Abstract
Treatment of articular cartilage lesions in the knee remains a challenge for the practising orthopaedic surgeon. A wide range of options are currently practised, ranging from conservative measures through various types of operations and, recently, use of growth factors and emerging gene therapy techniques. The end result of these methods is usually a fibrous repair tissue (fibrocartilage), which lacks the biomechanical characteristics of hyaline cartilage that are necessary to withstand the compressive forces distributed across the knee. The fibrocartilage generally deteriorates over time, resulting in a return of the original symptoms and occasionally reported progression to osteoarthritis. Our purpose in this study was to review the aetiology, pathogenesis and treatment options for articular cartilage lesions of the knee. At present, autologous cell therapies, growth factor techniques and biomaterials offer more promising avenues of research to find clinical answers.
Introduction
Articular cartilage lesions in weight-bearing joints often fail to heal on their own and may be associated with pain, loss of function and long-term complications such as osteoarthritis [13]. Osteochondral injuries are both naturally and therapeutically irreversible with current treatment parameters. Inferior repair commonly occurs, but stable regeneration of hyaline cartilage has never been documented [11]. Curl et al., in a review of 31,516 knee arthroscopies, reported that 63% of knees had chondral lesions (averaging 2.7 lesions per knee) and 20% had full-thickness lesions, with 5% of these occurring in patients less than 40 years of age [12]. Seventy-five percent of patients less than 40 years old had solitary lesions; the rest had multiple chondral lesions. Sixty-five percent of the whole group had accompanying meniscal or ligament lesions, mostly ACL tear [8, 12].
Treatment of articular cartilage lesions in the knee remains a challenge for the practising orthopaedic surgeon. Decisions about whether and how to treat an individual lesion are problematic [8]. A wide range of options are practised nowadays, ranging from conservative measures, through simple arthroscopic interventions, marrow tapping techniques, osteochondral auto/allo-grafting, cell-based techniques, growth factors and emerging gene therapy techniques [11]. Regardless of the treatment method or the origin of repair factors, the end result is generally a fibrous repair tissue (fibrocartilage) which lacks the biomechanical characteristics necessary to withstand the compressive factors distributed across the knee during articulation. This fibrocartilage generally deteriorates over time, resulting in return of the original symptoms and occasionally reported progression to osteoarthritis [11].
Our purpose is to review the pathogenesis and treatment options of articular cartilage lesions of the knee.
Aetiology of knee cartilage lesions
There are two distinct chondral injury phenotypes according to attributing factors: focal lesions and degenerative lesions. Focal lesions are well delineated defects, usually caused by trauma, osteochondritis dissecans or osteonecrosis. Degenerative defects are typically poorly demarcated and usually caused as a result of ligament instability, meniscal injuries, malalignment or osteoarthritis [11].
Trauma is the most common cause of osteochondral lesions. Usually it is caused by sports injury or accidents. The shearing force creates a stress fracture through cartilage matrix, and sometimes through subchondral bone. Patellar dislocation leads to osteochondral fracture through this mechanism and is responsible for 40–50% of osteochondral lesions around the femoral condyles [6]. It is most common in young active patients aged 20–40 years. Osteochondritis dissecans was first described by Konig in 1888. It is caused in 60% of patients by recurrent microtrauma to femoral condyles and is located in the lateral aspect of the medial femoral condyles in 85% of cases [5]. Osteonecrosis is thought to be primary (spontaneous/avascular) or secondary to various factors such as steroid therapy, post-meniscectomy, alcoholism, etc. [43]. Osteoarthritis is the most common cause of chondral lesions after age 40. Degenerative lesions are of different depths and shapes. Stiffening of subchondral bone results in less shock absorption and cartilage matrix breakdown [11]. Weight bearing enlarges the lesion and abrades the subchondral bone over time [11]. Loss of biomechanical function due to meniscal tears and loss of knee stability due to ligament damage (particularly the ACL) result in increased cartilage injury [49]. Lewandrowski et al. reported that articular cartilage lesions were accompanied by meniscal tears in 76% of cases and that longitudinal meniscal tears were significantly more associated with cartilage lesions than horizontal tears [33].
Due to this multifaceted aetiology, the role of prophylactic therapy is dubious. There is a small therapeutic window when cartilage damage is caused by meniscal or ligament damage [11].
Description of chondral lesions
For a good understanding of chondral lesions and suitable treatment policies, there is a need for a simple classification and qualification of the lesion. The grading system devised by Outerbridge is simple and clinically useful in daily practice and is the most used system (Table 1) [40]. Description of the lesion is based on accurate notation of the location (MFC, LFC), size (i.e. surface area), shape (circular, rectangular) and description of the walls (contained, partially contained or opened). The depth of the lesion is designated as mild (partial thickness), moderate (full thickness) or severe with extension into subchondral bone [8].
Table 1.
Classifications of chondral lesions [8]
| Outerbridge |
|---|
| Grade 0: normal articular cartilage |
| Grade I: softening, blistering or swelling of the cartilage |
| Grade II: partial thickness fissures and clefts <1 cm diameter |
| Grade III: full thickness fissures, to subchondral bone >1 cm diameter |
| Grade IV: exposed subchondral bone |
| International Cartilage Repair Society (ICRS) |
|---|
| I: superficial fissure |
| II: <50% depth |
| III: 50% to full thickness loss |
| IV: osteochondral lesion extends through bone |
| V: osteochondritis dissecans lesion (OCD) |
| VI: avascular necrosis (AVN) |
| Bauer–Jackson descriptive (I–IV traumatic/V–VI degenerative) |
|---|
| I: linear |
| II: stellate |
| III: chondral flap |
| IV: chondral crater |
| V: fibrillation |
| VI: exposed subchondral bone |
| International Cartilage Repair Society (ICRS) |
|---|
| I: superficial fissure |
| II: <50% depth |
| III: 50% to full thickness loss |
| IV: osteochondral lesion extends through bone |
| V: osteochondritis dissecans lesion (OCD) |
| VI: avascular necrosis (AVN) |
| Bauer–Jackson descriptive (I–IV traumatic/V–VI degenerative) |
|---|
| I: linear |
| II: stellate |
| III: chondral flap |
| IV: chondral crater |
| V: fibrillation |
| VI: exposed subchondral bone |
Clinical and radiological assessment of articular cartilage injury
Patients may suffer an insidious onset of pain with or without effusion, depending on the lesion aetiology. Others may have a progressive onset of joint-line and/or patellofemoral pain with occasional mechanical symptoms such as locking or catching. The most common clinical presentation of a full-thickness lesion is a loose body. It may be associated with an acute injury and a concomitant large knee effusion. This is the scenario in patellar dislocation or in dashboard injury [8].
A routine complete physical examination should be performed to rule out malalignment, meniscal tears, ligamentous instability or extensor mechanism problems [8, 11]. Usually, the examination does not elicit a distinct problem other than localised pain, effusion, locking or catching.
Routine plain radiographs, including AP, LAT, and standing PA flexion views, may reveal different findings, according to aetiology. Joint space narrowing, subchondral sclerosis or cysts will suggest an osteoarthritic origin. Osteochondritis dissecans defect can also be seen on plain radiographs, with or without loose body. Conventional radiography may reveal no changes even with full-thickness cartilage lesions [8].
The role of bone scans is still controversial. According to Dye and Chew, increased scintigraphic activity will reflect any significant joint injury, and it diminishes when the joint returns towards a normal state [15]. MR imaging remains the benchmark of articular cartilage lesions with associated soft tissue or bone changes [25]. The optimal resolution for the articular chondral surface is proton-density imaging of 3–4 mm sections and T2-weighted imaging with fat saturation sequences [25]. By using MRI, one can see bone structure, chondral lesions, meniscal or ligamentous pathology and bone marrow oedema (bone bruise). Arthroscopy is the gold standard and most accurate technique for diagnosing articular cartilage lesions [8].
Optional treatment modalities of articular cartilage injury
The treatment of chondral lesions depends on patient selection, daily and sport activities, age, aetiology, grade and quality of the lesion. Treatment options range from conservative, through arthroscopic or open surgical procedures.
Conservative treatment
The goal of conservative treatment is to reduce symptoms, not heal the lesion. It is considered in mild symptomatic cases or in cases with small lesions where surgery could do more harm than good [11]. The appropriate treatment for the asymptomatic patient with incidental finding of chondral injury is problematic. The only documentation of the natural history with a long-term follow-up was reported by Messner and Maleitus in 1996 [37]. They reported that 22 out of 28 patients with isolated chondral lesions had good or excellent results without treatment. Fourteen years later most of their patients had abnormal radiographic findings suggesting that asymptomatic lesions may deteriorate to permanent knee damage. The surgeon can consider one or more of several non-operative approaches according to symptomatology and severity of the lesion [17]. One approach is that of medications, such NSAID, analgesics, and hormones (oestrogen, growth hormone, etc). Next, there are several mechanical approaches including weight loss, rest, ice, canes, bracing, physical therapy. etc. Also, nutrition supply should be considered, with chondroprotective agents (glucoseamine & chondroitin phosphate, MSM, Omega-3) and calcium and vitamins, as well as intra-articular injections such as steroids and viscosupplementation (Synvisc, Ostenil, etc). To date, there has been no evidence of structural improvement with these conservative modalities [8].
Operative treatment
The various techniques available for surgical intervention result in reparative or restorative tissue response. The purpose of surgery is the regeneration of osteochondral defects to ultrastructural and biomechanical competence of hyaline cartilage. Unfortunately, in all surgical techniques, the repair tissue is fibrocartilagenous in nature with little hyaline cartilage restoration [38]. The basic idea is to adjust treatment to the individual patient, and to repair related and/or contributing problems before or with the treatment of chondral injuries, such as varus-valgus alignment, patellofemoral tracking, stability of cruciate and collateral ligaments and meniscal lesions [18]. Arthroscopy is the definitive surgical method, especially in the presence of chondral loose bodies; the goal is to restore and preserve function, alleviate pain and minimise progression to osteoarthritis. Surgical treatment for cartilage lesions is contraindicated in some cases as inflammatory arthropathy, unstable or malaligned joint, “kissing lesions” (bipolar), infection and obesity [36].
There are some surgical treatment modalities depending on surface area of the lesion, surgeon experience/preference and on financial capabilities [11]. These modalities are presented in Table 2.
Table 2.
Surgical options for cartilage lesions [8]
| Current generation |
|---|
| Arthroscopic lavage and debridement |
| Marrow tapping techniques |
| Abrasion arthroplasty |
| Subchondral drilling |
| Microfracture |
| Osteochondral autografting—mosaicoplasty |
| Osteochondral allografting |
| New generation |
|---|
| Autologous cell techniques |
| ACI & MACI |
| Growth factors |
| Gene therapies |
ACI autologous chondrocyte implantation, MACI matrix-induced autologous chondrocyte implantation
Arthroscopic lavage and debridement
First noted by Burman in 1935, washout of the injured synovial joints had been proven to be the best frontline treatment of chondral lesions [27]. Arthroscopic lavage washes out inflammatory mediators, loose cartilage and collagen debris that may lodge in the synovium and cause synovitis and effusion. Debridement of cartilage (chondroplasty) removes loose flaps or edges that mechanically impinge on the joint [8, 11]. Debridement chondroplasty may be done by several techniques including curettage, and mechanical debridement with a shaver, although this technique does not leave smooth cartilage and may cause more cartilage breakdown. Also, there is thermal debridement with radiofrequency RFE wand (thermal, ablation or laser). This technique causes chondrocyte death and matrix degeneration.
Jackson et al. reported symptomatic improvement in 45% of patients 3.5 years after arthroscopy, and measurable improvement in 80% [26]. They proved later that mechanical debridement is effective, with 88% immediate improvement, as well as 68% prolonged improvement [27, 28]. Arthroscopic therapy, on the other hand, may facilitate degenerative changes [11]. However, longer follow-up is necessary to decide whether this treatment carries the longevity of modern articular cartilage repair techniques.
Abrasion arthroplasty
Popularised in the early 1980s by Johnson, abrasion arthroplasty is indicated especially when there is an exposed sclerotic degenerative arthritic lesion, without femoro-tibial malalignment or high locomotive demands [11]. The aim is to debride the boundaries of the articular cartilage defect to sustain a uniformly contoured edge of fresh collagen, capable of adhering a fibrin clot [30]. Then, the subchondral bone is breached, allowing blood to perfuse into the defect forming a fibrin clot. The outcomes of abrasion arthroplasty vary among studies, and none exhibits consistent good or excellent results [11].
Subchondral drilling
Arthroscopic drilling was used first by Smillie and Dundee in 1957 [48]. It was popularised later by Pridie in 1959 [46]. After debridement of the lesion edges to a contained crater, the subchondral bone is drilled with a high speed drill through trabecular bone. Blood is allowed to perfuse into the defect forming a blood clot and initiating defect repair (Fig. 1). The repaired cartilage is seen to be a mix of hyaline and fibrocartilage [11, 46]. The main drawback of this technique is thermal necrosis. In 1991, Tippet et al. [53] reported that after more than five years follow-up, 70% of the patients had excellent results, 8% good and 22% fair to poor.
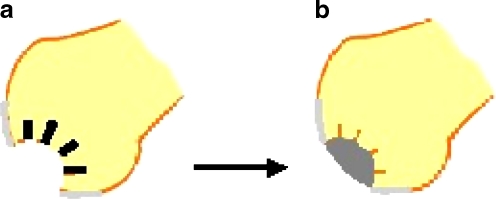
Fig. 1.
Subchondral drilling. a High speed drilling through trabecular bone. b Fibrin clot filling the crater
Microfracture
With the purpose of making a rough subchondral surface, which is attractive for fibrin clot, but without the thermal effects of a drill, Steadman et al. proposed the use of an arthroscopic awl to create several holes 3–4 mm apart [50]. In a series of more than 200 patients treated, with three to five years follow-up, they reported improvement in 75% of cases, stabilisation in 20% and deterioration in 5% [50]. Histological analysis of microfracture repair shows, as is the case with all marrow-tapping techniques, that a hybrid hyaline cartilage and fibrocartilage dominates the defect site [11, 50]. In a study of 85 patients with full thickness lesions treated with microfracture and 36 months follow-up, Kreuz et al. found improvement in all patients during the first 18 months. Deterioration began after 18 months and was significantly pronounced in patients older than 40 years. They concluded that results of microfracture are age-dependent and the best prognostic factor is age 40 or younger [32]. However, Alparslan et al. reported good results with improvement of function and activity after microfracture of full-thickness chondral lesions in 20 patients, with average age of 44 years, after 3.8 years follow-up [2].
Osteochondral autografting (OATS)—mosaicoplasty
For tasking regeneration of osteochondral defects, autografting is an obvious approach, due to same tissue and antigenicity, with a non-weight bearing area as a donor. The optimal patient is young with a medium-sized lesion (2.5–4 sq cm). Effectiveness is limited to the repair of focal defects and inability to restore degenerative lesions [9]. Arthroscopic debridement is followed by removal of unstable cartilage, aiming for a stable contained crater, preferably circular. The next step is measurement of surface area and cylindrical removal of subchondral bone, as close as possible to the lesion border. The next stage is harvesting cylindrical osteochondral plugs from a donor area (preferably NWB trochlear edges) to the same depth removed from the crater. Insertion of these plugs is performed, 1 mm apart, by a graduated tamp, allowing accurate depth. Postoperative rehabilitation starts with three to six weeks of non weight bearing, according to location and size of the lesion. CPM, passive and active ROM and muscle strengthening is crucial during this time. Partial weight bearing is allowed for another three to six weeks with a gradual increase, ending with full weight bearing.
Hangody et al., the developers of the mosaicoplasty technique, reported on a five-year follow-up study of 155 patients, of which 85 remained asymptomatic for the whole period. Histological samples illustrated incorporation of hyaline plugs with fibrocartilage grouting, as well as stable osseous integration [24]. These results were reproduced by Marcacci et al., after more than seven years follow-up, on 30 patients treated by mosaicoplasty [34].
Osteochondral allografting
Due to graft size limitation and donor site morbidity, further search led to “complication-free” allogeneic osteochondral graft. This may be used for medium to large, full-thickness lesions (>10 sq cm) after failure of other primary surgical procedures [8, 11]. Two types are used: shell (<1 cm subchondral bone based) and deep grafts. Fresh allografts, obtained within 24–72 hours, provide higher chondrocyte availability but carry a high risk for disease transmission. On the other hand, cryopreserved frozen allografts have reduced immunogenecity and disease transmission, but low chondrocyte availability. The best candidates are monopolar defects with bone loss such as osteochondritis dissecans, trauma, tumour or salvage situations. Worse results are with osteoarthritis, avascular necrosis, or bipolar defects [41].
Many studies have documented overall 85% success rates using free grafts, mainly for unipolar defects, as bipolar success is significantly diminished [21]. The benefits of this technique are that there is no donor site morbidity or size limitation, and grafting of mature hyaline cartilage. When performed, it is technically demanding and slow healing is expected. Weight bearing is restricted between six and 12 weeks, and contact sports are forbidden for six (femur) to 12 (tibia) months [21].
Autologous chondrocyte implantation (ACI)
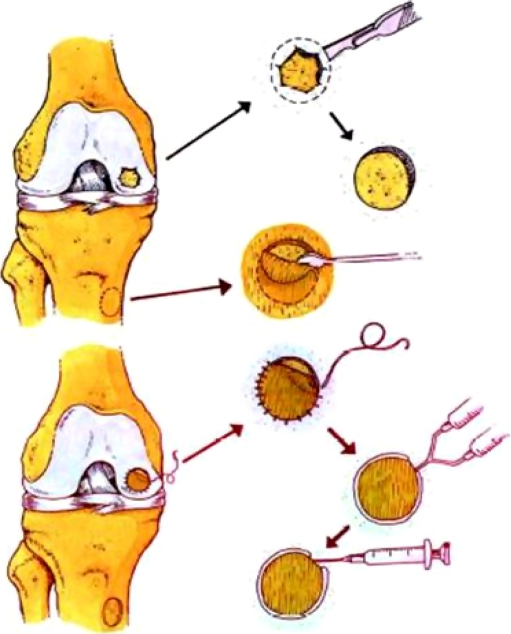
The use of human autologous chondrocyte implantation (ACI) was first documented by Mats Brittberg et al. in 1994. They reported on the success of deep cartilage defect treatment in 23 patients, using first generation ACI. Their results showed 87% good and excellent results in femoral condylar repair and 73% demonstration of hyaline-like cartilage upon microscopy performed in a second look arthroscopy and biopsy [7]. The process is performed in several stages. The first step is diagnostic arthroscopy and cartilage harvest. Second, chondrocyte cultivation is performed in a GMP laboratory for cell propagation for six weeks. Third, implantation surgery occurs, which is usually debridement and sizing of the cartilage defect, harvesting of the periosteal flap from the proximal tibia (ACI-P), suitable for the defect size, fixation of the flap and injection of the cultured chondrocytes before closing the last suture (Fig. 2).
Fig. 2.
Stages of ACI. Surgical options for early arthritis in young persons and athletes: the role of meniscus transplants, microfracture, osteoarticular transplants (OATs), autologous chondrocyte implantation (ACI) and osteotomy
ACI is indicated for the younger (aged 20–50 years) active patients with an isolated traumatic femoral chondral lesion, greater than 2–4 sq cm, with less than 3–6 mm depth, so initial repair of the subchondral base is not necessary [42]. A treatment algorithm was presented by a working group based on European and American literature and on peer-reviewed opinions of leading investigators in the field [55]. Accompanying ligamentous and meniscal lesions, joint malalignment and patellofemoral instability must be corrected concurrently. Newman, as quoted by Craig et al., reported on his experience with 100 ACI patients in 1998 after four years mean follow-up. He found 96% good and excellent results in focal femoral condyle lesions, 89% in patients with osteochondritis dissecans and 75% in ACL reconstructed patients [11].
In a Cartilage Repair Registry Report (vol 4, Genzyme Tissue Repair, Cambridge, MA, February 1998), the United Sates and European experience (not including Swedish) was reported on 891 transplants. Clinicians note good and excellent results in 86%. The complication rate was 12.6%, a second operation was required in 9.9% and failure was noted in 2%. The cumulative failure at two years was estimated at 5.8% [8]. Rauno-Ravina and Jato meta-analysed three clinical trials and nine case series. They found no evidence that ACI was more effective, or safe, than other conventional techniques [47]. This evaluation was performed because of several concerns raised regarding the interpretation of the results in the existing literature [39]. Some of these are absence of randomisation and controls, outcome analysis (knee scores) and absence of biochemical (collagen typing) and biomechanical data [39]. In addition, multiple staged procedures, large surgical incision, difficult access to certain areas for suturing, prolonged operation time, long rehabilitation time and periosteal flap complications (delamination, cell leak, peripheral hypertrophy and calcification problems with clinical “catching” of the knee), have encouraged researchers to search for alternative bioscaffolds that are less problematic [11]. In April 2007, Steinwachs and Kreuz reported on 63 patients treated by ACI. The chondrocyte suspension within the defect was covered with a type I/III collagen membrane (second generation ACI/ACI-C). Patients were evaluated preoperatively and at six, 18 and 36 months after surgery. After more than three years follow-up, the ICRS and modified Cincinnati scores showed significant improvement at all evaluation points. There was no patient with a symptomatic graft hypertrophy. They concluded that graft hypertrophy can be avoided by using a collagen membrane instead of periosteal flap [52]. These results were reproduced by Erggelet et al. by comparing two groups of patients, using periosteal flap in 42 and biodegradable collagen fleece (BioSeed-C) in 40 patients, at a minimum follow-up of two years [16].
It is accepted that the results of ACI were less favourable in patellofemoral joint lesions [13]. Handl et al. reported on six patients treated by ACI for deep chondral lesions in the patella. At an average 18 months follow-up, they found significant improvement in knee function compared to preoperative status and to the contralateral knee. MRI examinations showed graft incorporation in all patients [22]. The same results were also found by this group using ACI to treat chondral lesions in the talus bone [23].
Matrix-induced ACI (MACI)
Because of the complications of periosteal flap and surgical difficulties, researches continue looking for simpler procedures with less drawbacks. With ACI based surgery, a second generation of matrix-induced autologous chondrocyte implantation (MACI) was applied by Verigen company, as described by D’Anchise et al. [13]. The procedure is based on two structures. The first is a collagen membrane (types I/III) seeded with cultured autologous chondrocytes. The chondrocytes are seeded on the cambium side, which allows attachment and neomatrix synthesisation. The other side is a smooth, non-restrictive, hyaline-like surface allowing smooth glide of chondral surfaces. The second structure is fibrin glue, a mixture of fibrinogen and thrombin, that sticks the membrane to the chondral surface. It has been proven that the glue is a stable surface for chondral ingrowth, has potential for osteoinduction and allows chondral migration throughout the defect space [8]. The technique has two stages. First is a diagnostic arthroscopy for diagnosis and sizing of the lesion and harvesting of the chondral biopsy (1–2 cc). These chondrocytes are cultivated on a GMP laboratory and seeded on collagen membrane. The second stage is surgical, involving debridement of the cartilage defect to the subchondral plate, without bleeding [8, 13]. The next steps include shaping to a stable crater and sizing of the defect, shaping and sizing of the membrane accordingly, injecting the glue to the level of the cartilage surface, pressing the membrane with soft-side up to the level of the cartilage, removing air bubbles and cleaning of leaked glue, taking the knee through a range of movement to ensure there is no leak or delamination and closing the knee (Fig. 3). Reported benefits of MACI are no periosteal harvesting, suture free, good stability of implant, less invasive and early mobilisation.
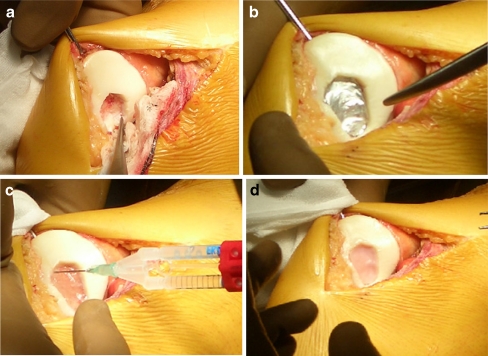
Fig. 3.
Surgical stage of matrix-induced autologous chondrocyte implantation (MACI) in patellar chondral lesion. a Debridement and shaping of chondral lesion. b Sizing of lesion for preparing of the membrane. c Filling the crater with fibrin glue. d Pressing the membrane and looking for air bubbles and glue leak before moving the knee
Several reports have appeared in the past few years on the benefits of MACI as the treatment of choice for chondral lesions. D’Anchise et al., in a two-year clinical and histological follow-up study, concluded that MACI is able to relieve pain and restore knee function, as well as apparently being capable of regenerating hyaline cartilage [13]. A prospective study using MACI was performed by Behrens et al. on 38 patients, with full-thickness cartilage defects. Twenty-five patients were followed up between two and five years, of which 11 were followed up for more than five years. Evaluation was done by four different scores as well as the results of six arthroscopies and biopsies obtained from four patients postoperatively. Three of the four scores showed significantly improved results. The Tegner-Lysholm score showed improvement which was not significant. Their conclusion was that MACI represents a suitable but cost-intensive alternative in the treatment of cartilage defects in the knee [3].
Ongoing studies are being done to prove the superiority of matrix-induced MACI over other techniques, especially ACI. In a randomised trial comparing ACI an MACI, Bentley et al. found good results in 60% of patients in both groups. However, at histology, the ICRS scores were marginally better in the ACI group [4]. The main drawbacks of the MACI treatment option are price, long rehabilitation time and short follow-up.
A solid implant with autologous chondrocyte, to repair chondral or osteochondral defects, is a new trend for ACI. In order to make implantation easier and to promote differentiation, an agarose-alginate matrix, seeded with chondrocytes, was developed and denominated Cartipatch (Tissue Bank of France, TBF). It combines arthroscopically harvested autologous chondrocytes with a 3D hydrogel scaffold of aragose and alginate that is derived from medical-grade algae. The resultant circular patches of different diameter are implanted through a miniarthrotomy. The patches adhere to the lesion bed without use of additional glue or suturing. Nowadays, it is evaluated in an ongoing phase III clinical trial.
Artificial chondroplasty
Several kinds of implants are used today, most are experimental, and are for focal chondroplasty, especially as a salvage procedure in elderly osteoarthritic patients. Focal chondroplasty by Co–Cr metallic implants, for the management of full-thickness cartilage defects in an animal model, was reported by Kirker-Head et al. [31]. After one year follow-up, the chondral lesions were much reduced by radiographs, related to their sizes at implantation day. Their results implied the safety, biocompatibility and functionality of the implant [31].
Future trends/research
As in other fields in surgical medicine, the future is being shaped by bioengineering and modification of stem cells. Several trends already exist and research continues to find simpler ways for treating all kinds of cartilage defects and produce hyaline or hyaline-like cartilage with biomechanical and biostructural properties similar to human cartilage. In 1994, using a rabbit model, Wakitani et al. reported that pluripotential stem cells, isolated from bone marrow, synovium or periosteom, could repair osseous and chondral defects [57]. In 1995, Grande et al. reported that mesenchymal stem cells repaired cartilage defects and subchondral bone [20].
Autologous matrix induced chondrogenesis (AMIC) is a single procedure, aimed at cartilage repair by the patient’s stem cells. The defect is prepared and followed by microfracture. Then, Chondrogel is sutured to the crater edge. Early good results were obtained by this technique, especially in bigger lesions and in early osteoarthritis. However, its superiority over microfracture alone is yet to be proven [51]. Another cell-based option to treat knee chondral defects uses biodegradable alginate beads containing human mature allogenic chondrocytes. Almqvist et al. reported on 21 patients treated by these beads after two years of follow-up. Evaluation was done by Western Ontario and McMaster Universities Osteoarthritis Index and a visual analog scale for pain. Postoperative biopsy samples were obtained from 13 patients after 12 months follow-up. A statistically significant clinical improvement became apparent after six months and continued during the 24 months of follow-up. Their conclusion was that alginate-based scaffold, containing human mature chondrocytes, is feasible and safe for treatment of knee cartilage defects, but not superior to other cartilage repair techniques [1].
Growth factors act as three-dimensional templates for cell migration and proliferation. Most experimental interest is in transforming growth factor (TGF) beta (mainly 1 and 3), a potent chondrogenic with osteoinductive properties [54], and bone morphogenetic protein (BMP) (mainly 2 and 7), a potent osteogenic but which aids in chondral and osseous proliferation and differentiation [14]. BMP-7 (Osteo Progenitor-1; OP-1) was found to be a potent osteogenic factor in the treatment of tibial fracture non-union [14].
Already by 1995, Vukicevic’ et al. stated that there is ample evidence that bone morphogenetic proteins are directly responsible for de novo cartilage and bone formation in vivo [56]. In 1997 Grgic’ et al. investigated the influence of OP-1 on healing full-thickness articular cartilage defects made in NZW rabbit knees [19]. Results indicated that OP-1 induced articular cartilage healing and regeneration of the joint surface, which contained cells resembling mature joint chondrocytes. This hypothesis was proven again in chondral defects created in sheep knees by Jelic et al. in 2001 [29]. Control cartilage defects were compared to others treated with low OP-1 dose and high OP-1 dose at three and six months. During this period, the control defects remained empty, while defects treated with OP-1 were filled with connective tissue and cartilage faster with the high dose OP-1 group. The authors suggested that a recombinant BMP stimulates ingrowth of mesenchymal cells into the chondral defects, which then transform into newly formed articular cartilage-like tissue. According to these findings, Pecina et al. suggested that hyaline cartilage formation by gene therapy induction, with cell implantation, might be the answer to the current limitations for cartilage treatment modalities, with the option of permanent solution for this entity [44, 45].
Gene therapy concentrates on manipulation of progenitor cells and chondrocytes to locally express genes encoding growth factors to enhance osteochondral repair. Mason et al. reported complete bone and articular cartilage regeneration at eight to 12 weeks. This was achieved by modification of mesenchymal stem cells retrovirally transfected and seeded with BMP-7 on polyglycolic acid scaffold in osteochondral defects of rabbit knees [35].
Bioscaffolds are biomaterials that act as three-dimensional templates for cellular propagation and growth factors seeding. These could be natural (collagens, hyaluronan, fibrin glue, etc.) or synthetic (carbon fiber, polyglycolic and polylactic acids, etc.) [11].
As a conclusion, for good regeneration of osteochondral defects, cells (cultured, fragments, mesenchymal stem cells, etc.), bioscaffolds (natural/synthetic), as well as chondroinductive (TGF) and osteoinductive (BMP) growth factors [10] are required. Malalignment, patellofemoral problems, meniscus tears and/or ligament instabilities should be treated before or simultaneously with cartilage resurfacing [18].
There is no “gold standard” in the treatment of cartilage defects or the choice of treatment option. Many algorithms are used, relying especially on surface area of the defect and on surgeon experience.
Rehabilitation depends on treatment mode used and on defect personality (classification and qualification). Return to functional work and sport is possible in all procedures, but takes different periods of time. More time is required to return to contact sports, especially after allograft procedures. Sometimes permanent moderation of activities should be considered [8].
Summary
Articular cartilage is a nearly frictionless system with unique biomechanical properties. Unfortunately, it’s intrinsic reparative process cannot cope with full-thickness injuries. The current reparative or restorative procedures provide an opportunity to return the surface to it’s normal or near normal status. At present, autologous cell therapies, growth factor techniques and biomaterials offer a more promising avenue of research to find clinical answers. We should always remember that many other factors can influence the necessity of treating these defects such as accompanying joint abnormalities, body weight, and activity level. Treatment options used should be suitable for the special patient and familiar to the treating surgeon. Most of these patients will return to functional activity or sports, but some of them will require life-long modification of their daily activities.
References
- 1.Almqvist KF, Dhollander AA, Verdonk PC, Forsyth R, Verdonk R, Verbruggen G. Treatment of cartilage defects in the knee using alginate beads containing human mature allogenic chondrocytes. Am J Sports Med. 2009;37(10):1920–1929. doi: 10.1177/0363546509335463. [DOI] [PubMed] [Google Scholar]
- 2.Alparslan B, Ozkan I, Acar U, Cullu E, Savk SO. The microfracture technique in the treatment of full-thickness chondral lesions of the knee. Acta Orthop Traumatol Turc. 2007;41(Suppl 2):62–69. [PubMed] [Google Scholar]
- 3.Behrens P, Bitter T, Kurz B, Russlies M. Matrix-induced autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13(3):194–202. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 4.Bentley G (2008) Advances in cartilage cell transplantation. Symposium on cartilage repair. 8th EFFORT congress, Nice, France, May 2008
- 5.Bianchi G, Paderni S, Tigani D, Mercuri M. Osteochondritis dissecans of the lateral femoral condyle. Chir Organi Mov. 1999;84(2):183–187. [PubMed] [Google Scholar]
- 6.Boden BP, Pearsall AW, Garrett WE, Jr, Feagin JA., Jr Patellofemoral instability: evaluation and management. J Am Acad Orthop Surg. 1997;5(1):47–57. doi: 10.5435/00124635-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Brittberg M, Faxen E, Peterson L. Carbon fibre scaffolds in the treatment of early knee osteoarthritis. A prospective 4-year follow-up of 37 patients. Clin Orthop. 1994;307:155–164. [PubMed] [Google Scholar]
- 8.Browne JE, Branch TP. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg. 2000;8(3):180–189. doi: 10.5435/00124635-200005000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Chang PC, Pradhan RM, Mitra AK, Sim CS, Tay BK. The results of autogenous tibial periosteal transplants for full thickness cartilage defects in the knee joints of pigs. Ann Acad Med Singapore. 1999;28(1):8–14. [PubMed] [Google Scholar]
- 10.Craig W. A current review on the biology and treatment of articular cartilage defects. J Musculoskelet Res. 2003;7:157–181. doi: 10.1142/S0218957703001125. [DOI] [Google Scholar]
- 11.Craig W, David JW, Ming HZ. A current review on the biology and treatment of the articular cartilage defects (part I & part II) J Musculoskelet Res. 2003;7(3&4):157–181. [Google Scholar]
- 12.Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456–460. doi: 10.1016/s0749-8063(97)90124-9. [DOI] [PubMed] [Google Scholar]
- 13.D’Anchise R, Manta N, Prospero E, Bevilacqua C, Gigante A. Autologous implantation of chondrocytes on a solid collagen scaffold: clinical and histological outcomes after two years of followup. J Orthop Traumatol. 2005;6:36–43. doi: 10.1007/s10195-005-0078-5. [DOI] [Google Scholar]
- 14.Desmyter S, Goubau Y, Benahmed N, Wever A, Verdonk R. The role of Bone morphogenetic protein-7 (osteogenic protein-1) in the treatment of tibial fracture non-unions. An overview of the use in Belgium. Acta Orthop Belg. 2008;74(4):534–537. [PubMed] [Google Scholar]
- 15.Dye SF, Chew MH. The use scintigraphy to detect increased osseous metabolic activity about the knee. Instr Course Lect. 1994;43:453–469. [PubMed] [Google Scholar]
- 16.Erggelet C, Kreuz PC, Mrosek EH, Schagemann JC, Lahm A, Ducommun PP, Ossendorf C (2009) Autologous chondrocyte implantation versus ACI using 3D-biodegradable graft for the treatment of large full-thickness cartilage lesions of the knee. Arch Orthop Trauma Surg. doi:10.1007/s00402-009-0957-y [DOI] [PubMed]
- 17.Farnworth L. Osteochondral defects of the knee. Orthopaedics. 2000;23(2):146–157. doi: 10.3928/0147-7447-20000201-15. [DOI] [PubMed] [Google Scholar]
- 18.Fritz J, Gaissmaier C, Schewe B, Weise K. Cartilage repair in the knee joint. Unfallchirurg. 2006;109(7):563–574. doi: 10.1007/s00113-006-1121-6. [DOI] [PubMed] [Google Scholar]
- 19.Grgic’ M, Jelic’ M, Basic’ V, Basic’ N, Pec’ina M, Vukicevic’ S. Regeneration of articular cartilage defects in rabbits by osteogenic protein-1 (bone morphogenetic protein-7) Acta Med Croatia. 1997;51(1):23–27. [PubMed] [Google Scholar]
- 20.Grande DA, Southerland SS, Manji R. Repair of articular cartilage defects using mesenchymal stem-cells. Tissue Eng. 1995;1:345–353. doi: 10.1089/ten.1995.1.345. [DOI] [PubMed] [Google Scholar]
- 21.Gross AE. Fresh osteochondral allografts for post-traumatic knee defects: surgical technique. Operat Tech Orthop. 1997;7:334–339. doi: 10.1016/S1048-6666(97)80037-7. [DOI] [Google Scholar]
- 22.Handl M, Trc T, Hanus M, Stastny E, Fricova-Poulova M, Neuwirth J, Adler J, Havranova D, Varga F. Therapy of severe chondral defects of the patella by autologous chondrocyte implantation. Acta Chir Orthop Traumatol Cech. 2006;73(6):373–379. [PubMed] [Google Scholar]
- 23.Handl M, Trc T, Hanus M, Stastny E, Fricova-Poulova M, Neuwirth J, Adler J, Havranova D, Varga F. Autologous chondrocyte implantation in the treatment of cartilage lesions of ankle joint. Acta Chir Orthop Traumatol Cech. 2007;74(1):29–36. [PubMed] [Google Scholar]
- 24.Hangody L, Kish G, Karpati Z. Osteochondral plugs: autogenous osteochondral mosaicoplasty for the treatment of focal chondral and osteochondral articular defects. Operat Tech Orthop. 1997;7:312. doi: 10.1016/S1048-6666(97)80035-3. [DOI] [PubMed] [Google Scholar]
- 25.Herzog RJ. Radiologic imaging in rehabilitation. In: Kibler WB, Herring SA, Press JM, Lee PA, editors. Functional rehabilitation of sports and musculoskeletal injuries. Gaithersburg: Aspen; 1998. pp. 20–56. [Google Scholar]
- 26.Hunziker EB (1992) Articular cartilage structure in humans and experimental animals. In: Kuettner KE, Peyron JP, Schleyerbach R, Hascall VC (eds) Articular cartilage and osteoarthritis, Elsevier Sciences Ltd, pp 183–199
- 27.Jackson RW. Arthroscopic treatment of degenerative arthritis. In: McGinty JB, editor. Operative arthroscopy. New York: Raven Press; 1991. pp. 319–323. [Google Scholar]
- 28.Jackson RW, Marans HJ, Silver RS. Arthroscopic treatment of degenerative arthritis of the knee. J Bone Joint Surg Br. 1998;70:332. [Google Scholar]
- 29.Jelic M, Pecina M, Haspl M, Kos J, Taylor K, Maticic D, McCartney J, Yin S, Rueger D, Vukicevic S. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-1) in sheep. Growth Factors. 2001;19(2):101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 30.Johnson LL. Arthroscopic abrasion arthroplasty. In: McGinty JB, Caspari RB, Jackson RW, Poehling GG, editors. Operative arthroscopy. 2. Lippincott-Raven: Philadelphia; 1996. pp. 427–446. [Google Scholar]
- 31.Kirker-Head CA, Sickle DC, Ek SW, McCool JC. Safety of, and biological and functional response to, a novel metallic implant for the management of focal full-thickness cartilage defects: preliminary assessment in an animal model op to 1 year. J Orthop Res. 2006;24(5):1095–1108. doi: 10.1002/jor.20120. [DOI] [PubMed] [Google Scholar]
- 32.Kreuz PC, Erggelet C, Steinwachs MR, Krause SJ, Lahm A, Niemeyer P, Ghanem N, Sudkamp N. Is microfracture of chondral defects in the knee associated with different results in patients aged 40 years or younger? Arthroscopy. 2006;22(11):1180–1186. doi: 10.1016/j.arthro.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 33.Lewandrowski K, Muller J, Schollmeier G. Concomitant meniscal and articular lesion in the femorotibial joint. Am J Sports Med. 1997;25:486–494. doi: 10.1177/036354659702500411. [DOI] [PubMed] [Google Scholar]
- 34.Marcacci M, Kon E, Delcogliano M, Filardo G, Busacca M, Zafagnini S. Arthroscopic autologous osteochondral grafting for cartilage defects of the knee: prospective study results at a minimum 7 years follow-up. Am J Sports Med. 2007;35(12):2014–2021. doi: 10.1177/0363546507305455. [DOI] [PubMed] [Google Scholar]
- 35.Mason JM, Grande DA, Barcia M, Grant R, Pergolizzi RG, Breitbart AS. Expression of human bone morphogenic protein-7 in primary rabbit periosteal cells: potential utility in gene therapy for osteochondral repair. Gene Ther. 1998;5(8):1098–1104. doi: 10.1038/sj.gt.3300703. [DOI] [PubMed] [Google Scholar]
- 36.Matthews LS (2004) Is there a role for radiofrequency-based ablation in the treatment of chondral lesions?; Roundtable discussion of the American Association of North America- AANA; published in Amer J Orthop 2005;154(8S):3–4 [PubMed]
- 37.Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiologic follow-upon 28 young athletes. Acta Orthop Scand. 1996;67:165–168. doi: 10.3109/17453679608994664. [DOI] [PubMed] [Google Scholar]
- 38.Odenbring S, Egund N, Lindstrand A, Lohmander LS, Wille’n H. Cartilage regeneration after proximal tibial osteotomy for medial gonarthrosis: an arthroscopic, roentgenographic and histologic study. Clin Orthop. 1992;277:210–216. [PubMed] [Google Scholar]
- 39.O’Driscoll SW, Marx RG, Beaton DE, Miura Y, Gallay SH, Fitzsimmons JS. Validation of a simple histological-histochemical cartilage scoring system. Tissue Eng. 2001;7(3):303–312. doi: 10.1089/10763270152044161. [DOI] [PubMed] [Google Scholar]
- 40.Outerbridge RE. The etiology of chondromalacia patella. J Bone Joint Surg Br. 1961;43:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 41.Ozturk A, Ozdemir MR, Ozkan Y. Osteochondral autografting (mosaicoplasty) in grade IV cartilage defects in the knee joint: 2- to 7-years results. Int Orthop. 2006;30(3):200–204. doi: 10.1007/s00264-005-0068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paletta GA, Jr, Manning T, Snell E, Parker R, Bergfeld J. The effect of allograft meniscal replacement on intraarticular contact area and pressure in the human knee: a biomechanical study. Am J Sports Med. 1997;25:692–698. doi: 10.1177/036354659702500519. [DOI] [PubMed] [Google Scholar]
- 43.Pate DV, Breazeale NM, Behr CT, Warren RF, Wickiewicz TL, O’Brien SJ. Osteonecrosis of the knee: current clinical concepts. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):2–11. doi: 10.1007/s001670050064. [DOI] [PubMed] [Google Scholar]
- 44.Pecina M, Jelic M, Ivkovic A, Hudetz D. Gene therapy applications in orthopaedics. Int Orthop. 2006;30(3):215–216. doi: 10.1007/s00264-005-0047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pecina M, Jelic M, Martinovic S, Haspl M, Vukicevic S. Articular cartilage repair: the role of bone morphogenetic proteins. Int Orthop. 2002;26(3):131–136. doi: 10.1007/s00264-002-0338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pridie AH. The method of resurfacing osteoarthritic knee. J Bone Joint Surg [Br] 1959;41:618–623. [Google Scholar]
- 47.Ruano-Ravina A, Jato DM. Autologous chondrocyte implantation: a systemic review. Osteoarthr Cartil. 2006;14(1):47–51. doi: 10.1016/j.joca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 48.Smillie IS. Treatment of osteochondritis dissecans. J Bone Joint Surg [Br] 1957;39:248. doi: 10.1302/0301-620X.39B2.248. [DOI] [PubMed] [Google Scholar]
- 49.Stanitski CL. Articular hypermobility and chondral injury in patients with acute patellar dislocation. Am J Sports Med. 1995;23:146–150. doi: 10.1177/036354659502300203. [DOI] [PubMed] [Google Scholar]
- 50.Steadman JR, Rodkey WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Operat Tech Orthop. 1997;7:300–307. doi: 10.1016/S1048-6666(97)80033-X. [DOI] [Google Scholar]
- 51.Steinwachs M (2008) Cell based articular cartilage repair strategies. Symposium on cartilage repair. 8th EFFORT congress, Nice, France, May 2008
- 52.Steinwachs M, Kreuz PC. Autologous chondrocyte implantation in chondral defects of the knee with a type I/III collagen membrane: a prospective study with a 3-year follow-up. . Arthroscopy. 2007;23(4):381–387. doi: 10.1016/j.arthro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Tippet JW. Articular cartilage drilling and osteotomy in osteoarthritis of the knee. In: McGinty JB, editor. Operative arthroscopy. New York: Raven; 1991. pp. 325–339. [Google Scholar]
- 54.Ueda H, Hong L, Yamamoto M, Shigeno K, Inoue M, Toba T, Yoshitani M, Nakamura T, Tabata Y, Shimizu Y. Use of collagen sponge incorporating transforming growth factor-beta1 to promote bone repair in skull defects in rabbits. Biomaterials. 2002;23(4):1003–1010. doi: 10.1016/S0142-9612(01)00211-3. [DOI] [PubMed] [Google Scholar]
- 55.Vanlauwe J, Almqvist F, Bellemans J, Huskin JP, Verdonk R, Victor J. Repair of symptomatic cartilage lesions of the knee: the place of autologous chondrocyte implantation. Acta Orthop Belg. 2007;73(2):145–158. [PubMed] [Google Scholar]
- 56.Vukicevic’ S, Stavijenic’ A, Pec’ina M. Discovery and clinical applications of bone morphogenetic proteins. Eur J Clin Chem Clin Biochem. 1995;33(10):661–671. doi: 10.1515/cclm.1995.33.10.661. [DOI] [PubMed] [Google Scholar]
- 57.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg [Am] 1994;76(4):579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]