Abstract
Cementless fixation depends on bone ingrowth for long-term success. Simvastatin as a lipid lowering agent has been demonstrated to have osteoanabolic effects. This study was designed to measure the possible effect of simvastatin on implant osseointegration. Bilateral femoral implantation of titanium cylinders was performed in 20 rabbits. Blood lipid levels were measured pre- and postoperatively. Scanning electron microscopy (SEM) was used to measure the percentage of the surface of each implant in contact with bone and mechanical pull-out testing was performed. The blood lipid levels were significantly reduced in the simvastatin group. Histomorphometric examination revealed increased bone ingrowth and mechanical examination showed increased interface strength in the simvastatin group. Mechanical and histological data showed superior stability and osseous adaptation at the bone/implant interface for the simvastatin group. We conclude that simvastatin has potential as a means of enhancing bone ingrowth, which is a key factor in the longevity of cementless implants.
Résumé
La fixation d’une prothèse sans ciment dépend de la réhabitation osseuse. La Simvastatine est un agent lipidique qui a un effet ostéo anabolique. Cette étude a pour but de montrer les effets de la Simvastatine sur l’ostéo intégration osseuse. Matériel et méthode : une implantation de cylindres de titane a été réalisée sur les deux fémurs de vingt lapins. Le taux de lipide a été mesuré en pré et post opératoire. L’examen en microscopique électronique a mesuré le pourcentage de la surface de réhabitation et des essais d’arrachage ont également été réalisés. Résultats : le niveau des lipides sanguins est réduit de façon significative dans le groupe de Simvastatine. L’histomorphométrie osseuse montre la croissance, l’orientation de la réhabitation et les tests mécaniques, l’augmentation de l’interface avec augmentation des forces nécessaires pour l’arrachage. En conclusion, les données mécaniques et histologiques montrent une stabilité supérieure dans le groupe Simvastatine. Nous pouvons conclure que la Simvastatine a un potentiel d’augmentation de la réhabitation osseuse facteur clé du succès à long terme des implants sans ciment.
Introduction
Osseointegration after primary stabilisation is of critical importance for the long-term outcome of joint replacement surgery. Although implant design, material and surgical technique were the main factors responsible for the primary stability, biomechanical forces, patient variables and surface coatings affect osseointegration [4]. Several materials have been used in order to accelerate and enhance this process, including surface coatings such as hydroxyapatite-coated implants or experimentally the use of growth factors [4, 6, 11, 19, 21].
Simvastatin is a hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and a potent lipid lowering drug [22]. In addition to a lipid lowering effect, it stimulates bone growth, mostly by increasing the expression of BMP-2 and 4, but it also has osteogenic effects independent from these factors [9, 22]. Although the detailed mechanism of this osteogenic action of simvastatin is still unclear, rho-kinase inhibition, differentiation of endothelial progenitor cells with Akt protein kinase, osteoblastic differentiation and its effect on vitamin K metabolism are possible explanations for the mode of action [9, 14, 16]. Clinically, simvastatin has also been shown to increase bone mineral density and reduce the incidence of osteoporotic fractures in several retrospective series [1, 15, 17].
Realising the potential for improving osseointegration, we wondered precisely how simvastatin may effect osseous response in an arthroplasty model by its stimulatory effect on bone growth. This hypothesis was tested in a small animal model of arthroplasty in which the influence of simvastatin was examined mechanically and histologically in bone growth.
Materials and methods
Bilateral distal femoral intramedullary implantation of titanium cylinders was performed in 20 skeletally mature male New Zealand white rabbits with a mean weight of 2.9 kg (range; 2.7–3.5 kg), after obtaining approval from the Animal Research Ethical Committee. General anaesthesia was induced by intramuscular administration of 80 mg/kg ketamine. Blood samples were obtained in order to measure the blood lipid levels. Both legs were prepped and draped. A median skin incision and a medial parapatellar approach was used to expose the femur. Gentle reaming to a diameter of 4.5 mm was performed in the intramedullary canal of the femur with a low-speed drill. An implant was inserted in a press-fit fashion into the bone tunnel. The implants were titanium alloy (Ti-6Al-4V) grit-blasted cylinders 10 mm long and 5 mm in diameter and specifically manufactured for this study. One end of the implant was threaded for fixation to an adapter for mechanical testing. Simvastatin was obtained from the manufacturer (Merck Sharpe Dohme, West Chester, Pennsylvania) as a pure compound. An alkaline hydrolysis method was used for the activation of simvastatin as previously described [23]. The experimental group received 50 mg/kg/day subcutaneous injections and the control group received the same volume of saline (1 cc) daily for six weeks. For histological and mechanical evaluation, all of the rabbits were sacrificed at six weeks with intravenous barbiturate. Blood chemistry analysis included total cholesterol and triglyceride levels. The joints were exposed and the femora were harvested en bloc. Anteroposterior and lateral radiographs of the specimens were taken immediately after harvesting. Each specimen from each rabbit was used for both stress testing and histological analysis. One femur wrapped in saline-soaked gauze was immediately frozen to −60°C and then used for the mechanical testing of fixation strength. Another femur was preserved for histological analysis.
For mechanical testing, the frozen femora were defrosted in physiological saline at room temperature. Tests were performed on a hydroelectric materials testing system (model 1123, Instron, Canton, Massachusetts). The implanted femur was embedded in acrylic cement and tested within one hour of preparation. The implant was pulled out of the potted bone at a constant rate of 1mm/min and the maximal force reached before failure was recorded. The maximal value was converted to megapascals (force divided by surface area of the implant).
For histological evaluation, the plugs were embedded in methylmethacrylate after fixation and immersion with alcohol. Three samples for each implant were sectioned (2 mm in thickness) using a low-speed diamond wheel saw microtome. After grinding and polishing one surface of each section, the specimens were coated with gold-palladium (Emitech K550x, England) and processed for backscattered scanning electron microscopic (BSEM) examination. A Leo 438VP scanning electron microscope (England) equipped with a backscattered electron (BSE) detector was used for BSEM imaging with accelerating voltages at 25 kV. Image grey-scale values were separated into three levels: bone, soft tissue and implant. Osseointegration has been defined as the direct contact between bone and implant [13]. The percentage of the surface of each implant in direct contact with bone determined the percent osseointegration [8, 24]. A total of six neighbouring images were obtained from each section to determine the total bone/implant area at 35× magnification (Fig. 1). Those six images were then assembled to reproduce the total bone/implant interface area for analysis (Fig. 2). In the region of interest, the total area of the three different structures were calculated by thresholding the grey levels using Zeiss LSM 510 software (Germany). Therefore, a calculation was made from: i) total implant surface; ii) total bone trabecular surface; iii) total area of soft tissues [18].
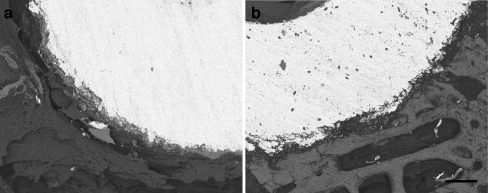
Fig. 1.
a Backscattered scanning electron microscopic (BSEM) image of the control group, demonstrating a lesser amount of osseointegration. 250 grey-level thresholding was implemented to differentiate the constituents in the bone/implant surface, where the implant surface was transformed into absolute black (grey level = 0.36, bar = 300 micrometres). b BSEM images of the simvastatin group sample, demonstrating a greater amount of osseointegration
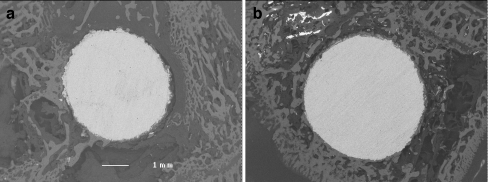
Fig. 2.
Six neighbouring images were taken with a backscattered electron detector (BSD) at 35× magnification to reconstruct a high-resolution panoramic image showing the entire bone/implant surface. Examination of the simvastatin group (b) revealed a greater percentage of bone growth when compared with the control group (a)
The mean and standard deviation values were calculated for all data. Comparisons of the histomorphometric data and mechanical data were performed using the Student’s t-test variance analysis and correlation analysis. p values of <0.05 were considered to be significant. The data obtained was analysed using SPSS (Statistical Package for Social Sciences) Windows release 11.5.
Results
All animals successfully completed the study and all groups had a minimum of six specimens available for complete analysis. The mean blood lipid levels pre- and postoperatively for the simvastatin-treated and control groups are presented in Table 1, including the total cholesterol and triglyceride levels. There was a significant decrease (p < 0.05) in the simvastatin-treated group. Radiological examination at the time of sacrifice revealed no translucent or sclerotic areas and no implant migration was noticed on the bone surface surrounding the implant. The average forces measured during pull-out testing with an Instron testing device were as follows; the mean applied distractive forces were 2.08 MPa (mean SD = 0.60) in the control group and 3.10 MPa (mean SD = 0.84) in the simvastatin group. The fixation strength of the simvastatin group was significantly higher (p < 0.05) than those of the control group. The results of the quantitative BESM examination revealed that the percentage bone growth was 22.5% (mean SD = 3.7) in the control group and 31.8% (mean SD = 5.2) in the simvastatin group (Fig. 2). These values were found to be significantly different (p < 0.05) between the two groups of animals. Overall, bone ingrowth was higher in the simvastatin group when compared to the control group.
Table 1.
Blood lipid levels in the simvastatin and control groups
| Simvastatin (mean, SD) | Control (mean, SD) | |||
|---|---|---|---|---|
| Preop. (mg/ml) | Postop. (mg/ml) | Preop. (mg/ml) | Postop. (mg/ml) | |
| Total cholesterol | 48 (±5.2) | 42 (±6.3) | 46 (±4.9) | 46.70 (±5.4) |
| Triglyceride | 103 (±13.0) | 90 (±16.7) | 99 (±10.4) | 95 (±12.8) |
Discussion
In the biological fixation of cementless arthroplasty, recipient bone is used to obtain a sufficient interference fit, which provides short-term stability to allow adequate ingrowth, thus, maintaining long-term fixation. In the field of prosthetic research, considerable efforts have been made to develop new techniques for the porous surface to enhance bone ingrowth, thereby, improving the longevity of joint replacements. Regional bone remodelling and success of the joint replacement can vary with the prosthesis design and surface modifications, which can be evaluated with efficient in vivo measurement techniques, such as quantitative computed tomography (CT)-assisted osteodensitometry [10, 11, 19, 20]. Current methods of enhancing bony apposition mainly include bioactive coats and texturing of the implant [2]. Clinically, these modifications with improved bone ingrowth capacity also had preventive effects on osteolysis, which is an important factor for longevity of the implant [21]. Additionally, several local factors have been reported to play a role on osseointegration, including transforming growth factor-B, osteoblasts and autogenous bone graft [12, 24]. However, all have remained experimental and none have gained wide clinical application.
Simvastatin, as a member of the lipid lowering statin family, can promote bone formation, as shown by Mundy et al. [17]. Statins have been shown to activate the promoter of the bone morphogenetic protein-2 (BMP-2) gene in osteoblasts and decrease the number of osteoclasts in vitro [17, 18]. BMP-2 is one of the most potent osteoconductive proteins involved in the recruitment, proliferation and differentiation of osteoprogenitor cells, resulting in bone formation [25]. Simvastatin also increases cancellous bone volume, bone formation rate (BFR) and cancellous bone compressive strength in vivo [16]. Our study was undertaken to test the hypothesis that simvastatin could be used to enhance bone ingrowth in joint replacement. The minimally loaded intramedullary implantation model, in male rabbits only, was chosen because the effect of the drug could be isolated from confounding factors, such as implant design, loading conditions and hormonal factors. Since orally administered statins are subject to hepatic first-pass metabolism, the bioavailability of statins to bone sites may be impaired, alkali hydrolysis and parenteral administration of simvastatin was preferred in our study [25]. The blood chemistry obtained before and after the study revealed a significant (p < 0.05) decrease in cholesterol levels, which was in concordance with an active compound in the circulation lowering the blood lipid levels.
Osseous integration can be evaluated with mechanical testing and radiological and histological examination. In mechanical testing, pull-out and push-out tests are the most frequently used tests for measuring the maximal force reached before failure. Pull-out tests are more suitable for intramedullary implantation models [7]. Feighan et al. [3], Goldberg et al. [5] and D’Lima et al. [2] reported interface strengths of approximately from 2 to 3 MPa in similar rabbit arthroplasty models designed for investigating the influence of surface blasting on implant fixation. All three authors demonstrated no significant differences in interface strengths among the different surface blasting techniques. In our study, the mechanical tests revealed significantly higher (p < 0.05) interface strengths in the drug group compared to the control group, suggesting a positive effect of simvastatin on osseointegration.
Histomorphological examination of osseointegration was usually performed with scanning electron microscopy in the backscattered mode [2, 8]. Goldberg et al. [5] demonstrated that 31% of their surface was in contact with bone and Feighan et al. [3] reported values between 30% and 35%. D’Lima et al. [2] reported different percentages from 30% to 58% in diaphyseal and metaphyseal samples in grit-blasted implants in analogous rabbit models. In our study, we were able to show that the bone to soft tissue-implant ratio was significantly (p < 0.05) higher in the simvastatin group (31.8%) when compared to the control group (22.5%). In the light of these findings, it seems reasonable to assume that the osteoanabolic effects of simvastatin on bone formation may also enhance osseointegration on metallic implants.
A migrated implant or the presence of radiolucent lines on plain roentgenograms are indicators of mechanical instability associated with failed osseointegration and poor clinical results [26]. There were no significant differences on radiological examination (radiolucent lines and implant migration) between the two groups; the absence of mechanical loading in our experimental model may be used as an explanation for this finding. Therefore, plain roentgenograms provided no valuable information regarding the bone ingrowth in our study. Overall, the histological analysis and mechanical testing of the implants support the conclusion that the implant was integrated well into the bone.
This study was subject to certain limitations. First, the animal model used here was developed to investigate the effect of materials and drugs and, therefore, avoids mechanical load, which is an important factor in the clinical setting. Second, the number of animals and histological specimens were limited. Histomorphometric, radiographic and mechanical data were all evaluated in view of the small sample size. Despite these shortcomings, good osseointegration with the implant was observed.
In this study, the overall osseointegration in the simvastatin group was significantly higher than that of the control group. Ultimately, controlled clinical trials are needed to determine the role of simvastatin for the enhancement of bone ingrowth and the prevention of early migration in humans, especially in cementless prostheses for severely osteoporotic patients.
We conclude that simvastatin has the potential to enhance osseointegration in a rabbit arthroplasty model. Mechanical and histological data showed superior stability and osseous adaptation at the bone/implant interface for the simvastatin group. Our new findings suggest that statins, such as simvastatin, may have potential in osseointegration enhancement in cementless fixation in total and revision joint replacement.
Acknowledgements
This work was supported by the Scientific Research Projects Fund of Ankara University (project no. 20040809179).
References
- 1.Chan KA, Andrade SE, Boles M, Buist DS, Chase GA, Donahue JG, Goodman MJ, Gurwitz JH, LaCroix AZ, Platt R. Inhibitors of hydroxymethylglutaryl-coenzyme A reductase and risk of fracture among older women. Lancet. 2000;355:2185–2188. doi: 10.1016/S0140-6736(00)02400-4. [DOI] [PubMed] [Google Scholar]
- 2.D’Lima DD, Lemperle SM, Chen PC, Holmes RE, Colwell CW., Jr Bone response to implant surface morphology. J Arthroplasty. 1998;13:928–934. doi: 10.1016/S0883-5403(98)90201-7. [DOI] [PubMed] [Google Scholar]
- 3.Feighan JE, Goldberg VM, Davy D, Parr JA, Stevenson S. The influence of surface-blasting on the incorporation of titanium-alloy implants in a rabbit intramedullary model. J Bone Joint Surg Am. 1995;77:1380–1395. doi: 10.2106/00004623-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Frosch KH, Sondergeld I, Dresing K, Rudy T, Lohmann CH, Rabba J, Schild D, Breme J, Stuermer KM. Autologous osteoblasts enhance osseointegration of porous titanium implants. J Orthop Res. 2003;21:213–223. doi: 10.1016/S0736-0266(02)00143-2. [DOI] [PubMed] [Google Scholar]
- 5.Goldberg VM, Stevenson S, Feighan J, Davy D. Biology of grit-blasted titanium alloy implants. Clin Orthop Relat Res. 1995;319:122–129. [PubMed] [Google Scholar]
- 6.Hayashi K, Mashima T, Uenoyama K. The effect of hydroxyapatite coating on bony ingrowth into grooved titanium implants. Biomaterials. 1999;20:111–119. doi: 10.1016/S0142-9612(98)00011-8. [DOI] [PubMed] [Google Scholar]
- 7.Hing KA, Best SM, Tanner KE, Bonfield W, Revell PA. Biomechanical assessment of bone ingrowth in porous hydroxyapatite. J Mater Sci Mater Med. 1997;8:731–736. doi: 10.1023/A:1018500309969. [DOI] [PubMed] [Google Scholar]
- 8.Holmes RE, Hagler HK, Coletta CA. Thick-section histometry of porous hydroxyapatite implants using backscattered electron imaging. J Biomed Mater Res. 1987;21:731–739. doi: 10.1002/jbm.820210605. [DOI] [PubMed] [Google Scholar]
- 9.Izumo N, Fujita T, Nakamuta H, Koida M. Lipophilic statins can be osteogenic by promoting osteoblastic calcification in a Cbfa1- and BMP-2-independent manner. Methods Find Exp Clin Pharmacol. 2001;23:389–394. doi: 10.1358/mf.2001.23.7.662123. [DOI] [PubMed] [Google Scholar]
- 10.Laine HJ, Puolakka TJS, Moilanen T, Pajamäki KJ, Wirta J, Lehto MUK. The effects of cementless femoral stem shape and proximal surface texture on ‘fit-and-fill’ characteristics and on bone remodeling. Int Orthop. 2000;24:184–190. doi: 10.1007/s002640000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leali A, Fetto JF. Preservation of femoral bone mass after total hip replacements with a lateral flare stem. Int Orthop. 2004;28:151–154. doi: 10.1007/s00264-004-0554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis CG, Jones LC, Hungerford DS. Effects of grafting on porous metal ingrowth. A canine model. J Arthroplasty. 1997;12:451–460. doi: 10.1016/S0883-5403(97)90202-3. [DOI] [PubMed] [Google Scholar]
- 13.Linder L, Carlsson A, Marsal L, Bjursten LM, Brånemark PI. Clinical aspects of osseointegration in joint replacement. A histological study of titanium implants. J Bone Joint Surg Br. 1988;70:550–555. doi: 10.1302/0301-620X.70B4.3403596. [DOI] [PubMed] [Google Scholar]
- 14.Maeda T, Matsunuma A, Kawane T, Horiuchi N. Simvastatin promotes osteoblast differentiation and mineralization in MC3T3-E1 cells. Biochem Biophys Res Commun. 2001;280:874–877. doi: 10.1006/bbrc.2000.4232. [DOI] [PubMed] [Google Scholar]
- 15.Meier CR, Schlienger RG, Kraenzlin ME, Schlegel B, Jick H. HMG-CoA reductase inhibitors and the risk of fractures. JAMA. 2000;283:3205–3210. doi: 10.1001/jama.283.24.3205. [DOI] [PubMed] [Google Scholar]
- 16.Montagnani A, Gonnelli S, Cepollaro C, Pacini S, Campagna MS, Franci MB, Lucani B, Gennari C. Effect of simvastatin treatment on bone mineral density and bone turnover in hypercholesterolemic postmenopausal women: a 1-year longitudinal study. Bone. 2003;32:427–433. doi: 10.1016/S8756-3282(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 17.Mundy G, Garrett R, Harris S, Chan J, Chen D, Rossini G, Boyce B, Zhao M, Gutierrez G. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 18.Oxlund H, Andreassen TT. Simvastatin treatment partially prevents ovariectomy-induced bone loss while increasing cortical bone formation. Bone. 2004;34:609–618. doi: 10.1016/j.bone.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Pitto RP, Mueller LA, Reilly K, Schmidt R, Munro J. Quantitative computer-assisted osteodensitometry in total hip arthroplasty. Int Orthop. 2007;31:431–438. doi: 10.1007/s00264-006-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosenthall L, Bobyn JD, Tanzer M. Bone densitometry: influence of prosthetic design and hydroxyapatite coating on regional adaptive bone remodelling. Int Orthop. 1999;23:325–329. doi: 10.1007/s002640050383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sanchez-Sotelo J, Lewallen DG, Harmsen WS, Harrington J, Cabanela ME. Comparison of wear and osteolysis in hip replacement using two different coatings of the femoral stem. Int Orthop. 2004;28:206–210. doi: 10.1007/s00264-004-0558-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnettler R, Alt V, Dingeldein E, Pfefferle HJ, Kilian O, Meyer C, Heiss C, Wenisch S. Bone ingrowth in bFGF-coated hydroxyapatite ceramic implants. Biomaterials. 2003;24:4603–4608. doi: 10.1016/S0142-9612(03)00354-5. [DOI] [PubMed] [Google Scholar]
- 23.Skaletz-Rorowski A, Lutchman M, Kureishi Y, Lefer DJ, Faust JR, Walsh K. HMG-CoA reductase inhibitors promote cholesterol-dependent Akt/PKB translocation to membrane domains in endothelial cells. Cardiovasc Res. 2003;57:253–264. doi: 10.1016/S0008-6363(02)00618-1. [DOI] [PubMed] [Google Scholar]
- 24.Skedros JG, Bloebaum RD, Bachus KN, Boyce TM. The meaning of gray levels in backscattered electron images of bone. J Biomed Mater Res. 1993;27:47–56. doi: 10.1002/jbm.820270107. [DOI] [PubMed] [Google Scholar]
- 25.Knoch F, Wedemeyer C, Heckelei A, Saxler G, Hilken G, Brankamp J, Sterner T, Landgraeber S, Henschke F, Löer F, Knoch M. Promotion of bone formation by simvastatin in polyethylene particle-induced osteolysis. Biomaterials. 2005;26:5783–5789. doi: 10.1016/j.biomaterials.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Vresilovic EJ, Hozack WJ, Rothman RH. Radiographic assessment of cementless femoral components. Correlation with intraoperative mechanical stability. J Arthroplasty. 1994;9:137–141. doi: 10.1016/0883-5403(94)90062-0. [DOI] [PubMed] [Google Scholar]