Abstract
The objectives of this paper were to look into the possible incidence of obvious soft tissue extension from Langerhans’ cell histiocytosis (LCH) of the spine in children and to evaluate the effects of chemotherapy for those patients. Eighteen patients with histopathological diagnosis of LCH were reviewed and nine with obvious paravertebral soft tissue extension were included in this study. Soft tissue extension was involved in the spinal canal and/or around the vertebral body in eight cases and posterior involvement was prevalent in one case. Eight patients experienced neurological symptoms. All received chemotherapy and one had surgical treatment. The mean follow-up time was 30.3 months. Soft tissue extension disappeared completely in all patients. No clinical evidence of disease was observed at the most recent follow-up. The incidence of LCH of the spine in children with obvious soft tissue extension was up to 50%. Chemotherapy is safe and effective, and surgical decompression was probably not necessary for most patients.
Résumé
Objectif de l’étude : vérifier l’incidence de la pénétration tissulaire d’une histiocytose, à cellules Langerhancienne (LCH) au niveau du rachis de l’enfant et évaluer les effets de la chimiothérapie. Matériel et méthode : 18 patients avec diagnostic histologique du LCH ont été revus. 9 présentaient une invasion évidente des tissus mous et ont été inclus dans cette étude. Résultat : l’extension tissulaire a été localisée au niveau du canal médullaire ou autour du corps vertébral chez 8 patients. Cette localisation a été uniquement postérieure chez un patient. Huit présentaient des signes neurologiques et tous ont reçu de la chimiothérapie et un seul un traitement chirurgical. Le suivi moyen a été de 30,3 mois. La pénétration tissulaire a complètement disparu chez tous les patients. Il n’y a pas eu de récidive clinique de la maladie au plus récent recul. Conclusion : l’incidence dans la LCH de la pénétration tissulaire au niveau du rachis de l’enfant, est de 50%, de la chimiothérapie est un traitement sûr et efficace, il n’est pas nécessaire d’envisager une décompression chirurgicale dans la plupart des cas.
Introduction
Langerhans’ cell histiocytosis (LCH) is a rare disease associated with a proliferation of Langerhans’ cells [1, 6] and encompasses three disparate diagnoses: Letterer-Siwe disease, Hand-Schüller-Christian disease and eosinophilic granuloma (EG) [7]. Up to 80% of LCH lesions in children are of EG type [2]. The incidence of spinal involvement varies from 6.5% [5] to 25% [17] of cases with LCH of the skeleton. Eighty percent of cases occur in children younger than 10 years of age [4].
LCH of the spine usually develops in the vertebral body with an osteolytic appearance and leads to what is classically known as “vertebra plana” [9, 15]. Reports on adjacent paravertebral soft tissue mass in LCH of the spine are rare in the literature [10, 16, 18, 25]. However, LCH may be more aggressive, which may make the lesions look like a malignancy, which appear as bubbly and lytic lesions, and expand posterior elements with a soft tissue mass [8]. Since the introduction of magnetic resonance imaging (MRI), soft tissue extension from the vertebral body of LCH into the spinal canal and paraspinal soft tissue has become a well known entity [3, 12, 20–22]. However, the real incidence of soft tissue extension remains unknown because reports are either case reports or reviews, which do not address the incidence of soft tissue extension from LCH of the spine in children.
Various treatments of LCH of the spine have been proposed, including prolonged bed rest, immobilisation with cast and brace, hormone, chemotherapy, radiation therapy and surgery [11, 13, 16]. The treatment of LCH of the spine in children with obvious soft tissue extension, especially those with neurological deficit, is still controversial. Most researchers suggested that the neural elements should be decompressed and then fused [13, 20]. Some investigators advocated immobilisation and radiation [12]. A few recommended hormone or chemotherapy [21, 22]. In those case reports and small case series, however, the most suitable treatment for the patients is unclear.
The purpose of this study was to look into the possible incidence of obvious soft tissue extension from LCH of the spine in children and to evaluate the effects of chemotherapy on these conditions.
Patients and methods
This study has been approved by the ethics committee of our hospital. Eighteen children under 15 years old with LCH of the spine, confirmed by histopathological examination, were treated at the authors’ institution between 2000 and 2006. All of the patients had radiography and magnetic resonance imaging (MRI), and 12 patients underwent computed tomography (CT) scanning. These images were reviewed independently by two radiological specialists. The patients who had obvious paravertebral and/or intraspinal (extra-osseous) soft tissue extension diagnosed consistently by both radiological specialists were included in this study.
Clinical information was collected from the records, including age, sex, medical history, symptoms, neurological findings, radiographs, CT and MR images. All of the patients had biopsy and histological findings. Treatment was identified as using a brace, steroids, chemotherapy or surgical treatment. None of the patients accepted radiotherapy. The ultrasonography of the liver and spleen was assessed. Hepatic function testing, complete blood count, urine osmolarity and erythrocyte sedimentation (ESR) were measured in all cases. Bone marrow aspiration and biopsy of the iliac crest were performed in four patients.
The Oucher pain scale (0–10) was used to assess the local and radicular pain before treatment, 2 weeks, 6 weeks and 3 months after treatment, and at final follow-up [24].
The shape of the collapse was categorised as either symmetrical (vertebra plana, involving the vertebral body uniformly) or asymmetrical (more collapse on one side or anterior wedging) in the radiographs. Paravertebral and intraspinal soft tissue extension and the involved element (vertebral body, posterior elements or both) were determined by CT and MRI scans.
Clinical data were obtained from admitting, readmitting records, follow-up interviews or phone reviews, and X-rays, MRI and/or CT.
Results
Clinical manifestations and radiographic findings
Of the 18 patients with LCH of the spine in children, nine (50%; seven boys and two girls) had obvious soft tissue extension in the paravertebral and/or intraspinal mass (Table 1). The average age of these patients at diagnosis was 7.7 years (range, 3.9–13 years). All patients had only one vertebra involved. In these patients, two were cervical, five were thoracic, one was lumbar and one was sacral. One patient had extraspinal skeletal lesions at the scapula and femoral neck.
Table 1.
Clinical and radiological data on nine children with Langerhans’ cell histiocytosis (LCH) of the spine with obvious soft tissue extension
| Case | Sex | Age at diagnosis (years) | Site of spine lesions | Pain site | Pain time | Neurological symptoms | Vertebral collapse | Site of extension | Treatment | Follow-up (months) | Clinical results |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 8.5 | T3 | Back | 1 month | Increased KJ, ankle clonus | Vertebra plana | Circumferential | Chemotherapy | 48 | Normal |
| 2 | M | 10 | T12 | Back | 10 month | Radicular pain, ankle clonus, increased KJ, Oppenheim sign (+) | Vertebra plana | Circumferential | Chemotherapy | 44 | Normal |
| 3 | M | 7 | C5 | Neck | 40 days | Radicular pain (right arm) | Lateral wedging | Paravertebral-unilateral | Chemotherapy | 40 | Normal |
| 4 | M | 4.3 | S1 | Lower back | 1 month | Radicular pain (right leg) | Lateral wedging | Paravertebral-unilateral | Chemotherapy | 37 | Normal |
| 5 | M | 3.9 | C2 | Neck | 1 month | – | No | Posterior to lamima | Chemotherapy | 28 | Normal |
| 6 | F | 9.8 | T7 | Back | 6 months | Paraplegia (Frankel B) | Anterior wedging | Circumferential | Chemotherapy+surgery | 26 | Mild kyphosis |
| 7 | M | 13 | T7 | Back | 10 days | Weakness of the lower extremities (4/5) | Lateral wedging | Paravertebral-unilateral | Chemotherapy | 18 | Normal |
| 8 | M | 5.7 | T4 | Back | 1 month | Paraplegia (Frankel C) | Vertebra plana | Spinal canal | Chemotherapy | 18 | Normal |
| 9 | M | 7 | L3 | Lower back | 1 month | Weakness of the left lower extremity (4/5) | Lateral wedging | Paravertebral-unilateral | Chemotherapy | 14 | Mild kyphosis |
The most common initial symptom was local pain in all patients. The Oucher pain scale ranged from 3 to 7 (mean 4.8). Three patients suffered radicular pain; the Oucher pain scale was from 3 to 8 (mean 5.3). Two patients had a history of mild trauma. Two patients had kyphosis (cases 6 and 9). Eight patients (88.9%) experienced neurological symptoms, including radicular pain in two patients, mild neurological deficits in three patients (mild weakness of the lower extremities, increased knee jerk, pyramidal tract signs, ankle clonus), radicular pain and mild neurological deficits in one, and severe neurological deficit in two patients.
All patients had radiographs and MRI, and six had CT scan. The level involved was a single vertebra. Only three vertebrae had symmetrical collapse of the vertebral body (vertebra plana); five vertebrae showed asymmetrical collapse (Fig. 1) and one patient had only involvement of the posterior elements of C2.
Fig. 1.
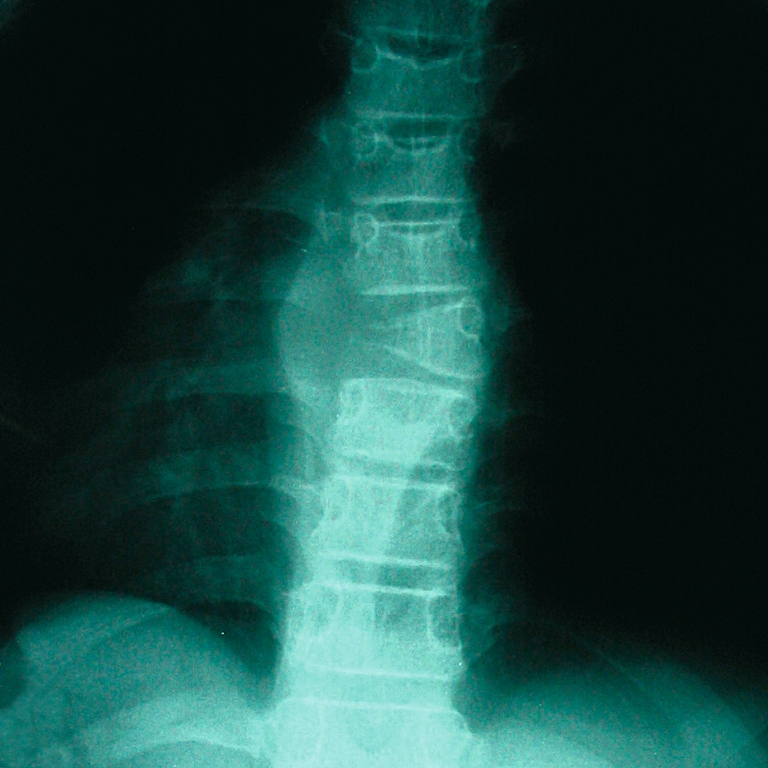
Atypical radiographical presentation of the vertebral involvement of Langerhans’ cell histiocytosis (LCH) of the spine in children with soft tissue mass. One lateral erosion with soft tissue mass of T7 (case 7)
Paravertebral soft tissue extension was seen in four patients’ plain radiographs. MRI and/or CT showed the main involvement in the vertebral body in four patients and only posterior elements in one. The rest of the patients showed involvement in the vertebral body and posterior elements.
Soft tissue extension was involved in the spinal canal or foramena and caused neurological compression in eight patients. The main involvement was in the spinal canal in one patient, around the vertebral body and intraspinal involvement in three patients (Fig. 2), one side of the vertebrae and intraspinal involvement in four patients, posterior involvement and no intraspinal compression in one patient (Fig. 3).
Fig. 2.
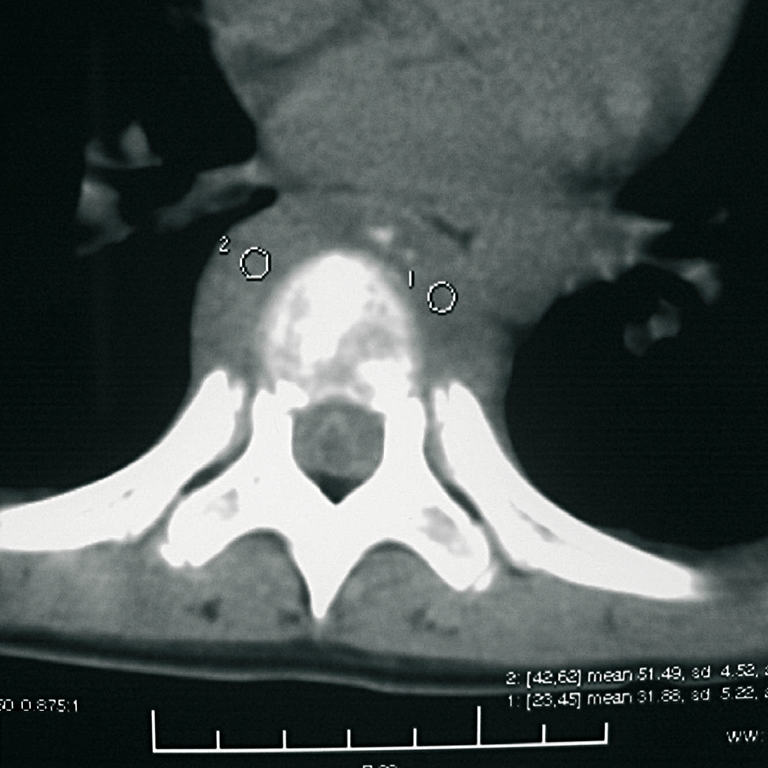
LCH of T7. Axial computed tomography (CT) image demonstrating circumferential soft tissue extension from the vertebral body (case 6)
Fig. 3.
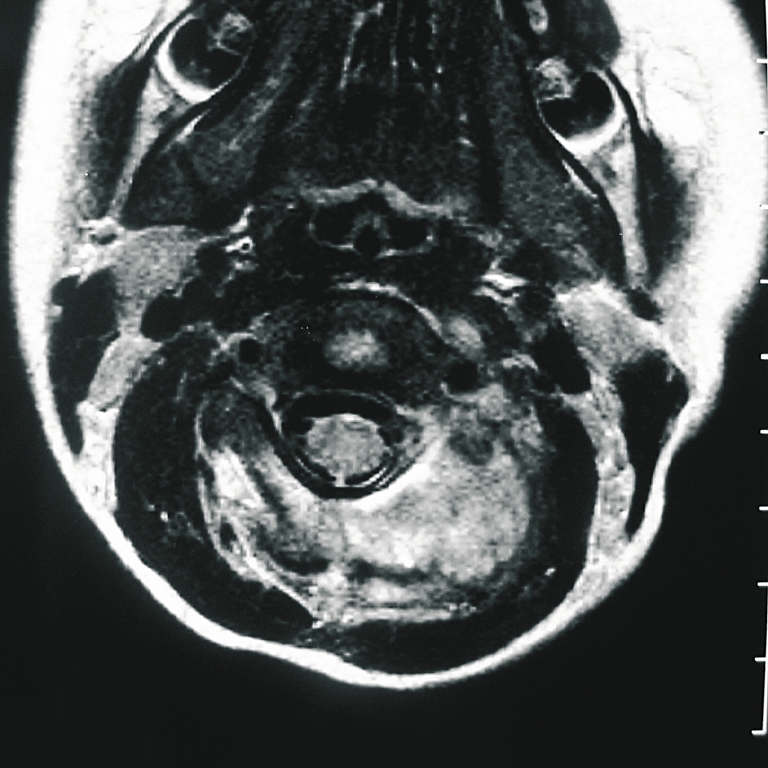
LCH of C2. Magnetic resonance imaging (MRI) view (T1-weighted with gadolinium-enhanced image) shows the prominent extension of soft tissue only into one side of the posterior arch (case 5)
Diagnosis, treatment and clinical responses
All cases were misdiagnosed, such as solid tumour in three, malignant solid tumours in three, lymphoma in one and tuberculosis in two, in other hospitals before admission to our unit. The initial radiological and clinical diagnoses in five patients were LCH in the authors’ institution, and four were misdiagnosed as lymphoma, aneurysmal bone cyst, tuberculosis and malignant solid tumour. The diagnosis was confirmed by biopsy in all patients (Fig. 4). Three patients’ specimens were obtained from an open biopsy operation and six patients’ specimens were obtained from needle biopsy. Of the needle biopsies, four were guided with CT and two were guided with ultrasonography. The ESR was found to be over 20 mm/h in two patients. There was no evidence of the involvement of organ systems.
Fig. 4.
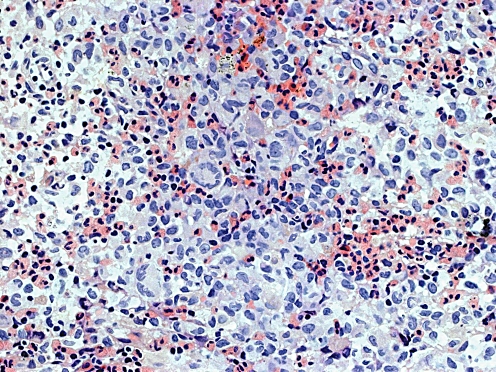
High-power photomicrograph shows Langerhans’ histiocytes and mixed numerous eosinophils. A few of the histiocytes have fused to form multinucleated giant cells (haematoxylin and eosin stain, ×400) (case 5)
All patients received chemotherapy, including vincristine, prednisone, methotrexate and 6-Mercaptopurine. The chemotherapy protocols are shown in Table 2. Only one patient had both surgical treatment and chemotherapy (case 6). Four cases received analgesic treatment before chemotherapy and withdrew analgesics at the beginning of chemotherapy. Six patients received additional bracing. Seven patients were bed-ridden for 1 to 4 weeks. One patient (case 7) had a pathological fracture involving the left femoral neck and received surgical treatment for the femoral neck fracture with curettage, bone graft and internal fixation. Two patients (case 1 and case 2) were misdiagnosed as tuberculosis and received antibiotics for two weeks, one in another hospital and one in ours. All of the patients had no serious side effect with chemotherapy. Three had vomiting and two had slight and transient elevation in hepatic enzymes when they received chemotherapy.
Table 2.
Chemotherapy agents and regimens used to treat LCH of the spine with obvious soft tissue extension in children
| Agents | Dose | Regimens |
|---|---|---|
| Vincristine | 0.5–1 mg/m2 | IV, per week (3 months) |
| IV, per two weeks (3 months) | ||
| IV, per four weeks (3 months) | ||
| Methotrexate | 5–10 mg/m2 | IV, per week (3 months) |
| IV, per two weeks (3 months) | ||
| IV, per four weeks (3 months) | ||
| Prednisone | 5 mg/m2 | Oral, per day (9 months) |
| 6-Mercaptopurine | 10 mg/m2 | Oral, per day (6 months) |
| 5 mg/m2 | Oral, per day (3 months) |
mg/m2=dose per body surface area; IV=intravenous
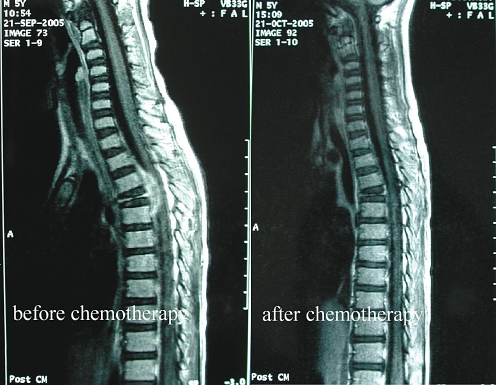
One patient (case 8) with severe neurological deficit was only treated by chemotherapy and a brace. Surprisingly, his neurological function rapidly improved on the second day of chemotherapy, then gradually continued to improve and completely recovered in three months. The prominent soft tissue mass, which intruded on the spinal canal, completely disappeared in one month after being given chemotherapy (Fig. 5). Due to severe neurological deficits and a major kyphosis after injury, one patient (case 6) had an emergency operation with posterior decompression, corpectomy, anterior fusion with cage and pedicle screw fixation, and additional posterior fusion. Her muscle function of the lower extremities began to recover one month postoperatively. For mild neurological deficits caused by soft tissue extension, case 9 only received chemotherapy, although the patient had kyphosis.
Fig. 5.
LCH of T4. MRI views (T1-weighted with gadolinium-enhanced image) shows the prominent soft tissue mass intruding into the spinal canal, which disappeared completely one month after chemotherapy (case 8)
Local pain and radicular pain was gradually relieved after the first cycle of chemotherapy. The Oucher pain scale of local pain was from 0 to 3 (mean 1.3) in 2 weeks, 0 to 2 (mean 0.8) in 6 weeks and 0 to 1 (mean 0.2) in 3 months after chemotherapy. The Oucher pain scale of radicular pain was 0 to 3 (mean 1.0) in 2 weeks and 0 in 6 weeks after chemotherapy. The patients with mild neurological deficits also began to recover after the first cycle of chemotherapy.
Follow-up varied from 14 to 48 months (mean 30.3 months). No clinical evidence of disease in all patients, such as pain or discomfort, was observed at the most recent follow-up. MRI scans were taken at the different follow-up times (one month to two years) after treatment in all patients. The MRI scans showed that obvious soft tissue extension completely disappeared in all patients. Only two patients were left with mild kyphosis but no symptoms. Neurological deficits completely recovered.
Discussion
The incidence of LCH of the spine in children with soft tissue extension was not made clear in the literature because most reports were case reports, small case series [12, 21, 22] or reviews mentioning the occurrence of soft tissue extension [20]. In this series, the incidence of LCH of the spine in children with soft tissue extension was 50%.
Although many investigators concluded that neurological symptoms were uncommon in patients with LCH of the spine in children [9, 11, 18, 25], neurological symptoms occurred in up to 88.9% of the patients with obvious soft tissue extension in this series. All of the patients with neurological symptoms had intraspinal or foraminal involvement with soft tissue masses, especially two cases with severe neurological deficits. Obviously, soft tissue extension caused the high incidence of neurological symptoms in the patients. Sanchez et al. [19] observed neurological deficits in 64% of patients with thoracic EG and in 75% of patients with lumbar EG, but only 14% of patients with thoracic and 12% with lumbar EG having muscular symptoms. Bertram et al. [3] performed a meta-analysis of 53 cases, which indicated that neurological symptoms were experienced in 33% of patients with cervical EG.
In this series, it is difficult to make a diagnostic decision. Of nine patients, only three had typical “vertebra plana” in roentgenograms. Five vertebrae showed asymmetrical collapse and one patient had only involvement of the posterior elements. The patients are easily misdiagnosed with malignant tumours, more aggressive tumours, lymphoma or tuberculosis. On the other hand, vertebra plana with soft tissue mass can be also seen in variety of spine tumours, including Ewing’s sarcoma, lymphoma, aneurysmal bone cyst, leukemia, malignant solid tumours and metastatic tumours [8–10, 13]. Spinal biopsy is necessary for a definitive diagnosis for those atypical cases, as in this series, although some authors insist that typical lesions in radiographs do not require biopsy and can be safely followed with serial radiography [25].
Various methods of treatment have been proposed, including prolonged bed rest and immobilisation with a spinal brace, chemotherapy, hormone, radiation therapy and surgery [11, 13, 16]. Since LCH of the spine as a single lesion is a benign tumour with a self-limiting process, typical lesions confined to the spine as single or dual lesions can be satisfactorily treated with conservative observation, wearing a spinal orthoses or casting with or without initial bed rest [16]. However, once the patients have obvious soft tissue extension, especially with the spinal canal involvement, the consequences of the disease will be a neurological disaster in the patients. For this situation, adequate management is needed to avoid the risk, but it remains unknown as to what kind of management is the most suitable for this kind of patient.
Most authors have recommended surgical treatment in the cases with neurological deficits [13, 20]. In our series, we only resorted to an emergency operation in the spine for one patient. The others received non-operative treatment in order to avoid major surgical intervention, especially to young patients.
Green et al. [12] successfully managed two patients with neural deficit using radiation therapy. They suggested that surgical decompression of these lesions was probably not necessary because any neural deficit would quickly disappear after biopsy and irradiation. In spite of the facts, there is still no clear evidence as to whether radiation therapy is beneficial to the patients [15, 20]. There is also a risk of secondary malignancy after radiotherapy [14]. Garg et al. [11] indicated that the risk associated with radiation therapy was unacceptable to give an overwhelmingly positive natural history of this disorder.
Although chemotherapy was proposed as a systemic treatment for multi-site bone lesions and multiple system involvements, there was no evidence as to whether chemotherapy is a good option in the management of LCH of the spine in children with soft tissue extension, especially the patients with neurological deficits. In this study, we showed that chemotherapy was able to rapidly reduce the intraspinal soft tissue mass and to relieve the local pain and radicular pain, even with spinal cord and nerve root compression. Eight of nine patients were cured only by chemotherapy. Moreover, only slight side effects were observed in this series of patients. This indicates that chemotherapy is a safe and effective method to treat patients with LCH of the spine in children with soft tissue extension, and surgical decompression is probably not necessary for most patients. These results were consistent with other reports in the treatment of LCH of the spine in children [21, 22]. Tanaka et al. [22] used prednisone and methotrexate to treat two of cases of LCH of the atlas, and they seem to be safe and effective. Tan et al. [21] treated four children with cervical LCH with only prednisone; all patients recovered completely and retained normal function of the cervical spine. Womer et al. [23] described using alternate-day prednisone and weekly methotrexate for low-risk LCH. These authors concluded that low-dose chemotherapy was safe and effective.
Based on the results in this series, we suggest a protocol for the management of spinal LCH in children with soft tissue extension as follows. If the patients have intraspinal involvement by soft tissue mass and no spinal instability, but are neurologically intact or have only minor neurological deficits, chemotherapy is recommended. If the patients have intraspinal involvement by soft tissue mass and no spinal instability, but moderate or severe neurological deficits, chemotherapy can be still the first option under careful monitoring of the neurological function. If the neurological deficits are more severe or the neurological function has not recovered after a short period of time, surgical treatment is recommended. If the patient has spinal instability or neurological deficits caused by kyphosis, surgical treatment is also recommended.
Conclusions
The incidence of Langerhans’ cell histiocytosis (LCH) of the spine in children with obvious soft tissue extension was up to 50% and most of the patients experienced neurological symptoms in this series. For these patients, chemotherapy is a safe and effective treatment. Surgical decompression is probably not necessary for most patients. Only with spinal instability or neurological deficits caused by kyphosis is surgical treatment recommended.
Acknowledgements
The authors thank Dr. Monroe I. Levine from the Center for Spinal Disorders in Denver, CO, for his help with this paper. This work was supported by the Provincial Natural Science Foundation of Guangdong (grant no. 06021290).
References
- 1.Aster J, Kumar V. White cells, lymph nodes, spleen, and thymus. In: Cotran RS, Kumar V, Collins T, Robbins SL, editors. Robbins pathologic basis of disease. 6. Philadelphia: Saunders; 1999. pp. 644–686. [Google Scholar]
- 2.Azouz EM, Saigal G, Rodriguez MM, et al. Langerhans’ cell histiocytosis: pathology, imaging and treatment of skeletal involvement. Pediatr Radiol. 2005;35:103–115. doi: 10.1007/s00247-004-1262-0. [DOI] [PubMed] [Google Scholar]
- 3.Bertram C, Madert J, Eggers C. Eosinophilic granuloma of the cervical spine. Spine. 2002;27:1408–1413. doi: 10.1097/00007632-200207010-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bilge T, Barut S, Yaymaci Y, et al. Solitary eosinophilic granuloma of the lumbar spine in an adult. Case report. Paraplegia. 1995;33:485–487. doi: 10.1038/sc.1995.107. [DOI] [PubMed] [Google Scholar]
- 5.Bunch WH. Orthopedic and rehabilitation aspects of eosinophilic granuloma. Am J Pediatr Hematol Oncol. 1981;3:151–156. doi: 10.1097/00043426-198100320-00006. [DOI] [PubMed] [Google Scholar]
- 6.Cheyne C. Histiocytosis X. J Bone Joint Surg Br. 1971;53:366–382. [PubMed] [Google Scholar]
- 7.Favara BE, Feller AC, Pauli M, et al. Contemporary classification of histiocytic disorders. The WHO Committee on Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29:157–166. doi: 10.1002/(SICI)1096-911X(199709)29:3<157::AID-MPO1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 8.Fowles JV, Bobechko WP. Solitary eosinophilic granuloma in bone. J Bone Joint Surg Br. 1970;52:238–243. [PubMed] [Google Scholar]
- 9.Garg S, Dormans JP. Primary tumor of the spine in children: a review from the Pediatric Musculoskeletal Tumor Program at the Children’s Hospital of Philadelphia. Univ Penns Orthop J. 2003;16:19–29. [Google Scholar]
- 10.Garg S, Dormans JP. Tumors and tumor-like conditions of the spine in children. J Am Acad Orthop Surg. 2005;13:372–381. doi: 10.5435/00124635-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Garg S, Mehta S, Dormans JP. Langerhans cell histiocytosis of the spine in children. Long-term follow-up. J Bone Joint Surg Am. 2004;86:1740–1750. doi: 10.2106/00004623-200408000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Green NE, Robertson WW, Jr, Kilroy AW. Eosinophilic granuloma of the spine with associated neural deficit. Report of three cases. J Bone Joint Surg Am. 1980;62:1198–1202. [PubMed] [Google Scholar]
- 13.Knoeller SM, Uhl M, Adler CP, et al. Differential diagnosis of benign tumors and tumor-like lesions in the spine. Own cases and review of the literature. Neoplasma. 2004;51:117–126. [PubMed] [Google Scholar]
- 14.Ladisch S, Gader H. Treatment of langerhans cell histiocytosis—evolution and current approaches. Br J Cancer Suppl. 1994;23:S41–S46. [PMC free article] [PubMed] [Google Scholar]
- 15.Levine SE, Dormans JP, Meyer JS, et al. Langerhans’ cell histiocytosis of the spine in children. Clin Orthop Relat Res. 1996;323:288–293. doi: 10.1097/00003086-199602000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Mammano S, Candiotto S, Balsano M. Cast and brace treatment of eosinophilic granuloma of the spine: long-term follow-up. J Pediatr Orthop. 1997;17:821–827. doi: 10.1097/00004694-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Mickelson MR, Bonfiglio M. Eosinophilic granuloma and its variations. Orthop Clin North Am. 1977;8:933–945. [PubMed] [Google Scholar]
- 18.Nesbit ME, Kieffer S, D’Angio GJ. Reconstitution of vertebral height in histiocytosis X: a long-term follow-up. J Bone Joint Surg Am. 1969;51:1360–1368. doi: 10.2106/00004623-196951070-00014. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez RL, Llover J, Moreno A, et al. Symptomatic eosinophilic granuloma of the spine. Orthopedics. 1984;7:1721–1726. doi: 10.3928/0147-7447-19841101-11. [DOI] [PubMed] [Google Scholar]
- 20.Scarpinati M, Artico M, Artizzu S. Spinal cord compression by eosinophilic granuloma of the cervical spine. Case report and review of the literature. Neurosurg Rev. 1995;18:209–212. doi: 10.1007/BF00383729. [DOI] [PubMed] [Google Scholar]
- 21.Tan G, Samson I, Wever I, et al. Langerhans cell histiocytosis of the cervical spine: a single institution experience in four patients. J Pediatr Orthop B. 2004;13:123–126. doi: 10.1097/00009957-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka N, Fujimoto Y, Okuda T, et al. Langerhans cell histiocytosis of the atlas. A report of three cases. J Bone Joint Surg Am. 2005;87:2313–2317. doi: 10.2106/JBJS.D.03008. [DOI] [PubMed] [Google Scholar]
- 23.Womer RB, Anunciato KR, Chehrenama M. Oral methotrexate and alternate-day prednisone for low-risk Langerhans cell histiocytosis. Med Pediatr Oncol. 1995;25:70–73. doi: 10.1002/mpo.2950250204. [DOI] [PubMed] [Google Scholar]
- 24.Yeh CH. Development and validation of the Asian version of the oucher: a pain intensity scale for children. J Pain. 2005;6:526–534. doi: 10.1016/j.jpain.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Yeom JS, Lee CK, Shin HY, et al. Langerhans’ cell histiocytosis of the spine. Analysis of twenty-three cases. Spine. 1999;24:1740–1749. doi: 10.1097/00007632-199908150-00016. [DOI] [PubMed] [Google Scholar]