Abstract
Effective therapies for the regeneration of large osteochondral defects are still lacking; however, various approaches have been used. We evaluated the efficacy of Escherichia coli-derived dimeric recombinant human BMP-2 (E-rhBMP-2) for the repair of large osteochondral defects in a rabbit model. Osteochondral defects made in the femoral patellar groove of the knee were treated by transplanting gelatin sponges onto which no or various doses of E-rhBMP-2 were loaded. The outcomes were compared with those of an untreated control group four, 12 and 24 weeks after transplantation. At early time points, the cartilage tissue was repaired in a dose-dependent manner, and bone repair was accelerated in the defects treated with high doses of E-rhBMP-2. At 24 weeks, the repair of cartilage tissue was better with E-rhBMP-2 treatment, even at low doses, than without E-rhBMP-2 treatment. Our findings suggest that the use of E-rhBMP-2 improves and accelerates the repair of osteochondral defects in a rabbit model.
Introduction
The repair of osteochondral defects is a challenge for orthopaedic surgeons [1], and several methods, including tissue engineering procedures, have been developed and used in an attempt to repair articular cartilage defects [2–5]. Autologous chondrocyte implantation is clinically possible in the United States, some European countries and Korea. However, such cell-based therapies [6–14] are very expensive because of the high cost of construction and maintenance of cell-processing facilities. Therapies based on cytokines, such as bone morphogenetic protein-2 (BMP-2), for the repair of damaged cartilage would be more clinically useful and convenient than cell-based therapies because they are less expensive and less invasive.
BMPs are members of the transforming growth factor-β (TGF-β) superfamily [15]. Recombinant human BMPs (rhBMPs) appear to be intimately involved in the growth and differentiation of mesenchymal cells to chondrocytes and osteoblasts and induce ectopic cartilage and bone formation [16, 17]. RhBMPs have also been shown to enhance production of articular cartilage matrix in vitro without inducing the formation of bone [18–20]. Sellers et al. reported that the repair of osteochondral defects 3 mm in diameter with the use of rhBMP-2 in a rabbit model [21] was acceptable at 24 weeks and one year after treatment [22]. Vukicevic et al. reported that rhBMP-7 (osteogenic protein-1) supported the maturation of embryonic chick sternal chondrocytes [23] and that bovine articular chondrocytes did not undergo hypertrophy when cultured in the presence of rhBMP-7 in vitro [24]. Furthermore, they reported regeneration of articular cartilage chondral defects promoted by rhBMP-7 in rabbits (3 mm diameter) [25] and sheep (10 mm diameter) [26] in vivo.
However, to our knowledge, treatment in that study involved only a single dose of rhBMP, and few studies of the dose-dependent effects of rhBMP in the repair of osteochondral defects have been conducted. Therefore, the optimal dose of rhBMP-2 for osteochondral repair has not been elucidated. Furthermore, the rhBMPs used in previous studies were produced by BMP gene-transfected mammalian cells, i.e. Chinese hamster ovary (CHO) cells [16, 17]. This type of rhBMP-2 (CHO-rhBMP-2) is very expensive because of limited yields from CHO cells. One possible way to solve this problem is to use chemically dimerised monomer rhBMPs derived from BMP-gene-transfected Escherichia coli (E-rhBMP-2), because this method is relatively inexpensive and yields high amounts of rhBMPs [27]. E-rhBMP-2 is different from CHO-rhBMP-2 in that one N-glycosylation is missing in E-rhBMP-2 [27]. We have reported that the induction of new bone formation by E-rhBMP-2 is almost the same as that of CHO-rhBMP-2 [28–30]. However, the repair of osteochondral defects with the use of E-rhBMP-2 has not been reported. In this study, we are the first to report the assessment of the dose-dependent effects of BMP-2 for osteochondral repair and the efficacy of E-rhBMP-2 for the repair of large osteochondral defects (5 mm in diameter), a diameter much larger than the diameters reported by Sellers et al. (3 mm in diameter) [21, 22].
Materials and methods
Animals
Thirty-three female Japanese white rabbits with a mean weight of 3.3 kg (range 2.9–3.7) were purchased from Japan SLC Inc. (Shizuoka, Japan). Each rabbit was housed in a separate cage with free access to water and standard food in strict accordance with the Institutional Guidelines for the Care and Use of Laboratory Animals of Osaka City University.
Operative procedures
Anaesthesia was induced in rabbits by a simultaneous intramuscular injection of ketamine (10 mg/kg body weight) and xylazine (3 mg/kg body weight). One full-thickness cylindrical defect, 5 mm in diameter and 5 mm in depth, was created in the articular cartilage of the knee joint, by means of a medial parapatellar incision, down to bleeding subchondral bone in the patellar groove of the femur using an electric drill equipped with a Steinman pin. The defects in both knees were either treated with the implants or left untreated. The implants were produced as follows: gelatin sponges (Spongel; Astellas Pharma Inc., Tokyo, Japan), 5 mm in diameter and 5 mm in height, were impregnated with 0, 1, 5, 10, 20 or 40 μg of E-rhBMP-2 in a buffer (5 mmol/l glutamic acid, 2.5% glycine, 0.5% sucrose and 0.01% Tween-80) and then lyophilised. The rabbits were allowed unrestrained movement within their cages immediately after recovery from anaesthesia.
Histological analysis
The specimens were fixed in 4% paraformaldehyde for 24 hours, decalcified with 0.5 M ethylenediaminetetraacetic acid (EDTA), followed by dehydration in a graded ethanol series and embedded in paraffin wax. Sagittal sections (5 μm thick) were cut and stained with haematoxylin and eosin or toluidine blue per standard procedures and examined by light microscopy.
Histological grading
The histological findings were scored according to a histological grading scale modified from that described by Wakitani et al. [6]; higher scores were assigned to higher-quality cartilage and subchondral bone repair. We also categorised the thickness of subchondral bone using a six-category scale with a maximum score of 18 points (Table 1).
Table 1.
Histological grading scale modified from that of Wakitani et al. [6]
| Grade | Description |
|---|---|
| A. Cell morphology | |
| 4 | Hyaline cartilage |
| 3 | Mostly hyaline cartilage |
| 2 | Mostly fibrocartilage |
| 1 | Mostly non-cartilage |
| 0 | Non-cartilage only |
| B. Matrix staining (metachromasia) | |
| 3 | Normal (compared with host) |
| 2 | Slightly reduced |
| 1 | Significantly reduced |
| 0 | No staining |
| C. Surface regularitya | |
| 3 | Smooth (>3/4) |
| 2 | Moderate (1/2 to <3/4) |
| 1 | Irregular (1/4 to <1/2) |
| 0 | Severely (<1/4) |
| D. Thickness of cartilageb | |
| 2 | >2/3 |
| 1 | 1/3 to 2/3 |
| 0 | <1/3 |
| E. Integration of donor to host adjacent cartilage | |
| 2 | Both edges integrated |
| 1 | One edge integrated |
| 0 | Neither edge integrated |
| F. Thickness of subchondral bonec | |
| 4 | >100% |
| 3 | 75–100% |
| 2 | 50–75% |
| 1 | 25–50% |
| 0 | <25% |
aTotal smooth area of the repaired cartilage compared with that of the entire area of the cartilage defect
bMean thickness of the repaired cartilage compared with that of the adjacent normal cartilage
cMean thickness of the repaired subchondral bone compared with that of the surrounding subchondral bone
Experiment 1
To determine the optimal dose of E-rhBMP-2 and to monitor short-term trends in the healing process of osteochondral defects, the rabbits were divided into seven groups. In group 1, the defects were untreated (control); in group 2, the defects were treated with a gelatin sponge without E-rhBMP-2 (control); and in groups 3–7, the defects were treated with a gelatin sponge to which 1, 5, 10, 20 or 40 μg of E-rhBMP-2 was added. Each group consisted of two rabbits (four knees). At four and 12 weeks after surgery, the rabbits were killed with an intravenous injection of ketamine; two knees from each group were examined macroscopically and histologically.
Experiment 2
To examine whether E-rhBMP-2 can accelerate and improve the repair of full-thickness osteochondral defects over a long term, rabbits were randomised into five groups. In group 1, the defects were untreated; in group 2, the defects were treated with a gelatin sponge without E-rhBMP-2; and in groups 3–5, the defects were treated with a gelatin sponge to which 1, 5 or 40 μg of E-rhBMP-2 was added. Each group consisted of three rabbits (six knees). At 24 weeks after surgery, the rabbits were killed as described above and the knees were examined macroscopically and histologically.
Statistical analysis
Fisher’s exact probability test was used for the statistical analysis and P value <0.05 was considered to be statistically significant.
Results
Four rabbits died of old age during the postoperative period. (We used very large and old rabbits for this study, because young rabbits have a better capacity for repair.) Knees from the rabbits that died were excluded from the experimental design.
Experiment 1
Macroscopic findings
We compared the regularity of the treated cartilage surfaces with that of normal articular cartilage around the defect in all seven groups (Fig. 1, upper panels). At four weeks after surgery, the surfaces of the defects treated with implants containing more than 5 μg of E-rhBMP-2 were completely covered with whitish granular tissue (Fig. 1d–g); however, the articular surfaces of the untreated defects and the defects treated with implants containing less than 1 μg of E-rhBMP-2 were concave, and the margin of the defect was clearly visible (Fig. 1a–c). At 12 weeks after surgery, white soft tissue had formed over all defects treated with more than 1 μg of E-rhBMP-2, whereas white soft tissue was observed only at the margin of the defect. Also, the central area of the defect was still concave in the untreated group and in the group treated with implants that did not contain E-rhBMP-2 (data not shown).
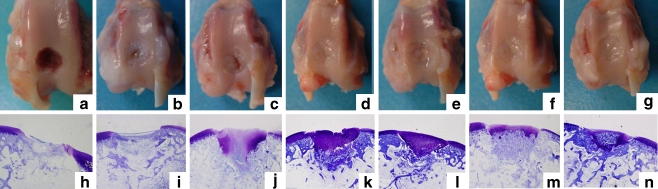
Fig. 1.
Macroscopic and histological findings 4 weeks after surgery. a and h Untreated defects. b and i Defects treated with the gelatin sponge only (no E-rhBMP-2). c and j Defects treated with 1 μg E-rhBMP-2. d and k Defects treated with 5 μg E-rhBMP-2. e and l Defects treated with 10 μg E-rhBMP-2. f and m Defects treated with 20 μg E-rhBMP-2. g and n Defects treated with 40 μg E-rhBMP-2. i-m Toluidine blue staining, original magnification ×20
Histological findings
Histological analysis of the toluidine blue-stained tissue sections was conducted to determine the level of repair in the defective knees. At four weeks after surgery, fibrous tissue-like repair was observed in both the untreated defect and the defect treated with an implant that did not contain E-rhBMP-2; no metachromasia was evident (Fig. 1h, i). However, the defects treated with more than 1 μg of E-rhBMP-2 were replaced by newly formed metachromasia-positive cartilage (Fig. 1j–n), and the repaired cartilage was thicker than the adjacent normal cartilage. Subchondral bone repair was observed in the defect treated with more than 5 μg of E-rhBMP-2 (Fig. 1k–n); the repaired cartilage was thinner in the defect treated with 20 or 40 μg of E–rhBMP-2 (Fig. 1m, n), and bone formation was observed in the repaired cartilage of the defect treated with 20 or 40 μg of E-rhBMP-2, the highest dose of E-rhBMP-2 (Fig. 1m, n).
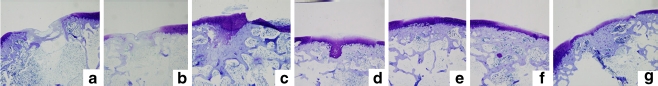
At 12 weeks after surgery, no metachromatic matrix was evident in the untreated defect or in the defect treated with an implant that did not contain E-rhBMP-2, and both defects were still concave (Fig. 2a, b). In contrast, metachromasia was observed on the surfaces of the repaired defects from the E-rhBMP-2-treated groups. The thickness of the repaired cartilage was dose-dependent at doses from 1 to 40 μg (Fig. 2c–g). The metachromasia-positive articular surfaces of the defects from the group treated with 40 μg of E-rhBMP-2 were thinner than the adjacent normal cartilage (Fig. 2g); whereas those from the group treated with 10 or 20 μg of E-rhBMP-2 showed better repair (Fig. 2e, f).
Fig. 2.
Histological findings 12 weeks after surgery. Toluidine blue staining, original magnification ×20. a Untreated defect. b Defect treated with the gelatin sponge only (no E-rhBMP-2). c Defect treated with 1 μg E-rhBMP-2. d Defect treated with 5 μg E-rhBMP-2. e Defect treated with 10 μg E-rhBMP-2. f Defect treated with 20 μg E-rhBMP-2. g Defect treated with 40 μg E-rhBMP-2
Histological scores
The histological findings four and 12 weeks after surgery were evaluated and rated using a histological grading scale (Table 1). At four weeks after surgery, the scores of untreated defects were 0 and 2, and those of the defects treated with a gelatin sponge without E-rhBMP-2 were 4 and 5. Those of the defects treated with a gelatin sponge with 1, 5, 10, 20 or 40 μg of E-rhBMP-2 were 9 and 9, 12 and 15, 12 and 14, 15 and 15, and 14 and 15, respectively. At 12 weeks after surgery, the scores of untreated defects were 3 and 4, and those of the defects treated with a gelatin sponge without E-rhBMP-2 were 5 and 8. Those of the defects treated with a gelatin sponge with 1, 5, 10, 20 or 40 μg of E-rhBMP-2 were 10 and 12, 15 and 16, 14 and 16, 13 and 14, and 13 and 14, respectively.
At 12 weeks after surgery, the group treated with 5 μg of E-rhBMP-2 had the highest score, and the scores of both control groups and of the groups treated with 1, 5 or 10 μg of E-rhBMP-2 were better than those at four weeks; however, the scores of the groups treated with 20 or 40 μg of E-rhBMP-2 were lower at 12 weeks than at four weeks.
Experiment 2
Macroscopic findings
At 24 weeks after surgery, smooth continuity of the articular surface between the surgical site and the surrounding cartilage was observed in defects treated with E-rhBMP-2, regardless of dose; however, the irregularity at the centre of the articular surface and the margin of the defect remained visible in the defects from both control groups (data not shown).
Histological findings
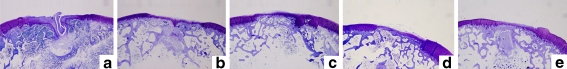
Although the degree of metachromasia-positive repaired tissue and bone formation was greater at 24 weeks than at four and 12 weeks in specimens from both control groups, clefts were still evident at the centre of the defects in five of six specimens (Fig. 3a, b). In contrast, the degree of repair in the defects treated with E-rhBMP-2, regardless of dose, was similar at all three time points on the basis of microscopic findings (Fig. 3c–e). The thickness of the repaired articular cartilage at 24 weeks was less than half that of the adjacent normal cartilage at 12 weeks.
Fig. 3.
Histological findings 24 weeks after surgery. Toluidine blue staining, original magnification ×20. a Untreated defect. b Defect treated with the gelatin sponge only (no E-rhBMP-2). c Defect treated with 1 μg E-rhBMP-2. d Defect treated with 5 μg E-rhBMP-2. e Defect treated with 40 μg rhBMP-2
Histological scores
At 24 weeks after surgery, the histological scores for the repaired articular surfaces of the osteochondral defects indicated a dose-dependent effect of E-rhBMP-2, and the histological scores of the groups treated with five and 40 μg of E-rhBMP-2 showed significant differences from the untreated defect groups (Fisher’s exact probability test); however, there were no significant differences between the E-rhBMP-2-treated groups (Table 2).
Table 2.
Histological scores 24 weeks after surgery for the repaired tissues treated with various doses (or no) E-rhBMP-2
| Rabbit number | Implants | ||||
|---|---|---|---|---|---|
| Empty | 0 μg | 1 μg | 5 μg | 40 μg | |
| 1 | 10 | 12 | 15 | 14 | 15 |
| 2 | 7 | 11 | 10 | 11 | 10 |
| 3 | 10 | 14 | 13 | 15 | 13 |
| 4 | 11 | 11 | 12 | 13 | 13 |
| 5 | 12 | 9 | 9 | 12 | 12 |
| 6 | 7 | 11 | 14 | 13 | 12 |
| Mean ± SD | 9.5 ± 2.1 | 11.3 ± 1.6 | 12.2 ± 2.3 | 13 ± 1.4* | 12.5 ± 1.6* |
‘Empty’ indicates untreated defects,‘0’ indicates defects treated with the gelatin sponge only (no E-rhBMP-2)
* Significantly different from the untreated defect group (P < 0.05)
Discussion
The findings of this study indicate that E-rhBMP-2 promotes the repair process of osteochondral defects, even at low doses. The repaired cartilage was thick at the early time point after surgery (four weeks). Enchondral ossification was promoted, and the repaired cartilage became thinner with time and with higher doses of E-rhBMP-2. At 24 weeks after surgery, there were no significant differences in cartilage repair between the groups treated with doses of 1–40 μg E-rhBMP-2. This finding might indicate that doses as low as 1 μg of E-rhBMP-2 are sufficient for repairing osteochondral defects in a rabbit model. We reported previously that more BMP is needed to repair bone defects in higher animals [31–34]. Thus, we estimate that higher amounts of E-rhBMP-2 are needed to repair human articular cartilage. When establishing the appropriate amount of E-rhBMP-2 for use in humans in the repair of articular cartilage defects, it should be borne in mind that high doses of BMP are not always necessary, as shown in our study.
The major disadvantage of using CHO-rhBMP-2 is its high cost. The lowest dose of E-rhBMP-2 was the most advantageous, because its cost is much lower than that of CHO-rhBMP-2. This low cost enables its widespread application in conventional orthopaedic surgery to enhance osteochondral healing and bone repair. Another disadvantage of rhBMP-2 is the increased risk of adverse events with the use of large doses [31–34]. In our study, no significant differences in histological appearance or scores were observed at 24 weeks between specimens treated with various doses of rhBMP-2. The successful osteochondral repair achieved with the low dose of E-rhBMP-2 suggests a decreased risk of adverse events. Sellers et al. reported no differences between the results at 24 weeks and those at one year after treatment with rhBMP-2 [21, 22]. Hence, we expect that the use of rhBMP-2 over longer periods of time will be successful.
Shapiro et al. reported that the thickness of repaired cartilage became half that of normal adjacent cartilage and that this thinning was due to an increase in new bone formation extending to the joint surface [35]. Thus, the thinning of the repaired cartilage in our study cannot be attributed to the effect of rhBMP-2 but the natural course. Furthermore, a cleft was observed at the centre of the defects not treated with E-rhBMP-2, whereas no cleft was visible in the defects treated with E-rhBMP-2. This finding suggests that the accelerated repair of subchondral bone provided support for the overlying cartilage and prevented the fissuring of the cartilage as a result of decreasing biomechanical instability [21]. Therefore, the ability of E-rhBMP-2 to accelerate and induce the rigid new subchondral bone would be important for the repair of the smooth articular surface. Moreover, from a clinical point of view, the formation of new subchondral bone extending to the joint surface and the thinning of the articular cartilage is not the ideal repair for osteochondral defects. The ideal repair (i.e. thick hyaline cartilage over the articular surface lined with thick subchondral bone) would involve a modified method in which bone formation is suppressed exclusively at the articular zone. Further investigation is needed to establish the ideal method for the repair of osteochondral defects.
In conclusion, the results indicate that a low dose of E-rhBMP-2 is effective at accelerating and improving the repair of osteochondral defects and should have a significant role in clinical applications.
Acknowledgements
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (project grants 16109009 and 1679085 to K.T. and grant 19791018 to Y.I.). The authors thank Ms. A. Inagaki, K. Hata, K. Kamei and Y. Hanamoto for their technical assistance.
References
- 1.Buckwalter JA, Mankin HJ. Articular cartilage repair and transplantation. Arthritis Rheum. 1998;41(8):1331–1342. doi: 10.1002/1529-0131(199808)41:8<1331::AID-ART2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 2.Pridie KH. A method of resurfacing osteoarthritis knee joints. J Bone Joint Surg Br. 1959;41:618–619. [Google Scholar]
- 3.Bert JM. Role of abrasion arthroplasty and debridement in the management of osteoarthritis of the knee. Rheum Dis Clin North Am. 1993;19(3):725–739. [PubMed] [Google Scholar]
- 4.Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;391:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 5.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10(6):432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 6.Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Bentley G, Greer RB. Homotransplantation of isolated epiphyseal and articular cartilage chondrocytes into joint surfaces of rabbits. Nature. 1971;230:385–388. doi: 10.1038/230385a0. [DOI] [PubMed] [Google Scholar]
- 8.Brittberg M, Nilsson A, Lindahl A, et al. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res. 1996;326:270–283. doi: 10.1097/00003086-199605000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. New England J Med. 1994;31:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 10.Chesterman PJ, Smith AU. Homotransplantation of articular cartilage and isolated chondrocytes. An experimental study in rabbits. J Bone Joint Surg Br. 1968;50(1):184–197. [PubMed] [Google Scholar]
- 11.Furukawa T, Eyre DR, Koide S, et al. Biochemical studies on repair cartilage resurfacing experimental defects in the rabbit knee. J Bone Joint Surg Am. 1980;62:79–89. [PubMed] [Google Scholar]
- 12.Hendrickson DA, Nixon AJ, Grande DA, et al. Chondrocyte-fibrin matrix transplants for resurfacing extensive articular cartilage defects. J Orthop Res. 1994;12:485–497. doi: 10.1002/jor.1100120405. [DOI] [PubMed] [Google Scholar]
- 13.Sato K, Urist MR. Bone morphogenetic protein-induced cartilage development in tissue culture. Clin Orthop Relat Res. 1984;183:180–187. [PubMed] [Google Scholar]
- 14.Vacanti CA, Kim W, Schloo B, et al. Joint resurfacing with cartilage grown in situ from cell-polymer structures. Am J Sports Med. 1994;22:485–488. doi: 10.1177/036354659402200408. [DOI] [PubMed] [Google Scholar]
- 15.Celeste AJ, Iannazzi JA, Taylor RG, et al. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Nat Acad Sci. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyons KM, Pelton RM, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- 17.Rosen V, Wozney JM, Wang EA, et al. Purification and molecular cloning of a novel group of BMPs and localization of BMP mRNA in developing bone. Connect Tissue Res. 1989;20:313–319. doi: 10.3109/03008208909023902. [DOI] [PubMed] [Google Scholar]
- 18.Luyten FP, Yu YM, Yanagishita M, et al. Natural bovine osteogenin and recombinant human bone morphogenetic protein-2B are equipotent in the maintenance of proteoglycans in bovine articular cartilage explant cultures. J Biol Chem. 1992;267:3691–3695. [PubMed] [Google Scholar]
- 19.Sailor LZ, Hewick RM, Morris EA. Recombinant human bone morphogenetic protein-2 maintains the articular chondrocyte phenotype in long-term culture. J Orthop Res. 1996;14:937–945. doi: 10.1002/jor.1100140614. [DOI] [PubMed] [Google Scholar]
- 20.Nawata M, Wakitani S, Nakaya H. Use of bone morphogenetic protein-2 and diffusion chambers to engineer cartilage tissue for the repair of defects in articular cartilage. Arthritis Rheum. 2005;52:155–163. doi: 10.1002/art.20713. [DOI] [PubMed] [Google Scholar]
- 21.Sellers RS, Peluso D, Morris EA. The effect of recombinant human bone morphogenetic protein-2 (rhBMP-2) on the healing of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1997;79:1452–1463. doi: 10.2106/00004623-199710000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sellers RS, Zhang R, Glasson SS, et al. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82:151–160. doi: 10.1302/0301-620X.82B1.10733. [DOI] [PubMed] [Google Scholar]
- 23.Chen P, Vukicevic S, Sampath TK. Osteogenic protein-1 promotes growth and maturation of chick sterna chondrocytes in serum-free cultures. The results demonstrate that OP-1, as the sole medium supplement, supports the maturation of embryonic chick sternal chondrocytes in vitro. J Cell Sci. 1995;108:105–114. doi: 10.1242/jcs.108.1.105. [DOI] [PubMed] [Google Scholar]
- 24.Chen P, Vukicevic S, Sampath TK. Bovine articular chondrocytes do not undergo hypertrophy when cultured in the presence of serum and osteogenic protein-1. Biochem Biophys Res Commun. 1993;197:1253–1259. doi: 10.1006/bbrc.1993.2612. [DOI] [PubMed] [Google Scholar]
- 25.Grgic M, Jelic M. Regeneration of articular cartilage defects in rabbits by osteogenic protein-1 (bone morphogenetic protein-7) Acta Med Croatica. 1997;51:23–27. [PubMed] [Google Scholar]
- 26.Jelic M, Pecina M, Haspl M, et al. Regeneration of articular cartilage chondral defects by osteogenic protein-1 (bone morphogenetic protein-7) in sheep. Growth Factors. 2001;19(2):101–113. doi: 10.3109/08977190109001079. [DOI] [PubMed] [Google Scholar]
- 27.Ruppert R, Hoffmann E, Sebald W. Human bone morphogenetic protein 2 contains a heparin-binding site which modifies its biological activity. Eur J Biochem. 1996;237:295–302. doi: 10.1111/j.1432-1033.1996.0295n.x. [DOI] [PubMed] [Google Scholar]
- 28.Kubler NR, Reuther JF, Faller G, et al. Inductive properties of recombinant human BMP-2 produced in a bacterial expression system. Int J Oral Maxillofac Surg. 1998;27(4):305–309. doi: 10.1016/S0901-5027(05)80621-6. [DOI] [PubMed] [Google Scholar]
- 29.Bessho K, Konishi Y, Kaihara S, et al. Bone induction by Escherichia coli-derived recombinant human bone morphogenetic protein-2 compared with Chinese hamster ovary cell-derived recombinant human bone morphogenetic protein-2. Br J Oral Maxillofac Surg. 2000;38(6):645–649. doi: 10.1054/bjom.2000.0533. [DOI] [PubMed] [Google Scholar]
- 30.Yano K, Hoshino M, Ohta Y et al (2009) Osteoinductive capacity and heat stability of recombinant human bone morphogenetic protein-2 produced by Escherichia coli and dimerized by biochemical processing. J Bone Miner Metab 27(3):355–363 [DOI] [PubMed]
- 31.Boden SD, Zdeblick TA, Sandhu HS, et al. The use of rhBMP-2 in interbody fusion cages. Definitive evidence of osteoinduction in humans: a preliminary report. Spine. 2000;25:376–381. doi: 10.1097/00007632-200002010-00020. [DOI] [PubMed] [Google Scholar]
- 32.Boden SD, Kang J, Sandhu H, et al. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: Volvo Award in clinical studies. Spine. 2002;27:2662–2673. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 33.Burkus JK, Gornet MF, Dickman CA, et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337–349. doi: 10.1097/00024720-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Johnsson R, Stromqvist B, Aspenberg P. Randomized radiostereometric study comparing osteogenic protein-1 (BMP-7) and autograft bone in human noninstrumented posterolateral lumbar fusion. Spine. 2002;27:2654–2661. doi: 10.1097/00007632-200212010-00004. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro F, Koide S, Glimcher MJ. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532–553. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]