Abstract
Metastatic pathological fractures of the proximal femur are increasingly treated by endoprosthetic proximal femoral replacement. We report the results and the costs incurred performing these procedures at our supra-regional sarcoma unit. Sixty-two patients underwent 63 proximal femoral replacements for metastatic bone disease over a seven-year period. Breast cancer was the most common primary pathology. One patient underwent a revision procedure for infection. Twenty-two patients suffered dislocations, most commonly those undergoing a conventional arthroplasty articulation. The estimated cost of a proximal femoral replacement is £18,002 at our centre. Less than half of this is reimbursed under Payment by Results. Endoprosthetic replacement of the proximal femur is an effective treatment of metastases, but is poorly reimbursed under current funding arrangements.
Introduction
Metastases are the most common malignancy of bone, and the skeleton is the third most common site for metastatic disease after the lung and the liver. As improvements in cancer therapies result in lengthier survival, there are increasing numbers of patients with skeletal metastases who develop fractures or impending fractures, often involving the proximal femur. Skeletal complications of malignancy will be detrimental to the patient’s quality of life.
The aim of surgical management of skeletal metastases is to provide stable fixation allowing immediate weight-bearing and a return to function [1]. With increased survival, conventional techniques of fracture management may fail due to metastasis progression or fracture non-union. Fracture healing is often poor in diseased or irradiated bone and the surgeon must take into account the fact that these fractures may not unite [3]. There has been, therefore, an increase in these lesions being treated by excision and proximal femoral replacement (PFR), performed in the majority of cases at a regional sarcoma centre.
We reviewed our experience of patients with metastatic bone disease that underwent resection and endoprosthetic proximal femoral replacement at our hospital over a seven-year period and assessed the financial implications of the procedure.
Patients and methods
We reviewed our experience of patients undergoing PFRs for metastatic disease at The Royal National Orthopaedic Hospital, Stanmore over a seven-year period (January 1998 to December 2005). Patients were identified from the tumour database and cross-referenced to operating theatre registers, as well as prosthesis and pathology databases, to enable full data capture. Demographic information, namely, age, gender, the primary lesion where known, the site of the lesion, and duration of follow-up period, were also noted. All patients referred to our institution were discussed in a multidisciplinary team setting, attended by oncologists, radiologists, orthopaedic surgeons and other allied health professionals.
Results
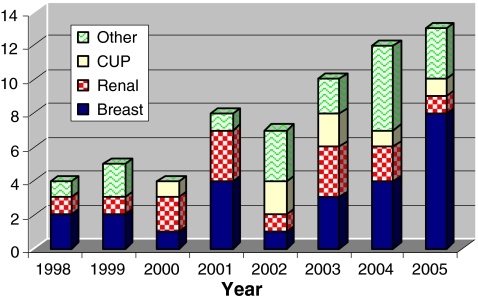
Sixty-three patients (39 female, 24 male) underwent PFR for metastatic disease under the care of one of four orthopaedic oncologists (SRC, TWRB, JAS, RCP). There was a year-on-year increase in PFRs performed (Fig. 1). Breast cancer was the most common primary malignancy, accounting for nearly 40% of the patients, followed by renal cancer and cancer of unknown primary (Table 1). The median length of stay was 25 days (range 9–127). With the introduction of a modular proximal femoral endoprosthesis, METS (Stanmore Implants Worldwide, London) in 2001, there was a reduction in length of stay (median, from 28 days to 24 days).
Fig. 1.
Annual numbers of proximal femoral replacements (PFRs) performed by diagnosis
Table 1.
Primary tumour pathology
| Primary | Number | Percentage |
|---|---|---|
| Breast | 25 | 39.7% |
| Renal | 14 | 22.2% |
| CUP | 7 | 11.1% |
| Lung | 4 | 6.3% |
| Prostate | 3 | 4.7% |
| Melanoma | 2 | 3.2% |
| Thyroid | 2 | 3.2% |
| Colorectal | 1 | 1.6% |
| Oesophagus | 1 | 1.6% |
| Leiomyosarcoma | 1 | 1.6% |
| Ovary | 1 | 1.6% |
| Plasmacytoma | 1 | 1.6% |
| Phaeochromocytoma | 1 | 1.6% |
Pathological fracture and previous surgery
Twenty-eight patients sustained pathological fractures prior to undergoing proximal femoral replacement. Of these, nine had undergone surgical stabilisation elsewhere (five intra-medullary nailing including spiral blade fixation, four compression hip screws).
Operative treatment
Where appropriate (e.g. the isolated metastasis), a core needle biopsy was performed to determine if the lesion was metastatic prior to undertaking definitive tumour resection.
All operations were performed in clean-air operating theatres with antibiotic prophylaxis at a single institution by the orthopaedic oncology team. Resection of metastatic lesions was performed in a similar manner to that for primary bone tumours. When possible, wide soft tissue margins were obtained and the shaft of the femur was transected at least 2 cm away from the most distal extent of the disease. In view of the fact that this is palliative surgery, important neurovascular structures were usually preserved at the expense of wide margins in order to maximise functional outcome. In patients with intraarticular spread of tumour we performed conventional joint replacement surgery and did not attempt extraarticular resections. The femoral reconstruction was achieved by using either a custom-made endoprosthesis (Centre for Biomedical Engineering, Institute of Orthopaedics, Stanmore, UK) or a modular endoprosthesis, METS (Stanmore Implants Worldwide, Stanmore, UK). The femoral stem was cemented and a hydroxyapatite collar used with the METS prosthesis in the majority of cases. In patients whose disease spared the greater trochanter, this was osteotomised and reattached to the endoprostheses. The proximal femoral METS endoprostheses contains a spiked, hydroxyapatite-coated shoulder with two screw holes for this specific purpose (Fig. 2). This enables gluteus medius and minimus to be reattached, thereby preserving abductor function. Where the greater trochanter was not salvageable, a soft tissue reconstruction was performed.
Fig. 2.

METS proximal femoral replacement showing trochanteric reattachment plate (courtesy of Stanmore Implants Worldwide, UK)
Reconstruction of the acetabulum was achieved using standard hip replacement acetabular prostheses (either cemented or uncemented) in 53 cases, a bipolar femoral head (Biomet, Bridgend) in eight cases and a large metal-on-metal resurfacing type acetabular prosthesis in two cases.
The patients were then observed for 24 hours on the high dependency unit with the leg initially in a trough and then treated in “slings and springs” until they had regained abductor control. Mobilisation then commenced with full weight-bearing with the physiotherapists in an abduction hip brace for six weeks.
Complications
Dislocation was the most frequent complication, occurring in 22 patients (35%), and this was more common with conventional acetabular cups than with either bipolar head (12.5%) or large head metal-on-metal articulations (0%). For conventional acetabular cups, the incidence of dislocation was higher with a 28-mm articulation (52%) than a 32-mm articulation (30%). Nine dislocations were treated by closed reduction and 11 by open reduction. All the isolated dislocations occurred in the early postoperative period (first six weeks). Two patients suffered multiple dislocations, one of whom underwent acetabular augmentation.
Four patients suffered infective complications. One patient underwent a one-stage revision for infection, two patients had washout and debridements and one was readmitted for intravenous antibiotics. A further patient required evacuation of an haematoma immediately postoperatively. The overall re-operation rate was 38% (24 of 63 hips).
Outcome
At latest follow-up 18 patients were still alive at a mean follow-up of 30 months (range 10–48). The median patient survival was 23 months. Survival based on pathology is given in Table 2. Twelve patients (20%) survived less than six months. The pathologies of these patients were three breast, three unknown primary, two renal and one each of prostate, plasmacytoma, melanoma and lung. Of the patients to survive less than six months, eight had sustained pathological fractures (67%) compared with an overall figure of 44% (28 of 63). Only one of these eight had undergone previous surgery.
Table 2.
Survival by diagnosis
| Pathology | Alive | Dead | ||
|---|---|---|---|---|
| n | Mean survival time (range) | n | Mean survival time (range) | |
| Breast (N = 25)a | 10 | 32 (21–42 months) | 15a | 26 (2–85 months) |
| Renal (N = 14) | 2 | 12, 13 months | 12 | 24 (3–74 months) |
| CUP (N = 7) | 0 | N/A | 7 | 9 (2–27 months) |
| Lung (N = 4) | 1 | 10 monthsb | 3 | 1, 7, and 20 months |
| Prostate (N = 3) | 1 | 34 months | 2 | 3 and 48 months |
| Overall (N = 63)a | 18 | 30 (10–48 months) | 45a | 21 (1–85 months) |
a Some cases bilateral
b Lost to more recent follow-up
No patient required a subsequent amputation. One prosthesis required revision for infection. All other prostheses have remained in situ.
Costs
Table 3 is an attempt to quantify the costs of the inpatient episode. It only represents the duration of the stay at the tertiary centre. It does not include any stay or preliminary investigations at the referring centre. Nor does it include the cost of any complications, rehabilitation, adjuvant therapy or indirect costs such as loss of wages. The estimate for operating theatre time was based on the timings of five consecutive PFRs, the first figure representing the anaesthetic and set-up time and the second surgical time. Costs of a hospital bed are higher in a tertiary centre than a district general hospital. The overall cost of a PFR for metastatic disease is approximately £18,000.
Table 3.
Costs of a typical proximal femoral replacement with a metal-on-metal articulation. For bipolar heads the cost of the prosthesis is £1435 less and for a conventional articulation £1616 less
| Parameter | Quantity | Unit cost | Subtotal |
|---|---|---|---|
| Prosthesis | 1 | £4052 | £4052 |
| Ward (days) | 24 | £384 | £9216 |
| HDU (days) | 1 | £1021 | £1021 |
| Theatre (min) | 54 + 144 | £14.46 | £2863 |
| Imaging | 4 X-rays, 1 MRI | £25 per X-ray £150 MRI |
£250 |
| Bloods, A/B, LMWH | Various | - | £600 |
| Total cost | £18002 |
Discussion
The British Orthopaedic Association guidelines for the management of metastatic bone disease and those of the Breast Speciality Group of the British Association of Surgical Oncology [1, 12] suggest that reconstruction for metastases should provide immediate stability, last the lifetime of the patient, stabilise the entire bone and assume that any fracture will not unite.
Proximal femoral replacement is an effective treatment for metastases of the proximal femur [6, 9, 11]. It allows achievement of the pre-requisites of metastasis surgery, namely, stable fixation allowing immediate weight-bearing and a return to function.
We have previously reported satisfactory functional outcome in patients undergoing proximal femoral replacement for metastases with an average MSTS score of 72.3% (range 57–83%) and a mean TESS score of 68.4% (range 46–84%) [10]. This functional outcome is similar to other series of proximal femoral replacements [2, 4, 7, 8].
Eighteen (29%) of our patients were still alive at a mean of 31 months postoperatively. The remainder died at a mean of 30 months, highlighting the need for a stable, long-lasting reconstruction.
Our dislocation rate is unquestionably high. There is no doubt that increasing the head–neck ratio with either bipolar heads or large head metal-on-metal articulations reduces the dislocation rate. Other authors have recommended bipolar heads ahead of conventional articulations for reasons of stability [7].
Implant costs for a Stanmore METS PFR stem are in the region of £2200. Modular prostheses are cheaper than custom made. Cemented cups with small heads are cheaper than bipolar or large head metal-on-metal articulations, but complications, particularly dislocations, in our series were more frequent. The two sides of the debate, head–neck ratio versus complication rate, need to be balanced. For patients with a poorer prognosis, e.g. in our series cancer of unknown primary and lung cancer, a bipolar head would seem to be appropriate. For those with an anticipated lengthier survival, the large head size is an alternative. This only emphasises the role of the multidisciplinary team in patient care.
Massive endoprosthetic replacement has been demonstrated by Grimer et al. to be cost effective for bone sarcomas [5]. Our estimated cost of a PFR is £18,000. The tariff reimbursement is £7185 (HRG H71, 2007–2008). This represents a loss of some £10,000 per case. With the increases in PFR for metastasis seen yearly, the financial burden on sarcoma units is ever-increasing. This financial dichotomy needs to be addressed to enable the gold standard treatments to be employed.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.British Orthopaedic Association (2001) Metastatic bone disease: a guide to good practice. British Orthopaedic Association, London
- 2.Farid Y, Lin PP, Lewis VO, Yasko AW (2006) Endoprosthetic and allograft-prosthetic composite reconstruction of the proximal femur for bone neoplasms. Clin Orthop Relat Res (442):223–229 [DOI] [PubMed]
- 3.Gainor BJ, Buchert P (1983) Fracture healing in metastatic bone disease. Clin Orthop Relat Res (178):297–302 [PubMed]
- 4.Gerrand CH, Currie D, Grigoris P, Reid R, Hamblen DL. Prosthetic reconstruction of the femur for primary bone sarcoma. Int Orthop. 1999;23(5):286–290. doi: 10.1007/s002640050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grimer RJ, Carter SR, Pynsent PB. The cost-effectiveness of limb salvage for bone tumours. J Bone Joint Surg. 1997;79-B(4):558–561. doi: 10.1302/0301-620X.79B4.7687. [DOI] [PubMed] [Google Scholar]
- 6.Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30(6):452–457. doi: 10.1007/s00264-006-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Menendez LR, Ahlmann ER, Kermani C, Gotha H (2006) Endoprosthetic reconstruction for neoplasms of the proximal femur. Clin Orthop Relat Res (450):46–51 [DOI] [PubMed]
- 8.Ogilvie CM, Wunder JS, Ferguson PC, Griffin AM, Bell RS (2004) Functional outcome of endoprosthetic proximal femoral replacement. Clin Orthop Relat Res (426):44–48 [DOI] [PubMed]
- 9.Orlic D, Smerdelj M, Kolundzic R, Bergovec M. Lower limb salvage surgery: modular endoprosthesis in bone tumour treatment. Int Orthop. 2006;30(6):458–464. doi: 10.1007/s00264-006-0193-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park DH, Jaiswal PK, Al-Hakim W, Aston WJ, Pollock RC, Skinner JA, Cannon SR, Briggs TW (2007) The use of massive endoprostheses for the treatment of bone metastases. Sarcoma 2007:62151 [DOI] [PMC free article] [PubMed]
- 11.Sarahrudi K, Hora K, Heinz T, Millington S, Vecsei V. Treatment results of pathological fractures of the long bones: a retrospective analysis of 88 patients. Int Orthop. 2006;30(6):519–524. doi: 10.1007/s00264-006-0205-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Breast Speciality Group of the British Association of Surgical Oncology British Association of Surgical Oncology Guidelines: the management of metastatic bone disease in the United Kingdom. Eur J Surg Oncol. 1999;25(1):3–23. doi: 10.1053/ejso.1998.0593. [DOI] [PubMed] [Google Scholar]