ± 0.07 | 5 ± 22 | 23(96) | 6(96) | ||
| CPE = 0.20 ± 0.13 | 107 ± 76 | 52(90) | 20(90) | ||
| Garcia-Rey et al. [8] | Penetration rate | Linear (mm/y) | |||
| p < 0.001 | |||||
| HXLPE = 0.006 ± 0.007 | |||||
| CPE = 0.038 ± 0.013 | |||||
| Glyn-Jones et al. [9] | Wear rate Penetration |
Linear wear (mm/y) | Total penetration (mm) | ||
| p = 0.012 | p = 0.0184 | ||||
| HXLPE = 0.03 ± 0.06 | 0.35 ± 0.14 | ||||
| CPE = 0.07 ± 0.05 | 0.45 ± 0.19 | ||||
| Martell et al. [15] | Penetration rate | 2D linear (mm/y) | 2D volume (mm3/y) | 3D linear | 3D volume |
| p = 0.001 | p = 0.049 | p = 0.005 | p = 0.199 | ||
| HXLPE = 0.12 ± 0.05 | 62.07 ± 34.15 | 0.14 ± 0.07 | 62.72 ± 30.48 | ||
| CPE = 0.20 ± 0.10 | 90.89 ± 52.74 | 0.29 ± 0.17 | 101.77 ± 62.71 | ||
| Triclot et al. [23] | Penetration rate | Linear (mm/y) | Volume (mm3/y) | ||
| p = 0.0027 | p = 0.0058 | ||||
| HXLPE = 0.025 ± 0.128 | 29.24 ± 44.08 | ||||
| CPE = 0.106 ± 0.109 | 53.32 ± 48.68 | ||||
HXLPE highly cross-linked polyethylene, CPE conventional polyethylene, M/L medial(+)/lateral(-), P/D proximal(+)/distal(-), A/P anterior(+)/posterior(-)
aThe outcomes can not be expressed as mean and standard deviation due to lack of the exact number of participants analysed in the study
bData from two studies reported in one article
No failures related to polyethylene liners were reported in the studies included. Two studies compared wear of Marathon polyethylene liners with that of Enduron liners. Clavert et al. [4] reported significant differences between the two groups in linear (p = 0.002), 3D linear (p = 0.012), and volumetric wear rate (p = 0.017). Similarly, Engh et al. [7] reported that the Marathon group was significantly lower in the 2D linear and volumetric wear rate than those in the Enduron group (p < 0.001 and p < 0.001). Meanwhile, they found significantly lower incidence of any osteolysis (p < 0.001) and osteolysis more than 1 cm2 (p = 0.002) in the Marathon group.
Three studies reported the penetration results of Durasul polyethylene liners compared with Sulene liners. Digas et al. [5] found that the Durasul group was significantly lower in proximal and total head penetration (p < 0.001 and p < 0.001), not in medial and anterior head penetration (p = 0.3 and p = 0.5). In another randomised trial, Garcia-Rey et al. [8] reported that there was a significant difference in penetration rate (p < 0.001) in favour of the Durasul group. Similarly, Triclot et al. [23] described that the Durasul group had significantly lower linear and volumetric penetration rate (p = 0.0027 and p = 0.0058).
Two studies compared penetration of Longevity polyethylene liners with that of conventional liners. Digas et al. [5] found that the Durasul group was significantly lower in proximal and total head penetration (p < 0.001 and p < 0.001), but not in medial and anterior head penetration (p = 0.2 and p = 0.4). Gly-Jones et al. [9] reported that the Longevity group was significantly superior to the control group in linear wear rate (p = 0.012) and total penetration (p = 0.0184).
Comparing Crossfire polyethylene liners with conventional liners, Martell et al. [15] reported significantly lower penetration rates in 2D linear (p = 0.001), 2D volumetric (p = 0.049), and 3D linear (p = 0.005) types. They also noted that the Crossfire group had showed a trend for reduction in 3D volumetric wear rate.
Discussion
We have summarised the best evidence from randomised, controlled trials comparing highly cross-linked polyethylene with conventional polyethylene in total hip arthroplasty. Although the quality of the trials included were different, all highly cross-linked polyethylene groups had a significantly lower wear or penetration than conventional polyethylene groups. This shows that highly cross-linked polyethylene can significantly reduce wear.
In a prospective non-randomised study, Dorr et al. [6] reported a significantly lower linear wear rate of 0.029 ± 0.02 mm/y in the Durasul group compared with 0.065 ± 0.03 mm/y in the Sulene group after the initial bedding-in penetration. This was consistent with the results from this systematic review. Similarly, in two retrospective comparative studies with five-year follow-up, Olyslaegers et al. [17] and Rajadhyaksha et al. [19] reported an almost 51% and 74% reduction of wear rate, respectively, for highly cross-linked polyethylene after the bedding-in period.
There are some important limitations to note in our work. First, there was significant clinical and methodological heterogeneity between the studies included. They differed with respect to outcomes, methods of radiological evaluation, methodological quality, manufacturing process of highly cross-linked polyethylene, implants, fixation methods and surgical techniques, which prevented data from being pooled. Second, due to the novelty of highly cross-linked polyethylene, the follow-up periods were short, which prevented us from making firm decisions about some important results such as incidence of osteolysis and revision total hip arthroplasty. Third, conflict of interest is an issue that requires special consideration. In this review, authors in seven studies received or will receive benefits from commercial parties. Some studies [3, 22] reported that the research more likely favoured the sponsor’s product when an investigator had a financial interest in or funding from companies.
Conclusions
This systematic preliminary review suggests that highly cross-linked polyethylene has significantly less wear than conventional polyethylene. Further follow-up is necessary to determine the safety and incidence of osteolysis of highly cross-linked polyethylene.
Footnotes
The authors have not received nor will receive benefits from any source for this study.
References
- 1.Berry DJ, Harmsen WS, Cabanela ME, et al. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Callaghan JJ, Templeton JE, Liu SS, et al. Results of Charnley total hip arthroplasty at a minimum of thirty years. A concise follow-up of a previous report. J Bone Joint Surg Am. 2004;86:690–695. doi: 10.2106/00004623-200404000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Chren MM, Landefeld CS. Physicians’ behavior and their interactions with drug companies. JAMA. 1994;271:684–689. doi: 10.1001/jama.271.9.684. [DOI] [PubMed] [Google Scholar]
- 4.Clavert GT, Devane PA, Fielden J et al (2008) A double-blind, prospective, randomized controlled trial comparing highly cross-linked and conventional polyethylene in primary total hip arthroplasty. J Arthroplasty. doi:10.1016/j.arth.2008.02.011 [DOI] [PubMed]
- 5.Digas G, Karrholm J, Thanner J, et al. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop. 2007;78(6):746–754. doi: 10.1080/17453670710014518. [DOI] [PubMed] [Google Scholar]
- 6.Dorr LD, Wan Z, Shahrdar C, et al. Clinical performance of a Durasul highly cross-linked polyethylene acetabular liner for total hip arthroplasty at five years. J Bone Joint Surg Am. 2005;87(8):1816–1821. doi: 10.2106/JBJS.D.01915. [DOI] [PubMed] [Google Scholar]
- 7.Engh CA, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip arthroplasty using cross-linked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty. 2006;21:17–25. doi: 10.1016/j.arth.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, et al. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br. 2008;90(2):149–153. doi: 10.1302/0301-620X.90B2.19887. [DOI] [PubMed] [Google Scholar]
- 9.Glyn-Jones S, Mclardy-Smith P, Gill HS, et al. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br. 2008;90(5):556–561. doi: 10.1302/0301-620X.90B5.20545. [DOI] [PubMed] [Google Scholar]
- 10.Graeter JH, Nevins R. Early osteolysis with Hylamer acetabular liners. J Arthroplasty. 1998;13:464–466. doi: 10.1016/S0883-5403(98)90016-X. [DOI] [PubMed] [Google Scholar]
- 11.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 12.Higgins JPT, Altman DG (2008) Assessing risk of bias in included studies. In: Higgins JPT, Green S (eds) Cochrane handbook for systematic reviews of intervention version 5.0.0. The Cochrane Collaboration. Available via DIALOG. http://www.cochrane-handbook.org
- 13.Kurtz SM, Muratoglu OK, Evans M, et al. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials. 1999;20:1659–1688. doi: 10.1016/S0142-9612(99)00053-8. [DOI] [PubMed] [Google Scholar]
- 14.Livingston BJ, Chmell MJ, Spector M, et al. Complications of total hip arthroplasty associated with the use of an acetabular component with a Hylamer liner. J Bone Joint Surg Am. 1997;79:1529–1538. doi: 10.2106/00004623-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Martell JM, Verner JJ, Incavo SJ. Clinical performance of a highly cross-linked polyethylene at two years in total hip arthroplasty: a randomized prospective trial. J Arthroplasty. 2003;18:55–59. doi: 10.1016/S0883-5403(03)00341-3. [DOI] [PubMed] [Google Scholar]
- 16.Muratoglu OK, Bragdon CR, O’Connor DO, et al. A novel method of cross-linking ultra-high-molecular-weight polyethylene to improve wear, reduce oxidation, and retain mechanical properties. J Arthroplasty. 2001;16:149–160. doi: 10.1054/arth.2001.20540. [DOI] [PubMed] [Google Scholar]
- 17.Olyslaegers C, Defoort K, Simon JP, et al. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty. 2008;23(4):489–494. doi: 10.1016/j.arth.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Oparaugo PC, Clarke IC, Malchau H, et al. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand. 2001;72:22–28. doi: 10.1080/000164701753606644. [DOI] [PubMed] [Google Scholar]
- 19.Rajadhyaksha AD, Brotea C, Cheung Y et al (2008) Five-year comparative study of highly cross-linked (crossfire) and traditional polyethylene. J Arthroplasty. doi:10.10.16/j.arth. 2007. 09015 [DOI] [PubMed]
- 20.Saikko V, Calonius O, Keranen J. Wear of conventional and cross-linked ultra-high-molecular-weight polyethylene acetabular cups against polished and roughened CoCr femoral heads in a biaxial hip stimulator. J Biomed Mater Res. 2002;63:848–853. doi: 10.1002/jbm.10471. [DOI] [PubMed] [Google Scholar]
- 21.Sochart DH. Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop Relat Res. 1999;363:135–150. doi: 10.1097/00003086-199906000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Stelfox HT, Chua G, O’Rourke K, et al. Conflict of interest in the debate over calcium-channel antagonists. N Engl J Med. 1998;338:101–106. doi: 10.1056/NEJM199801083380206. [DOI] [PubMed] [Google Scholar]
- 23.Triclot P, Grosjean G, Masri F, et al. A comparison of the penetration rate of two polyethylene acetabular liners of different levels of cross-linking. A prospective randomised trial. J Bone Joint Surg Br. 2007;89:1439–1445. doi: 10.1302/0301-620X.89B11.19543. [DOI] [PubMed] [Google Scholar]
- 24.Wroblewski BM, Fleming PA, Siney PD. Charnley low-frictional torque arthroplasty of the hip. 20-to-30 year results. J Bone Joint Surg Br. 1999;81:427–430. doi: 10.1302/0301-620X.81B3.9521. [DOI] [PubMed] [Google Scholar]
- 25.Wu TX, Liu GJ. The concepts, design, practice and reports of allocation concealment and blinding. Chin J Evid-based Med. 2007;73:222–225. [Google Scholar]