Abstract
Inhibition of N-methyl-D-aspartate (NMDA) receptors has been viewed as a primary cause of xenon anesthesia, yet the mechanism is unclear. Here, we investigated interactions between xenon and the ligand-binding domain (LBD) of a NMDA receptor and examined xenon-induced structural and dynamical changes that are relevant to functional changes of the NMDA receptor. Several comparative molecular dynamics simulations were performed on two X-ray structures representing the open- and closed-cleft LBD of the NMDA receptor. We identified plausible xenon action sites in the LBD, including those nearby agonist sites, in the hinge region, and at the interface between two subunits. The xenon binding energy varies from −5.3 to −0.7 kcal/mol. Xenon's effect on the NMDA receptor is conformation-dependent and is produced through both competitive and non-competitive mechanisms. Xenon can promote cleft opening in the absence of agonists and consequently stabilizes the closed channel. Xenon can also bind at the interface of two subunits, alter the inter-subunit interaction, and lead to a reduction of the distance between GT-links. This reduction corresponds to a rearrangement of the channel toward a direction of pore size decreasing, implying a closed or desensitized channel. In addition to these non-competitive actions, xenon was found to weaken the glutamate binding, which could lead to low agonist efficacy and appear as competitive inhibition.
Keywords: Xe, anesthesia mechanism, anesthetics, ligand-binding domain, competitive and non-competitive inhibition, open- and closed-cleft conformations
INTRODUCTION
Xenon, an inert gas, can produce general anesthesia. N-methyl-D-aspartate (NMDA) receptors have been recognized as major molecular targets in xenon anesthesia on the basis of potent xenon inhibition of these receptors.1,2 Despite the evidence of xenon action on NMDA receptors, the mechanism of action is still under debate. One speculation was that xenon disrupted normal function of the NMDA receptors by blocking their channels,3 but many experiments suggest that xenon does not act as a classical open-channel blocker at the NMDA receptor.4,5 A competitive mechanism of xenon action was also proposed based on the data of molecular modeling and electrophysiology measurements.6 Although the competition of xenon with glycine for the agonist binding site was considered as a major cause of inhibition on NMDA receptors, the possibility of non-competitive xenon inhibition was not excluded.6 A more comprehensive understanding on how xenon modulates NMDA receptors is desired.
Biological functions of NMDA receptors are far beyond being the molecular targets of xenon. They play critical roles in synaptic plasticity and memory function.7,8 Dysfunction of NMDA receptors has been linked to excitotoxity and neurological disorders, such as epilepsy, schizophrenia, Parkinson's and Huntington's diseases.9 NMDA receptors are heterotetrameric cation channels, mostly composed by NR1 and NR2 subunits. Each subunit has an extracellular amino-terminal domain (ATD) and ligand-binding domain (LBD), three transmembrane (TM1, TM2, and TM3) segments, a P loop, and an intracellular C-terminus (CTD). The segments S1 and S2 in LBD form a venus-flytrap structure and define the region for agonist recognition. In an intact NMDA receptor, S1 connects to TM1, while S2 links TM2 and TM3 on its two ends (see Fig. S1 in the Supporting Information). Simultaneous binding of neurotransmitters glycine and glutamate to the respective of NR1 and NR2 is required for activation of NMDA receptors. The S1S2 cleft is closed upon agonist binding and results in ion channel opening. The whole process can go to the opposite direction upon the binding of antagonists.10 At present there are only a limited number of high-resolution structures for certain parts of the receptor, such as ATD and LBD. The crystal structures of LBD and its complexes with an agonist or an antagonist provide an excellent base to reveal the underlying cause of xenon inhibition.
Several critical issues have been addressed in our study. Where does xenon interact with the LBD? How differently does xenon interact with the open- and closed-cleft LBDs? Does xenon compete with agonist binding? What mechanisms can account for non-competitive inhibition? To answer these questions, we performed multiple parallel MD simulations on the X-ray structures of the open- (PDB code: 1PBQ) and closed-cleft (PDB code: 2A5T) LBDs in the absence and presence of xenon. We found that xenon could weaken the agonist binding to some extent, but it imposes more profound effects via a non-competitive manner on the LBD in different S1S2 cleft conformations. In the open-cleft conformation, where the native agonists were absent, xenon enhanced the cleft opening in LBD and virtually stabilized the closed-channel conformation. In the closed-cleft conformation, xenon was found residing at the interface of two subunits. Xenon induced disturbance to the interplay between the adjacent subunits was able to inhibit the channel opening. Collectively, the study has offered many molecular details to formulate a mechanistic explanation for xenon inhibition on NMDA receptors.
METHODS
All MD simulations were performed using NAMD2.611 with CHARMM2712 force field parameters at Pittsburgh Supercomputer Center. The xenon parameter published by Verlet and Weis13 was used. Two X-ray structures of the NMDA receptor were chosen to represent the open-cleft (PDB codes: 1PBQ) and closed-cleft (PDB codes: 2A5T) conformations. 1PBQ contains two NR1 subunits (referred here as NR1A and NR1B);10 the antagonist DCKA was removed in the simulations. 2A5T is a dimer consisting of one glycine-bound NR1 subunit and one glutamate-bound NR2 subunit.14 The missing residues within the loop regions were patched using MODELLER15 In this study, the opened or closed system refers to the conformation of the S1S2 cleft rather than the ion channel. Initial xenon locations were decided based on the results from docking using Autodock4.016 and a previous study.6 Besides the LBD of the NMDA receptor, the open system had 18929 TIP3P water, 50 Na+ and 54 Cl−, and the close system had 18135 TIP3P water molecules along with 49 Na+ and 51 Cl−. Both open and closed systems had a final ion concentration of 200mM. Dimensions of the open- and closed-cleft systems were 116 × 79 × 68 and 82 × 78 × 94 Å, respectively. A total of four systems were prepared for MD simulations, including open and closed systems in the absence (control) and presence of xenon atoms. All the systems underwent energy minimization for 50000 time steps and equilibration with Cα restraint changing from 50 to 0 kcal/mol over 200 ps. Subsequently, 20-ns MD simulations were performed under NPT (1 atm and 303 K) for all four systems. Langevin dynamics was applied to maintain constant temperature. A cutoff of 10 Å was used for non-bonded interactions. Particle Mesh Ewald method was used for long-range electrostatic interactions. The time step for integration was 1fs. And the data were saved every 1 ps. Xenon binding energies were calculated using free energy perturbation (FEP) method17,18 implemented in NAMD2.6.11 The coordinates and velocities at the end of 20-ns simulations in the presence of xenon were used for the FEP calculations. For each binding site, xenon atom was gradually annihilated with λ decreased from 1 (fully interaction) to 0 (no interaction). The decrement step was 0.025 for λ in the ranges of 1 to 0.8 or 0.2 to 0, and 0.05 otherwise. There were 28 separate λ windows for each calculation. With a 2-fs time step, each window underwent 10-ps equilibration followed by 100-ps data collection. To avoid the end point catastrophes resulting from annihilation of xenon atom, a radius-shifting coefficient of 4.0 Å2 was used, and electrostatics scaled linearly with λ ranging from 1 to 0.5, while was completely turned off for λ less than 0.5. The same FEP calculation was performed on xenon atom in a water box. The calculated binding energies resulted from subtracting the FEP calculation in the protein from the one in water. A considerable artifact induced by the net charge on the glutamate ligand in FEP calculations prohibited the use of the same method for calculating ligand binding energies. Thus, the binding energies for two ligands (glycine and glutamate) were estimated using Autodock4.0.16
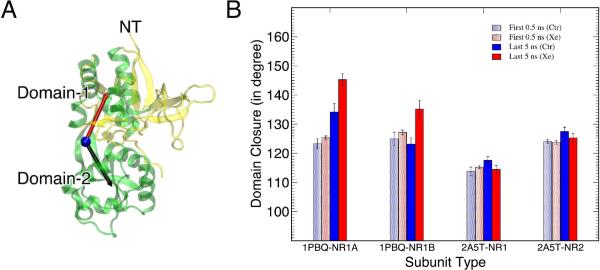
Domain-1 and Domain-2 (Fig. 4 A and Fig. S1) are named following the convention used in the literature.10 The domain closure was defined in Fig. 4 A by the angle between Domain-1 and Domain-2. Only the helical regions of Domain-1 and the whole Domain-2 were used for measurements of domain closure.
Figure 4.
(A) The structure of a ligand-binding domain (green) showing the definition of the domain closure. The blue sphere represents the hinge, the red and black vectors point to the center of mass of Domain 1 and Domain 2, respectively. The angle between the two vectors defines the domain closure. To prevent the bias from flexible loops, only the α helices of Domain 1 were included in the calculation of the center of mass. (B) Comparison of the domain closure in the open-cleft (1PBQ-NR1A & 1PBQ-NR1B) and closed-cleft (2A5T-NR1 & 2A5T-NR2) conformations in the absence (blue) or presence (red) of xenon at the first 0.5-ns (light color) and last 5-ns (dark color) MD simulations. Mean standard deviations were calculated using 50 or 500 frames from the first 0.5-ns or last 5-ns simulation, respectively.
The normalized water exchange rate surrounding a xenon atom, Rex, was calculated by
| (1) |
where T is the total time considered, A is the surface area of a 5-Å radius sphere, NA and NB are the total number of different water molecules over T and the average number of water molecules at each time frame within 5 Å of the xenon atom, respectively.
Root mean square fluctuation (RMSF) was used to measure the protein flexibility for the fast motions during the simulation. Gaussian network model (GNM)19,20 was used to evaluate the protein flexibility for the slow global dynamics, in which each residue was represented by the Cα atom and a 10-Å cutoff was chosen to build the contact matrix. Only the three slowest GNM modes for the structures after 20-ns MD simulations were included in the mean square fluctuation (MSF) analysis.
RESULTS AND DISCUSSION
Xenon binding in LBD of the NMDA receptor
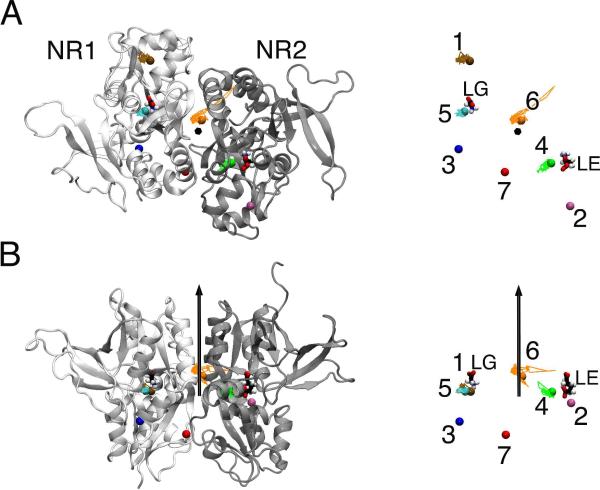
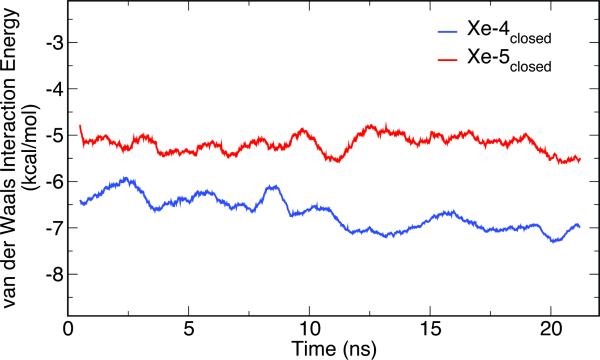
The closed-cleft system seemed have more stable xenon-binding sites. Over the course of 20-ns simulation, Xe-5open and Xe-6open in NR1B moved within the cleft between Domain-1 and Domain-2, but the rest four xenon atoms originally in the open-cleft LBD migrated into water. (see Fig. S2). In contrast, four out of seven xenon atoms stayed inside of the closed-cleft LBD of the NMDA receptor during the entire simulation. As shown in Fig. 1, Xe-4closed and Xe-5closed were nearby the glutamate agonist and glycine coagonist, respectively. Xe-1closed was within the NR1 subunit and Xe-6closed resided at the interface of NR1 and NR2. Their displacements over the 20-ns simulation were much smaller than those by Xe-5open and Xe-6open (see Fig. S3), suggesting more favorable xenon binding in the closed-cleft structure. Table 1 summarizes the xenon binding energies within the LBDs of the NMDA receptor at the end of the simulations. It is notable that Xe-4closed had much lower binding energy than Xe-5closed, though both barely moved away from their initial locations near the agonist sites. The volume from Connolly's surface was measured using the CASTp online server (http://sts.bioengr.uic.edu/castp/calculation.php).21 The difference in the cavities sizes, 346 Å3 for the Xe-4closed site versus 54 Å3 for the Xe-5closed, might have contributed to the difference in their binding energies. The optimal van der Waals interaction distances between xenon and other atoms range from ~ 3.5 to 4.2 Å. The cavities for Xe-4closed and Xe-5closed sites roughly correspond to a radius of 4.3 and 2.3 Å. The van der Waals interaction is therefore more favorable to Xe-4closed than Xe-5closed, as shown in Fig. 2. Besides the enthalpy difference, entropy effect can also contribute to their 4.65 kcal/mol binding energy difference. A large volume allows more translational degree of freedom. This contribution, however, can only account for ~ 1 kcal/mol gain, estimated by −RT ln(V1/V2). Another entropy contribution to higher binding affinity for Xe-4closed likely resulted from the increased side-chains flexibility of the surrounding residues in the presence of xenon. The trajectories of Xe-4closed annihilation in the FEP calculation indeed revealed that number of hydrogen bonds in the cavity was reduced considerably when the interaction of Xe-4closed with its surrounding was fully counted. Several snapshots displayed in Fig. 3 demonstrate the loss of hydrogen bonding in the presence of Xe-4closed. Conversely, Xe-5closed was unable to receive such an entropy gain to its binding energy, considering that the size of the cavity for Xe-5closed is about the same as the volume of a single xenon atom (~45 Å3). In other words, Xe-5closed has a less chance to modify the surrounding residues' flexibility, being trapped within a confined region. Clearly, entropy can play an important role in xenon binding in addition to the van der Waals interaction.
Figure 1.
Top (A) and side (B) views of xenon trajectories in the closed-cleft ligand-binding domain of the NMDA receptor (PDB code: 2A5T) over a 20-ns simulation. The black vector represents a pseudo two fold symmetric axis between the two subunits, NR1 (white) and NR2 (gray). The agonist glycine (LG) and glutamate (LE) are represented in stick. Initial xenon locations in the simulation are marked with spheres. Seven xenon atoms: Xe-1 in brown, flanked by helix-F and helix-G of NR1; Xe-2 in pink, flanked by helix-F and helix-G of NR2; Xe-3 in blue, flanked by helix-I and helix-K of NR1. Xe-4 (green) and Xe-5 (cyan) are next to the glutamate and glycine agonists, respectively. Xe-6 (orange) and Xe-7 (red) are located at the interface of NR1 and NR2. The xenon trajectories are shown in solid lines with the time step of 10 ps. For clarity, on the right, the xenon atoms and two agonists are labeled. The trajectories for Xe-2, Xe-3, and Xe-7 are not shown because they moved out of the protein during the simulation.
TABLE 1.
The calculated binding energies for xenon atoms stable in the closed- and open-cleft ligand-binding domain of NMDA receptors
| Protein Structure Model | Label | Calculated Binding Energy* (kcal/mol) |
|---|---|---|
| The open-cleft model (1PBQ) | Xe-5 | −2.24 |
| Xe-6 | 0.04 | |
|
|
||
| The closed-cleft model (2A5T) | Xe-1 | −2.62 |
| Xe-4 | −5.32 | |
| Xe-5 | −0.67 | |
| Xe-6 | −2.93 | |
The binding energies were calculated using FEP method. Xenon binding energy in water is 1.45 ± 0.04 Kcal/mol.
Figure 2.
The van der Waals interaction energies between Xe-4closed and Xe-5closed and their neighboring residues within 7 Å in the closed-cleft conformation of the NMDA receptor during the 20-ns simulation. The averaged van der Waals interaction energy computed for the last 5 ns is −7.05 (±0.68) kcal/mol for Xe-4closed and −5.28 (±1.08) kcal/mol for Xe-5closed, respectively.
Figure 3.
Snapshots from the FEP calculation when the interaction of Xe-4closed (green sphere) with its surrounding was completely annihilated (A); partially annihilated (B); and fully included (C). The more the xenon interactions are included, the less hydrogen bonding (black dash line) around the glutamate (colored by atom type in transparent surface). All the residues within 5 Å away from glutamate are shown in stick. In the FEP calculation, a xenon atom is annihilated gradually, which leads to the xenon atom migrating out its original site (A) due to the disappearing interaction with other atoms. To evaluate the change of the original site in this process, we picked the relative stable glutamate, ~ 3 Å to Xe-4closed in (C), as the reference of this site.
Xenon binding sites are largely amphiphilic. Water molecules could be found nearby all the xenon binding sites, such as that shown in Fig. 3. Two quantities were examined for quantifying local water effect on xenon binding. One was the tendency of water exchange at xenon binding sites by calculating total number of water molecules encountered within 5 Å of a xenon atom over the course of last 5-ns simulation. Another was the hydration level of individual xenon site by calculating the average number of water molecules within 5 Å of a xenon atom in each time frame during the simulation. The ratio of these two quantities normalized by time and the surface area of 5-Å sphere, as defined in Eq. 1, provided a water exchange rate. A lower exchange rate often corresponded to a more confined environment with less exposure to bulk water. It appeared that the calculated xenon binding energies had a good correlation with the normalized water exchange rates (see Fig. S4). More stable binding was found for xenon in an environment where water had less exchange.
In the crystal structure of the closed-cleft LBD, the number of water molecules next to the agonist glutamate and glycine was 8 and 2, respectively. A similar water distribution was observed in the simulation. Average number of water molecules around Xe-4closed (adjacent to the glutamate co-agonist) was also about 4 times as that around Xe-5closed (nearby the glycine agonist). In comparison to the glycine-bound NR1, the glutamate-bound NR2 subunit has a larger pocket with more water in the cleft. However, more water did not mean more water exchange. Indeed, most water molecules around the Xe-4closed site were trapped in the pocket.
Xenon enhanced the cleft opening in the open-cleft conformation
Change in the openness of the S1S2 cleft is a key element for the channel status of NMDA receptors.10 Antagonists, such as DCKA, prevent the S1S2 cleft from closing and lead to a subsequent closed channel. Conversely, binding to agonists, such as glycine and glutamate, closes the S1S2 cleft and induces the ion channel opening. To evaluate if xenon affects the S1S2 cleft openness, we compared the domain closure of the open- and closed-cleft systems in the absence or presence of xenon at the beginning and in the end of 20-ns MD simulations (Fig. 4). The domain closure was measured by the angle between two vectors: one vector pointing to the center of Domain-2, the other pointing toward the center of all the helices in Domain-1, and both originating from the hinge of S1S2 cleft, as illustrated in Fig. 4 A. Clearly, xenon had no significant effect on the domain closure of the NR1 and NR2 subunits in the closed-cleft conformation. Over 20-ns simulations in the absence or presence of xenon, both subunits remained their domain closure of ~115° and ~127°, respectively. Conversely, the open-cleft conformation of NR1A or NR1B experienced additional ~10° openness in the presence of xenon comparing to that in the control system for the last 5-ns simulations (Fig. 4 B).
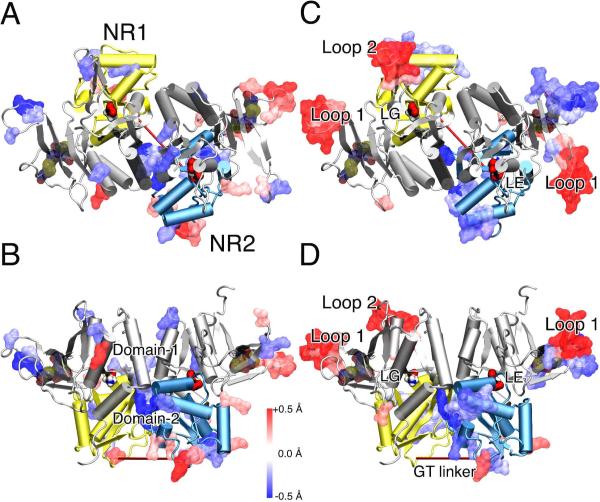
More profound xenon effect on the open-cleft conformation was seemingly unexpected, given that there was fewer number of xenon binding in the open-cleft conformation. However, the susceptibility to xenon perturbation may be determined by the intrinsic dynamical property of a protein conformation. RMSF of the Cα atoms were calculated for the last 5 ns simulations of both the open- and closed-cleft control systems, showing an overall larger RMSF in the open than the closed system. MSF of three lowest modes of GNM was also obtained for all the systems to assess the slower motion of the protein, revealing more dynamical regions in the open conformation than the closed one (see Fig. S5). Taken together, the open-cleft conformation is more flexible and its intrinsic flexibility has made the open-cleft conformation more susceptible to xenon perturbation. In the closed-cleft conformation, residues in the flexible regions often experienced more profound motional changes in the presence of xenon. As shown in Fig. 5, residues in loop 1 and loop 2 changed more significantly in their RMSF and MSF than residues in a more rigid region. The agonist binding at the cleft made the hinge region inflexible and xenon had no visible impact to the cleft openness. On the other hand, because of intrinsic higher flexibility in the open-cleft system, even transient xenon interactions (lasted only ~6 ns) with the S1S2 cleft promoted the domain opening (Fig. 4 B). Apparently, xenon with a low affinity yet high mobility could induce a profound change in protein conformation as long as the protein is flexible enough for the change. The openness of the S1S2 cleft enhanced by xenon in the open-cleft conformation virtually promotes the channel to remain closed and affects the channel conductance.
Figure 5.
Difference of backbone flexibility between the xenon and control systems for the ligand-binding domain of the NMDA receptor in the closed-cleft conformation (cartoon) is highlighted using a color gradient: from the most increased flexibility (red) to the most decreased flexibility (blue). (A) Top and (B) site views of the RMSF changes in the presence and absence of xenon over the last 5-ns simulations. (C) Top and (D) site views of the changes based on GNM analysis in the mean square fluctuation of the three lowest motions in the systems with and without xenon atoms. The glycine (LG) and glutamate (LE) agonists are presented in solid surface. Domain-1 is colored silver in both subunits. Domain-2 is colored in yellow in NR1 and light blue in NR2. The disulphide bonds in Loop 1 are highlighted in transparent surface and colored by atom type. Xenon atoms were not included in the GNM network, as explained in the Supporting Information by Fig. S6.
Xenon inhibition to the closed-cleft conformation
Although xenon did no produce obvious effect on the openness of the closed-cleft conformation, it might be able to change the function of NMDA receptors via two pathways: altering the binding of native agonists in LBD and interfering the interaction between NR1 and NR2 subunits.
The respective binding energies for glutamate and glycine agonists were −8.87 and −5.64 kcal/mol in the control system, and −6.02 and −6.29 kcal/mol in the presence of xenon. These computed energy values were comparable to the experimental measurements, −7.46 kcal/mol for glutamate22,23 and −8.79 kcal/mol for glycine,24 considering a potential ~ 2 to 3 kcal/mol error of AutoDock calculation.25,26 Although it may not be meaningful to compare differences within the standard deviation, the relative comparison for two highly similar systems still provides some useful information, such as in our case for the same ligand in the highly resembled protein conformations in the presence and absence of xenon. The binding energy for the glycine agonist in the system with xenon was similar to that in the control system (−6.29 vs. −5.64 kcal/mol), indicating that Xe-5closed adjacent to the glycine agonist had no strong impact to the agonist binding. This seems to be a good example that xenon does not act as a competitive antagonist for channel inhibition. However, a binding energy increase of 2.85 kcal/mol for the glutamate coagonist near Xe-4closed (−6.02 vs. −8.87 kcal/mol) sent a different message. A significant loss of hydrogen bonding around glutamate in the presence of Xe-4closed (Fig. 3) also suggested that xenon could weaken the agonist binding. Nevertheless, neither glycine nor glutamate had sizeable displacement from their initial locations over the 20-ns simulation. Taken together, xenon nearby the agonists can perturb the agonist binding to some degrees, but it is probably not strong enough to replace the agonists from their binding sites.
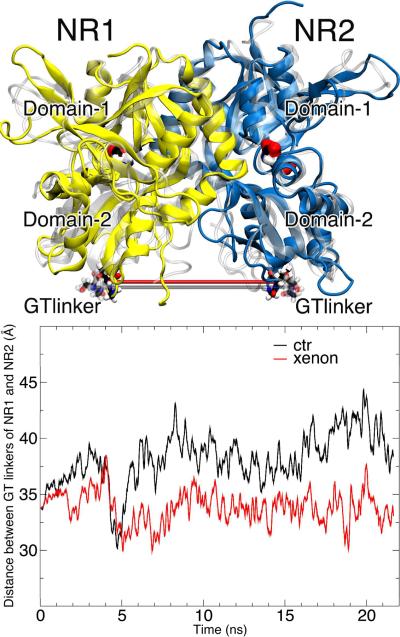
Xenon (Xe-6closed) modulation at the interface between NR1 and NR2 subunits of the closed-cleft conformation is another possible mechanism accounting for xenon inhibition. A kinetics study has shown that coupling between the two subunits is vital in the channel gating of NMDA receptors.27 Such a coupling can be measured by the distance between GT linkers of two subunits, as depicted in Fig. 6. In an intact receptor, a GT linker is where S1 and S2 segments are respectively linked to the TM1 and TM2 domains in a subunit (see Fig. S1). A recent experimental study on a glutamate receptor compellingly showed that the dimmer rearrangement in the LBD from a non-desensitized state to a desensitized state correlated with a ~ 10 Å reduction in the GT linkers' distance.28 Thus, the distance between the GT linkers of two subunits gives a measure on the channel state. A shorter distance correlates with a tendency of the channel shifting to the closed or desensitized state. Figure 6 shows that the distance between two GT linkers in the closed-cleft conformation is ~ 5 Å shorter in the xenon system than in the control system. How did Xe-6closed at the interface contribute to this change? In the control system, NR1 hinge region interacted with NR2 K-helix through the salt bridge between R247 in NR1 and E274 in NR2. In the presence of Xe-6closed, this interaction was perturbed and new pairs of salt bridge or hydrogen bonding were formed, including R247 in NR1 paired with E131 at the NR2 and H272 in NR1 paired with Y236 in NR2. The change in the pattern of electrostatic interactions (shown in Fig. S7) occurred between ~ 5 to 15 ns and was favorable to the inter-subunit shear motion such that the GT-linkers' distance was reduced. Changes of interactions at the hinge region, even small, can cause a noticeable change to the tip of the subunit, such as the GT linker. Though Xe-6closed most likely caused the change of GT-linkers' distance for directly modulating the interaction between two adjacent subunits, we do not exclude the possibility of this change as a result of a collective effect of other xenon atoms. This finding also brought up the importance of appropriately assigned protonation states for the involved residues, which can be explored in future studies. From the experimental point of view, this intersubunit interaction can be examined by mutating the suggested residues or by changing the protonation states under different PH conditions to see whether functioning of this channel is indeed affected.
Figure 6.
(A) The locations of the GT linkers in the initial (transparent in white) and final (solid) structures of NR1 (yellow) and NR2 (blue) in the simulation. The two agonists are presented in surface and colored by atom types. (B) The distance between the GT linkers of NR1 and NR2 subunits over 20-ns simulations in the absence (black) or presence (red) of xenon atoms. A GT linker is where the ligand-binding domain is connected with the TM domains of the NMDA receptor (see Fig. S1).
CONCLUSIONS
Our study has revealed molecular details relating to xenon inhibition. These details remain challenging to be illustrated experimentally, but are essential for a mechanistic understanding about xenon inhibition of NMDA receptors. The conclusion of the study is consistent with the previous experimental suggestion6 that two types of inhibition, competitive and non-competitive, exist in xenon action on NMDA receptors. In addition, our study has demonstrated that xenon exerts its inhibition effect on open- and closed-cleft LBD in different ways.
For the closed-cleft conformation, xenon is more likely to act in a non-competitive manner. The evidence of competitive inhibition is rather circuitous than direct. Despite signs of weakening glutamate binding and hydrogen bonding at the agonist site, neither agonist moved away from their binding sites over the simulations. No functional-related conformational change in LBD could be attributed to aforementioned changes in glutamate binding over the 20-ns simulations. In contrast, within the same simulation time, the evidence for non-competitive xenon inhibition appeared. Our simulations first demonstrated that the channel pore size, proportional to the distance of GT linkers, could be modulated by xenon binding at the interface of two subunits. It appeared that xenon modification of a few pairs of inter-subunit hydrogen bonding is likely to lead a measurable change in the NMDA receptor channel.
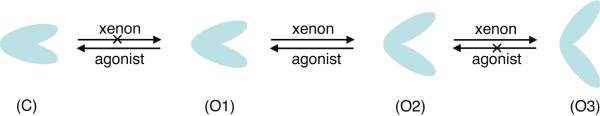
For the open-cleft LDB, the enlarged cleft opening in the presence of xenon sends at least two messages. First, the profound cleft opening reduces the likelihood for agonist binding and consequently hinders channel activation. Depending on the openness of the LBD cleft, this inhibition can be in either a competitive or non-competitive manner, as explained in Fig. 7. Second, it does not require xenon with high binding affinity to make the open cleft more open. The majority of xenon atoms in the open cleft had transient interaction with LBD, but it seemed sufficient to add an additional ~10° to the cleft.
Figure 7.
A diagram showing how xenon affects the domain closure of the ligand-binding domain (LBD) of the NMDA receptor. Multiple conformations exist with various degrees of domain closure, such as C, O1, O2 and O3. Agonist binding initiates the S1S2 cleft closing (C) and the channel opening. Xenon may weaken the agonist binding, but is unable to replace the agonist and open the cleft. Xenon can also inhibit the channel in a non-competitive manner by altering the interaction between NR1 and NR2 (not shown here). For LBD without agonists, xenon may promote the cleft opening from O1 to O2 or from O2 to O3. When the S1S2 cleft is widely opened (O3) by xenon, the separation between two domains is too large to allow agonist binding at the hinge region of the S1S2 cleft. This is another format of non-competitive xenon inhibition. The competitive inhibition can occur between two intermediate states O1 and O2: xenon promotes the transition from O1 to O2, while agonists work in the opposite direction.
A recent kinetics study by Kussius and Popescu suggested the existence of multiple resting states of NMDA receptors.29 Our MD simulations corroborate the suggestion and demonstrate multiple intermediates can exist between the closed- and open-cleft conformations at the equilibrium. Figure 7 summarizes xenon's action on the different conformations of LBD. Collectively, both competitive and non-competitive mechanisms are involved in xenon inhibition of NMDA receptors.
Supplementary Material
ACKNOWLEDGMENT
This research was supported in part by the National Science Foundation through TeraGrid resources provided by the Pittsburgh Supercomputing Center. TeraGrid systems are hosted by Indiana University, LONI, NCAR, NCSA, NICS, ORNL, PSC, Purdue University, SDSC, TACC, and UC/ANL. This research was also supported by grants from the National Institutes of Health (R01GM066358, R01GM056257, and R37GM049202).
Footnotes
Supporting Information Available Seven figures are presented as the supporting materials. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- (1).Franks NP, Dickinson R, de Sousa SLM, Hall AC, Lieb WR. How does xenon produce anaesthesia? Nature. 1998;396:324. doi: 10.1038/24525. [DOI] [PubMed] [Google Scholar]
- (2).Sanders RD, Franks NP, Maze M. Xenon: no stranger to anaesthesia. Brit. J. Anaesth. 2003;91:709–717. doi: 10.1093/bja/aeg232. [DOI] [PubMed] [Google Scholar]
- (3).Colloc'h N, Santos JSD, Retailleau P, Vivares D, Bonnete F, et al. Protein crystallography under xenon and nitrous oxide pressure: comparison with in vivo pharmacology studies and implications for the mechanism of inhaled anesthetic action. Biophys. J. 2007;92:217–224. doi: 10.1529/biophysj.106.093807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).de Sousa SLM, Dickinson R, Lieb WR, Franks NP. Contrasting synaptic actions of the inhalational general anesthetics isoflurane and xenon. Anesthesiology. 2000;92:1055–1066. doi: 10.1097/00000542-200004000-00024. [DOI] [PubMed] [Google Scholar]
- (5).Weigt HU, Adolph O, Georgieff M, Georgieff EM, Fohr KJ. Evidence that xenon does not produce open channel blockade of the NMDA receptor. J. Neurophysiol. 2008;99:1983–1987. doi: 10.1152/jn.00631.2007. [DOI] [PubMed] [Google Scholar]
- (6).Dickinson R, Peterson BK, Banks P, Simillis C, Martin JC, A. C, et al. Competitive inhibition at the glycine site of the N-methyl-D-aspartate receptor by the anesthetics xenon and isoflurane: evidence from molecular modeling and electrophysiology. Anesthesiology. 2007;107:756–767. doi: 10.1097/01.anes.0000287061.77674.71. [DOI] [PubMed] [Google Scholar]
- (7).Reasor JD, Poe GR. Learning and memory during sleep and anesthesia. Int. Anesthesiol. Clin. 2008;46:105–129. doi: 10.1097/AIA.0b013e318181e513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Forman SA, Chin VA. General anesthetics and molecular mechanisms of unconsciousness. Int. Anesthesiol. Clin. 2008;46:43–53. doi: 10.1097/AIA.0b013e3181755da5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- (10).Furukawa H, Gouaux E. Mechanisms of activation, inhibition and specificity: crystal structures of the NMDA receptor NR1 ligand-binding core. Embo. J. 2003;22:2873–2885. doi: 10.1093/emboj/cdg303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Phillips JC, Rosemary B, Wang W, Gumbart J, Tajkhorshid E, et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).MacKerell AD, Jr., Bashford D, Bellott M, Dunbrack RL, Jr., Evanseck JD, et al. All-atom empirical potential for molecular modeling and dynamics studies of proteins. J. Phys. Chem. B. 1998;102:3586–3616. doi: 10.1021/jp973084f. [DOI] [PubMed] [Google Scholar]
- (13).Verlet L, Weis JJ. Perturbation theory for the thermodynamic properties of simple liquids. Mol. Phys. 1972;24:1013–1024. [Google Scholar]
- (14).Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangement and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- (15).Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–1773. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Huey R, Morris GM, Olson AJ, Goodsell DS. A semiempirical free energy force field with charge-based desolvation. J. Comput. Chem. 2007;28:1145–1152. doi: 10.1002/jcc.20634. [DOI] [PubMed] [Google Scholar]
- (17).Zacharias M, Straatsma TP, McCammon JA. Separation-shifted scaling, a new scaling method for Lennard-Jones interactions in thermodynamic integration. J. Chem. Phys. 1994;100:9025–9031. [Google Scholar]
- (18).Chipot C, Pearlman DA. Free energy calculations. The long and winding gilded road. Mol. Simulat. 2002;28:1–12. [Google Scholar]
- (19).Bahar I, Atilgan AR, Erman B. Direct evaluation of thermal fluctuations in proteins using a single-parameter harmonic potential. Fold. Des. 1997;2:173–181. doi: 10.1016/S1359-0278(97)00024-2. [DOI] [PubMed] [Google Scholar]
- (20).Yang LW, Liu X, Jursa CJ, Holliman M, Rader AJ, et al. iGNM: a database of protein functional motions based on Gaussian Network Model. Bioinformatics. 2005;21:2978–2987. doi: 10.1093/bioinformatics/bti469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dundas J, Ouyang Z, Tseng J, Binkowski A, Turpaz Y, Liang J. CASTp: computed atlas of surface topography of proteins with structural and topographical mapping of functionally annotated residues. Nucleic Acids Research. 2006;34:W116–W118. doi: 10.1093/nar/gkl282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Nahum-Levy R, Tam E, Shavit S, Benveniste M. Glutamate but not glycine agonist affinity for NMDA receptors is influenced by small cations. J. Neurosci. 2002;22:2550–2560. doi: 10.1523/JNEUROSCI.22-07-02550.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Maier W, Schemm R, Grewer C, Laube B. Disruption of interdomain interactions in the glutamate binding pocket affects differentially agonist affinity and efficacy of N-methyl-D-aspartate receptor activation. J. Biol. Chem. 2007;282:1863–1872. doi: 10.1074/jbc.M608156200. [DOI] [PubMed] [Google Scholar]
- (24).Nilsson A, Duan J, Mo-Boquist LL, Benedikz E, Sundstrom E. Characterisation of the human NMDA receptor subunit NR3A glycine binding site. Neuropharmacology. 2007;52:1151–1159. doi: 10.1016/j.neuropharm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- (25).Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comp. Chem. 1998;19:1639–1662. [Google Scholar]
- (26).Trott O, Olson A. Software news and update AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comp. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Hansen KB, Naur P, Kurtkaya NL, Kristensen AS, Gajhede M, et al. Modulation of the dimer interface at ionotropic glutamate-like receptor delta2 by D-serine and extracellular calcium. J. Neurosci. 2009;29:907–917. doi: 10.1523/JNEUROSCI.4081-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- (29).Kussius CL, Popescu GK. Kinetic basis of partial agonism at NMDA receptors. Nat. Neurosci. 2009;12:1114–1120. doi: 10.1038/nn.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.