Abstract
The objective of this study was to compare elastic intramedullary nailing (EIN) with dynamic skeletal traction spica casting (DSTSC) in terms of postoperative radiographic angulations, length of hospital stay, and cost in a resource-limited setting. We prospectively studied 51 children, five to twelve years of age, with femoral fractures treated with either EIN (n = 26) or DSTSC (n = 25). Children treated with EIN had significantly longer hospital stays (17 ± 8.0 days) than those treated with DSTSC (6.0 ± 2.5 days). Financial constraints in acquiring supplies caused a significant increase in time from admission to surgery (EIN 9.5 ± 2.3 days; DSTSC 1.1 ± 0.3 days), and cost was about 400% higher for EIN compared with DSTSC. At twelve weeks follow-up, all patients in both groups had acceptable radiographic angulations. In resource-limited healthcare settings, DSTSC is an effective alternative to EIN with comparable post-op radiographic angulations, decreased hospital stays, and lower cost.
Résumé
Le but de cette étude est de comparer l’enclouage par clou élastique intramédullaire (EIN) et la traction dynamique avec plâtre (DSTSC). Des examens post-opératoires ont été réalisés sur les radiographies mesurant l’angulation, la longueur de l’hospitalisation et son coût. Méthode: nous avons réalisé une étude prospective sur 51 enfants de 5 à 12 ans, présentant une fracture fémorale traitée soit par clou élastique intramédullaire (26 patients) soit par traitement orthopédique DSTSC (25 patients). Résultats, les patients traités par clou élastique ont eu une hospitalisation beaucoup plus longue (de 7 à ± 8 jours) que ceux traités orthopédiquement par le DSTSC (6 ± 2,5 jours) avec des contraintes financières plus importantes et des durées de séjour entraînant un surcoût de 400% plus important pour le clou comparé au traitement orthopédique. A 12 semaines de suivi tous les patients ont eu un résultat radiographique tout à fait acceptable en termes d’angulation. En conclusion: en période de ressource limitée pour la santé, le traitement orthopédique est une alternative efficace face à l’enclouage par clou élastique intramédullaire notamment en ce qui concerne l’angulation post-opératoire, la diminution de la durée moyenne de séjour et la diminution des coûts.
Introduction
Treatment using traction with spica casting is less expensive and better tolerated in young children (<five years of age), whereas many studies recommend that more skeletally mature adolescents (>fourteen years of age) be treated with standard intramedullary femoral nailing [5, 7, 18]. For children between the ages of five and fourteen, there are a variety of surgical and nonsurgical treatment options, but there is no agreement on the optimal modality [2, 4, 12, 13, 17, 18]. Disadvantages associated with traditional reduction with spica casting include a lengthy hospital stay in addition to potential physical, socioeconomic, and psychological damage caused by cast-immobilised children requiring significant outpatient care [15, 21–23]. Elastic intramedullary nailing (EIN) has gained popularity as an effective treatment for stabilising paediatric femoral fractures in place of traction with spica casting to reduce hospital stay and the need for patient immobilisation [5, 19, 24]. This decrease in hospital stay and earlier return to ambulation potentially diminishes the physical and psychosocial strain imposed by femoral fractures to both the family and patient [3, 11, 14]. However, problems with EIN include hardware cost, need for an image intensifier and dedicated operating room, possible nail migration, and local irritation of muscle and skin overlying the distal aspect of the nails.
Resource-limited hospitals face a unique set of challenges that require treatments that take into account outcome variables, complications, and resource availability as well as local socioeconomic factors. Treatments should be simple to apply, rely on local resources, have few complications, and facilitate early discharge. We previously published initial phase I results of a novel dynamic skeletal traction spica cast (DSTSC) system we developed in a resource-limited setting. This approach was inexpensive and easy to use with low complications, short hospital stays, and satisfactory clinical and radiographic outcomes [10]. In our study, we further modified this DSTSC system (phase II) in order to improve ambulation and decrease cost. The purpose of this prospective study was to determine whether EIN or DSTSC is a more effective treatment modality for paediatric femoral fractures in a resource-limited setting by analysing differences in postoperative radiographic angulations, length of hospital stay, and cost between groups. Our experimental hypothesis was that DSTSC would be an effective alternative to EIN in a resource-limited environment with similar postoperative radiographic angulations but decreased hospital stay and lower cost.
Materials and methods
The study protocol was approved by the institutional review board at the Davao Medical Center (DMC) in Davao City, Mindanao, Philippines before data collection was initiated, and informed consent was obtained from all patients prior to treatment. We used a modified version of our previous phase I DSTSC system [10] which we refer to as phase II. For phase II, we maintained the length of the spica cast to above the knee on the injured leg, but shortened the spica cast on the noninjured leg to above the knee rather than down to the toes in order to: (1) allow more freedom of motion for the normal side knee, ankle, and foot, (2) reduce plaster cost, and (3) determine if reduction stability would be compromised if the cast was reduced to above the knee on the normal side.
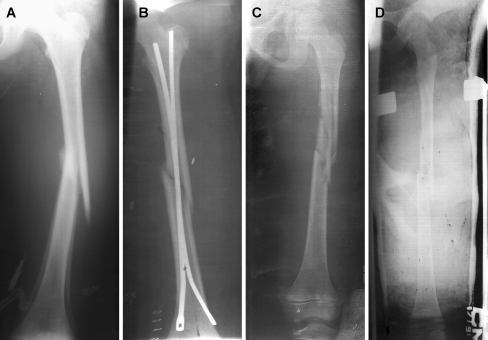
From 2002–2006, all children between the ages of five and 12 with a femoral fracture at DMC were enrolled in this study and treated with either EIN or DSTSC. Treatment was assigned by flipping a coin in order to reduce selection bias, and treatment was administered by the same two surgeons to reduce individual variability. Patient demographics, injury characteristics, radiographic angulations, complications, length of hospital stay, and total cost for EIN and DSTSC were recorded for data analysis. A medium effect size was anticipated based on preliminary radiographic findings and initial hospital stay and cost data collected for EIN and DSTSC [6]. A power calculation was performed based on our expected effect sizes between EIN and DSTSC in terms of time to discharge and cost [8], and minimal major differences were defined as a two-fold longer hospital stay and a two-fold greater cost for EIN compared with DSTSC. A sample size of 25 for each group was determined to be adequate to achieve a power level of 80% (β < 0.2). A total of 51 patients (five to 12 years of age) were enrolled in this study, 26 treated with EIN and 25 with DSTSC. Patients with multiple fractures (n = 3), type II or III open fractures (n = 2), pathological fractures (n = 1), neuromuscular disease (n = 1), or incomplete clinical or radiographic data (n = 4) were excluded. There were no major differences in fracture characteristics between groups (Table 1), and injury presentation and postoperative radiographs showed that all fractures healed with appropriate angulations (Fig. 1a–d). Mean initial traction force for DSTSC was 4.6 ± 0.5 kg with appropriate bone segment overlap postoperatively and at follow-up (range 1.0–1.9 cm).
Table 1.
Patient demographics, injury characteristics, duration of hospital stay, radiographic findings, complications, and total cost for 26 patients treated with EIN and 25 patients treated with DSTSC (values given as mean ± SD )
| Patient characteristics | EIN (n = 26) | DSTSC (n = 25) |
|---|---|---|
| Age (y) | 8.7 ± 1.8 | 7.3 ± 2.7 |
| Gender (M/F) | 20 M, 6 F | 21 M, 4 F |
| Open/closed | 20 closed, 6 open | 19 closed, 6 open |
| Winquist class (1, 2, 3) | 10 W1, 12 W2, 4 W3 | 10 W1, 13 W2, 2 W3 |
| Type (trans, comm, spir, obliq) | 18 trans, 4 comm, 4 obliq | 5 trans, 8 spir, 12 obliq |
| Prox/mid/dist 1/3 of femur | 3 prox, 21 mid, 2 dist | 10 prox, 15 mid |
| Left/right | 16 L, 10 R | 19 L, 6 R |
| Mechanism | 15 Fall, 11 MVA | 18 Fall, 7 MVA |
| Initial tx (skin/skel tract) | 16 Skin tract, 10 Skel tract | – |
| Traction (kg) | – | 4.6 ± 0.5 |
| Time to surgery (days) | 9.5 ± 2.3 | 1.1 ± 0.3* |
| Time from surgery to discharge (days) | 7.5 ± 3.4 | 4.9 ± 2.4 |
| Total hospital stay (days) | 17 ± 8.5 | 6.0 ± 2.5* |
| Injury presentation A/P film (°) | 15 ± 8.1 | 7.9 ± 9.1 |
| Varus/valgus | Varus | Varus |
| Injury presentation lateral film (°) | 16 ± 10 | 15 ± 12 |
| Anterior/posterior | Posterior | Anterior |
| Injury presentation overlap (cm) | – | 1.9 ± 0.9 |
| Postoperative A/P film (°) | 0.7 ± 1.4 | 5.4 ± 6.8 |
| Varus/valgus | Varus | Varus |
| Postoperative lateral film (°) | 1.5 ± 1.9 | 1.9 ± 6.9 |
| Anterior/posterior | Anterior | Anterior |
| Postoperative overlap (cm) | – | 1.0 ± 0.3 |
| 12 wk follow-up A/P film (°) | 0.4 ± 0.9 | 1.9 ± 6.9 |
| Varus/valgus | Varus | Varus |
| 12 wk follow-up lateral film (°) | 1.2 ± 1.4 | 5.4 ± 6.8 |
| Anterior/posterior | Anterior | Anterior |
| 12 wk follow-up overlap (cm) | – | 1.0 ± 0.3 |
| Complications | 1 nail migration, 2 skin irritation | 2 pin-tract infections |
| Total cost ($) | $844 | $216* |
trans transverse, comm comminuted, spir spiral, obliq oblique, skel skeletal, prox proximal, dist distal
*p < 0.05
Fig. 1.
A representative anteroposterior radiograph of a femoral fracture in a study patient before surgical intervention (a) and after elastic intramedullary nailing (EIN) (b). A representative anteroposterior radiograph of a femoral fracture in a study patient before (c) and after dynamic skeletal traction spica casting (DSTSC) (d)
The standard technique for EIN in paediatric patients with femoral fractures has been described in the literature [9]. We performed all EIN procedures in a retrograde fashion through the distal aspect of the femur. Lateral and anteromedial incision sites were chosen 2–2.5 cm proximal to the distal femoral physis or at the level of the superior border of the patella. Nail length was estimated based on radiographs to allow the medial nail to extend into the femoral neck level and the lateral nail to the greater trochanteric apophysis. We determined limb alignment and rotation based on radiographs and clinical examination, respectively, in addition to wound conditions and any other complications.
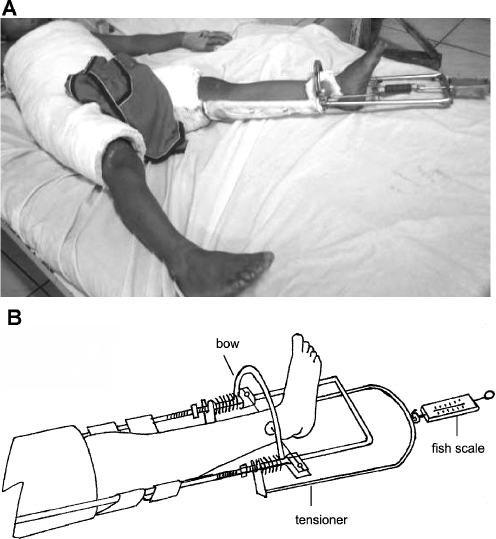
For phase II DSTSC, patients were placed in Buck’s traction on admission and then immediately placed in a DSTSC apparatus using ketamine sedation. Under sterile conditions, a Kirschner wire (0.062 in) was placed through the distal tibia anterior to the fibula at a distance 5–7 cm proximal to the tip of the lateral malleolus for skeletal traction. Xeroform (InvaCare, Elyria, OH) gauze was then applied followed by a felt pad which was secured using disc plates and Jurgan pin balls (Jurgan Development & Mfg, Madison, WI) to prevent lateral pin migration. The Kirschner wire was then attached to a traction bow and placed under tension. While maintaining manual traction, the patient was placed in a half hip spica cast with the fractured side and normal leg both casted above the knee (Fig. 2a,b). Femurs were positioned according to fracture level and were abducted 35–45°, externally rotated 10–15°, and flexed 20–30° (up to 45° for proximal fractures). The knee joint was free on the normal side and kept in full extension on the fracture side. The length of the traction brace was adjusted based on the child’s size, and the device was incorporated into a spica cast with plaster. Sheet wadding was applied to the brace at points of contact with the cast to facilitate later removal.
Fig. 2.
a A photo of a study patient in a phase II DSTSC apparatus. b A schematic line drawing of the phase II DSTSC system on the injured leg side
The traction brace was aligned with the proper rotation along the longitudinal axis of the extremity, and then a traction bow was attached to the brace along with a tensioning device and 18-gauge wire. Traction was provided by a coiled steel spring, and the device was tensioned by turning the wing nut until the desired amount overlap was achieved on radiographs. Traction force was measured using a handheld, spring-loaded scale, and approximately 3.5–5.5 kg of traction was needed to achieve proper fracture overlap. The injured leg was supported by a cloth hammock while in the traction device and a few drops of 70% alcohol were placed at the pin sites through the overlying dressings. After discharge, patients were evaluated in the outpatient clinic and traction was adjusted until healing was sufficient to remove the apparatus and pin under sedation (about three to four weeks). Healing was typically achieved after eight to ten weeks, and after removal of the DSTSC system, patients used crutches if necessary to aid in walking for one month.
A satisfactory outcome for EIN and DSTSC was defined as a healed fracture with acceptable alignment and angulation with patient returning to full activity, or until complications had resolved. Acceptable varus/valgus angulation, anteroposterior angulation, and shortening were set at 10°, 15°, and 15 mm, respectively, for children five to ten years of age [16]. For children ages 11 and older, acceptable angulations and shortening were set at 5°, 10°, and 10 mm, respectively [16]. Initial presentation injury films were used to grade fracture severity according to the Winquist classification system [25] and to determine alignment and angular deformities. Postoperative and follow-up films were analysed for any changes in fracture alignment, angulation, and length. Radiographs of all patients were measured and re-measured by three separate examiners to verify the recorded data.
Total costs for EIN and DSTSC were calculated by adding together the respective costs for hardware (titanium nails, traction brace, Kirschner wire), supplies (sutures, plaster, dressings), operative anaesthesia, radiographs, medications (antibiotics), and hospital stay. Cost for a pair of titanium nails (Synthes, West Chester, PA; Howmedica, Mahway, NJ) for EIN was estimated based on current market prices. Physician fees did not factor into total cost calculations because DMC is a Philippine government hospital in which physicians are given a fixed income and not paid per procedure. Lost individual wages of parents or caretakers tending to injured children were not factored into total cost as a result of the highly variable nature of personal incomes and how each family balanced the time dedicated to patient care.
Statistical analysis was performed on study outcome variables (postoperative radiographic angulations, length of hospital stay, and cost) for EIN and DSTSC treatment groups using two-tailed unpaired Student’s t test (GraphPad Prism, San Diego, CA). Power analysis based on expected effect sizes between EIN and DSTSC determined that a sample size of 25 per group prior to study initiation was adequate to achieve a power level of 80% (β < 0.2) (GraphPad StatMate, San Diego, CA). All of the data presented are given as means ± SD.
Results
Total hospital stay was longer (p = 0.015) for EIN than for DSTSC (17 ± 8.0 days vs. 6.0 ± 2.5 days). Differences in hospital stay were mainly attributable to longer (p = 0.001) waiting periods from injury presentation to surgical intervention (EIN 9.5 ± 2.3 days; DSTSC 1.1 ± 0.3 days), whereas time from surgery to discharge was comparable between groups (EIN 7.5 ± 3.4 days; DSTSC 4.9 ± 2.4 days). Increased time from presentation to surgery was primarily due to financial constraints in acquiring necessary supplies.
Total costs were higher (p = 0.001) for EIN compared with DSTSC. Cost for a pair of titanium elastic nails for EIN was estimated to be $600 based on current market prices and the locally manufactured DSTSC device was $26. Total cost for EIN was $844, which is about 400% more than for DSTSC ($216). Physician fees did not factor into total costs as DMC is a Philippine government hospital where physicians are paid a fixed salary. Unlike EIN, DSTSC can be performed without an operating room and the DSTSC device has a one-time local manufacturing cost and can be completely reused with other patients with the exception of the Kirschner wire. Lost personal income of patient caretakers was not factored into cost calculations as a result of high individual variability, but this would have increased the cost difference between EIN and DSTSC given the average eleven-day longer hospital stay for EIN.
Radiographic angulations were similar between groups on presentation, postoperatively, and at follow-up with no differences in results between three separate examiners. EIN and DSTSC had similar postop varus/valgus angulations on anteroposterior X-rays (EIN 0.7° ± 1.4° varus; DSTSC 5.4° ± 6.8° varus) as well as similar anteroposterior angulations on lateral films (EIN 1.5° ± 1.9° anterior; DSTSC 1.9° ± 6.9° anterior). No additional changes in radiographic angulations were noted between groups at follow-up. Complication rates between groups were comparable with one case of nail migration and two cases of skin irritation for EIN and two pin-tract infections for DSTSC. All complications resolved without further surgical intervention.
Discussion
The goal of this study was to determine whether EIN or DSTSC is a more effective treatment for paediatric femoral fractures in a resource-limited setting by analysing differences in postoperative radiographic angulations, duration of hospital stay, and cost. Our results supported our experimental hypothesis that DSTSC has similar postoperative radiographic angulations but decreased hospital stay and lower cost in comparison with EIN, thus making it an effective alternative to EIN in a resource-limited environment.
A main limitation of this study was lack of patient follow-up beyond twelve weeks due to patient financial and logistical difficulties. Although all patients had follow-up at six and twelve weeks, we would have liked to have had follow-up at regular intervals up to a year after surgery. Since we were unable to examine patients after twelve weeks of follow-up, we cannot comment on the precise final alignment, angulation, or length of fractures. However, given the acceptable radiographic angulations seen in both groups at follow-up, we believe that our findings are valid and that it is unlikely that any of the patients experienced significant changes in healed fracture characteristics after their last visit. Another limitation to our study was the longer waiting time to obtain necessary equipment for EIN compared with DSTSC due to large cost differences; it is Philippine government hospital policy that patients pay upfront for hospital expenses. These financially-driven waiting times are a key outcome measure in our study setting since financial and social factors greatly influence access to healthcare in developing nations.
There are several problems with EIN that are magnified by the lack of resources in a developing nation such as cost of materials, need for an image intensifier and operating room, and anaesthetic risks in addition to general risks such as nail slippage. Few if any studies have investigated improved techniques to traditional traction with spica casting as an alternative treatment to EIN in resource-limited settings. As a result of the lack of studies investigating alternative, low-cost solutions for paediatric femoral fractures, we first described a novel DSTSC system which we showed to be easy to use and cost-effective with low complications, short hospital stays, and appropriate clinical and radiographic outcomes [10]. We expanded upon these results by modifying our previous DSTSC design while also comparing it directly to EIN in terms of radiographic outcomes, hospital stay, and cost. In our study, EIN was about 400% more expensive than DSTSC, totalling $844 compared with $216, and these cost differences greatly influenced times from injury presentation to surgery as patients needed more time to find the required financial resources.
DSTSC offers several advantages over traditional methods of traction with spica casting (Table 2). Constant traction force may be applied since the traction pin is not incorporated into the cast, and there is less chance of pin breakage or lateral motion. The force of traction may be adjusted as muscle spasm subsides, and the risk of skin complications should be less in comparison to techniques using skin traction. The traction apparatus may also be reused and pin placement for distal tibial traction can be achieved without image intensification. In addition, pin placement need not be parallel to the mechanical axis of the limb and does not involve risk of physeal injury or recurvatum deformity as has been noted with proximal tibial traction [1, 20].
Table 2.
Comparison of dynamic skeletal traction spica casting (DSTSC) with various traction and spica casting techniques for treatment of pediatric femoral shaft fractures
| Type of traction treatment | Initial management/in-hospital stay | Treatment course |
|---|---|---|
| Traditional traction | Skin traction (txn) with pulleys and weights | Six to eight weeks or until complete healing and return to function |
| Other traditional traction | Preliminary skin txn | Two to three weeks (skin or skeletal txn below tibial tubercle, trans-femoral condyles) followed by spica cast, discharged as outpatient until fracture heals, cast removed |
| Static traction spica 90/90 cast | Hip and knee flexed at (90°) with skeletal txn applied through the femoral condyles in this position | Two to three weeks followed by spica cast in this position incorporating the pin in the cast, discharged as out patient until fracture heals |
| Other static traction casts | Preliminary skin txn | One to two days then skeletal txn pins at the femoral condyles or below tibial tubercle incorporated into the spica casts, discharged when fracture heals, pins removed earlier as outpatient. Cast can also be applied incorporating tape to the skin to apply txn (dynamic) but this is typically not well maintained |
| Dynamic skeletal traction spica cast (DSTSC) | Preliminary temporary skin txn | One to two days skeletal txn pin inserted at distal tibia as feasible, incorporated into spica cast with the DSTSC txn apparatus, discharged in four in-hospital days as outpatient, followed until fracture heals. DSTSC is the only continuously controlled txn cast in contrast to static casts and can be used with varying cast lengths |
Overall, it is critical that treatments in resource-limited settings take into account clinical and radiographic outcome measures, complications, and resource availability in addition to the local socioeconomic and political factors. Treatments should be easy to apply, rely on local resources, have few complications, facilitate early discharge, and involve minimal cost to the patient and hospital [10]. Results from our comparison study of phase II DSTSC with EIN show that DSTSC has similar postoperative radiographic outcomes as EIN, but with significantly shorter hospital stays and lower cost. Therefore, we conclude that DSTSC is an effective treatment option in place of EIN for paediatric femoral fractures in settings of resource-limited health care.
Acknowledgements
We would like to thank Dr. Ronald Tangente, Dr. Espiridion Reyes, and the entire Department of Orthopaedic Surgery at the Davao Medical Center for their invaluable help and support in completing this study.
References
- 1.Bjerkreim I, Benum P. Genu recurvatum: a late complication of tibial wire traction in fractures of the femur in children. Acta Orthop Scand. 1975;46:1012–1019. doi: 10.3109/17453677508989291. [DOI] [PubMed] [Google Scholar]
- 2.Buckley SL (1997) Current trends in the treatment of femoral shaft fractures in children and adolescents. Clin Orthop Relat Res 60–73 [DOI] [PubMed]
- 3.Buechsenschuetz KE, Mehlman CT, Shaw KJ, et al. Femoral shaft fractures in children: traction and casting versus elastic stable intramedullary nailing. J Trauma. 2002;53:914–921. doi: 10.1097/00005373-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Canale ST, Tolo VT. Fractures of the femur in children. Instr Course Lect. 1995;44:255–273. [PubMed] [Google Scholar]
- 5.Carey TP, Galpin RD (1996) Flexible intramedullary nail fixation of pediatric femoral fractures. Clin Orthop Relat Res 110–118 [DOI] [PubMed]
- 6.Cohen J. Statistical power analysis for the behavioral sciences. Inc., Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 7.Czertak DJ, Hennrikus WL. The treatment of pediatric femur fractures with early 90–90 spica casting. J Pediatr Orthop. 1999;19:229–232. doi: 10.1097/00004694-199903000-00018. [DOI] [PubMed] [Google Scholar]
- 8.Dupont WD, Plummer WD., Jr Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-M. [DOI] [PubMed] [Google Scholar]
- 9.Flynn JM, Hresko T, Reynolds RA, et al. Titanium elastic nails for pediatric femur fractures: a multicenter study of early results with analysis of complications. J Pediatr Orthop. 2001;21:4–8. doi: 10.1097/00004694-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Gracilla RV, Diaz HM, Penaranda NR, et al. Traction spica cast for femoral-shaft fractures in children. Int Orthop. 2003;27:145–148. doi: 10.1007/s00264-003-0443-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greisberg J, Bliss MJ, Eberson CP, et al. Social and economic benefits of flexible intramedullary nails in the treatment of pediatric femoral shaft fractures. Orthopedics. 2002;25:1067–1070. doi: 10.3928/0147-7447-20021001-18. [DOI] [PubMed] [Google Scholar]
- 12.Griffin PP, Green WT. Fractures of the shaft of the femur in children: treatment and results. Orthop Clin North Am. 1972;3:213–224. [PubMed] [Google Scholar]
- 13.Hansen ST. Internal fixation of children’s fractures of the lower extremity. Orthop Clin North Am. 1990;21:353–363. [PubMed] [Google Scholar]
- 14.Heinrich SD, Drvaric DM, Darr K, et al. The operative stabilization of pediatric diaphyseal femur fractures with flexible intramedullary nails: a prospective analysis. J Pediatr Orthops. 1994;14:501–507. doi: 10.1097/01241398-199407000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Hughes BF, Sponseller PD, Thompson JD. Pediatric femur fractures: effects of spica cast treatment on family and community. J Pediatr Orthop. 1995;15:457–460. doi: 10.1097/01241398-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Kasser JR, Beaty JH. Rockwood and Wilkins’ fractures in children. Philadelphia: Lippincott Williams and Wilkins; 2001. [Google Scholar]
- 17.Kissel EU, Miller ME. Closed ender nailing of femur fractures in older children. J Trauma. 1989;29:1585–1588. doi: 10.1097/00005373-198911000-00020. [DOI] [PubMed] [Google Scholar]
- 18.Levy J, Ward WT. Pediatric femur fractures: an overview of treatment. Orthopedics. 1993;16:183–190. doi: 10.3928/0147-7447-19930201-12. [DOI] [PubMed] [Google Scholar]
- 19.McCartney D, Hinton A, Heinrich SD. Operative stabilization of pediatric femur fractures. Orthop Clin North Am. 1994;25:635–650. [PubMed] [Google Scholar]
- 20.Miller PR, Welch MC (1978) The hazards of tibial pin replacement in 90–90 skeletal traction. Clin Orthop Relat Res 97–100 [PubMed]
- 21.Newton PO, Mubarak SJ. Financial aspects of femoral shaft fracture treatment in children and adolescents. J Pediatr Orthop. 1994;14:508–512. doi: 10.1097/01241398-199407000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Reeves RB, Ballard RI, Hughes JL. Internal fixation versus traction and casting of adolescent femoral shaft fractures. J Pediatr Orthop. 1990;10:592–595. doi: 10.1097/01241398-199009000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Ryan JR. 90–90 skeletal femoral traction for femoral shaft fractures in children. J Trauma. 1981;21:46–48. doi: 10.1097/00005373-198101000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Sanders JO, Browne RH, Mooney JF, et al. Treatment of femoral fractures in children by pediatric orthopedists: results of a 1998 survey. J Pediatr Orthop. 2001;21:436–441. doi: 10.1097/00004694-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Winquist RA, Hansen ST., Jr Comminuted fractures of the femoral shaft treated by intramedullary nailing. Orthop Clin North Am. 1980;11:633–648. [PubMed] [Google Scholar]