Abstract
Objective
To evaluate the effects of surgical weight loss on hepatic lipid peroxidation levels and cytochrome P-450 protein expression in patients with nonalcoholic fatty liver disease (NAFLD).
Summary Background Data
NAFLD and nonalcoholic steatohepatitis (NASH) affect hepatic cytochrome P-450 (CYP) protein expression and activity, and CYP2E1 may play a role in the pathogenesis of NAFLD and NASH through induction of oxidative stress and lipid peroxidation. NAFLD and NASH are associated with increased systemic lipid peroxidation levels and elevated hepatic CYP2E1 activity, but hepatic CYP3A4/5 activity is decreased.
Methods
Liver biopsies from 20 patients with NAFLD who underwent bariatric surgery were obtained intraoperatively and at 15 ± 7 months following surgery. Hepatic malondialdehyde (MDA) levels (a marker of lipid peroxidation), CYP2E1 and CYP3A4/5 protein expression, and steatosis, as a percent of total area, were measured by immunohistochemistry followed by digital image quantitation.
Results
Following weight loss, as reflected by reduced BMI (54 ± 9 vs. 37 ± 9 kg/m2; P < 0.001), features of the metabolic syndrome, grade and stage of liver disease, and liver histology were all significantly improved (P < 0.01). Hepatic MDA staining (35 ± 18% vs. 23 ± 14%; P = 0.02), CYP2E1 protein content (68 ± 9% vs. 56 ± 11%; P < 0.001), and steatosis (17 ± 7% vs. 2 ± 3%; P < 0.001) were significantly reduced following weight loss. CYP3A4/5 protein content was unchanged (57 ± 13% vs. 55 ± 13%; P = 0.433). The reduction in lipid peroxidation was independently associated with changes in CYP2E1 protein expression after bariatric surgery (r = 0.477; P = 0.033).
Conclusion
Elevations in hepatic lipid peroxidation and CYP2E1 expression that are seen in NAFLD improve significantly with weight loss induced by bariatric surgery.
Nonalcoholic fatty liver disease (NAFLD) has become one of the most common liver diseases in developed countries. Fatty liver encompasses an entire spectrum of disease, from accumulation of lipid (simple steatosis) to the more progressive nonalcoholic steatohepatitis (NASH) associated with fibrosis, necrosis, inflammation, and ultimately cirrhosis. As obesity and metabolic syndrome are both strongly associated with NAFLD, a logical therapeutic avenue for the treatment of fatty liver is weight loss. Indeed, it has been demonstrated that weight loss following bariatric surgery results in significant improvement in features of NAFLD and metabolic syndrome as well as normalization of liver histology.1–5
Although surgical-induced weight loss can effectively improve liver histology in NAFLD, the pathogenesis of NAFLD and NASH is not well understood. One hypothesis is that oxidative stress and inflammation, leading to increased lipid peroxidation, may play a central role in the development of NASH. It has been shown that levels of lipid peroxidation are increased in human NASH6–9 and suggested that the pro-inflammatory and pro-fibrotic aldehyde end products of lipid peroxidation (malondialdehyde [MDA] and 4-hydroxynonenal) can potentially account for all of the histologic features observed in NASH.10 For example, compared with liver tissue from normal individuals and patients with NAFLD, liver tissue from NASH patients demonstrates elevated markers of lipid peroxidation,7 and there is a significant correlation between hepatic lipid peroxidation levels and hepatic fibrosis in patients across the spectrum of NAFLD.9
One important source of lipid peroxidation and oxidative stress in the liver is cytochrome P-450 2E1 (CYP2E1), a microsomal enzyme involved in fatty acid hydroxylation that is capable of initiating the process of lipid peroxidation which may be important in the pathogenesis of NASH. Expression and activity of CYP2E1 is increased in human NAFLD and NASH11–14 and in animal models of NASH.15,16 Although the exact role of CYP2E1 in the pathogenesis of NAFLD and NASH is unclear, it is known that CYP2E1 can undergo futile cycling in the absence of substrates and is therefore capable of producing large amounts of reactive oxygen species, including superoxide anions, hydroxyl radicals, and hydrogen peroxides that can induce cellular injury and/or death.6 Mechanistic studies have demonstrated a direct link between increased CYP2E1 activity and hepatocyte injury that operates through a pathway involving oxidative stress. This is evidenced by both induction of CYP2E1 with pharmacological agents and overexpression of CYP2E1 producing heightened sensitivity and increased cell death in response to ethanol and fatty acids, and the ability of both CYP2E1 inhibitors and antioxidants to block apoptosis induced by these agents.17,18
Although alterations in lipid peroxidation levels and CYP2E1 expression and activity in human NAFLD and NASH have been characterized, little information is available regarding other important CYP enzymes in liver disease and drug metabolism, such as the CYP3A subfamily. CYP3A (including the CYP3A4, CYP3A5, fetal CYP3A7, and CYP3A43 isoforms) is the most abundant CYP in the human body and is responsible for the metabolism of more than 50% of drugs that are currently available.19,20 A representative list of medications that are metabolized by the CYP3A family and CYP2E1 is shown in Table 1. In general, chronic liver diseases such as cirrhosis are associated with decreased clearance of drugs, including several substrates of CYP3A.21 This is attributed to both decreased blood flow to hepatocytes as well as decreased functional capacity of hepatocytes. Although examination of CYP3A activity and expression in NAFLD and NASH is very limited, Weltman et al reported a decrease in CYP3A immunostaining in liver sections from patients with NASH compared with healthy controls12 and Leclercq et al have shown that in a nutritionally-induced animal model of hepatic steatosis, lipid accumulation was accompanied by a significant reduction in both CYP3A protein expression and activity.22 In humans, we recently demonstrated that steatosis is associated with a significant reduction in hepatic CYP3A activity in vitro, but that CYP3A protein expression is unchanged.23 Additional studies are clearly needed to further characterize hepatic CYP3A protein expression and activity in humans with fatty liver disease.
TABLE 1.
Medications Metabolized by CYP3A and CYP2E1
| CYP3A | CYP2E1 |
|---|---|
| Macrolide antibiotics | Anesthetics |
| Clarithromycin | Enflurane |
| Erythromycin | Halothane |
| Antiarrythmics | Isoflurane |
| Quinidine | Methoxyflurane |
| Benzodiazepines | Sevoflurane |
| Diazepam | Others |
| Midazolam | Acetaminophen |
| Immune modulators | Aniline |
| Cyclosporine | Benzene |
| Tacrolimus | Dacarbazine |
| HIV antivirals | Dapsone |
| Indinavir | Chlorzoxazone |
| Ritonavir | Ethanol |
| Saquinavir | Isoniazid |
| Antihistamines | N,N-dimethylformamide |
| Astemizole | Theophylline |
| Terfenadine | Toluene |
| Calcium channel blockers | Trimethadione |
| Felodipine | |
| Nifedipine | |
| Verapamil | |
| HMG CoA reductase inhibitors | |
| Atorvastatin | |
| Lovastatin | |
| Simvastatin | |
| Steroids | |
| Estradiol | |
| Hydrocortisone | |
| Progesterone | |
| Testosterone | |
| Other | |
| Caffeine | |
| Cocaine | |
| Codeine | |
| Dapsone | |
| Dexamethasone | |
| Dextromethorphan | |
| Fentanyl | |
| Gleevec | |
| Haloperidol | |
| Irinotecan | |
| Lidocaine | |
| Methadone | |
| Propranolol | |
| Quinine | |
| Risperidone | |
| Tamoxifen | |
| Taxol | |
| Terfenadine | |
| Vincristine |
In the current study, we measured levels of hepatic lipid peroxidation and CYP2E1 and CYP3A protein expression and distribution before and after bariatric surgery-induced weight loss. In this longitudinal study, we also considered patient clinical characteristics (including measures of fatty liver disease and metabolic syndrome), histologic findings, serum parameters, and patient comorbidities and demographics for correlative studies within our well-characterized group of patients.
METHODS
The Institutional Review Boards of the University of Pittsburgh and Indiana University School of Medicine reviewed this study protocol and approved the study as being consistent with the principles of the 1975 Declaration of Helsinki. All patients gave a written informed consent prior to their participation in the study.
Subjects
Patients investigated in this study were previously included in a paper that described in detail the liver histologic changes following bariatric surgery in morbidly obese individuals.1 Seventy patients planning to undergo weight loss surgery with a pre-existing diagnosis of NAFLD were prospectively identified through the University of Pittsburgh Bariatric Surgery Center Clinical Database, and findings from the first study included striking improvements in hepatic steatosis, inflammation, and fibrosis in concert with a substantial reduction in components of the metabolic syndrome.1 In this follow-up substudy, a cohort of 20 randomly chosen patients from the original study were included. All patients were diagnosed with NAFLD and had paired liver biopsies at the time of bariatric surgery and subsequently at varying intervals following the surgical procedure. Criteria used to establish the diagnosis of NAFLD included one or more of the following: gross features of fatty liver at ultrasonographic examination, intraoperative visual assessment of the liver, or histologic evaluation of liver specimens obtained at the time of operation. Exclusion criteria included a history of alcoholism (consumption of >20 g alcohol per day), evidence of autoimmune hepatitis, chronic hepatitis B or C infection, human immunodeficiency virus (HIV), genetic hemochromatosis, alpha 1 antitrypsin deficiency, Wilson disease, or the use of known hepatotoxic drugs. Other exclusion criteria included patients for whom no repeat biopsy was performed, or if the time interval between initial and repeat liver biopsies was less than 3 months.
Surgical weight-loss operations were carried out by the laparoscopic approach in all patients. The operations included Roux-en-Y gastric bypass, laparoscopic adjustable gastric band, and sleeve gastrectomy. The type of operation performed was based on patient preference and, in the rare case of physical factors such as dense intestinal adhesions or a very large liver resulting in an unacceptable risk for gastric bypass (in which case a sleeve gastrectomy would be performed), on surgeon decision during the course of surgery. Patient follow-up was scheduled for every 3 months with laboratory evaluation every 6 months until weight loss had stabilized, then at least once per year.
Serum Studies
Preoperative and follow up studies included complete blood counts, urinalysis, serum chemistries, nutritional indices, and pregnancy test (in women <50 years old). Liver function tests were analyzed on a Dade-Behring Dimension RXL Chemistry analyzer (Deerfield, IL). Serum parameters included alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Also measured were fasting plasma glucose and biochemical components of the lipid panel including total cholesterol, serum triglycerides, high density lipoprotein (HDL), and low density lipoprotein (LDL).
Anthropometric Measurements and Comorbidities/Metabolic Syndrome
All patients were evaluated extensively, including a health history, physical examination, and nutritional and psychiatric evaluation. The anthropometric measurements obtained for this study included height, weight, and body mass index (BMI).
All patients were screened for diabetes using the American Diabetes Association criteria, including fasting plasma glucose ≥126 mg/dL. Comorbidities associated with diabetes and obesity were recorded.
The presence of metabolic syndrome was determined based on the guidelines proposed by the Third Report of the National Cholesterol Education Program Adult Treatment Panel (ATP III).24 The ATP III clinical definition of the metabolic syndrome requires the presence of 3 or more of the following: (a) waist circumference >102 cm for men and >88 cm in women; (b) triglyceride level ≥150 mg/dL; (c) high density lipoprotein (HDL) level <40 mg/dL in men and <50 mg/dL in women; (d) systolic blood pressure ≥130 mm Hg or diastolic pressure ≥85 mm Hg; or (e) fasting plasma glucose ≥110 mg/dL.
Resolution of comorbidities was defined as normalization of blood pressure (hypertension), fasting plasma glucose (diabetes), and total/LDL/HDL cholesterol and triglyceride numbers (dyslipidemia) to desirable/optimal targets recommended by the ATP III.
Liver Biopsies
Liver biopsies were obtained intraoperatively (near the end of the surgical procedure) and at follow-up percutaneously with the use of the TruCut biopsy device (Bard Maxcore, Covington, GA). Intraoperatively, the TruCut device was advanced through the anterior abdominal wall in the region of the epigastrium, under laparoscopic vision, into the left lobe of the liver. Deployment of the device resulted in the acquisition of at least a 10-mm core of hepatic tissue. Repeat biopsies were immediately taken if specimens were unsatisfactory. At follow-up, liver biopsies were obtained either in the course of second operations such as laparoscopic exploration or laparoscopic cholecystectomy, or percutaneously, ultrasound-guided, in the gastrointestinal department. A 15-gauge biopsy needle (Microvasive, Natick, MA), which allows for a long core of at least 1 cm, was used for the ultrasound-guided technique.
Liver Histology and Immunohistochemistry
Liver biopsies were processed routinely in the clinical histology laboratory. Formalin-fixed, paraffin-embedded histologic sections were stained with hematoxylin and eosin (H&E) and Masson Trichrome stains for microscopic evaluation. The biopsies were evaluated by an experienced hepatopathologist who was blinded to patient characteristics and biopsy sequence. Three features of NAFLD/NASH were graded histologically according to the modified Brunt classification25: steatosis, inflammation, and fibrosis. Steatosis was graded on a scale from 0 to 4 according to the amount of fat that was present throughout the lobules: 0, none (<1%); 1, 1%–25%; 2, 26%–50%; 3, 51%–75%; and 4, >75%. Inflammation was graded on a scale of 0–3: 0, none; 1, mild (scattered lymphocytes or small clusters within portal tracts and lobules); 2, moderate (the same as grade 1 but with increased portal and lobular inflammation with lobular macrophages and/or neutrophils); and 3, severe (the same as grade 2 but with more intense inflammation, including several collections of inflammatory cells in the lobules, concentrated around zone 3). Fibrosis was staged on a scale of 0 – 4: 0, none; 1, centrilobular pericellular fibrosis; 2, periportal and pericellular fibrosis; 3, bridging fibrosis; and 4, cirrhosis. Liver disease grade (scored from 0–3 based on the amount of inflammation) and liver disease stage (scored from 0 – 4 based on the amount of fibrosis) were also determined by the hepatopathologist.1
Immunohistochemistry was performed on 4-μm thick sections cut from formalin-fixed paraffin-embedded tissue. Briefly, the sections were deparaffinized and heat-induced antigen retrieval was carried out with EDTA in a pressure cooker. Endogenous peroxidase was quenched by incubating with hydrogen peroxidase. Sections were incubated with anti-MDA antibody (Abcam, Inc, Cambridge, MA), anti-CYP2E1 antibody (LifeSpan Biosciences, Seattle, WA), or anti-CYP3A4/5 antibody (Gentest, Woburn, MA) and subsequently with a secondary antibody (EnVision+ from DAKO, Carpintenia, CA). The reaction was developed using streptavidin labeled with horse radish peroxidase and DAB as the chromogen. Immunohistochemical staining and steatosis was digitally quantitated and expressed as a percent of total liver biopsy area using SPSS Sigma Scan Pro 5.0 software (SPSS Inc, Chicago, IL).
Statistical Analysis
Basic descriptive statistics, including means, standard deviations (SD), ranges, and percentages were used to characterize the study patients. Because the data lacked a normal distribution, the Wilcoxon Signed Rank Test was used to compare patients before and after bariatric surgery. Spearman rank correlations were used to detect the associations between lipid peroxidation levels, CYP2E1 and CYP3A4/5 protein content, and steatosis and patient clinical characteristics and comorbidities, histologic findings, and serum parameters. Wherever appropriate, stepwise regression analysis was performed to take into account the linear effect of several independent variables predicting the dependent variables (lipid peroxidation levels and CYP2E1 protein content). Statistical analyses were performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, IL). A P value < 0.05 was considered statistically significant.
RESULTS
Patient Demographics and Liver Histology
The patient demographics, comorbidities, and results of the serum studies and liver histology scoring are shown in Table 2. There were significant reductions in BMI, fasting glucose, lipids, and liver enzyme levels, as well as improvements in comorbidities and liver histology following weight loss. Although most parameters were significantly improved after surgery, there was only a trend for reduction of LDL cholesterol and elevation of HDL cholesterol following weight loss.
TABLE 2.
Characteristics of Patients Studied Before and After Bariatric Surgery-Induced Weight Loss (n = 20)
| Preoperative | Postoperative | P | |
|---|---|---|---|
| Age (yr) | 43 ± 9 | NA | |
| Females/males | 12/8 | NA | |
| Time between biopsies (mo) | 15 ± 7 | NA | |
| BMI (kg/m2) | 54 ± 9 | 36 ± 9 | <0.001 |
| Fasting glucose (mg/dL) | 126 ± 34 | 97 ± 24 | 0.001 |
| AST (IU/L) | 30 ± 13 | 24 ± 9 | 0.059 |
| ALT (IU/L) | 43 ± 20 | 34 ± 12 | 0.044 |
| Lipids* | |||
| Total cholesterol (mg/dL) | 206 ± 52 | 178 ± 49 | 0.02 |
| Triglycerides (mg/dL) | 195 ± 92 | 117 ± 68 | <0.001 |
| HDL cholesterol (mg/dL) | 41 ± 14 | 44 ± 14 | 0.183 |
| LDL cholesterol (mg/dL) | 130 ± 39 | 116 ± 40 | 0.078 |
| Metabolic syndrome (%) | 12 (60) | 0 (0) | 0.001 |
| Hypertension (%) | 12 (60) | 2 (10) | 0.002 |
| Diabetes mellitus (%) | 7 (35) | 1 (5) | 0.014 |
| Insulin use (%) | 2 (10) | 1 (5) | 0.317 |
| Dyslipidemia (%) | 18 (90) | 5 (25) | <0.001 |
| Liver histology scores | |||
| Steatosis | 2.6 ± 0.7 | 0.7 ± 0.8 | <0.001 |
| Inflammation | 1.7 ± 0.7 | 0.9 ± 0.5 | 0.001 |
| Fibrosis | 1.6 ± 1.0 | 1.3 ± 1.0 | 0.035 |
| Disease grade | 2.2 ± 0.7 | 0.9 ± 0.4 | <0.001 |
| Disease stage | 1.7 ± 1.0 | 1.3 ± 1.0 | 0.011 |
Values are expressed as mean ± SD or n (%).
Excludes 2 patients missing postoperative lipid measures (n = 18).
In addition to the hepatopathologist’s scoring of hepatic lipid content, liver steatosis was also quantitated using H&E staining followed by digital quantification. After weight loss, there was a significant reduction in hepatic steatosis measured both by the hepatopathologist (fat amount score 2.6 ± 0.7 vs. 0.7 ± 0.8; P < 0.001) and by digital quantification (17 ± 7% vs. 2 ± 3%; P < 0.001). Further, there was a strong correlation between these 2 independent measures of hepatic lipid content, both before surgery (r = 0.677; P = 0.001) and when quantitating the reduction in steatosis following weight loss (r = 0.560; P = 0.010).
Immunohistochemical Analyses
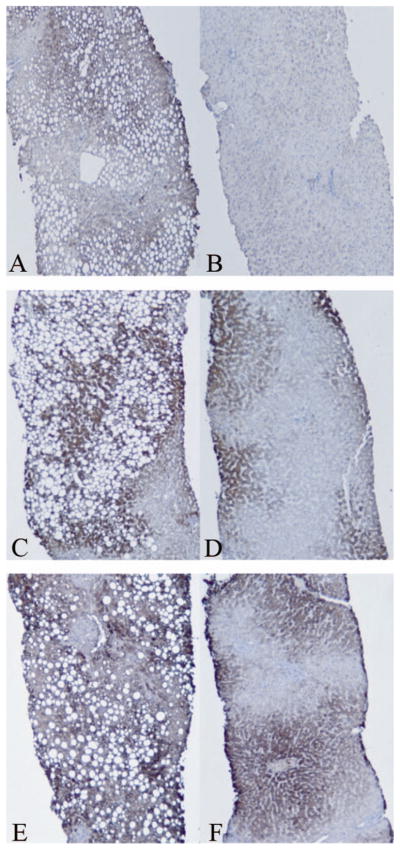
Immunohistochemistry and digital image quantification was used to measure hepatic lipid peroxidation and protein content of CYP2E1 and CYP3A4/5 before and after bariatric surgery (Table 3), and a set of representative images before and after surgery are shown in Figure 1. Lipid peroxidation, as measured by MDA staining, was reduced 34% after surgery. Steatosis and NAFLD are associated with elevated levels of lipid peroxidation (Fig. 1; Panel A) and this is significantly reduced following weight loss (Panel B). CYP2E1 protein expression was significantly reduced by 17% following weight loss. Prior to surgery (Fig. 1; Panel C), there is extensive steatosis surrounded by intense CYP2E1 staining. Following surgery (Fig. 1; Panel D), liver CYP2E1 protein expression is significantly decreased. Weight loss did not significantly affect CYP3A4/5 expression. Examination of CYP3A4/5 protein distribution showed that CYP3A4/5 expression is not closely associated with lipid droplets before weight loss (Fig. 1; Panel E) and has a concentrated, zonal expression pattern following normalization of liver histology (Panel F).
TABLE 3.
Immunohistochemical Assessment of Liver Biopsies Before and After Bariatric Surgery-Induced Weight Loss (n = 20)
| Preoperative | Postoperative | P | |
|---|---|---|---|
| Malondialdehyde (MDA) content | 34.9% ± 18.3% | 22.9% ± 13.8% | 0.015 |
| CYP2E1 protein content | 68.0% ± 9.1% | 56.3% ± 11.1% | <0.001 |
| CYP3A4/5 protein content | 57.1% ± 12.7% | 55.1% ± 12.9% | 0.433 |
| Steatosis | 16.6% ± 6.9% | 2.5% ± 3.2% | <0.001 |
Values are expressed as mean ± SD and as a percent of total liver biopsy area.
FIGURE 1.
Immunohistochemical staining of liver biopsies before and after bariatric surgery. Lipid peroxidation levels (measured by malondialdehyde [MDA] staining) are greater prior to bariatric surgery (Panel A) and are significantly reduced following weight loss (Panel B). Prior to surgery (Panel C), there is extensive steatosis surrounded by intense CYP2E1 staining. Following surgery and weight loss (Panel D), CYP2E1 protein expression is significantly decreased. CYP3A4/5 protein content is unchanged following bariatric surgery (compare Panels E and F), despite the improvements in liver histology and steatosis after weight loss.
Correlative Studies
Univariate analysis revealed a significant correlation between baseline MDA staining and hepatic steatosis (r = 0.533; P = 0.011), presence of dyslipidemia (r = 0.491; P = 0.028), grade of inflammation (r = 0.453; P = 0.045), grade of fibrosis (r = 0.519; P = 0.019), stage of liver disease (r = 0.508; P = 0.022), total cholesterol level (r = −0.616; P = 0.006), and triglycerides (r = −0.548; P = 0.019). Upon stepwise regression, grade of inflammation (r = 0.448; P = 0.014), grade of fibrosis (r = 0.492; P = 0.008), and total cholesterol levels (r = −0.374; P = 0.036) were identified as significant independent predictors of hepatic lipid peroxidation levels before surgery. The change in MDA staining after weight loss was correlated with the change in hepatic steatosis (r = 0.454; P = 0.044) and the change in CYP2E1 protein expression (r = 0.475; P = 0.034). As depicted in Figure 2, stepwise analysis showed that only the change in CYP2E1 protein content was independently associated with changes in lipid peroxidation levels after surgery (r = 0.477; P = 0.033).
FIGURE 2.
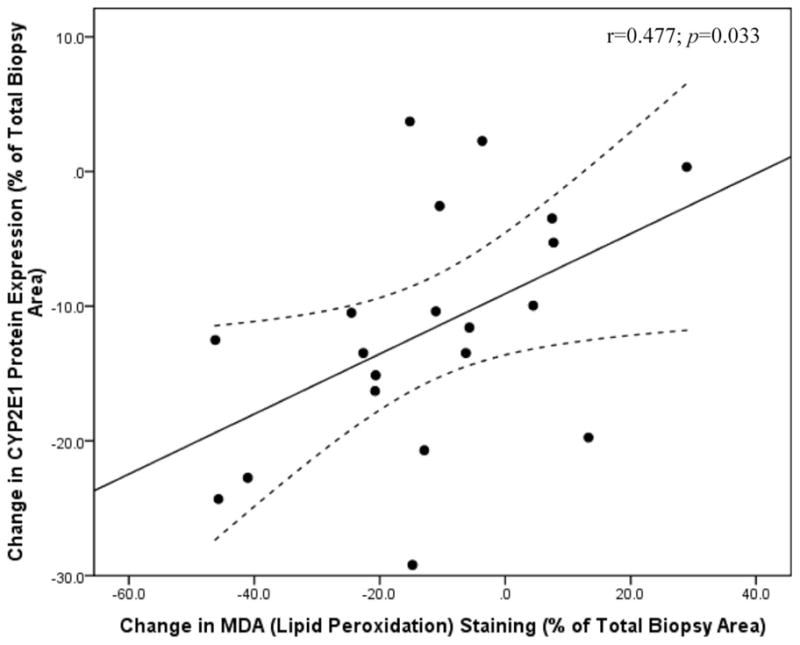
Changes in hepatic lipid peroxidation levels following weight loss are independently associated with changes in CYP2E1 protein expression (r = 0.477; P = 0.033). Lipid peroxidation levels (measured by malondialdehyde [MDA] staining) and CYP2E1 protein content was measured in liver biopsies taken before and after weight loss by immunohistochemistry followed by digital image quantification and is represented as the percent (%) of the total liver biopsy area that stained positive for MDA or CYP2E1.
Univariate analysis showed a significant correlation between CYP2E1 protein expression and CYP3A4/5 protein content before surgery (r = 0.564; P = 0.010), age (r = −0.747; P < 0.001), and ALT levels before surgery (r = 0.515; P = 0.020). Upon stepwise regression analysis, baseline CYP3A4/5 protein expression (r = 0.501; P = 0.005) and age (r = −0.571; P = 0.002) were identified as significant independent predictors of hepatic CYP2E1 protein content before weight loss. For the change in hepatic CYP2E1 protein expression following surgery and weight loss, univariate analysis revealed a significant association with the change in MDA staining (r = 0.475; P = 0.034) and resolution of dyslipidemia (r = 0.634; P = 0.005). As shown in Figure 3, stepwise analysis identified only resolution of dyslipidemia as an independent predictor of changes in CYP2E1 protein expression following weight loss (r = 0.572; P = 0.013).
FIGURE 3.
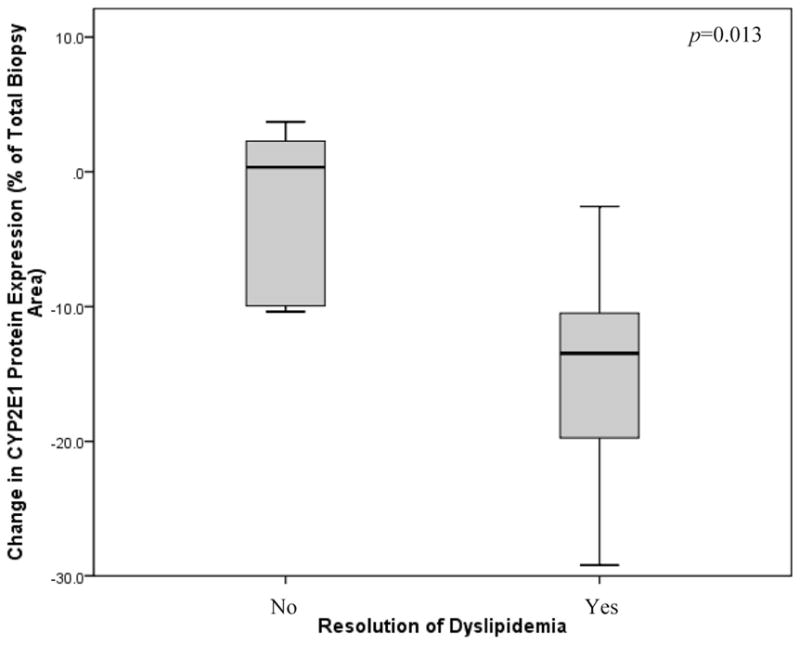
Changes in hepatic CYP2E1 protein expression following weight loss are independently associated with resolution of dyslipidemia (P = 0.013). CYP2E1 protein content was measured in liver biopsies by immunohistochemistry followed by digital image quantification and is represented as the percent (%) of the total liver biopsy area that stained positive for CYP2E1. Of the 20 subjects who participated in the study, 18 had dyslipidemia prior to bariatric surgery. After weight loss, resolution of dyslipidemia was observed in 13 patients (72%) but remained in 5 subjects (28%).
For baseline CYP3A4/5 protein content, univariate analysis revealed a significant relationship with only CYP2E1 expression before surgery (r = 0.564; P = 0.010), as shown in Figure 4.
FIGURE 4.
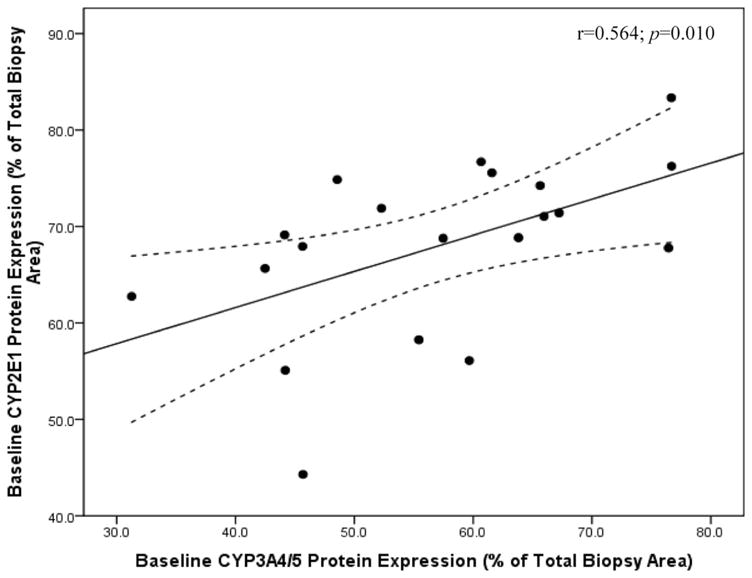
Baseline hepatic CYP3A4/5 protein expression is independently associated with hepatic CYP2E1 protein content prior to bariatric surgery and weight loss (r = 0.564; P = 0.010). Hepatic CYP protein content was measured by immunohistochemistry followed by digital image quantitation of protein expression in 20 subjects before and after bariatric surgery-induced weight loss. CYP protein expression is represented as the percent (%) of the total liver biopsy area that stained positive for the CYP protein of interest.
DISCUSSION
Bariatric surgery has proven to be the most effective tool for substantial and sustained weight loss in response to the current obesity epidemic.26 According to the American Society for Metabolic and Bariatric Surgery, the number of bariatric surgery procedures is rising exponentially from an estimated 16,000 in the early 1990s to an estimated 205,000 procedures in 2007, and this number continues to increase. It is imperative that the effects of this surgery and the resulting weight loss are adequately investigated. The goal of this study was to investigate, in a well-characterized group of patients with NAFLD, the changes in hepatic lipid peroxidation and CYP2E1 and CYP3A4/5 protein expression following bariatric surgery and weight loss. Using immunohistochemical techniques, we were able to observe the levels and protein expression patterns of both CYP2E1 and CYP3A4/5 in liver tissue before and after weight loss and explore associations with several clinical characteristics (including measures of fatty liver disease and metabolic syndrome), histologic findings of liver biopsies, serum parameters, and patient comorbidities and demographics. Overall, we found that hepatic lipid peroxidation levels, CYP2E1 protein expression, and steatosis were significantly reduced following weight loss, but CYP3A4/5 protein content was unchanged.
Not surprisingly, liver histology was significantly improved following weight loss and there were substantial improvements in hepatic steatosis. These findings are in agreement with several previous studies demonstrating that weight loss following bariatric surgery is associated with a reduced prevalence and severity of liver disease.1–5 In addition to the conventional method of quantifying steatosis through grading by a hepatopathologist, in this study we developed a novel technique to quantitate hepatic steatosis. Liver biopsies from patients were H&E stained and then subjected to digital image quantification. Using this strategy, we were able to quantitate the percent of the total liver area that contained lipid droplets (steatosis). A diagnosis of NAFLD is made when the liver is found to contain ≥10% fat, while a normal liver should not contain more than 5% fat. Prior to surgery, our NAFLD cohort had an average of 17% steatosis that was reduced by 85% following surgery, to 2%. Furthermore, the strong correlation between our novel measure of hepatic lipid content and the hepatopathologist’s grading of liver steatosis, both before surgery and in quantitating the reduction of steatosis following weight loss, reinforces the validity of our method.
The reduction in MDA staining after weight loss was independently associated with changes in CYP2E1 protein expression. This relationship is not surprising considering the central role that CYP2E1 plays in induction of lipid peroxidation in the liver.16 This association provides additional support for the hypothesis that elevated hepatic CYP2E1 expression and activity may play a central role in the pathogenesis of NAFLD and NASH through induction of oxidative stress. We also observed a significant reduction in hepatic CYP2E1 protein content following weight loss, confirming previous in vivo findings by Emery et al.14 Our results are also in agreement with previous animal and human studies demonstrating that NAFLD, NASH, type 2 diabetes, and obesity are all independently associated with elevations in hepatic CYP2E1 expression and activity.11–14,27–29 The distribution of hepatic CYP2E1 protein in normal liver is confined mainly to acinar zone 3 and induction of this enzyme with alcohol increases CYP2E1 content also in zones 1 and 2.30,31 Our results demonstrate a similar pattern, with elevated CYP2E1 immunostaining before weight loss throughout all acinar zones and being highly associated with the distribution of lipid droplets. Following weight loss and a reduction in lipid peroxidation levels and hepatic steatosis, and as NAFLD and liver histology improved, CYP2E1 immunostaining was significantly reduced, but was not completely confined to zone 3 as would be expected in healthy liver.
The reduction in hepatic CYP2E1 protein content following weight loss was independently associated with resolution of dyslipidemia. Interestingly, this relationship was not predicted by changes in any of the individual components of the lipid panel examined, likely due to our small sample size. Based on the distribution of CYP2E1 prior to surgery, in direct proximity with lipid droplets, we expected that changes in CYP2E1 protein content after surgery would be associated with changes in steatosis. Interestingly, we did not observe such an association, but the significant reduction in lipid peroxidation levels was independently associated with the decreased hepatic CYP2E1 protein content observed after surgery.
Covariate analysis revealed a significant relationship between hepatic CYP2E1 and CYP3A4/5 protein expression before weight loss. To our knowledge, this is the first time that a significant positive relationship between CYP2E1 and CYP3A protein content has been identified in human liver samples. Previous study of the interindividual variation in several cytochrome P450 forms in human liver microsomes revealed that CYP3A is the only enzyme whose content was correlated with total CYP450 content,32 suggesting that this is not the result of a nonspecific up-regulation of all hepatic cytochrome enzymes. There is also some evidence that corresponding changes in CYP2E1 and CYP3A may occur. For example, it is known that ethanol consumption can induce not only CYP2E1, but also CYP3A in primary cultures of human hepatocytes33 and in the intestine.34 Furthermore, George et al have shown that there is an age-related decline in the overall hepatic cytochrome P450 content, with a specific reduction in CYP2E1 and CYP3A by 5% and 8% per decade of life, respectively.35 However, other studies dispute this reduction in overall cytochrome P450 content with age36 and the age-related decline in CYP3A activity.37 In our baseline (cross-sectional) study, there was a significant negative relationship between baseline hepatic CYP2E1 content and increasing age, but there was no effect of age on hepatic CYP3A4/5 protein expression prior to surgery.
We have previously reported that hepatic steatosis is associated with a decrease in vitro CYP3A activity, but not CYP3A protein content, as tested using human liver microsomes.23 In the current study, we confirmed these findings and observed no change in CYP3A expression after weight loss, when hepatic steatosis was significantly reduced. A reduction in CYP3A protein activity, but not protein content, suggests that a post-translational mechanism may be responsible for this change. Alcohol-mediated regulation of CYP2E1 has been reported to function through a similar protein stabilization mechanism.38 A limitation of the current study was that we did not have enough liver tissue to perform cytochrome P450 activity assays. Complete clarification of the relationship between hepatic steatosis and CYP3A activity/function warrants further study.
Some additional limitations of our study require discussion. First, we were unable to gather medication and dietary restriction data for the subjects. For example, we observed a significant reduction in CYP2E1 following weight loss, despite the possibility that some subjects may have had greater CYP2E1 expression induced by alcohol consumption. This is also true for potential confounding dietary factors such as St. John’s Wort and grapefruit juice that may affect CYP3A expression levels; however, we did not observe any significant changes in CYP3A4/5 protein content so this was not a major concern for this particular study. It is also important to note that statin usage was likely in this patient cohort, especially prior to surgery, and we were unable to obtain this information. We would like to point out that almost all statins (except pravastatin) are metabolized by CYP3A, but, to our knowledge, no statin medications are significant inducers or inhibitors of CYP2E1 or CYP3A. Second, a common limitation for studies such as ours is the relatively small sample size (n = 20 for the overall analysis and n = 18 for the lipid profile analysis) and the inability to obtain enough liver tissue to perform CYP2E1 or CYP3A activity assays, mRNA expression measurements, or Western blot analyses to quantitate protein expression. This is due to the fact that obtaining liver biopsies from humans, especially “healthy” subjects such as at our postoperative timepoint, is difficult. Third, liver biopsies were taken after the patients had been subjected to systemic anesthetic gases and the liver had sustained local manipulation.1 The concern with this procedure is the potential for an inflammatory response in the liver that could have manifest itself in the immunohistochemical and/or histologic analyses. However, all biopsies were obtained in an identical manner and any acute physical manipulation of the liver should have had no effect on lipid peroxidation levels or hepatic cytochrome P450 expression. Furthermore, although certain anesthetic agents are substrates for CYP2E1, to our knowledge, none act as significant inhibitors or inducers of CYP2E1 or CYP3A. Fourth, it is important that we acknowledge the lack of blinding in the digital quantification of the immunohistochemical staining, as liver histology is strikingly different before and after bariatric surgery. To prevent this from being a confounding factor for our study results, we standardized staining thresholds for measuring protein expression to prevent bias. Finally, this follow-up substudy included 20 randomly chosen patients from the original cohort of 70 individuals.1 We acknowledge the possibility that these 20 patients are not representative of the entire bariatric cohort, but believe this is unlikely due to the fact that similar changes in histology and clinical measures were observed in this group of 20 patients and in the entire cohort of 70 patients.
In conclusion, hepatic lipid peroxidation levels are decreased following bariatric surgery and CYP2E1 but not CYP3A4/5 protein expression levels are significantly reduced. There is a strong relationship between the reductions in lipid peroxidation and CYP2E1 protein expression after bariatric surgery. These findings have important implications for both the reduction of liver disease and potential changes in drug metabolism and/or interactions following bariatric surgery-induced weight loss.
Acknowledgments
The authors thank Dr. Romil Saxena and Dr. Michael J. Morton for their assistance with the immunohistochemical techniques.
Supported in part by K24 DK072101 (to N. C.) and supported by Clinical Pharmacology Training Grant to Indiana University (T32 GM08425) (to L.N.B.).
Footnotes
L.N.B., C.J.T., R.S., R.V., P.S., M.R., A.K., N.C., and S.G.M. made equal contributions to conception/design of study, acquisition of data, and analysis and interpretation of data, drafting and revising the manuscript, and gave final approval for submission.
N.C. has financial consulting agreements with several pharmaceutical companies but none pose a potential conflict.
References
- 1.Mattar SG, Velcu LM, Rabinovitz M, et al. Surgically-induced weight loss significantly improves nonalcoholic fatty liver disease and the metabolic syndrome. Ann Surg. 2005;242:610–620. doi: 10.1097/01.sla.0000179652.07502.3f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverman EM, Sapala JA, Allepman HD. Regression of hepatic steatosis in morbidly obese persons after gastric bypass. Am J Clin Path. 1995;104:23–31. doi: 10.1093/ajcp/104.1.23. [DOI] [PubMed] [Google Scholar]
- 3.Frantzides CT, Carlson MA, Moore RE, et al. Effect of body mass index on non-alcoholic fatty liver disease in patients undergoing minimally invasive bariatric surgery. J Gastrointest Surg. 2004;8:849–855. doi: 10.1016/j.gassur.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 5.Kral JG, Thung SW, Biron S, et al. Effects of surgical treatment of the metabolic syndrome on liver fibrosis and cirrhosis. Surgery. 2004;135:48–58. doi: 10.1016/j.surg.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 7.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 8.Chalasani N, Deeg MA, Crabb DW. Systemic levels of lipid peroxidation and its metabolic and dietary correlates in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2004;99:1497–1502. doi: 10.1111/j.1572-0241.2004.30159.x. [DOI] [PubMed] [Google Scholar]
- 9.MacDonald GA, Bridle KR, Ward PJ, et al. Lipid peroxidation in hepatic steatosis in humans is associated with hepatic fibrosis and occurs predominantly in acinar zone 3. J Gastroenterol Hepatol. 2001;16:599–606. doi: 10.1046/j.1440-1746.2001.02445.x. [DOI] [PubMed] [Google Scholar]
- 10.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634–1636. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 11.Chalasani N, Gorski JC, Asghar MS, et al. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 12.Weltman MD, Farrell GC, Hall P, et al. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 13.Chtioui H, Semela D, Ledermann M, et al. Expression and activity of the cytochrome P450 2E1 in patients with nonalcoholic steatosis and steatohepatitis. Liver Int. 2007;27:764–771. doi: 10.1111/j.1478-3231.2007.01524.x. [DOI] [PubMed] [Google Scholar]
- 14.Emery MG, Fisher JM, Chien JY, et al. CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology. 2003;38:428–435. doi: 10.1053/jhep.2003.50342. [DOI] [PubMed] [Google Scholar]
- 15.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 16.Leclercq IA, Farrell GC, Field J, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu D, Cederbaum AI. Ethanol and arachadonic acid produce toxicity in hepatocytes from pyrazole-treated rats with high levels of CYP2E1. Mol Cell Biochem. 2000;204:157–167. doi: 10.1023/a:1007064706101. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Galleano M, Cederbaum AI. Cytotoxicity and apoptosis produced by arachidonic acid in Hep G2 cells overexpressing human cytochrome P4502E1. J Biol Chem. 1997;272:14532–14541. doi: 10.1074/jbc.272.23.14532. [DOI] [PubMed] [Google Scholar]
- 19.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 20.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Morgan DJ, McLean AJ. Clinical pharmacokinetic and pharmacodynamic considerations in patients with liver disease. An update. Clin Pharmacokinet. 1995;29:370–391. doi: 10.2165/00003088-199529050-00005. [DOI] [PubMed] [Google Scholar]
- 22.Leclercq I, Horsmans Y, Desager JP, et al. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. J Hepatol. 1998;28:410–416. doi: 10.1016/s0168-8278(98)80314-0. [DOI] [PubMed] [Google Scholar]
- 23.Kolwankar D, Vuppalanchi R, Ethell B, et al. Association between nonalcoholic hepatic steatosis and hepatic cytochrome P-450 3A activity. Clin Gastroenterol Hepatol. 2007;5:388–393. doi: 10.1016/j.cgh.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 25.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 26.Pories WJ, MacDonald KG, Jr, Morgan EJ, et al. Surgical treatment of obesity and its effect on diabetes: 10-y follow up. Am J Clin Nutr. 1992;55:582S–585S. doi: 10.1093/ajcn/55.2.582s. [DOI] [PubMed] [Google Scholar]
- 27.Favreau LV, Malchoff DM, Mole J, et al. Responses to insulin by two forms of rat hepatic microsomal cytochrome P-450 that undergo major (RLM6) and minor (RLM5b) elevations in diabetics. J Biol Chem. 1987;262:14319–14326. [PubMed] [Google Scholar]
- 28.Raucy JL, Lasker JM, Kraner JC, et al. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 29.Dong Z, Hong J, Ma Q, et al. Mechanism of induction of cytochrome P450ac (P450j) in chemically-induced and spontaneously diabetic rats. Arch Biochem Biophys. 1988;263:29–35. doi: 10.1016/0003-9861(88)90610-8. [DOI] [PubMed] [Google Scholar]
- 30.Tsutsumi M, Lasker JM, Shimizu M, et al. The intralobular distribution of ethanol-inducible P450IIE1 in rat and human liver. Hepatology. 1989;10:437–446. doi: 10.1002/hep.1840100407. [DOI] [PubMed] [Google Scholar]
- 31.Buhler R, Lindros KO, von Boguslawsky K, et al. Perivenous expression of ethanol-inducible cytochrome P450IIE1 in livers from alcoholics and chronically ethanol-fed rats. Alcohol Alcohol Suppl. 1991;1:311–315. [PubMed] [Google Scholar]
- 32.Snawder JE, Lipscomb JC. Interindividual variance of cytochrome P450 forms in human hepatic microsomes: correlation of individual forms with xenobiotic metabolism and implications in risk assessment. Regul Toxicol Pharmacol. 2000;32:200–209. doi: 10.1006/rtph.2000.1424. [DOI] [PubMed] [Google Scholar]
- 33.Kostrubsky VE, Strom SC, Wood SG, et al. Ethanol and isopentanol increase CYP3A and CYP2E in primary cultures of human hepatocytes. Arch Biochem Biophys. 1995;322:516–520. doi: 10.1006/abbi.1995.1495. [DOI] [PubMed] [Google Scholar]
- 34.Liangpunsakul S, Kolwankar D, Pinto A, et al. Activity of CYP2E1 and CYP3A enzymes in adults with moderate alcohol consumption: a comparison with nonalcoholics. Hepatology. 2005;41:1144–1150. doi: 10.1002/hep.20673. [DOI] [PubMed] [Google Scholar]
- 35.George J, Byth K, Farrell GC. Age but not gender selectively affects expression of individual cytochrome P450 proteins in human liver. Biochem Pharmacol. 1995;50:727–730. doi: 10.1016/0006-2952(95)00192-3. [DOI] [PubMed] [Google Scholar]
- 36.Schmucker DL, Woodhouse KW, Wang RK, et al. Effects of age and gender on in vitro properties of human liver microsomal monooxygenases. Clin Pharmacol Ther. 1990;48:365–374. doi: 10.1038/clpt.1990.164. [DOI] [PubMed] [Google Scholar]
- 37.Hunt CM, Westerkam SR, Stave M, et al. Human hepatic cytochrome P4503A activity in the elderly. Mech Ageing Dev. 1992;64:189–199. doi: 10.1016/0047-6374(92)90106-n. [DOI] [PubMed] [Google Scholar]
- 38.Raucy J, Carpenter SP. CYP2E1. In: Levy RH, Thummel KE, Trager WF, et al., editors. Metabolic Drug Interactions. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 95–114. [Google Scholar]