Abstract
Although not consistently replicated, a substantial number of studies suggest that left-handers have larger callosal regions than right-handers. We challenge this notion and propose that callosal size is not linked to left-handedness or right-handedness per se but to the degree of handedness lateralization. To test this hypothesis, we investigated the thickness of the corpus callosum in a large data set (n=361). We analyzed the correlations between callosal thickness and the degree of handedness lateralization in 324 right-handers and 37 left-handers at 100 equidistant points across the corpus callosum. We revealed significant negative correlations within the anterior and posterior midbody suggesting that larger callosal dimensions in these regions are associated with a weaker handedness lateralization. Significant positive correlations were completely absent. In addition, we compared callosal thickness between moderately lateralized left-handers (n=37) and three equally sized groups (n=37) of right-handers (strongly, moderately, and weakly lateralized). The outcomes of these group analyses confirmed the negative association between callosal size and handedness lateralization, although callosal differences between right- and left-handers did not reach statistical significance. This suggests that callosal differences are rather small, if examined as a dichotomy between two handedness groups. Future studies will expand this line of research by increasing the number of left-handers to boost statistical power, and by combining macro- and micro-structural, as well as functional and behavioral measurements to identify the biological mechanisms linking callosal morphology and handedness lateralization.
Keywords: corpus callosum, lateralization, handedness, MRI
Introduction
Novel findings in the mid-eighties indicated a significant relationship between callosal size and handedness, with larger corpora callosa in left-handers and ambidextrous individuals than in right-handers (Witelson, 1985). This observation was followed by a substantial number of studies investigating the link between callosal morphology and handedness. Several analyses confirmed the larger callosal dimensions in left-handers or non-consistent right-handers (Witelson, 1989; Denenberg et al., 1991; Witelson and Goldsmith, 1991; Habib et al., 1991; Driesen and Raz, 1995; Moffat et al., 1998; Tuncer et al., 2005; Josse et al., 2008). However, other studies did not detect any significant differences between right- and left-handers or non-consistent right-handers (Kertesz et al., 1987; O'Kusky et al., 1988; Steinmetz et al., 1992; Hines et al., 1992; Clarke and Zaidel, 1994; Steinmetz et al., 1995; Jancke et al., 1997; Preuss et al., 2002; Luders et al., 2003; Anstey et al., 2007). Interestingly, a few investigations, although rare, even revealed larger callosal sizes in right-handers (Hopper et al., 1994; Jancke et al., 1997; Westerhausen et al., 2004). A number of explanations may account for these discrepancies in results from different studies, such as different sample sizes, different methods for measuring hand preference and/or performance, different handedness classifications and cut-off scores for handedness categories, different callosal measurements and definitions of callosal subregions, and the (non-)adjustments of callosal measures for individual brain size.
Another largely uninvestigated source for discrepancy appears to be the degree of individual handedness lateralization within and across studies. More specifically, the anatomy of the corpus callosum has been advocated as a potential marker for functional lateralization (Clarke et al., 1993; Morton and Rafto, 2006; Josse et al., 2008). Thus, if the degree and not the direction of lateralization mattered the most, and if a subject sample contained right- and left-handers with rather similar degrees of lateralization, studies might have revealed no differences in callosal size. In contrast, if the sample contained right- and left-handers with largely differing degrees of lateralization, studies might have revealed differences between both groups. If we assume that a weaker functional lateralization is associated with an increased demand on inter-hemispheric communication (e.g., via callosal fibers), then callosal dimensions should be increased in less lateralized individuals (provided that callosal size correlates positively with the amount of information transferred between hemispheres). Since left-handers are usually less lateralized than right-handers (Driesen and Raz, 1995; Corballis, 2009), one might predict that most studies would report larger callosal dimensions in left-handers, rather than in right-handers. This seems to have led to the overly general conclusion that left-handers have larger callosal regions than right-handers.
We challenge this notion and propose that callosal size is not linked to left-handedness or right-handedness per se but to the degree of handedness lateralization (Corballis, 2009). Thus, if we were to compare more lateralized right-handers with less lateralized left-handers (the typical constellation), we should detect larger callosal dimensions in left-handers. In contrast, if we were to compare more lateralized left-handers with less lateralized right-handers (the atypical constellation), we should detect larger callosal dimensions in right-handers. To test this hypothesis, we generated three different samples of right- and left-handers: Two of these samples resembled the typical and the atypical constellations described above; the third sample contained right- and left-handers who had similar degrees of handedness lateralization. We then applied a surface-based mesh-modeling technique to compare the thickness of the corpus callosum between right- and left-handers at 100 equidistant points across the entire midsagittal surface. In addition, we conducted point-wise correlation analyses to establish whether callosal thickness and degree of handedness lateralization are significantly linked. We hypothesized larger callosal dimensions in less lateralized individuals regardless of the direction of their handedness.
Methods
Subjects
The overall sample of the present study included 361 healthy subjects (156 men and 205 women), ranging between 44 and 49 years. This sample was drawn from the PATH Through Life Project designed to study the risk and protective factors for normal aging, dementia and other neuropsychiatric disorders (Anstey et al., 2005). The present study focuses on the middle-age sample of the PATH Project, composed of 2530 individuals randomly selected from the population of Canberra, Australia. A subsample of 656 participants were offered a magnetic resonance imaging (MRI) scan, which 503 accepted, and 431 eventually completed (Wen et al., 2009). The reasons for not undergoing MRI after having initially agreed included subsequent withdrawal of consent, medical conditions contradicting MRI, and claustrophobia or other anxiety about the procedure. There were no differences in age, sex, and years of education between those who had an MRI scan and those who did not. One scan was lost due to a technical fault, giving a total number of 430 scans. For the current analysis, another 69 scans were excluded due to movement artifacts, poor scan quality, neurological disorders and missing handedness data, leaving 361 scans. The study was approved by the ethics committees of the Australian National University, Canberra and the University of New South Wales, Sydney, Australia. All participants gave written informed consent to be included in this study.
Handedness Measurement
Handedness1 was determined based on the Edinburgh Handedness Inventory (EHI; Oldfield, 1971). The EHI entails reporting hand use for ten actions such as writing, striking a match, holding a broom, etc. on a five-point scale (−2 always left, −1 mostly left, 0 either, +1 mostly right, +2 always right). A handedness coefficient ranging from −1 (exclusively left-handed) to +1 (exclusively right-handed) is computed by averaging all scores and dividing by 2. The resulting values ranged between −1 and +1 and revealed (i) the direction of handedness, with negative values indicating left-handedness and positive values indicating right-handedness; and (ii) the degree of handedness, with values closer to −1 and +1 indicating a stronger lateralization than values closer to 0.
Image Acquisition
T1-weighted 3-D structural MRI images were acquired in coronal plane using a Fast Field Echo (FFE) sequence on a 1.5 Tesla Gyroscan scanner (ACS-NT, Philips Medical Systems, Best, The Netherlands). About mid-way through this study, for reasons outside the researchers' control, the original scanner (scanner A) was replaced with a similar Philips scanner (scanner B). The scanning parameters were kept essentially the same. The first 163 subjects were scanned on scanner A with the following parameters: TR = 8.84 ms, TE = 3.55 ms, 8° flip angle, matrix size = 256×256, slices = 160, field of view (FOV) = 256×256 mm. Slices were contiguous with a slice thickness of 1.5 mm. For the remaining 268 subjects scanned on scanner B, the TR = 8.93 ms and TE = 3.57 ms values were slightly different in order to improve image quality, but all other parameters were exactly the same. To ensure the reliability and compatibility of the data, we compared the subjects scanned on the two scanners on socio-demographic and imaging parameters. There were no significant differences on age or years of education, but significantly more women were scanned on scanner B than A (p=0.003). The gray matter (GM), white matter (WM), and cerebrospinal fluid (CSF) volumes obtained from the two scanners did not differ significantly.
Image Preprocessing and Callosal Outlining
Images were placed into the Talairach standard space using automated 6-parameter rigid-body transformations (Collins et al., 1994) to correct for differences in head alignment. In addition, automated radio-frequency bias field corrections were applied (Sled et al., 1998) to correct for intensity drifts caused by magnetic field inhomogeneities. The corpus callosum was then outlined automatically based on the Chan-Vese model for active contours (Chan and Vese, 2001) using the LONI pipeline processing environment (Rex et al., 2003). This resulted in two midsagittal callosal segments (i.e., the upper and lower callosal boundaries) for each subject, as detailed elsewhere (Luders et al., 2006a; Luders et al., 2006b; Luders et al., 2007). Subsequently, each callosal segment was overlaid onto the MR image from which it had been extracted and visually inspected to insure that automatically generated callosal outlines followed precisely the natural course and boundaries of the corpus callosum. Contours that did not match this criterion were corrected manually by one rater (N.C.).
Callosal Thickness Measurements
To obtain highly localized measures of callosal thickness, anatomical surface-based mesh modeling methods were employed (Thompson et al., 1996a; Thompson et al., 1996b). That is, the upper and lower callosal boundaries were resampled at regular intervals to render the discrete points comprising the boundaries spatially uniform. Then, a new segment (i.e., the medial core) was automatically created by calculating a spatial average 2D curve from 100 equidistant surface points representing the upper and lower callosal boundaries. Finally, the distances between 100 surface points of the medial core and the 100 corresponding surface points of both the upper and the lower callosal boundaries were computed. These regional distances indicate callosal thickness with a high spatial resolution (i.e., at 100 locations distributed evenly over the callosal surface).
Brain Volume Measurements
To measure brain and tissue volumes, the T1-weighted images were processed with Freesurfer (http://surfer.nmr.mgh.harvard.edu/; Fischl et al., 2002; Fischl et al., 2004) and automatically separated into the different tissue types. Using the tissue-classified partitions, GM, WM, and CSF volumes were determined in dm3 as the sum of voxels representing GM, WM, and CSF, respectively. Intracranial volume (ICV) was calculated by summing up GM, WM, and CSF volumes.
Overall Sample and Subsamples
The overall sample of this study (n=361) contained 324 right-handers and 37 left-handers (i.e., 10.25%), which corresponds to the usual prevalence of left-handedness in the normal population (Annett, 1973; Raymond et al., 1996). Sample-specific descriptive statistics are provided in Table 1. In addition, we created four equally-sized subgroups of interest: one left-handed (LH) group (moderately lateralized) and three right-handed (RH) groups (strongly, moderately, and weakly lateralized). The number of subjects in each group (n=37) and the ratio of males to females (16:21), was determined based on the total number of available left-handers (n=37) and their gender distribution (16 males; 21 females). The moderately lateralized LH group (moderate LH) contained left-handers with EHI scores ranging from −1.0 to −0.05 (mean: −0.60; SD: 0.30). The moderately lateralized RH group (moderate RH) was created to resemble the moderate LH group as closely as possible, where the lowest EHI scores started only at 0.2 (rather than at 0.05). Thus, the moderate RH group contained right-handers with EHI scores ranging from 0.2 to 1.0 (mean: 0.62; SD: 0.27). In contrast, the strongly lateralized RH group (strong RH) contained right-handers with the highest EHI scores of 1.0 (mean: 1.0; SD: 0.00). The weakly lateralized RH group (weak RH) contained the right-handers with the lowest EHI ranging from 0.2 to 0.5 (mean: 0.34; SD: 0.08). Group-specific descriptive statistics are provided in Table 1. The four subgroups were used to generate three specific subsamples (n=74) containing right- and left-handers with either similar degrees of handedness lateralization (moderate RH / moderate LH) or different degrees of handedness lateralization (strong RH / moderate LH; weak RH / moderate LH). Right- and left-handers in all three specific samples did not differ significantly with respect to the ratio of males and females, age, and brain size.
Table 1.
Sample-specific descriptive statistics
| Group | Subjects | Males : Females | Mean ± SD for Age (in years) |
Mean ± SD for ICV (in dm3) |
|---|---|---|---|---|
| Overall Sample | ||||
| Total | 361 | 156 : 205 | 46.88 ± 1.37 | 1.54 ± 0.16 |
| RH | 324 | 140 : 184 | 46.85 ± 1.38 | 1.54 ± 0.15 |
| LH | 37 | 16 : 21 | 47.16 ± 1.32 | 1.55 ± 0.17 |
| Subgroups | ||||
| Moderate LH | 37 | 16 : 21 | 47.16 ± 1.32 | 1.55 ± 0.17 |
| Weak RH | 37 | 16 : 21 | 46.97 ± 1.30 | 1.56 ± 0.17 |
| Moderate RH | 37 | 16 : 21 | 46.97 ± 1.42 | 1.52 ± 0.15 |
| Strong RH | 37 | 16 : 21 | 46.86 ± 1.40 | 1.58 ± 0.18 |
RH = Right-handers; LH = Left-handers
SD = Standard Deviation
ICV = Intracranial Volume
Statistical Analyses
First, we converted the directional EHI-handedness measures into absolute values (i.e., all final EHI-scores were positive). Then, we calculated the correlation coefficients between the absolute EHI-scores and callosal thickness at 100 surface points within the overall sample (n=361), while co-varying for ICV. Subsequently, using the three specific subsamples (n=74), we compared callosal thickness between right- and left-handers (a) when right-handers were more lateralized (strong RH versus moderate LH), (b) when left-handers were more lateralized (weak RH versus moderate LH), and (c) when right- and left-handers were similarly lateralized (moderate RH versus moderate LH), while co-varying for ICV.
Supplemental Analysis
To explore the possibility that correlations between callosal thickness and handedness lateralization might be affected by the direction of handedness, we tested for an interaction between the degree of handedness lateralization (i.e., the absolute handedness scores) and the direction of handedness (i.e., left- versus right-handed), while co-varying for ICV. Given the small sample size of left-handers (n=37) this analysis is considered exploratory.
Results
Correlations
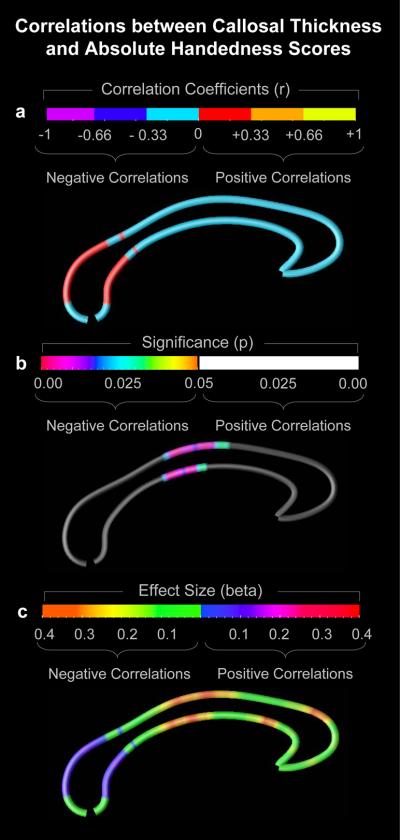
As demonstrated in Figure 1a, we detected negative correlations between callosal thickness and absolute handedness scores across almost the entire corpus callosum, except for the splenium. Negative correlations imply a greater callosal thickness with decreasing handedness lateralization, regardless of the direction of handedness. As further demonstrated (Figure 1b), negative correlations were significant at p≤0.05 across the anterior and posterior midbody. There were no significant positive correlations. When mapping effect sizes (Figure 1c), negative correlations were pronounced across the anterior and posterior midbody, and in the callosal anterior third.
Figure 1.
Correlations between callosal thickness and handedness lateralization within the overall sample. (a) The callosal map and color bar encode the r-values that depict the magnitude and direction of correlations between absolute handedness scores and callosal thickness. (b) The callosal map and color bar encode the p-values that depict significant negative correlations at p≤0.05. (c) The callosal map and color bar encode the beta effect sizes that depict the magnitude and direction of the correlation. The posterior region of the corpus callosum is located on the left; the anterior region points to the right.
Group Comparisons
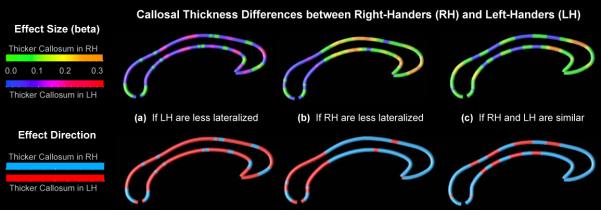
There were no significant differences when comparing callosal thickness between right- and left-handers, in any of the three specific comparisons (significance maps not shown). However, as illustrated in Figure 2, when mapping effect sizes (top panel) and illustrating the direction of group effects (bottom panel), differences between handedness groups became apparent with thicker callosal regions in subjects who are less lateralized. More specifically, when comparing more lateralized right-handers with less lateralized left-handers (strong RH versus moderate LH), we detected a predominantly larger thickness in left-handers (Figure 2a). In contrast, when comparing more lateralized left-handers with less lateralized right-handers (moderate LH versus weak RH), we detected a predominantly larger thickness in right-handers (Figure 2b), although parts of the splenium and isthmus were still thicker in left-handers. When comparing similarly lateralized right- and left-handers (moderate RH versus moderate LH), we detected a regular pattern of alternating directional effects across the callosal surface. Interestingly, this pattern did not extend into regions surrounding the posterior and anterior bend of the corpus callosum, and the splenium and anterior third were larger in right-handers.
Figure 2.
Callosal thickness differences between right- and left-handers within the three specific samples. (a) More lateralized right-handers versus less lateralized left-handers (strong RH versus moderate LH). (b) More lateralized left-handers versus less lateralized right-handers (moderate LH versus weak RH). (c) Similarly lateralized right- and left-handers (moderate RH versus moderate LH). Top: The callosal maps and color bar encode the beta effect sizes that depict the magnitude and direction of the group differences. Bottom: The callosal maps and color bar encode the direction of the group differences, regardless of its magnitude.
Supplemental Findings
We detected a significant interaction between the degree and direction of handedness lateralization suggesting a different relationship between callosal thickness and handedness lateralization in right- and left-handers. However, the observed differential effect was confined to a small region of the splenium (Supplemental Figure 1). This region did not overlap with any of the regions where significant negative correlations between callosal thickness and handedness lateralization were detected (Figure 1).
Discussion
We investigated the morphology of the corpus callosum with high regional specificity to resolve the question whether there is a link between callosal size and the degree of handedness lateralization. We hypothesized that callosal regions would be larger in individuals with less lateralization regardless of the direction of their handedness. In agreement with our hypothesis, we revealed significant negative correlations between callosal thickness and absolute handedness scores. Negative correlations imply an increasing callosal thickness with decreasing handedness lateralization. This supports the assumption that greater functional lateralization is associated with decreased demands on inter-hemispheric communication (e.g., via callosal fibers) which, in turn, is reflected in decreased callosal dimensions.
Our findings (partly) contrast with Moffat et al. (1998) who did not detect any correlations between callosal size and the degree of handedness (examined in left-handers only); with Habib (1991) who detected positive correlations between callosal size and handedness degree (examined as absolute values); and with Witelson and Goldsmith (1991) who detected negative correlations between callosal size and handedness degree (examined as directional values)2. These discrepancies in findings might be due to various differences across studies including (but not limited to) the following: the number of subjects (n=16 vs. n=22 vs. n=53 vs. n=361*3), the modality of the imaging data (i.e., post mortem vs. in vivo*), the a priori definition of callosal segments (i.e., radial vs. perpendicular parcellation schemes vs. no a priori definition*), the callosal measurement of interest (i.e., segment-specific area vs. point-wise thickness*), the tests employed for measuring handedness (i.e., Annett vs. Waterloo vs. Edinburgh*), the inclusion of brain size corrections (i.e., not applied vs. applied*). The dependency of study outcomes on these factors should not be underestimated, especially since it was previously demonstrated that a re-analysis of the same data revealed different outcomes solely due to applying different measurements related to handedness and callosal morphology (Kertesz et al., 1987; Denenberg et al., 1991). Importantly, to circumvent the risk of defining callosal sections with controversial fiber distribution (Hofer and Frahm, 2006), to avoid shaped-induced biases (Tomaiuolo et al., 2007), and also to increase the spatial resolution of callosal measurements, the current study did not rely on any parcellation scheme. Instead, we applied a well-validated approach to compute 100 point-wise indicators for callosal thickness across the whole corpus callosum.
Our assumption that callosal size is a marker for handedness lateralization regardless of handedness direction was further substantiated when we compared right- and left-handers directly. As suspected, when we compared less lateralized left-handers with strongly lateralized right-handers, the majority of callosal regions were larger in left-handers. In contrast, when we compared less lateralized right-handers with more lateralized left-handers, callosal regions were predominantly larger in right-handers. Finally, when we compared similarly lateralized right- and left-handers, we detected a regular pattern of alternating directional effects across large parts of the corpus callosum. The same inverse relationship between callosal size and handedness lateralization is also implied in outcomes of other studies revealing larger callosal regions in left-handers or in non-consistent right-handers (i.e., individuals who are typically less lateralized) than in consistent right-handers (Witelson, 1989; Denenberg et al., 1991; Witelson and Goldsmith, 1991; Habib et al., 1991; Driesen and Raz, 1995; Moffat et al., 1998; Josse et al., 2008).
Regional Specificity
Significant negative correlations between callosal thickness and handedness lateralization were confined to callosal sections across the anterior and posterior midbody. Pronounced negative correlations became also evident in the callosal anterior third when mapping effect sizes. In agreement with respect to the affected callosal segments, previous studies revealed handedness effects in the callosal anterior third (Habib et al., 1991; Westerhausen et al., 2004; Josse et al., 2008), the anterior midbody (Denenberg et al., 1991; Habib et al., 1991; Hopper et al., 1994; Jancke et al., 1997; Westerhausen et al., 2004; Josse et al., 2008), and the posterior midbody (Witelson, 1989; Hopper et al., 1994; Jancke et al., 1997; Moffat et al., 1998; Westerhausen et al., 2004).
Post mortem studies as well as in vivo examinations based on fiber tractography suggest that the anterior and posterior midbodies are involved in transferring motor information (Witelson, 1989; Hofer and Frahm, 2006; Park et al., 2008). Thus, the smaller callosal thickness in individuals with a strong hand preference is possibly associated with a diminished inter-hemispheric communication between the hand areas of the motor cortex, or between the motor cortices in general. Supporting this idea, a study in musicians has shown that professional pianists and string instrument players (i.e., individuals highly skilled in bi-manual motor coordination) had larger corpora callosa (Schlaug et al., 1995). However, a positive link between callosal size and number of crossing fibers has been confirmed mainly for small diameter fibers, while motor regions are, perhaps, rather connected through large diameter fibers (Aboitiz et al., 1992). Thus, the observed negative correlation between callosal size and handedness lateralization might be driven by individual brain organization and the degree of functional lateralization, in general (Witelson, 1989). For example, some studies provide evidence that ambidextrous people or left-handers (who are usually less lateralized with respect to handedness) are also less lateralized with respect to language functions (Pujol et al., 1999; Soros et al., 1999; Knecht et al., 2000). Interestingly, the current study also revealed pronounced negative correlations between callosal size and handedness within the callosal anterior which contains a high number of small diameter fibers (Aboitiz et al., 1992). These anterior callosal sections are not specifically related to handedness, but are known to connect prefrontal regions (Witelson, 1989; Hofer and Frahm, 2006; Park et al., 2008). Since prefrontal regions are largely involved in processing higher-order cognitive information (Jung and Haier, 2007), the observed negative link in anterior callosal regions might provide a hint that these higher-order cognitive processes are also differently organized in individuals with different degrees of handedness lateralization.
Surprisingly, we also detected group effects (albeit not significant) in a direction that tended to contradict the proposed negative correlation between callosal size and handedness lateralization. For example, we observed a thicker splenium in strongly lateralized left-handers. Thus, it could be that other functions, regulated by parietal, temporal, and occipital regions (Witelson, 1989; Hofer and Frahm, 2006; Park et al., 2008), have been re-organized in an opposing manner (i.e., a larger thickness in more lateralized individuals) as a consequence of handedness lateralization in order to minimize interference among different neuronal networks and optimize brain function. While this hypothesis requires testing in future analyses, the apparent non-conformity of the observed association between handedness and callosal morphology re-emphasizes the existence of region-specific relations (rather than a single overall relationship). That is, different segments of the corpus callosum appear to be differently linked to the degree of functional lateralization, as also implied in a previous study analyzing the relationship between corpus callosum size and various anatomical asymmetries (Luders et al., 2003).
Magnitude and Significance
In our correlation analysis we established that there is a significant negative link between callosal size and the lateralization of handedness, regardless of handedness direction. This link was confirmed when we compared right- and left-handers and systematically altered the degree of lateralization in both handedness groups. Although the direction of the group effects were largely in agreement with our hypothesis (i.e., larger callosal regions in less lateralized individuals), none of them was significant. This agrees with prior studies that also failed to reveal any significant differences between right- and left-handers (Kertesz et al., 1987; Steinmetz et al., 1992; Hines et al., 1992; Clarke and Zaidel, 1994; Steinmetz et al., 1995; Jancke et al., 1997; Preuss et al., 2002; Luders et al., 2003; Anstey et al., 2007). The diminutiveness of the group effect is in further agreement with studies looking at associations between callosal morphology and other functional lateralizations. For example, Josse et al. reported that the effect of callosal size on language lateralization “was not affected by whether the effects of handedness was factored out or not” (Josse et al., 2008).
Altogether, this seems to suggest that callosal differences between right- and left-handers, although existent, are rather small. Even so, group effects might become stronger when handedness groups differ in the degree of their lateralization. Thus, it is possible that previous studies which compared similarly lateralized right- and left-handers did not reveal group effects, while studies which compared differently lateralized right- and left-handers were able to detect significant regional differences. For example, even studies which consciously excluded individuals with only little degrees of lateralization, such as larger than −7 and smaller than +7 on a 14-point scale (Westerhausen et al., 2004), might have included right- and left-handers with different degrees of lateralization in the upper range (e.g., more lateralized left-handers in studies which detected larger callosal regions in right-handers).
Limitations and Implications for Future Studies
We revealed negative correlations between callosal thickness and the degree of handedness lateralization, and we observed increased callosal dimensions in less lateralized groups of right- and left-handers (i.e., regardless of the direction of handedness). These outcomes agree with our hypothesis that a weaker functional lateralization is associated with increased callosal dimensions. However, replication using a larger number of left-handers is clearly needed to boost statistical power. This will determine whether the established negative correlations truly exist regardless of the direction of handedness. Moreover, when comparing handedness groups, increasing the number of right- and left-handers will resolve whether the current study was only underpowered (and therefore did not reveal any significant differences between right- and left-handers), or whether the differences are rather negligible in general, if examined as a dichotomy between two handedness groups. Regardless, future studies of handedness will ideally determine both the direction and the degree of handedness lateralization, rather than analyzing individuals of merely opposite laterality (Corballis, 2009).
The current study was based on handedness information acquired via administering questionnaires only. As handedness is best predicted by combining “preference measures and several performance measures that tap into different elements of motor performance” (Brown et al., 2006), future studies might consider complementing self-reports of handedness with observational measures of hand preference or with actual measures of hand performance, such as indicated by pegboard or finger-tapping tasks. The current study generated a subsample of right- and left-handers with similar degrees of handedness lateralization (i.e., −0.6 and +0.6). Although closely matched, these two mean handedness scores represent rather moderate degrees of handedness. It would be interesting to find out if the observed regular pattern of alternating directional effects can be replicated in extreme right- and left-handers, classified as −1 and +1.
Last but not least, further research is necessary to establish whether links between callosal size and handedness are directly driven by callosal motor pathways, or whether laterality of other functions might contribute to the observed negative correlations. Alternatively, callosal thickness and functional lateralization of handedness might be completely devoid of any causal relationship with each other, but simply underlie the same developmental mechanisms (Habib et al., 1991). Future studies will expand this line of research by complementing indicators of callosal macro-structure with descriptors of callosal micro-structure, possibly based on callosal fiber tracking using diffusion tensor imaging (DTI) (Westerhausen et al., 2004). In addition, DTI and functional MRI may be combined to investigate the relationship between callosal organization and cortical activity across hemispheres (Putnam et al., 2008).
Supplementary Material
The colored section marks a region with evidence of interaction between degree and direction of handedness laterality. The posterior region of the corpus callosum is located on the left; the anterior region points to the right.
Acknowledgements
The study was supported by NHMRC of Australia Grant No. 973302, 179805, 157125, and by the National Institutes of Health through the NIH Roadmap for Medical Research, Grant U54 RR021813 entitled Center for Computational Biology (CCB). Nicolas Cherbuin is funded by NHMRC Research Fellowship No. 471501. Kaarin Anstey is funded by NHMRC Research Fellowship No. 366756. Additional support was provided by NIH grants P41 RR013642, M01 RR000865, R01 EB007813, R01 EB008281, and R01 EB008432, the Australian National Computational Infrastructure processing grant and by the Australian Academy of Science. The authors are grateful to Alen Zamanyan, Ivo Dinov, Anthony Jorm, Bryan Rodgers, Helen Christensen, Chantal Reglade-Meslin, Patricia Jacomb, Karen Maxwell, Andrew Janke, and the PATH interviewers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Since the EHI questionnaire does not reveal anything about the quality of left- and right-hand performance, it might be more precise to use the term “hand preference”. However, to be consistent with the existing literature, we will use the term “handedness” instead throughout the manuscript.
Witelson and Goldsmith also detected negative correlations when focusing on absolute values. Thus, their outcomes agree with findings of the current study. However, Witelson and Goldsmith concluded that there were too few individuals with handedness scores less than zero to justify treating magnitude and direction as two separate dimensions (Witelson and Goldsmith, 1991).
The asterisks denote the setup for the current study.
Reference List
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Annett M. Handedness in families. Ann.Hum.Genet. 1973;37:93–105. doi: 10.1111/j.1469-1809.1973.tb01817.x. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Dear K, Christensen H, Jorm AF. Biomarkers, health, lifestyle, and demographic variables as correlates of reaction time performance in early, middle, and late adulthood. Q.J.Exp.Psychol.A. 2005;58:5–21. doi: 10.1080/02724980443000232. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Mack HA, Christensen H, Li SC, Reglade-Meslin C, Maller J, Kumar R, Dear K, Easteal S, Sachdev P. Corpus callosum size, reaction time speed and variability in mild cognitive disorders and in a normative sample. Neuropsychologia. 2007;45:1911–1920. doi: 10.1016/j.neuropsychologia.2006.11.020. [DOI] [PubMed] [Google Scholar]
- Brown SG, Roy EA, Rohr LE, Bryden PJ. Using hand performance measures to predict handedness. Laterality. 2006;11:1–14. doi: 10.1080/1357650054200000440. [DOI] [PubMed] [Google Scholar]
- Chan TF, Vese LA. Active contours without edges. IEEE Trans.Image Process. 2001;10:266–277. doi: 10.1109/83.902291. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Lufkin RB, Zaidel E. Corpus callosum morphometry and dichotic listening performance: individual differences in functional interhemispheric inhibition? Neuropsychologia. 1993;31:547–557. doi: 10.1016/0028-3932(93)90051-z. [DOI] [PubMed] [Google Scholar]
- Clarke JM, Zaidel E. Anatomical-behavioral relationships: corpus callosum morphometry and hemispheric specialization. Behav.Brain Res. 1994;64:185–202. doi: 10.1016/0166-4328(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J.Comput.Assist.Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Corballis MC. The evolution and genetics of cerebral asymmetry. Philos.Trans.R.Soc.Lond B Biol.Sci. 2009;364:867–879. doi: 10.1098/rstb.2008.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg VH, Kertesz A, Cowell PE. A factor analysis of the human's corpus callosum. Brain Res. 1991;548:126–132. doi: 10.1016/0006-8993(91)91113-f. [DOI] [PubMed] [Google Scholar]
- Driesen NR, Raz N. The influence of sex, age, and handedness on corpus callosal morphology: A meta-analysis. Psychobiology. 1995;23:240–247. [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der KA, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Habib M, Gayraud D, Oliva A, Regis J, Salamon G, Khalil R. Effects of handedness and sex on the morphology of the corpus callosum: a study with brain magnetic resonance imaging. Brain Cogn. 1991;16:41–61. doi: 10.1016/0278-2626(91)90084-l. [DOI] [PubMed] [Google Scholar]
- Hines M, Chiu L, McAdams LA, Bentler PM, Lipcamon J. Cognition and the corpus callosum: verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav.Neurosci. 1992;106:3–14. doi: 10.1037//0735-7044.106.1.3. [DOI] [PubMed] [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited--comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Hopper KD, Patel S, Cann TS, Wilcox T, Schaeffer JM. The relationship of age, gender, handedness, and sidedness to the size of the corpus callosum. Acad.Radiol. 1994;1:243–248. doi: 10.1016/s1076-6332(05)80723-8. [DOI] [PubMed] [Google Scholar]
- Jancke L, Staiger JF, Schlaug G, Huang Y, Steinmetz H. The relationship between corpus callosum size and forebrain volume. Cereb.Cortex. 1997;7:48–56. doi: 10.1093/cercor/7.1.48. [DOI] [PubMed] [Google Scholar]
- Josse G, Seghier ML, Kherif F, Price CJ. Explaining function with anatomy: language lateralization and corpus callosum size. J.Neurosci. 2008;28:14132–14139. doi: 10.1523/JNEUROSCI.4383-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of Intelligence: Converging Neuroimaging Evidence. 2007 doi: 10.1017/S0140525X07001185. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Polk M, Howell J, Black SE. Cerebral dominance, sex, and callosal size in MRI. Neurology. 1987;37:1385–1388. doi: 10.1212/wnl.37.8.1385. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–2518. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Bilder RM, Thompson PM, Szeszko PR, Hamilton L, Toga AW. Positive correlations between corpus callosum thickness and intelligence. Neuroimage. 2007;37:1457–1464. doi: 10.1016/j.neuroimage.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb.Cortex. 2006a;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Toga AW. Gender effects on callosal thickness in scaled and unscaled space. Neuroreport. 2006b;17:1103–1106. doi: 10.1097/01.wnr.0000227987.77304.cc. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb.Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Hampson E, Lee DH. Brain. Pt 12. Vol. 121. 1998. Morphology of the planum temporale and corpus callosum in left handers with evidence of left and right hemisphere speech representation; pp. 2369–2379. [DOI] [PubMed] [Google Scholar]
- Morton BE, Rafto SE. Corpus callosum size is linked to dichotic deafness and hemisphericity, not sex or handedness. Brain Cogn. 2006;62:1–8. doi: 10.1016/j.bandc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- O'Kusky J, Strauss E, Kosaka B, Wada J, Li D, Druhan M, Petrie J. The corpus callosum is larger with right-hemisphere cerebral speech dominance. Ann.Neurol. 1988;24:379–383. doi: 10.1002/ana.410240305. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim JJ, Lee SK, Seok JH, Chun J, Kim DI, Lee JD. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum.Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss UW, Meisenzahl EM, Frodl T, Zetzsche T, Holder J, Leinsinger G, Hegerl U, Hahn K, Moller HJ. Handedness and corpus callosum morphology. Psychiatry Res. 2002;116:33–42. doi: 10.1016/s0925-4927(02)00064-1. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Putnam MC, Wig GS, Grafton ST, Kelley WM, Gazzaniga MS. Structural organization of the corpus callosum predicts the extent and impact of cortical activity in the nondominant hemisphere. J.Neurosci. 2008;28:2912–2918. doi: 10.1523/JNEUROSCI.2295-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond M, Pontier D, Dufour AB, Moller AP. Frequency-dependent maintenance of left handedness in humans. Proc.Biol.Sci. 1996;263:1627–1633. doi: 10.1098/rspb.1996.0238. [DOI] [PubMed] [Google Scholar]
- Rex DE, Ma JQ, Toga AW. The LONI Pipeline Processing Environment. Neuroimage. 2003;19:1033–1048. doi: 10.1016/s1053-8119(03)00185-x. [DOI] [PubMed] [Google Scholar]
- Schlaug G, Jancke L, Huang Y, Staiger JF, Steinmetz H. Increased corpus callosum size in musicians. Neuropsychologia. 1995;33:1047–1055. doi: 10.1016/0028-3932(95)00045-5. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans.Med.Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Soros P, Knecht S, Imai T, Gurtler S, Lutkenhoner B, Ringelstein EB, Henningsen H. Cortical asymmetries of the human somatosensory hand representation in right- and left-handers. Neurosci.Lett. 1999;271:89–92. doi: 10.1016/s0304-3940(99)00528-5. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Jancke L, Kleinschmidt A, Schlaug G, Volkmann J, Huang Y. Sex but no hand difference in the isthmus of the corpus callosum. Neurology. 1992;42:749–752. doi: 10.1212/wnl.42.4.749. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Staiger JF, Schlaug G, Huang Y, Jancke L. Corpus callosum and brain volume in women and men. Neuroreport. 1995;6:1002–1004. doi: 10.1097/00001756-199505090-00013. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW. Three-dimensional statistical analysis of sulcal variability in the human brain. J.Neurosci. 1996a;16:4261–4274. doi: 10.1523/JNEUROSCI.16-13-04261.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996b;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, Scapin M, Di PM, Le NP, Fadda L, Musicco M, Caltagirone C, Collins DL. Gross anatomy of the corpus callosum in Alzheimer's disease: regions of degeneration and their neuropsychological correlates. Dement.Geriatr.Cogn Disord. 2007;23:96–103. doi: 10.1159/000097371. [DOI] [PubMed] [Google Scholar]
- Tuncer MC, Hatipoglu ES, Ozates M. Sexual dimorphism and handedness in the human corpus callosum based on magnetic resonance imaging. Surg.Radiol.Anat. 2005;27:254–259. doi: 10.1007/s00276-004-0308-1. [DOI] [PubMed] [Google Scholar]
- Wen W, Sachdev PS, Li JJ, Chen X, Anstey KJ. White matter hyperintensities in the forties: their prevalence and topography in an epidemiological sample aged 44-48. Hum.Brain Mapp. 2009;30:1155–1167. doi: 10.1002/hbm.20586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos SS, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res.Cogn Brain Res. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Witelson SF. The brain connection: the corpus callosum is larger in left-handers. Science. 1985;229:665–668. doi: 10.1126/science.4023705. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112(Pt 3):799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Goldsmith CH. The relationship of hand preference to anatomy of the corpus callosum in men. Brain Res. 1991;545:175–182. doi: 10.1016/0006-8993(91)91284-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The colored section marks a region with evidence of interaction between degree and direction of handedness laterality. The posterior region of the corpus callosum is located on the left; the anterior region points to the right.