Abstract
We have made three types of poly (DL-lactide-co-glycolide) (PLG) scaffolds (porosity: scaffold I 80 ± 0.9%, II 85 ± 0.8%, III 92 ± 0.7%; compression module determined with 10% strain: scaffold I 0.26 MPa, II 0.091 MPa, III 0.0047 MPa). Osteochondral defects made in the femoral condyle of rabbits were treated with these scaffolds and the possibilities of cartilage repair were investigated histologically. At post-operative weeks 6 and 12, histological scores in the groups of scaffolds II and III were significantly higher than the score in the group of scaffold I. Scaffolds II and III, which have higher porosity than scaffold I, allow better migration of bone marrow cells and better replacement of the scaffold with bone and cartilage than scaffold I. This study suggests that higher porosity allowing bone marrow cells to migrate to the scaffold is important in repairing osteochondral defects.
Résumé
Nous avons réalisé trois types de montage PLG (DL lactide co glycolide) avec des porosités différentes (montage I à 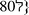 0,9%, montage II à 87
0,9%, montage II à 87 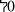 ,8%, montage III
,8%, montage III 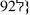 0,7%). Avec le module de compression a été imposée une tension de 10% avec 0,26 MPa au niveau de la pièce I, 0,091 Mpa en montage II et 0,0047 MPa en montage III). Un defect ostéochondral réalisé dans le condyle fémoral d’un lapin a été traité par ces trois procédés avec une possibilité de réparation cartilagineuse. Cette possibilité a été évaluée histologiquement. A 6 et 12 semaines postopératoires, le score histologique dans les groupes II et III sont significativement plus élevés que dans le groupe I. Les pièces II et III qui ont une plus grande porosité que la I permettent une meilleure migration des cellules de la moelle osseuse et une meilleure réparation de l’os et du cartilage que dans le montage I. Cette étude nous permet de penser qu’une porosité plus importante permet la migration plus facile de ces cellules de moelle osseuse lors de la réparation des défects ostéochondraux.
0,7%). Avec le module de compression a été imposée une tension de 10% avec 0,26 MPa au niveau de la pièce I, 0,091 Mpa en montage II et 0,0047 MPa en montage III). Un defect ostéochondral réalisé dans le condyle fémoral d’un lapin a été traité par ces trois procédés avec une possibilité de réparation cartilagineuse. Cette possibilité a été évaluée histologiquement. A 6 et 12 semaines postopératoires, le score histologique dans les groupes II et III sont significativement plus élevés que dans le groupe I. Les pièces II et III qui ont une plus grande porosité que la I permettent une meilleure migration des cellules de la moelle osseuse et une meilleure réparation de l’os et du cartilage que dans le montage I. Cette étude nous permet de penser qu’une porosité plus importante permet la migration plus facile de ces cellules de moelle osseuse lors de la réparation des défects ostéochondraux.
Introduction
Articular cartilage has a poor capacity for repair and there have been many attempts to treat articular cartilage lesions by procedures such as subchondral drilling, abrasion arthroplasty, microfracture techniques, autologous osteochondral grafts, periosteal-perichondral grafts or transplantation of autogeneic or allogeneic chondrocytes; however, repairing large osteochondral lesions remains challenging [1–4, 11, 13, 15]. Recent cartilage regeneration techniques based on tissue engineering principles have reported generally good results [1, 3, 4, 6, 7, 9, 19–21]. To transplant cultured cells into osteochondral lesions, scaffolds providing structural support are needed. Several bioabsorbable and bioactive scaffolds, which are compositions of synthetic polymer, calcium phosphate, hyaluronan, growth factors and cultured cells, have been developed for the repair of osteochondral defects [6–8, 12, 17–19].
It has been reported that full-thickness cartilage defects of a small size can be regenerated by erupted bone marrow cells containing osteoprogenitor cells [4, 16]. In contrast, when a full-thickness osteochondral defect, 6 mm both in diameter and in depth, is made in a goat femoral condyle, bone marrow cells containing osteoprogenitor cells erupting from the bottom of the defect cannot fill and cannot repair the defect [10]. It is speculated that an osteochondral defect of a critical size cannot be filled with reparative tissue and has deleterious effects leading to the creation of a large defect in the subchondral bone. Therefore, we have developed a new bioabsorbable scaffold made from a synthetic polymer, poly (DL-lactide-co-glycolide) (PLG) [14]. The PLG scaffold has multiple pores, to which the erupted bone marrow cells can attach, and has the possibility to repair the full-thickness osteochondral defects of a critical size made in a rabbit without using the cultured cells, thus preventing the collapse of the surrounding articular cartilage and subchondral bone.
In this study, to make an ideal scaffold for cartilage and bone regeneration, we focused on porosity and mechanical property of the PLG scaffold and investigated how they influence the healing of an osteochondral defect. We made three types of PLG scaffolds differing in porosity and mechanical properties and analysed the repair of osteochondral defects in rabbits using these PLG scaffolds.
Material and methods
Scaffolds
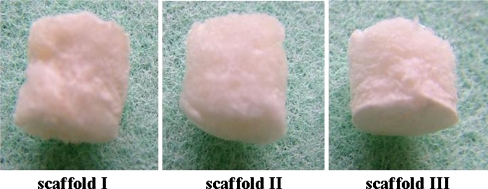
The biodegradable scaffolds used in this study were composed of PLG and were cylindrical in shape, of 5 mm in diameter and 5 mm in length. To make the scaffold, PLG was purchased from Absorbable Polymers International, Inc. (Pelham, AL, USA). The molar ratio of lactide to glycolide was 50:50 and the inherent viscosity was 1.08 dl/g measured at 30°C in hexafluoroisopropanol. Dimethyl sulfoxide (DMSO) was purchased from Wako Pure Chemical Industries Ltd. (Osaka, Japan). The PLG and DMSO were used without further purification. In order to make three types of the PLG scaffold, PLG was dissolved in three different concentrations of DMSO to give 15, 10 and 5% (weight by volume). The polymer dope was poured into a container and freeze-dried. The freeze rate was controlled at the rate of 1°C/min temperature decrease and freeze-dried in vacuo; in this way three types of porosity with differing mechanical properties were obtained as PLG scaffolds (Fig. 1). Lyophilized PLG scaffolds were soaked in 70/30 (volume by volume) of ethanol-water mixed solution and washed by the same solution to extract residual DMSO. Thereafter the PLG scaffolds were used in the animal experiment. Prior to the animal experiment, the porosity and mechanical properties of the three types of scaffolds were evaluated.
Fig. 1.
Three types of PLG scaffolds
The total porosity (Π) of the three types of PLG scaffolds was measured by gravimetry according to the equation:
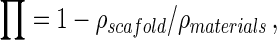 |
where ρmaterials was the density of the material from which the scaffold was made and ρscaffold was the apparent density of the scaffold measured by dividing the weight by the volume of the scaffold. The porosity of each scaffold was 80 ± 0.9% at scaffold I, 85 ± 0.8% at scaffold II and 92 ± 0.7% at scaffold III, presented in percentage (Table 1).
Table 1.
Porosity and mechanical properties of the synthetic polymer PLG scaffolds
| Porosity (%) | 10% strain compression module (MPa) | |
|---|---|---|
| Scaffold I | 80 ± 0.9 | 0.26 ± 0.08 |
| Scaffold II | 85 ± 0.8 | 0.091 ± 0.05 |
| Scaffold III | 92 ± 0.7 | 0.0047 ± 0.004 |
Compression modules of the scaffolds were determined with 10% strain in triplicate at room temperature using the EZ Test (Shimadzu Manufacturing Company Limited, Osaka, Japan) tensile tester operating at 0.5 mm/min. The compression module of each scaffold was 0.26 MPa at scaffold I, 0.091 MPa at scaffold II and 0.0047 MPa at scaffold III (Table 1).
Surgical procedures
Fifty-four skeletally mature female Japanese white rabbits (Kitayama LABES, Nagano, Japan) were used in this study. The average weight was 3.1 kg (range: 2.7–3.6 kg). The research protocol of this experiment was approved by the Animal Research Committee of Kobe University Graduate School of Medicine.
All operations were performed on the right knee of the rabbits under general anaesthesia from an intravenous pentobarbital sodium solution (30 mg/kg body weight). In each rabbit, the right limb was disinfected and 3 ml of 1% lidocaine was injected subcutaneously into the medial parapatellar region. The knee joint was opened with a medial parapatellar approach, the patella was dislocated laterally and the surface of the femoropatellar groove was exposed. A full-thickness cylindrical defect of 5 mm in diameter and 5 mm in depth was created in the patellar groove of the right femoral condyle, which contacts the patella when the knee is flexed at 90°, using the Osteochondral Autograft Transfer System (OATS; Arthrex, Naples, FL, USA). After irrigating the joint with sterile isotonic saline, rabbits were randomly allocated into three groups depending on the type of scaffold which was used to repair the defect: the defect was repaired by transplantation of PLG scaffolds I, II or III. After repairing the defect, the patella was reduced and the joint capsule and skin were sutured in separate layers. Post-operatively, all rabbits were allowed immediate free cage movement. At post-operative weeks 3, 6 and 12, six rabbits from each group were killed with an intravenous dose of sodium pentobarbital and the femoral condyles were taken and examined macroscopically and histologically.
Histological analysis
After macroscopic examination, the femoral condyles were fixed in 4% paraformaldehyde for 24 hours, decalcified with 0.25 mol/l ethylenediaminetetraacetic acid in phosphate-buffered saline at pH 7.5, dehydrated in graded alcohol solutions and embedded in paraffin wax. Sagittal sections (7 µm thick) were cut through the entire thickness of the defect, stained with haematoxylin and eosin or toluidine blue and examined by light microscopy. The histological findings were scored according to a histological grading scale modified from the scale described by Wakitani et al. [20]. This scale contained five categories with a high score of 14 points (Table 2). Each section was scored by three blinded examiners (RI, TK and NT) who had no knowledge of the treatment group or follow-up time of the specimens being analysed. The statistical significance of the difference in the histomorphometric measurements was determined by Welch’s t-test and p values < 0.05 were considered statistically significant.
Table 2.
Histological grading scalea
| Category | Points |
|---|---|
| A: Cell morphology | |
| Hyaline cartilage | 4 |
| Mostly hyaline cartilage | 3 |
| Mostly fibrocartilage | 2 |
| Mostly non-cartilage | 1 |
| Non-cartilage only | 0 |
| B: Matrix staining (metachromasia) | |
| Normal (compared with host) | 3 |
| Slightly reduced | 2 |
| Significantly reduced | 1 |
| No staining | 0 |
| C: Surface regularityb | |
| Smooth (> 3/4) | 3 |
| Moderate (1/2–3/4) | 2 |
| Irregular (1/4–1/2) | 1 |
| Severely irregular (< 1/4) | 0 |
| D: Thickness of cartilagec | |
| > 2/3 | 2 |
| 1/3–2/3 | 1 |
| < 1/3 | 0 |
| E: Integration of donor to host adjacent cartilage | |
| Both edges integrated | 2 |
| One edge integrated | 1 |
| Both edges not integrated | 0 |
| Total | Maximum 14 |
aModified from the scale described by Wakitani et al. [20]
bTotal smooth area of the reparative cartilage compared with the entire area of the cartilage defect
cAverage thickness of the reparative cartilage compared with that of the surrounding cartilage
Results
Macroscopic findings
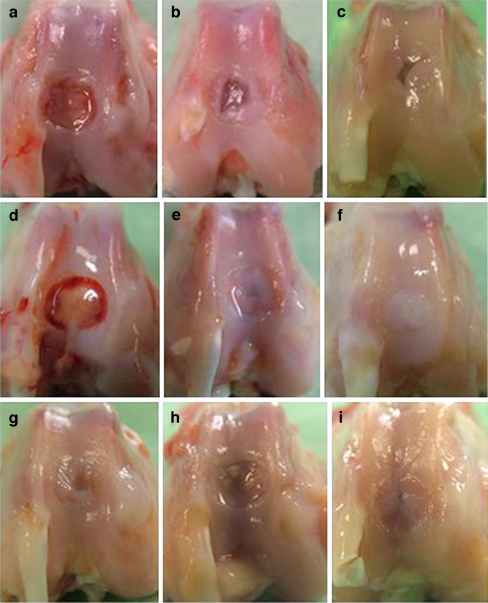
At post-operative week three, the macroscopic appearances of the study groups were very similar. White reparative tissue was observed at the margin of the defect; the central area of the defect was still depressed (Fig. 2a,d and g). At 6 weeks after surgery, the central area of the defect was slightly depressed but reparative tissue had increased compared with post-operative week three, and the scaffold II group (Fig. 2e) showed a better repair than the groups of scaffolds I and III (Fig. 2b and h). After 12 weeks, defects repaired with the groups of scaffolds II and III (Fig. 2f and i) were almost completely covered with reparative tissue, but a cleft remained in the defect in the scaffold I group (Fig. 2c).
Fig. 2.
Macroscopic findings of the femoral condyle. The defects were repaired with PLG scaffold I (a, b and c), scaffold II (d, e and f) and scaffold III (g, h and i). At post-operative weeks 3 (a, d and g), 6 (b, e and h) and 12 (c, f and i). At post-operative week 3, the three groups were very similar and reparative tissue filled the defects, but the central area of the defects was still depressed (a, d and g). At post-operative week 6, reparative tissue had increased compared with post-operative week 3 in the three groups (b, e and h). After 12 weeks, the defects were almost covered with reparative tissue in scaffolds II and III (f and i) but the cleft still remained in the defects in scaffold I (c)
Histological findings
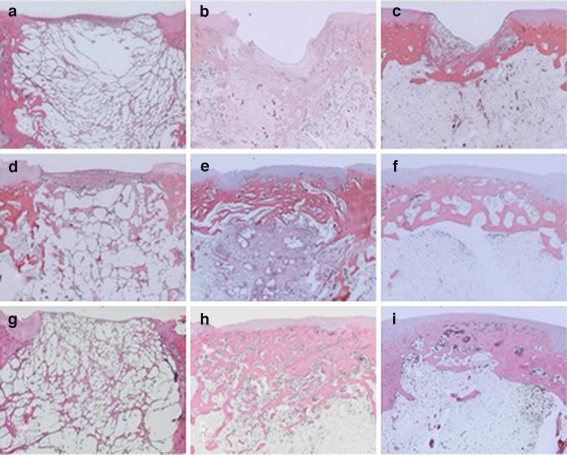
At post-operative week three, the defects were covered with newly formed fibrous tissue and some remnants of the scaffold structures were observed in all groups (Fig. 3a,d and g).
Fig. 3.
Histological findings stained with haematoxylin and eosin. The defect was repaired with PLG scaffold I (a, b and c), scaffold II (d, e and f) and scaffold III (g, h and i). At post-operative weeks 3 (a, d and g), 6 (b, e and h) and 12 (c, f and i). Original magnification ×25. At post-operative week 3, the defects were covered with newly formed fibrous tissue and some remnants of the PLG scaffold structures were present in the defect in all groups (a, d and g). At post-operative week 6 in the scaffold I group, most of the PLG scaffold was absorbed and had been replaced by some fibrous tissue, but a defect was observed (b). In the scaffold II and III groups, most of the PLG scaffold was absorbed and had been replaced by newly formed bone at the subchondral zone and by cartilaginous tissue at the articular surface of the defect (e and h). At post-operative week 12, a defect was still observed in the scaffold I group (c). In the scaffold II and III groups, most of the PLG scaffold had been absorbed and the defect was repaired with regenerated bone and cartilage (f and i)
At post-operative week six in the scaffold I group, most of the scaffold was absorbed and had been replaced by some fibrous tissue, but a large defect was observed (Fig. 3b). In the scaffold II and III groups, most of the scaffold was absorbed and had been replaced by newly formed bone at the subchondral zone and reparative tissue with cartilaginous tissue had been regenerated at the articular surface of the defect (Fig. 3e and h). The repaired articular surface of the scaffold II and III groups was smooth compared with that of the scaffold I group.
At post-operative week 12, in the scaffold I group, a large defect was still observed as had been seen at post-operative week six (Fig. 3c). In the scaffold II and III groups, most of the PLG scaffold was absorbed and replaced by newly formed bone and by regenerated cartilage (Fig. 3f and i). The surface of the defect was repaired with articular cartilage with smooth surface. At the subchondral bone area, regenerated bone had been remodelled. The thickness of regenerated cartilaginous tissue had increased and its regularity at the articular surface had been improved. Better organised subchondral bone formation was also observed, compared with that seen at post-operative week six.
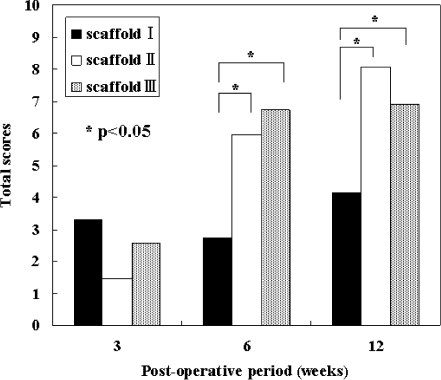
Histological scores
At post-operative weeks 3, 6 and 12, histological findings were evaluated by a histological grading scale (Table 2). The scores at each post-operative time point in each scaffold group are presented in Table 3 and the total scores are summarised in Fig. 4. At post-operative week three, there was no significant difference in scores among the three scaffold groups. However, at post-operative weeks six and 12, the total scores of the groups of scaffolds II and III were significantly higher than the score of the scaffold I group.
Table 3.
Histological scores with SD (range)
| Time after surgery (weeks) | Evaluation category | ||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | Total scores | ||
| Scaffold I | 3 | 1.3 ± 0.8 (0–2) | 0.6 ± 0.5 (0–1) | 1.2 ± 0.8 (0–2) | 0 ± 0 (0) | 0.3 ± 0.5 (0–1) | 3.3 ± 2.0 (0–6) |
| 6 | 1.0 ± 1.3 (0–3) | 0.5 ± 0.8 (0–2) | 0.6 ± 1.0 (0–3) | 0.3 ± 0.5 (0–1) | 0.3 ± 0.6 (0–2) | 2.7 ± 3.8 (0–11) | |
| 12 | 1.5 ± 1.3 (0–3) | 1.0 ± 0.9 (0–2) | 0.7 ± 1.0 (0–3) | 0.2 ± 0.4 (0–1) | 0.7 ± 0.7 (0–2) | 4.1 ± 3.9 (0–11) | |
| Scaffold II | 3 | 1.0 ± 0.8 (0–2) | 0.5 ± 0.5 (0–1) | 0.7 ± 0.5 (0–1) | 0 ± 0 (0) | 0 ± 0 (0) | 2.2 ± 1.5 (0–4) |
| 6 | 1.8 ± 0.9 (0–3) | 1.2 ± 0.5 (0–2) | 1.6 ± 1.1 (0–3) | 0.4 ± 0.5 (0–1) | 0.9 ± 0.7 (0–2) | 5.9 ± 3.0 (1–11) | |
| 12 | 2.5 ± 0.8 (1–4) | 1.6 ± 0.9 (0–3) | 2.2 ± 0.6 (1–3) | 0.8 ± 0.8 (0–2) | 1.0 ± 0.8 (0–2) | 8.0 ± 3.6 (4–14) | |
| Scaffold III | 3 | 0.8 ± 1.0 (0–2) | 0.3 ± 0.6 (0–1) | 1.7 ± 0.6 (1–3) | 0.2 ± 0.4 (0–1) | 0.2 ± 0.4 (0–1) | 3.2 ± 2.6 (1–9) |
| 6 | 2.2 ± 1.0 (0–3) | 1.3 ± 1.0 (0–3) | 1.6 ± 1.2 (0–3) | 0.6 ± 0.5 (0–1) | 1.0 ± 0.8 (0–2) | 6.7 ± 4.3 (0–12) | |
| 12 | 1.9 ± 1.0 (0–3) | 1.1 ± 0.6 (0–2) | 2.6 ± 0.6 (1–3) | 0.1 ± 0.3 (0–1) | 1.1 ± 0.8 (0–2) | 6.9 ± 2.7 (2–10) | |
Fig. 4.
Total histological scores. At post-operative weeks 3, 6 and 12, total histological finding scores, evaluated by a histological grading scale in Table 2 and presented in Table 3, are summarised. At post-operative week 3, there was no significant difference in scores among the three PLG scaffold groups. However, at post-operative weeks 6 and 12, the total scores in the groups of the scaffolds II and III were significantly higher than the score in the scaffold I group
Discussion
In this study, an osteochondral defect 5 mm in diameter, made in the femoral condyle of a rabbit, was treated with three types of PLG scaffolds differing in porosity and mechanical properties, the highest porosity of a PLG scaffold being scaffold III and the lowest porosity being scaffold I. At post-operative week three, the defects were covered with newly formed fibrous tissue, and some remnants of the PLG scaffold structures were present in the defects. There were no significant differences observed in the histological findings and the scores among the PLG scaffolds I, II and III. At post-operative weeks six and 12, in the scaffold I group, most of the PLG scaffold had been absorbed and replaced with fibrous tissue; however, a cleft of regenerated cartilage tissue had appeared in the defect. By contrast, in the groups treated with scaffolds II and III, most of the PLG scaffold was absorbed and had been replaced by newly formed bone at the subchondral zone and by regenerated cartilaginous tissue at the articular surface of the defects. The scores of the scaffold II and III groups were significantly higher than the score of the scaffold I group. PLG scaffolds II and III repaired the full-thickness osteochondral defects of a critical size made on the rabbit femoral condyle without using the cultured cells.
Most of the current approaches for repairing articular cartilage using tissue engineering use a combination of bioabsorbable scaffold as a carrier of cells and appropriate cell populations such as chondrocytes or mesenchymal stem cells [6, 7, 9, 19, 21, 22]. However, the long period of time and the system needed for cell culture, as well as the potential risk of disease transmission, have been cited as clinical disadvantages for the widespread use of these modalities.
In repairing the full-thickness osteochondral defect, exogenous cells may not be required because bone marrow cells erupted from the bottom of the osteochondral defect have a potential to differentiate to cartilage and bone, when the osteochondral defect was treated with the optimal scaffold combined with several factors: multiphasic bioabsorbable scaffold containing both the region for bone repairing and the region for cartilage repairing [5], amorphous calcium phosphate/poly (L-lactic acid) combined with fibroblast growth factor [8], hyaluronan- and polyester-based scaffolds [17], a composite of interconnected porous hydroxyapatite and synthetic polymer combined with bone morphogenetic protein-2 [18] and synthetic PLG scaffold [14]. In this study, we demonstrated that the PLG scaffolds II and III, which are of higher porosity than scaffold I, are able to repair full-thickness osteochondral defects of a critical size with a single material (cultured cell-free model). We speculate that the cells erupted from the underlying bone marrow attach to the PLG scaffold implanted in the osteochondral defect. Thereafter, the cells attached to the articular surface of the PLG scaffold regenerate cartilage tissue under the influence of synovial fluid and surrounding articular cartilage. The cells attached to the deep zone of the PLG scaffold regenerate to bone during absorption of the PLG scaffold. The porous PLG scaffold may play an important role in holding erupted bone marrow cells in the osteochondral defect, which leads to the regeneration of cartilage and bone while the PLG is absorbed.
Porosity, rate of degradation and mechanical properties of the materials play an important role in bone and cartilage regeneration by using biomaterial scaffolds [5, 12, 17]. Lower porosity stimulates osteogenesis by suppressing cell proliferation and forcing cell aggregation in vitro; however, higher porosity and larger pore size result in greater bone ingrowths to the scaffold but diminish the mechanical properties in vivo [12]. The optimal scaffold should be quickly replaced by bone in the subchondral area and by articular cartilage in the cartilage area during degradation of the scaffold [17]. Moreover, it is better that the scaffold prolongs the presence of regenerated cartilage and delays endochondral ossification of the regenerated cartilage.
In this study, the PLG scaffolds II and III repaired full-thickness osteochondral defects of a critical size made in a rabbit without using the cultured cells better than scaffold I. As scaffold I has the lowest porosity and the highest mechanical property, scaffold I may be optimal for supporting subchondral bone surrounding the defect and preventing collapse of the surrounding cartilage and subchondral bone; however, it might also prevent migration of the bone marrow cells erupted from the defect, delay absorbance of the scaffold and result in occurrence of a cleft. By contrast, in the PLG scaffolds II and III, which have a higher porosity than scaffold I, migration of bone marrow cells erupted from the defect and replacement of the scaffold with subchondral bone and articular cartilage might occur quickly. In this animal experiment, the benefit of higher porosity, which resulted in migration of bone marrow cells erupted from the defect, is thought to override the mechanical property of the scaffold in supporting the defect.
In conclusion, we have demonstrated cartilage repair with a PLG scaffold without using cultured cells in a rabbit model. Our results suggest that absorbable synthetic scaffolds with higher porosity lead the bone marrow cells to migrate and result in repairing the osteochondral defect during absorption of the scaffold without using the cultured cells. Further investigation of in vivo outcomes in another model may lead to the application of this technology for repair of human cartilage.
Acknowledgements
We thank C. Satoh for producing the PLG scaffold and M. Nagata for assistance with the histological experiments. This study was partially supported by Scientific Research Grant of Kobe University Graduate School of Medicine in 2006 and Scientific Research Grant of Shinryokukai in 2006.
Contributor Information
Risa Ikeda, Phone: +81-78-3825985, FAX: +81-78-3516944.
Hiroyuki Fujioka, Phone: +81-78-3825985, FAX: +81-78-3516944, Email: hfujioka@med.kobe-u.ac.jp.
Issei Nagura, Phone: +81-78-3825985, FAX: +81-78-3516944.
Takeshi Kokubu, Phone: +81-78-3825985, FAX: +81-78-3516944.
Narikazu Toyokawa, Phone: +81-78-3825985, FAX: +81-78-3516944.
Atsuyuki Inui, Phone: +81-78-3825985, FAX: +81-78-3516944.
Takeshi Makino, Phone: +81-78-3825985, FAX: +81-78-3516944.
Hiroaki Kaneko, Phone: +81-78-3825985, FAX: +81-78-3516944.
Minoru Doita, Phone: +81-78-3825985, FAX: +81-78-3516944.
Masahiro Kurosaka, Phone: +81-78-3825985, FAX: +81-78-3516944.
References
- 1.Boopalan PR, Sathishkumar S, Kumar S, Chittaranjan S. Rabbit articular cartilage defects treated by allogenic chondrocyte transplantation. Int Orthop. 2006;30:357–361. doi: 10.1007/s00264-006-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouwmeester SJM, Beckers JMH, Kuijer R, Linden AJ, Bulstra SK. Long-term results of rib perichondrial grafts for repair of cartilage defects in the human knee. Int Orthop. 1997;21:313–317. doi: 10.1007/s002640050175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop Relat Res. 1997;342:254–269. doi: 10.1097/00003086-199709000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Frenkel SR, Bradica G, Brekke JH, Goldman SM, Ieska K, Issack P, Bong MR, Tian H, Gokhale J, Coutts RD, Kronengold RT. Regeneration of articular cartilage—evaluation of osteochondral defect repair in the rabbit using multiphasic implants. Osteoarthritis Cartilage. 2005;13:798–807. doi: 10.1016/j.joca.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 6.Grigolo B, Roseti L, Fiorini M, Fini M, Giavaresi G, Aldini NN, Giardino R, Facchini A. Transplantation of chondrocytes seeded on a hyaluronan derivative (Hyaff-11) into cartilage defects in rabbits. Biomaterials. 2001;22:2417–2424. doi: 10.1016/S0142-9612(00)00429-4. [DOI] [PubMed] [Google Scholar]
- 7.Guo X, Wang C, Zhang Y, Xia R, Hu M, Duan C, Zhao Q, Dong L, Lu J, Song YQ. Repair of large articular cartilage defects with implants of autologous mesenchymal stem cells seeded into β-tricalcium phosphate in a sheep model. Tissue Eng. 2004;10:1818–1829. doi: 10.1089/ten.2004.10.1818. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Yang D, Yan W, Shi Z, Feng J, Gao Y, Weng W, Yan S. Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly (L-lactic acid) hybrid materials. Biomaterials. 2007;28:3091–3100. doi: 10.1016/j.biomaterials.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Ito Y, Ochi M, Adachi N, Sugawara K, Yanada S, Ikada Y, Ronakorn P. Repair of osteochondral defect with tissue-engineered chondral plug in a rabbit model. Arthroscopy. 2005;21:1155–1163. doi: 10.1016/j.arthro.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DW, Lalor PA, Aberman HM, Simon TM. Spontaneous repair of full-thickness defects of articular cartilage in a goat model. A preliminary study. J Bone Joint Surg Am. 2001;83:53–64. doi: 10.1302/0301-620X.83B7.12756. [DOI] [PubMed] [Google Scholar]
- 11.Johnson LL. Clinical methods of cartilage repair. Arthroscopic abrasion arthroplasty. A review. Clin Orthop Relat Res. 2001;391 Suppl:S306–S317. doi: 10.1097/00003086-200110001-00028. [DOI] [PubMed] [Google Scholar]
- 12.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–5491. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 13.Menche DS, Vangsness CT Jr, Pitman M, Gross AE, Peterson L (1998) The treatment of isolated articular cartilage lesions in the young individual. In: Cannon WD Jr (ed) AAOS Instructional Course Lectures 47, pp 505–515 [PubMed]
- 14.Nagura I, Fujioka H, Kokubu T, Makino T, Sumi Y, Kurosaka M. Repair of osteochondral defect with a new porous synthetic polymer scaffold. J Bone Joint Surg Br. 2007;89:258–264. doi: 10.1302/0301-620X.89B2.17754. [DOI] [PubMed] [Google Scholar]
- 15.Oztürk A, Ozdemir MR, Ozkan Y. Osteochondral autografting (mosaicplasty) in grade IV cartilage defects in the knee joint: 2- to 7-year results. Int Orthop. 2006;30:200–204. doi: 10.1007/s00264-005-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Solchaga LA, Temenoff JS, Gao J, Mikos AG, Caplan AI, Goldberg VM. Repair of osteochondral defects with hyaluronan- and polyester-based scaffolds. Osteoarthritis Cartilage. 2005;13:297–309. doi: 10.1016/j.joca.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Tamai N, Myoui A, Hirao M, Kaito T, Ochi T, Tanaka J, Takaoka K, Yoshikawa H. A new biotechnology for articular cartilage repair: subchondral implantation of a composite of interconnected porous hydroxyapatite, synthetic polymer (PLA-PEG), and bone morphogenetic protein-2 (rhBMP-2) Osteoarthritis Cartilage. 2005;13:405–417. doi: 10.1016/j.joca.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y, Ohgushi H, Fukuchi T, Sato M. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly-lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Willers C, Chen J, Wood D, Xu J, Zheng MH. Autologous chondrocyte implantation with collagen bioscaffold for the treatment of osteochondral defects in rabbits. Tissue Eng. 2005;11:1065–1076. doi: 10.1089/ten.2005.11.1065. [DOI] [PubMed] [Google Scholar]
- 22.Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy. 2007;23:178–187. doi: 10.1016/j.arthro.2006.09.005. [DOI] [PubMed] [Google Scholar]