Abstract
Platelet-rich plasma (PRP), a platelet concentrate made of autogenous blood, has been used to improve bone and soft tissue defect healing in recent years. The aim of this study was to assess the effect of PRP on articular cartilage defects in a rabbit model. Forty-eight osteochondral defects created in the femoropatellar groove were (a) left untreated, (b) treated with autogenous PRP in a poly-lactic-glycolic acid (PLGA), or (c) with PLGA alone. Platelets were enriched 5.12-fold compared to normal blood in the PRP. After four and 12 weeks, the explanted tissue specimens were assessed by macroscopic examination, micro-computed tomography, and histological evaluation. Macroscopic examination, micro-computed tomography and histology of the newly formed cartilage and bone in the defect differ significantly between the PRP-treated and the untreated groups, and stimulatory effect of PRP on osteochondral formation was observed. In conclusion, PRP in PLGA improves osteochondral healing in a rabbit model.
Electronic supplementary material
The online version of this article (doi:10.1007/s00264-009-0793-2) contains supplementary material, which is available to authorized users.
Introduction
It has been well established that articular cartilage has a limited capacity for self repair due to the low cellular mitotic activity of chondrocytes and its avascularity. Large cartilage defects often fail to heal spontaneously and may result in progressive deterioration and eventually osteoarthritis [4]. In recent years, autologous chondrocyte transplantation and autologous osteochondral grafting have become popular in the clinical routine and have demonstrated the ability to promote the restoration of cartilage for patients with localised articular cartilage defects in some clinical trials [2, 18]. However, the limited sources of transplants and donor site morbidity are inevitable drawbacks.
Based on the tissue-engineering approach, the use of bioactive agents such as recombined growth factors in an appropriate carrier to help the body to heal itself has become a focus of attention [9, 10]. Several growth factors, especially transforming growth factor-β (TGF-β) [10], basic fibroblast growth factor (bFGF) [9], and bone morphogenetic protein [3, 5, 12, 17] have been proven to be effective for cartilage tissue regeneration. But some disadvantages of recombined growth factors are also obvious, such as short shelf life and high price. Furthermore, only a few growth factors are available for clinical use at present.
When the clinical application of growth factors was considered, platelet-rich plasma (PRP), as a rich source of autologous growth factors, can be regarded as an alternative approach. PRP contains a number of these growth factors (platelet derived growth factor [PDGF], TGF-β, bFGF, insulin growth factor [IGF], vascular endothelial growth factor [VEGF], and epithelial cell growth factor [ECGF]) in its natural composition [9]. Because it can be used autogenously, it poses no risk of transmissible diseases. Furthermore, PRP can easily be obtained on the day of surgery by two centrifugation steps from autogenous whole blood. PRP has been extensively investigated for bone regeneration and soft tissue healing and many reports claim a positive effect of PRP [22]. Thus, if PRP growth factors were also effective for cartilage tissue, PRP could be an attractive clinical source for osteochondral tissue regeneration.
For in vivo application of PRP, we used PLGA as a carrier for PRP growth factors, since PLGA was investigated for repair of the whole thickness cartilage defect and showed promising biocompatibility and mechanical properties [21].
Materials and methods
Preparation of PRP
Blood samples were obtained from New Zealand rabbits weighing 2.8–3.2 kg. The Animal Research Committee of Shanghai Jiao Tong University School of Medicine approved all procedures. PRP was prepared using two centrifugation techniques, as previously reported [11]. Briefly, rabbits were anaesthetised, 16 ml of whole blood was drawn from each subject into tubes containing 4 ml of acid citrate dextrose-A solution as an anticoagulant. An aliquot was removed from each tube to determine the platelet count. The tubes were then spun in a laboratory centrifugation apparatus (6800; Kubota, Tokyo, Japan) at 4°C for 15 min at 800 rpm, and all plasma was transferred to new sterile tubes to be further centrifuged at 4°C for 15 min at 2000 rpm. The supernatant plasma was discarded and the remaining approximately 0.8 ml of plasma and precipitated platelet was designated PRP. Platelet counts were also performed for samples of PRP. To measure the concentration of growth factors secreted from PRP and circulating plasma obtained from centrifugation of peripheral whole blood, enzyme-linked immunosorbent assay (ELISA) was performed (n = 7 in each group) according to previous studies [11].
Preparation of microporous PLGA scaffolds
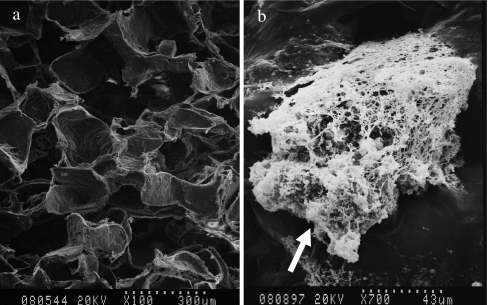
PLGA copolymer was purchased from Boehringer Ingelheim Pharma GmbH (Ingelheim, Germany). PLGA scaffolds were fabricated as previously described [13]. The microstructure of the scaffold was observed by scanning electron microscopy (SEM) (Fig. 1a).
Fig. 1.
SEM photographs of the porous PLGA scaffold and PRP/PLGA composite. a The micropore structure of the PLGA scaffold. b The activated platelets (white arrow) were embedded in the interspaces of the scaffold
Loading of PRP
A loading volume consisting of 20 µl human lyophilized thrombin (4 IU/ml; Sigma, USA), reconstituted in 10% CaCl2 solution (Merck, Germany), and 80 µl of PRP mixture was sequentially pipette loaded onto each PLGA scaffold placed in a well of a 24-well plate. PRP clotted upon contact with the thrombin-CaCl2 solution in the scaffold incubation at 37°C. Scanning electron microscopy (SEM) was used to verify the attachment of PRP onto the PLGA scaffold (Fig. 1b).
Surgical implantation
A full-thickness defect (diameter 5 mm, thickness 4 mm) was created through the articular cartilage and subchondral bone of the patellar groove in 16 rabbits (the PRP from them had been prepared as mentioned above and from the same rabbit) using a drill equipped with a 5-mm diameter drill bit. The PRP/PLGA was implanted to fill the cartilage defect on one knee while PLGA was used on the contralateral knee. Eight additional age-matched rabbits were used as a control group with full-thickness defects on both knees and no treatment. The animals were returned to their cages and allowed to move freely without joint immobilisation. The rabbits were sacrificed at four and 12 weeks after transplantation. Each cartilage defect area was evaluated both macroscopically and histologically. The sample size was eight for each group at each time point.
Macroscopic examination
At necropsy, the gross appearance of the defects was assessed, taking into consideration the surface characteristics of the repair and its continuity with the host tissue, as well as the presence of osteoarthritic changes throughout the joint.
Histological assessment
The distal femoral component was fixed and decalcified with 10% EDTA solution. Blocks were prepared for processing using coronary dissection of the defect centre. Samples were then dehydrated, embedded, cut and stained with toluidine blue and Safranin O/Fast green staining. Sections were examined in a blinded manner and independently scored by three investigators according to the O’Driscoll histological grading scale (Table 1). To detect expression of type II collagen, immunohistochemical evaluation was performed using the avidin-biotin immunoperoxidase method with an antibody raised against type II collagen (Merck, Germany).
Table 1.
Histological grading
| Category | Points |
|---|---|
| 1. Tissue morphology | |
| Hyaline cartilage | 4 |
| Mainly hyaline cartilage | 3 |
| Mixed hyaline and fibrocartilage | 2 |
| Mostly fibrocartilage | 1 |
| Fibrous tissue | 0 |
| 2. Matrix staining | |
| Normal or near normal | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| 3. Surface regularity | |
| Smooth and intact | 3 |
| Surface fissures (<25% neo-surface thickness) | 2 |
| Deep fissures (25–99% neo-surface thickness) | 1 |
| Complete disruption of the neo-surface | 0 |
| 4. Structure integrity | |
| Normal | 2 |
| Slight disruption, including cysts | 1 |
| Severe disintegration | 0 |
| 5. Thickness of neo-formed cartilage | |
| Similar to normal adjacent cartilage | 2 |
| Less than normal cartilage | 1 |
| No cartilage | 0 |
| 6. Bonding to adjacent cartilage | |
| Bonded at the both ends of graft | 2 |
| Bonded at one end, or partially at both ends | 1 |
| Not bonded | 0 |
| 7. Chondrocyte clustering | |
| No clusters | 2 |
| <25% of the cells | 1 |
| 25–100% of the cells | 0 |
| 8. Hypocellularity | |
| Normal | 3 |
| Slight | 2 |
| Moderate | 1 |
| Severe | 0 |
| 9. Degenerative changes in adjacent cartilage | |
| Normal cellularity, no clusters, normal staining | 3 |
| Normal cellularity, mild clusters, moderate staining | 2 |
| Mild or moderate hypocellularity, slight staining | 1 |
| Severe hypocellularity, poor or no staining | 0 |
| 10. Inflammation | |
| No inflammation | 2 |
| Slight inflammation | 1 |
| Strong inflammation | 0 |
| Total | 26 |
Micro-CT imaging
Specimens obtained at 12 weeks (three animals each) were evaluated with micro-computed tomography (µ-CT; GE micro-CT system, USA) for analysis of bone ingrowth into and around the defect.
Statistical analysis
Data were collected from triplicate or quadruplicate parallel samples and were expressed as the mean ± standard deviation (SD). Statistical analysis was performed using SPSS 11.5 software for windows student version. Statistically significant values were defined as p < 0.05 or p < 0.01 based on one-way analysis of variance (ANOVA).
Results
Assessment of the prepared PRP
The mean number of counted thrombocytes in the peripheral blood was 24.53 × 104/µl. The mean PRP platelet count was 125.59 × 104/µl. The concentration of thrombocytes in PRP increased 5.12-fold according to the mechanical count. Analysis of growth factor concentrations in PRP samples and circulating plasma samples using ELISA revealed that levels of growth factors were significantly higher in PRP samples (Table 2).
Table 2.
Growth factor levels in plasma preparations
| Plasma preparations | PDGF-BB (ng/ml) | TGF-β1 (ng/ml) | bFGF (pg/ml) | VEGF (pg/ml) |
|---|---|---|---|---|
| Circulating plasma (n = 7) | 5.67 ± 1.51 | 18.92 ± 5.7 | 21.04 ± 6.12 | 32.08 ± 6.68 |
| Platelet rich plasma (n = 7) | 29.5 ± 4.5a | 115.45 ± 34.8a | 77.34 ± 21.3a | 168.8 ± 36.87a |
PDGF platelet derived growth factor, TGF-β1 transforming growth factor beta 1, bFGF basic fibroblast growth factor, VEGF vascular endothelial growth factor
Values are expressed as the mean ± standard deviation
a Represents values statistically lower than circulating plasma levels, P < 0.01.
Macroscopic observations
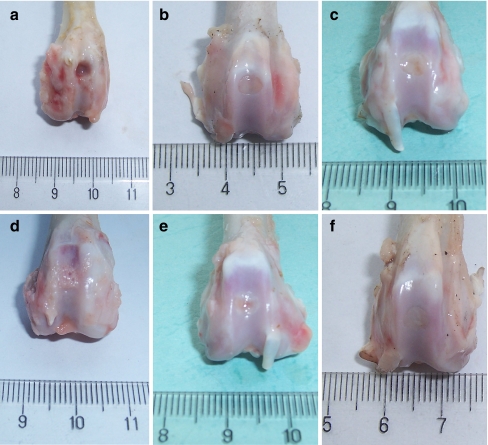
No signs of major wound infection, limited range of motion nor synovitis were found in any rabbits. Four weeks after implantation, the defects in the PRP/PLGA and PLGA groups were quite similar in gross appearance. They were filled with shiny translucent repair tissue with clearly discernable margins. A slight concavity could be observed towards the central area of the repair tissue. The defects in the control group remained empty or were sometimes covered with a thin reddish-brown granular tissue (Fig. 2a–c).
Fig. 2.
Gross appearance of the defects four and 12 weeks postoperatively in the control group (a, d), the PLGA group (b, e), and the PRP/PLGA group (c, f). At four weeks (a–c) and 12 weeks (d–f)
At 12 weeks, the regenerated tissue in the PRP/PLGA group was opaque white and seemed integrating well with the adjacent cartilage. No obvious margins could be distinguished (Fig. 2f). In the PLGA group, the defects were filled with the intermingling of fibrous and fibrocartilaginous tissue. The boundary was still clear. The central area of the defect was depressed (Fig. 2e). In the control group, the surface of reparative tissue was irregular; signs of osteoarthritis surrounding the defect were observed. The defect was filled with reddish-brown granular tissue that protruded into the joint (Fig. 2d).
Histological observations
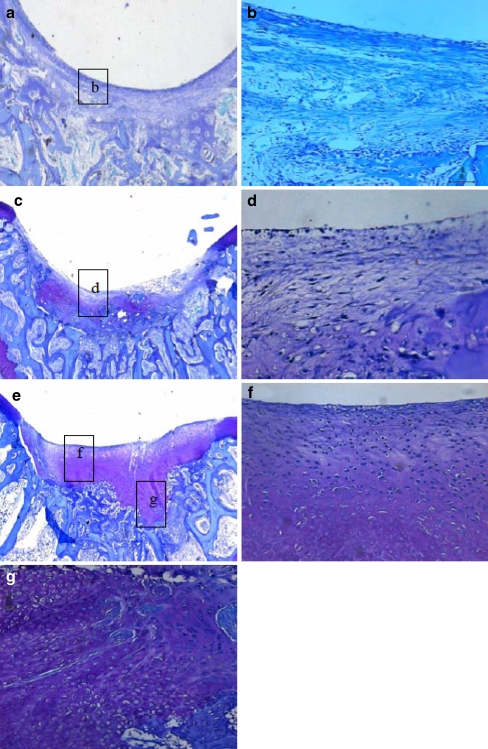
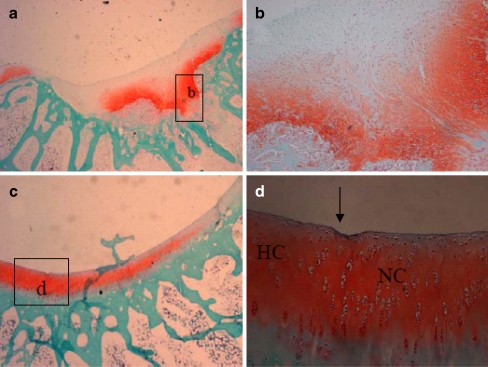
At four weeks, the defects in the PLGA group were filled with fibrous or immature repair tissue (Fig. 3c, d). No obvious cartilaginous extracellular matrix could be identified by Safranin O/Fast green and toluidine blue staining. However, in the PRP/PLGA group a remarkable difference with a much higher extent of neo-chondrogenesis was seen within the cartilage defect void (Fig. 3e–g). Safranin O/Fast green also revealed a high content of glycosaminoglycans of the ECM and the cellular morphology showed round and oval chondrocyte-like cells within lacunae (Fig. 4a,b). There were more cells infiltrated into the PRP/PLGA group compared with that in the PLGA group. The defects in the control group were filled with fibrous tissue with some new bone formation identified around it (Fig. 3a,b).
Fig. 3.
Microphotographs of defects in the PRP/PLGA group (c, f, g), the PLGA group (c, d), and the control group (a, b) four weeks after surgery. Toluidine blue staining; original magnification: 25 × (a, c, e) and 200× (b, d, f, g)
Fig. 4.
Microphotographs of the defects in the PRP/PLGA group four weeks (a, b) and 12 weeks (c, d) after surgery. Safranin O/Fast green staining; original magnification: 25× (a, c) and 200× (b, d). b Photomicrograph of the lower two-thirds of the cartilage repair tissue filling the defects void in the PRP/PLGA group four weeks after treatment. The cellular morphology showed round and oval cells within lacunae surrounded by an extracellular matrix presenting an intense safranin O staining due to a high content of glycosaminoglycans. These characteristics indicated a chondrogenic phenotype of the migrated cells within the PRP/PLGA. Towards the joint cavity the cartilage regenerates, showing a more fibrous tissue character. d Newly-formed elongated cells were arranged parallel to the surface in the superficial zone and spherical cells were arranged in a columnar form in the deep zone similar to host articular cartilage. HC host cartilage, NC newly formed cartilage
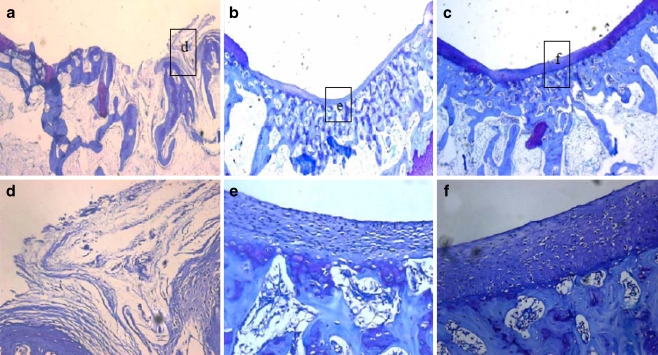
Twelve weeks after implantation, the repair tissue in the control group was mainly fibrous (Fig. 5a, d). Deep fissures could be observed which disrupted the whole structure of the newly-formed tissue. Restoration of the subchondral bone was quite insufficient. In the PRP/PLGA group, the defects were fully filled with regenerated tissue similar to hyaline cartilage. Elongated cells were arranged parallel to the surface in the superficial zone and spherical cells were arranged in a columnar form in the deep zone similar to normal articular cartilage (Fig. 4c, d). Most of the surface of the repair tissue was covered with a layer of cartilage, which integrated well with the surrounding native cartilage (Fig. 5c). An abundance of cartilaginous extracellular matrix could be identified by toluidine blue staining and safranin-O staining (Figs. 4c, d and 5f), and positive immunohistochemical staining of Col II could also be observed (supplement 1). Chondrocytes, accompanied with a typical structure of lacunae, were apparent in the regenerated area. Sometimes, in terms of subchondral bone repair, a continuous layer of trabecular bone was well formed below the cartilage. In the PLGA group, the scaffolds had been completely absorbed, the repair tissue in the defect was mainly fibrocartilage with little cartilaginous extracellular matrix identified by toluidine blue staining (Fig. 4b, e).
Fig. 5.
Microphotographs of the defects in the PRP/PLGA group (c, f), the PLGA group (b, e), and the control group (a, d) 12 weeks postoperatively. Toluidine blue staining; original magnification: 25× (a–c) and 200× (d–f)
Semiquantitative histological scoring
Total score values and individual parameter values of each treatment group are summarised in Table 3. From the data, we found that PRP-treated implants show significantly higher mean total and individual score values compared to both untreated controls and PLGA alone.
Table 3.
Histological scores of repair tissue at four and 12 weeks follow-up
| Score parameter | Four weeks | Twelve weeks | ||||
|---|---|---|---|---|---|---|
| Control group | PLGA group | PRP/PLGA group | Control group | PLGA group | PRP/PLGA group | |
| Tissue morphology | 0.0 ± 0.0 | 0.7 ± 0.5 | 1.8 ± 0.4** | 0.0 ± 0.0 | 1.2 ± 0.4 | 2.5 ± 0.8*** |
| Matrix staining | 0 .0 ± 0 .0 | 0.4 ± 0.2 | 1.5 ± 0.8** | 0.0 ± 0.0 | 0.7 ± 0.7 | 2.3 ± 0.5*** |
| Surface regularity | 1.2 ±0.3 | 1.5 ± 0.5 | 1.6 ± 0.7 | 1.5 ± 0.3 | 1.6 ±0.6 | 2.0 ± 0.0 |
| Structure integrity | 0.6 ± 0.4 | 1.0 ± 0.3 | 1.3 ± 0.5 | 0.7 ± 0.3 | 2.0 ± 0.8 | 2.0 ± 0.0 |
| Thickness of neo-formed cartilage | 0.0 ± 0.0 | 0.7 ± 0.2 | 1.2 ± 0.4 | 0.0 ± 0.0 | 1.0 ± 0.5 | 1.5 ± 0.6 |
| Bonding to adjacent cartilage | 1.0 ± 0.3 | 1.4 ±0.5 | 1.3 ± 0.4 | 1.0 ± 0.4 | 1.5 ±0.3 | 2.0 ± 0.7 |
| Chondrocyte clustering | 2.0 ± 0.4 | 2.0 ± 0.8 | 2.0 ± 0.5 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.4 |
| Hypocellularity | 2.0 ± 0.0 | 2.0 ± 0.5 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.3 ± 0.2 | 2.6 ± 0.5 |
| Degenerative changes in adjacent cartilage | 2.0 ± 0.5 | 2.0 ± 0.2 | 2.0 ± 0.3 | 1.8 ± 0.6 | 2.5 ±0.8 | 3.0 ± 0.5 |
| Inflammation | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 | 2.0 ± 0.0 |
| Total | 10.8 ± 1.9 | 13.7 ± 3.7 | 16.7 ± 4.0** | 11.0 ± 1.6 | 16.8 ± 4.3* | 21.9 ± 4.3* *** |
Ten individual parameters are listed according to the O’Driscoll grading scale
All values are given as the mean and standard deviation for each parameter
Statistical differences were determined by using bivariate χ2 tests (two-sided, with significance limit p < 0.05 without alpha-adjustment)
*p < 0.05 vs 4 weeks
**p < 0.05 vs. PLGA group and control group at 4 weeks
***p < 0.05 vs. PLGA group and control group at 12 weeks
Micro-CT imaging
The difference in the quantity of bone ingrowth between the PRP-treated group and the nontreated group was significant at 12 weeks (supplement 1). Analysis of bone volume showed that a larger amount of subchondral bone formed in the PRP-treated group than in the nontreated group.
Discussion
The hypothesis for this study was that PRP promotes osteochondral healing in a critical-size osteochondral defect on PLGA. The data demonstrate that the addition of PRP had a significantly positive effect on osteochondral formation as shown on histology and on µ-CT.
As is known, to enter widespread clinical use, tissue repair and regeneration technologies must not only be scientifically sound but also cost effective and well suited to clinical application. However, the last two of these requirements tend to be ignored. Recently, in response to this, Evans et al. [7] introduced a new concept known as facilitated endogenous repair, which relies on harnessing the intrinsic regenerative potential of endogenous tissues using molecular stimuli to initiate reparative processes in situ. Our study is a good example of this concept.
PRP has several advantages over recombinant growth factors or products of animal origin. First, as a point of care approach, the autologous preparation of PRP avoids the complex regulatory pathway. In addition, safety issues, such as immunological reactions or carcinogenesis, are of much less concern. Finally, the costs of using PRP would be considerably less than those associated with the use of recombinant proteins. Since exogenous cells are unnecessary thus simplifying the whole process, PRP has significant benefits in clinical use.
The use of autologous PRP, which serves as a source of growth factors, has gained wide acceptance in several surgical applications such as bone regeneration in periodontal and maxillofacial surgery, orthopaedic surgery, otolaryngology and plastic surgery [8, 15, 16, 19]. Recently, some studies had suggested that PRP stimulate either cell proliferation or matrix metabolism by articular chondrocytes in vitro [1]. However, few studies have investigated the potential benefits for chondral applications in vivo; therefore this study focussed on the effect of PRP on large osteochondral defects in vivo.
Qualitative and/or quantitative alterations of the platelets may affect the regenerative potential of platelet-rich plasma. In our study, PRP preparations concentrated over 1 million/ml platelets and therefore could be recognised as ‘‘therapeutic PRP’’ [11, 15]. Furthermore, in this study, PRP, compared to plasma, had a statistically significant five-fold increase in PDGF-BB, a six-fold increase in TGF-β1, a 5.2-fold increase in VEGF expression and a significant 3.7-fold increase in bFGF expression. Thus, we consider our PRP preparations adequate for investigating its effects on osteochondral defects.
In this study, PRP incorporated in PLGA and the PLGA alone were compared for cartilage regeneration ability. Our results demonstrated that when PRP was applied, most of the defects were filled with a well-established layer of cartilage tissue, with abundance of cartilaginous extracellular matrix, and Col II accumulation was observed. However, when PRP was not used, the regenerated tissue turned to fibrocartilage with little cartilaginous extracellular matrix formation detected by histological examination. The different outcomes suggested the remarkable healing properties of PRP for the repair of osteochondral defects in vivo. We believe that there are several reasons to account for the cartilage healing process. First, when PRP incorporated in PLGA scaffolds fill the defects, the growth factors are released as the PLGA biodegrades. In general, most growth factors undergo rapid degradation when injected directly in soluble form to the injury site, so they could not sustain biological activity in vivo. PRP growth factors are different from recombined growth factors, they are usually stored in a-granules of platelet in latent form. When platelets are activated, these factors are gradually secreted from platelets [15]. Second, chondrocytes derived from the surrounding normal cartilage were activated and migrated into the defect. A recent study found platelet-rich plasma could stimulate articular chondrocyte proliferation and matrix biosynthesis [1]. Third, bone marrow-derived mesenchymal stromal cells or other multi-potent cells were activated and migrated into the defect together. These cells migrated into the defects and gradually replaced the PLGA. In our study, round cells and rich cartilage ECM formations were seen as early as four weeks, and progress towards cartilage occurred gradually. PRP growth factors further stimulated these migrated cells, which eventually directly matured to cartilage. These results were also confirmed by Anja Drengk′s study demonstrating chondrogenic and proliferative effect of PRP on mesenchymal stem cells [6].
In addition, healing of the underlying subchondral area of the defect is quite important because the existence of subchondral bone is critical to support the overlying neocartilage tissue. In our study, bone volume analysis measured in µ-CT demonstrated that PRP induced regeneration of subchondral bone into and around the defect. In contrast, the nontreated group and the control group exhibited little bone formation into and around the defect. Moreover, histological results demonstrated that a continuous layer of trabecular bone was well-formed below the cartilage with clusters of new bone formation. However, less bone formation was observed inside the composites whether in PLGA or the control group. These outcomes indicate that the PRP promotes bone ingrowth. A number of experimental [19] and clinical [14, 20] studies demonstrating beneficial effects of PRP on bone defect healing reported results similar to ours; they all found that PRP could enhance the bone formation.
Conclusion
PRP yielded better osteochondral formation than the empty PLGA scaffold after 12 weeks. PRP incorporated in PLGA could successfully resurface the defect with cartilage and restore the subchondral bone in the rabbit model.
Electronic supplementary material
Below is the linked to the electronic supplementary material
(DOC 2748 kb)
(DOC 916 kb)
(DOC 5194 kb)
Acknowledgements
This study was supported by the Key Project Foundation of the Ministry of Health of P.R. China.
References
- 1.Akeda K, An HS, Okuma M, Attawia M, Miyamoto K, Thonar EJ, Lenz ME, Sah RL, Masuda K. Platelet-rich plasma stimulates porcine articular chondrocyte proliferation and matrix biosynthesis. Osteoarthr Cartil. 2006;14:1272–1280. doi: 10.1016/j.joca.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Boopalan PR, Sathishkumar S, Kumar S, Chittaranjan S. Rabbit articular cartilage defects treated by allogenic chondrocyte transplantation. Int Orthop. 2006;30:357–361. doi: 10.1007/s00264-006-0120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borovecki F, Pecina Slaus N, Vukicevic S. Biological mechanisms of bone and cartilage remodelling-genomic perspective. Int Orthop. 2007;31:799–805. doi: 10.1007/s00264-007-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckwalter JA, Mankin HA. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41:1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drengk A, Zapf A, Stürmer EK, Stürmer KM, Frosch KH (2008) Influence of platelet-rich plasma on chondrogenic differentiation and proliferation of chondrocytes and mesenchymal stem cells. Cells Tissues Organs. Aug 11. [Epub ahead of print] [DOI] [PubMed]
- 7.Evans CH, Palmer GD, Pascher A, et al. Facilitated endogenous repair: making tissue engineering simple, practical, and economical. Tissue Eng. 2007;13:1987–1993. doi: 10.1089/ten.2006.0302. [DOI] [PubMed] [Google Scholar]
- 8.Farrag TY, Lehar M, Cerhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117:157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 9.Gotterbarm T, Richter W, Jung M, Berardi Vilei S, Mainil-Varlet P, Yamashita T. An in vivo study of a growth-factor enhanced, cell free, two-layered collagen-tricalcium phosphate in deep osteochondral defects. Biomaterials. 2006;27:3387–3395. doi: 10.1016/j.biomaterials.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 10.Holland TA, Bodde EW, Baggett LS, Tabata Y, Mikos AG, Jansen JA. Osteochondral repair in the rabbit model utilizing bilayered, degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds. J Biomed Mater Res A. 2005;75:156–167. doi: 10.1002/jbm.a.30379. [DOI] [PubMed] [Google Scholar]
- 11.Ishida K, Kuroda R, Miwa M, Tabata Y, Hokugo A, Kawamoto T. The regenerative effects of platelet-rich plasma on meniscal cells in vitro and its in vivo application with biodegradable gelatin hydrogel. Tissue Eng. 2007;13:1103–1112. doi: 10.1089/ten.2006.0193. [DOI] [PubMed] [Google Scholar]
- 12.Jelic M, Pecina M, Haspl M, et al. Regeneration of articular chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth factors. 2001;19:101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 13.Kim SH, Yoon SJ, Choi B, Ha HJ, Rhee JM, Kim MS. Evaluation of various types of scaffold for tissue engineered intervertebral disc. Adv Exp Med Biol. 2006;585:169–181. doi: 10.1007/978-0-387-34133-0_12. [DOI] [PubMed] [Google Scholar]
- 14.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/S1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 15.Marx RE. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Nikolidakis D, Jansen JA. The biology of platelet-rich plasma and its application in oral surgery: literature review. Tissue Eng Part B Rev. 2008;14:249–258. doi: 10.1089/ten.teb.2008.0062. [DOI] [PubMed] [Google Scholar]
- 17.Pecina M, Jelic M, Martinovic S, et al. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26:131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Jt Surg Am. 2003;85:17–24. doi: 10.1302/0301-620X.85B1.13948. [DOI] [PubMed] [Google Scholar]
- 19.Kasten P, Vogel J, Geiger F, Niemeyer P, Luginbuhl R, Szalay K. The effect of platelet-rich plasma on healing in critical-size long-bone defects. Biomaterials. 2008;29:3983–3992. doi: 10.1016/j.biomaterials.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Schuckert KH, Jopp S, Teoh SH (2008) Mandibular defect reconstruction using three-dimensional polycaprolactone scaffold in combination with platelet-rich plasma and recombinant human bone morphogenetic protein-2: de novo synthesis of bone in a single case. Tissue Eng Part A. Sep 3. [Epub ahead of print] [DOI] [PubMed]
- 21.Uematsu K, Hattori K, Ishimoto Y, Yamauchi J, Habata T, Takakura Y. Cartilage regeneration using mesenchymal stem cells and a three-dimensional poly lactic-glycolic acid (PLGA) scaffold. Biomaterials. 2005;26:4273–4279. doi: 10.1016/j.biomaterials.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 22.Wiltfang J, Kloss FR, Kessler P, Nkenke E, Schultze-Mosgau S, Zimmermann R. Effects of platelet-rich plasma on bone healing in combination with autogenous bone and bone substitutes in critical-size defects. Clin Oral Implants Res. 2004;15:187–193. doi: 10.1111/j.1600-0501.2004.00980.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the linked to the electronic supplementary material
(DOC 2748 kb)
(DOC 916 kb)
(DOC 5194 kb)