Abstract
The compound (–)-epigallocatechin-3-gallate (EGCG) is the major catechin found in green tea [Camellia sinensis L. Ktze. (Theaceae)]. This polyphenolic compound and several related catechins are believed to be responsible for the health benefits associated with the consumption of green tea. The potential health benefits ascribed to green tea and EGCG include antioxidant effects, cancer chemoprevention, improving cardiovascular health, enhancing weight loss, protecting the skin from the damage caused by ionizing radiation, and others. The compound EGCG has been shown to regulate dozens of disease-specific molecular targets. Many of these molecular targets are only affected by concentrations of EGCG that are far above the levels achieved by either drinking green tea or consuming moderate doses of green tea extract-based dietary supplements. In spite of this, well-designed double-blinded controlled clinical studies have recently demonstrated the efficacy of green tea extracts and purified EGCG products in patients. Therefore, this review highlights results from what the authors believe to be some of the most clinically significant recent studies and describes current developments in the stereoselective total synthesis of EGCG.
Keywords: Camellia sinensis, green tea, catechins, flavonols, epigallocatechin-3-gallate, EGCG, clinical trials, cancer chemoprevention
1. Introduction
The natural product (–)-epigallocatechin-3-gallate (EGCG, 16) is the major polyphenolic constituent found in green tea [dried fresh leaves of the plant Camellia sinensis L. Ktze. (Theaceae) (Bettuzzi et al., 2005, Demeule et al., 2002). Several other polyphenolic compounds known as catechins are also found in lower abundance in green tea. These other catechins include (–)-epicatechin-3-gallate (ECG), (–)-epigallocatechin (EGC), (–)-epicatechin (EC) and (+)-catechin. More than 50% of the mass of this catechin combination is composed of EGCG and a vast body of scientific research suggests that EGCG (and other catechins) is responsible for the majority of the potential health benefits attributed to green tea consumption.
A recent search of the literature revealed more than 8000 citations that relate to the chemistry, bioactivity, production, and potential health benefits of green tea. Of these, over 4000 references pertain to EGCG and other natural products found in green tea (SciFinder/Medline, 2006). These citations can be classified into the following categories: 1) chemical analysis or characterization of green tea components; 2) epidemiological reports of various populations that consume green tea products; 3) evaluations of the antioxidant effects of green tea catechins; 4) examinations of the biomedical potential of green tea components using in vitro models; 5) biochemical studies that investigate the effects of green tea catechins on specific enzymes and biochemical systems that are believed to be potential molecular targets for various diseases and chemoprevention; 6) patents on the methods of preparation or utility of green tea and EGCG products; 7) a relatively small number of investigations that have documented the in vivo health-promoting potential of green tea extracts or purified compounds using animal models; and 8) emerging reports on the potential health benefits of EGCG from well-controlled and double-blinded clinical studies.
There remain several major challenges to interpret the clinical relevance of the hundreds of studies that examine the effects of EGCG on various in vitro disease-related molecular targets and in vivo models for potential health benefits. The overwhelming majority of in vitro studies find that EGCG inhibits a vast array of biomedically relevant molecular targets and disease-related cellular processes at relatively high concentrations (reviewed in: Boik, 2001, Doss et al., 2005, Adhami et al., 2003, Haslam, 1996, Khan et al., 2006, Conney, 2003). These include in vitro anticancer molecular targets and tumor cell cytotoxicity studies conducted at test concentrations that typically range from about 10 to 1000 μM. By contrast, a relatively small number of studies have shown that EGCG can inhibit certain biomedically important molecular targets such as DNA methyltransferases (Lee et al., 2005), squalene epoxidase (Abe et al., 2000), antiapoptotic Bcl-2 proteins (Leone et al., 2003), and vascular endothelial growth factor receptor (VEGFR) signaling (Lamy et al., 2002) at sub-micromolar concentrations. Pharmacokinetic studies conducted in humans indicate that the physiologically relevant serum concentrations of EGCG may be in the high nanomolar range (Henning et al., 2004, Chow et al., 2003, Ullmann et al., 2003). Therefore, high micromolar concentrations are unlikely to be established in the bloodstream of individuals that simply drink green tea or ingest only two to three 200 mg capsules of green tea extract (GTE) each day. Yet, epidemiological studies continue to suggest that there may be significant health benefits associated with drinking green tea (Bushman, 1998). This is further supported by animal studies that indicate the consumption of green tea and green tea products with high levels of EGCG and other catechins may have a significant effect on the prevention of tumors, cardiovascular disease, and other medical conditions. Meanwhile, considerable speculation has arisen to “fit” the results from in vitro studies that demonstrate the activities of EGCG on most of the molecular-targets and the tumor cell cytotoxic effects exerted by EGCG and GTE at concentrations that are far above the physiologically relevant range. This apparent discrepancy has brought forth a number of possible explanations. It has been suggested that the effects of EGCG may be more synergistic when combined with other catechins than previously thought (Suganuma et al., 1999). It is also believed that EGCG (and other tea catechins) may be metabolically activated to form more potent and effective bioactive compounds. Others speculate that EGCG may accumulate in tissues over time to produce cellular concentrations that are much higher than those have been observed in clinical serum samples. Alternatively, the simplest explanation is that the effects of EGCG on many of its reported molecular targets are merely high-concentration effects or experimental artifacts that reflect the propensity of catechins and other polyphenolic substances to chelate metals and bind proteins in a nonselective manner (reviewed in Haslam, 1996). This is the main reason that the high-throughput pharmaceutical screening community has considered polyphenols and other tannins to be “nuisance” compounds that must either be removed from test samples or dereplicated prior to extensive evaluation in protein-based bioassay systems (i.e., enzyme or receptor) (Cardellina et al., 1993). If this is the case, only a relatively small number of the numerous molecular mechanistic studies reported for EGCG and other green tea products actually reflect physiologically relevant processes.
It is conceivable that many of the effects observed with micromolar concentrations of EGCG are relevant to the potential benefits, side effects, and/or toxicity of either high-dose or mega-dose GTE and EGCG therapy (Pisters et al., 2001, Zhou et al., 2004). Likewise, the tumor cell-specific cytotoxic effects produced by high micromolar concentrations of EGCG may not represent phenomena that are physiologically relevant to dietary green tea consumption, but may be indicative of effects that may be achieved with high-dose supplementation of EGCG and other catechins. For these reasons, the authors have selected not to discuss many aspects of EGCG research that have been summarized elsewhere (Doss et al., 2005, Adhami et al., 2003, Haslam, 1996, Khan et al., 2006, Conney, 2003).
It is not the intention of the authors to provide a comprehensive coverage of the immense body of EGCG research, nor is the objective of this review to summarize the large number of different areas of EGCG research currently taking place. The authors simply desire to describe current developments in EGCG catechin chemistry and discuss the results from what the authors believe to be some of the most clinically significant recent studies. A number of recently published clinical efficacy studies have been conducted with purified EGCG and various products that contain EGCG (i.e., various regular and decaffeinated green tea extracts). Some of these have been well-designed double-blinded and properly controlled studies. The authors have selected not to include those studies that lack appropriate controls or where there is no means to differentiate between the specific effects of EGCG and the effects of caffeine in green tea extract products.
2. EGCG bioavailability in human subjects
Recent clinical studies have examined the pharmacokinetic profile of EGCG oral administration by healthy subjects. Ullmann and coworkers (2003) examined the safety, tolerability, and pharmacoketic properties of single dose administration of EGCG that ranged from 50 mg to 1600 mg. Only at oral doses of more than one gram EGCG, were greater than 1 μM maximal plasma EGCG concentrations observed (1600 mg dose, Cmax = 3392 ng/ml, range: 130 - 3392 ng/ml). Peak concentrations were reached between 1.3 – 2.2 h. The plasma kinetics of both free EGCG and total EGCG (free EGCG plus conjugated EGCG metabolites) were assessed at intervals for a period of 26 hours following administration. The mean total EGCG area under the concentration-time curve from 0 h to infinity AUC(0-∞) ranged from 442 to 10,368 ng•h/ml and the mean terminal elimination half-life t1/2z ranged from 1.9 to 4.6 h. Doses of purified EGCG up to 1600 mg were generally well tolerated. Chow and coworkers (2003) evaluated the safety and plasma kinetics of multiple-dose administration of purified EGCG and the decaffeinated green tea extract known as polyphenon E. This study examined once-daily and twice-daily dosing regimens of EGCG and polyphenon E over a four-week period. As observed in the previous study, EGCG intake at the doses of 400 and 800 mg established peak serum concentrations of both free and total EGCG at the high nanomolar range. However, an increase in EGCG bioavailability was observed after chronic 800 mg administration. Daily EGCG administration produced only minor gastrointestinal side effects.
3. UV-Injury and photoaging of the skin
Studies in animal models suggest that antioxidant and anti-inflammatory polyphenols in green tea extracts may reduce some of the harmful effects following exposure to UV radiation (Katiyar et al., 1995). Elmets and coworkers (2000) applied extracts of green tea polyphenols (GTP) or purified catechins (EGCG, EGC, EGC or EC) onto the skin of healthy volunteers. The treated sites were then exposed to a dose of simulated solar radiation at two times the minimum level that was required to produce erythema in untreated skin (2 minimal erythema dose or 2 MED). The UV-treated tissues were examined for erythema, UV-induced DNA damage, reduction in UV-sensitive Langerhans cells, and the presence of sunburn cells. Pretreatment of the skin with a 5% solution of GTP dramatically reduced the UV-induced erythema response (>80% reduction), reduced the number of sunburned cells (66% reduction), increased the survival of Langerhans cells (58% reconstitution of population), and reduced the UV-induced DNA damage (45% reduction). The only purified green tea polyphenols that were able to exert a similar UV-protective effect were EGCG and ECG, the catechins that possess gallate ester moieties. The spectrophotometric profile of EGCG and other green tea polyphenolics indicated that they do not absorb UVB and therefore, the UV-protective effects are not due to sunscreen-like effects.
A separate clinical study found that daily oral administration of either 400 or 800 mg of EGCG did not protect against UV-induced erythema (Chow et al., 2003). Similarly, a recent clinical study examined the ability of either topical or oral administration of green tea extract products to reduce the appearance of photoaging skin (Chiu et al., 2005). Forty women with moderate photoaging were randomized and treated with either topical or oral green tea extracts over an eight-week period. No clinically significant differences in the cutaneous signs of photoaging were observed. While topical administration of either EGCG or green tea extracts did appear to protect against UV-induced damage to the skin (Elmets et al., 2000), initial clinical indications suggest that oral EGCG administration may not be similarly effective and that EGCG and related compounds may protect, rather than repair damaged tissue.
4. Cancer chemoprevention
Reports from several ongoing clinical studies on the cancer chemopreventive potential of EGCG and green tea products have begun to emerge. Spurred by previous results that indicated EGCG could inhibit cervical cancer cell growth in vitro through the regulation of gene expression, cell cycle progression, and apoptosis (Ahn et al., 2003a), Ahn and coworkers (2003b) investigated the clinical efficacy of various EGCG and green tea preparations on human cervical lesions. A total of ninety women with human papilloma virus (HPV) infected cervical lesions were divided into four treatment groups and one control group. Patients in the treatment groups each received one of the following regimens: 1) a local application two times per week of an ointment formulated from the great tea extract preparation polyphenon E for 12 weeks; 2) a 200 mg daily oral dose of polyphenon E for 8-12 weeks; 3) both local and oral polyphenon E for 12 weeks; and 4) a 200 mg daily oral dose of EGCG for 8-12 weeks. The cervical lesions were evaluated before, during and after treatment by Pap smear cytology and other procedures that included a DNA-RNA hybrid capture method for HPV DNA detection. Positive responses included an overall decrease in the severity of lesions, loss of HPV DNA in the cervical lesions that were previously tested positive, and a complete loss of cervical lesions confirmed by tissue biopsy. Overall, 35 out of the 51 patients treated with either EGCG or green tea extract preparations showed a clinically significant positive response. The greatest number of patients responded positively to the topical polyphenon E, with or without supplemental oral polyphenon E (75% response). Over half of the patients that received either oral polyphenol E alone or purified EGCG also showed a significant response. Few side effects were observed in the patients treated with either EGCG or other green tea products. In contrast, only 4 out of the 39 untreated control group showed a positive clinical response. While a small number of the patients in the untreated group had some degree of improvement, a significantly larger portion of these patients showed no improvement or progressed to more advanced stage disease.
Another particularly encouraging study was a recently reported one-year proof-of-principle clinical trial for prostate cancer chemoprevention with oral green tea catechins (Bettuzzi et al., 2005). High-grade prostate intraepithelial neoplasia (HG-PIN) is considered the main premalignant lesion for prostate cancer. Approximately 30% of the patients with HG-PIN develop prostate cancer within one year. Sixty male volunteers with HG-PIN were selected for this double-blinded and placebo-controlled study that involved daily oral consumption of three 200 mg decaffeinated green tea catechin (GTC) preparations (600 mg total). The major component of these GTC preparations was (–)-EGCG (51.88%), followed by (–)-EC (12.24%), (–)-ECG (6.12%), and (–)-EGC (5.5%). Nine of the 30 patients (30%) given placebo developed prostate cancer within the one-year time course of the study. Meanwhile, only one out of the 30 patients (3%) that were given the daily GTC treatment developed prostate cancer. While this study did not establish the long-term benefit(s) of GTC consumption for the chemoprevention of prostate cancer, it is quite remarkable that a 90% reduction in the rate of HG-PIN-positive men developing prostate cancer was achieved among the patients that consume 600 mg GTC daily for one-year. No significant side effects were noted with the consumption of the GTC preparations at the daily doses that were administered.
5. Chemical synthesis of EGCG
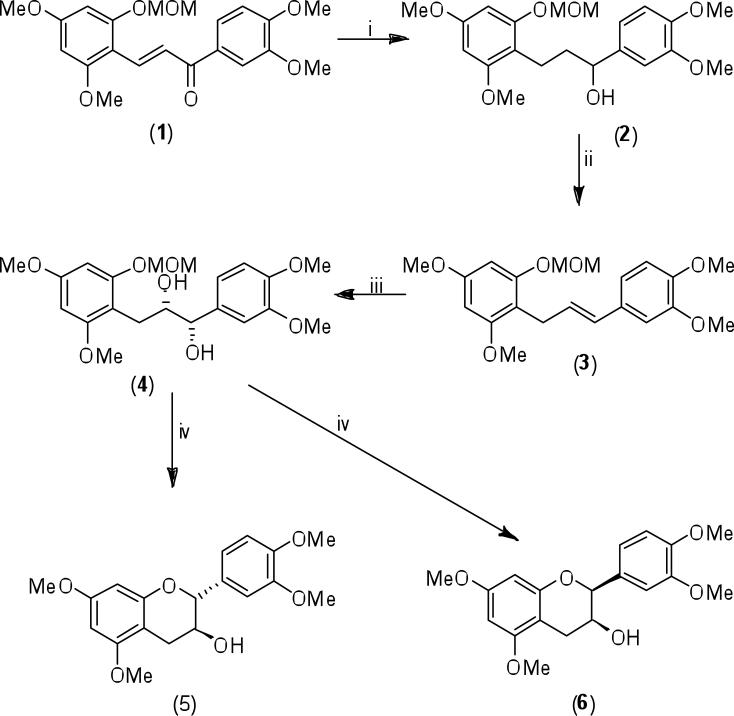
In spite of the progress in the stereoselective total syntheses of natural products over the last several decades, the first stereoselective synthesis of a series of flavan-3-ol permethylaryl ethers, and eventually of the free phenolic analogs, was only recently developed (Van Rensburg et al., 1997a, 1997b; Nel et al., 1999). Such a synthetic protocol is based upon the transformation of retro-chalcones into 1,3-diarylpropenes, which are then subjected to asymmetric dihydroxylation. The resulting diarylpropane-1,2-diols serve as chirons for essentially enantiopure flavan-3-ols. The protocol is demonstrated in Scheme 1 for the synthesis of the (+)-catechin (5) and (+)-ent-epicatechin (6) permethylaryl ethers.
Scheme 1.
Reagents and conditions: i) Pd-H2/EtOH, then NaBH4/EtOH; ii) SOCl2, then 1,8-DBU/CH2Cl2, reflux; iii) AD-mix-α, ButOH/H2O (1:1 v/v), MeSO2NH2, 0° C; iv) 3M HCl, MeOH/H2O (3:1 v/v).
The (E)-retro-2-methoxymethylchalcone methyl ether (1) was transformed by consecutive reduction and dehydration of the ensuing alcohol (2) to exclusively afford the (E)-1,3-diarylpropene (3) in ca 70% overall yield. Asymmetric dihydroxylation of the (E)-propene (3) with AD-mix-α (Kolb et al., 1994) afforded the (+)-(1S,2S)-syn-diol (4) in ca 85% yield and high optical purity (99% enantiomeric excess). Simultaneous deprotection and cyclization of the diol (4) with 3M HCl in methanol, yielded the 2,3-trans-(+)-catechin derivative (5) (ca 50% yield) and, for the first time, the (+)-2,3-cis-ent-epicatechin (6) (ca 20% yield). (-)-ent-Catechin and (-)-epicatechin, the enantiomers of (5) and (6), respectively, were accessible via utilization of AD-mix-β instead of AD-mix-α in step iii, Scheme1 (Van Rensburg et al., 1997b). The four enantiopure free phenolic diastereomers (+)-catechin, (-)-epicatechin, (-)-ent-catechin, and (+)-ent-epicatechin were available in comparable yields via utilization of methoxymethyl protection of the phenolic hydroxyl groups (Nel et al., 1999).
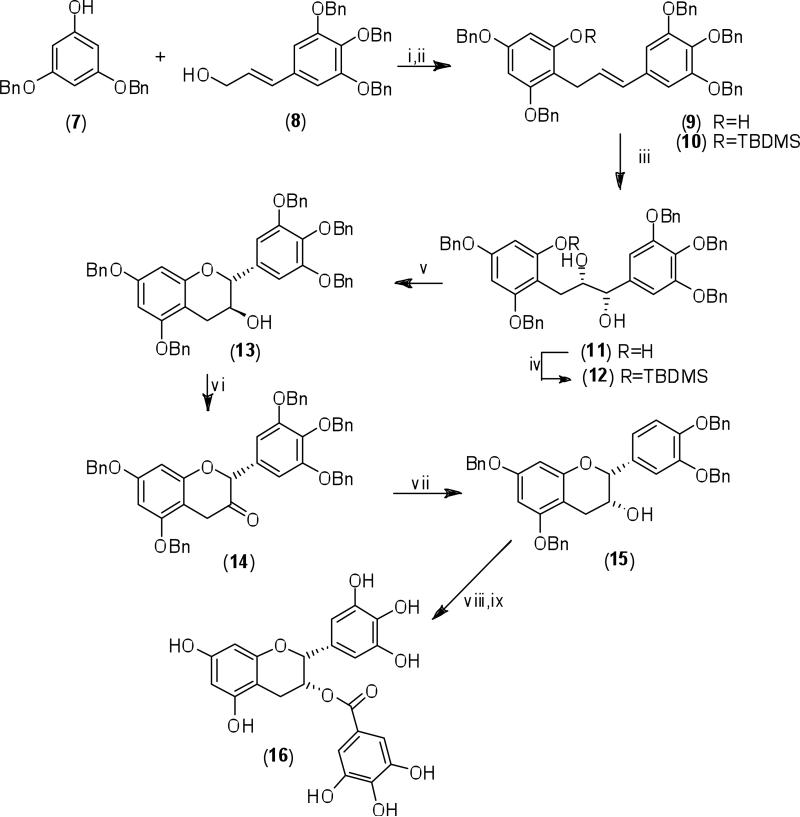
Li and Chan (2001) essentially applied the same protocol to synthesize (-)-epigallocatechin-3-gallate (16) and its enantiomer (+)-ent-epigallocatechin-3-gallate (Scheme 2). The (E)-1,3-diarylpropene (9) was synthesized by coupling of O-benzyl protected phloroglucinol (7) and (E)-cinnamyl alcohol (8). Asymmetric dihydroxylation of the TBDMS protected diarylpropene (10) using AD-mix-α afforded the (1S,2S)-syn-diol (11) which was deprotected to give phenol (12), and the latter subsequently cyclized to afford (+)-penta-O-benzylgallocatechin (13). This was converted into (-)-penta-O-benzylepigallocatechin (15) by Dess-Martin oxidation into the 3-ketoflavan (14) and subsequent stereoselective reduction of the latter. The compound EGCG (16) was then accessable via a simple acylation using 3,4,5-tri-O-benzylbenzoyl chloride, followed by deprotection via reductive de-O-benzylation. (+)-ent-Epigallocatechin-3-gallate was available via simply replacing AD-mix-α with AD-mix-β in the asymmetric dihydroxylation step.
Scheme 2.
Reagents and conditions: i) H2SO4(SiO2)/CH2Cl2/CS2/rt; ii) TBDMSCl/imidazole/DMF, rt; iii) AD-mix-α/CH3SO2NH2/H2O/t-BuOH, 0° C; iv) TBAF/THF, rt; v) CH(OEt)3/PPTS/CH2Cl2, rt; vi) Dess-Martin periodinane/CH2Cl2, rt; vii) L-selectride/THF, -78°C→ rt; viii) 3,4,5-tris(benzyloxy)benzoyl chloride/DMAP/CH2Cl2, rt; ix) H2/Pd(OH)2/MeOH/THF, rt.
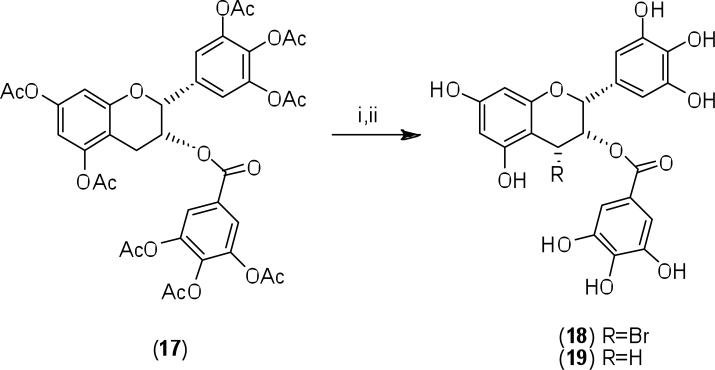
Noteworthy also is the synthesis of (-)-[4-3H]epigallocatechin-3-gallate in order to probe its metabolic fate in rats following intravenous administration (Kohri et al., 2001). Bromination of the per-O-acetyl derivative (17) of EGCG gave the 4-bromo derivative (18) with unspecified configuration at C-4. Simultaneous reduction (tritiation) and deprotection of (18) with NaB3H4/NaBH4 in methanol, afforded the (-)-[4-3H]EGCG (19), again without specification of the C-4 configuration.
6. Summary
Dozens of studies have demonstrated that non-physiologically relevant high concentrations of EGCG can potentially interfere with many disease-related biochemical processes in vitro. By contrast, EGCG potently and specifically inhibits a small number of important molecular targets at concentrations that may be achieved by consuming green tea or EGCG-rich dietary supplements. In spite of the attention paid to the large number of pharmacological activities associated with concentrations of EGCG that are physiologically irrelevant, the newest clinical studies support some of the potential heath benefits that have be ascribed to the consumption of green tea and EGCG. Recent methods developed for the stereoselective total synthesis of EGCG, and structurally related catechins, could provide new sources of these compounds for investigational and biomedical use.
Scheme 3.
Reagents and conditions: i) NBS/AIBN in CCl4, reflux; ii) NaB3H4/MeOH, rt, then NaBH4/MeOH
Acknowledgements
Support for this effort was provided by the NIH/NCI CA98787-3, DOD W81XWH-05-1-0119 and NOAA NURP/ NIUST NA16RU1496.
Abbreviations
- AD
asymmetric dihydroxylation
- AIBN
2,2'-azobis(isobutyronitrile)
- DBU
1,8-diazabicyclo[5.4.0]undec-7-ene
- DMAP
dimethylaminopyridine
- EC
(–)-epicatechin
- ECG
(–)-epicatechin-3-gallate
- EGC
(–)-epigallocatechin
- EGCG
(–)-epigallocatechin-3-gallate
- GTC
green tea catechin
- GTE
green tea extract
- HG-PIN
high-grade prostate intraepithelial neoplasia
- HPV
human papilloma virus
- MED
minimal erythema dose
- PPTS
pyridinium p-toluenesulfonate
- rt
room temperature
- TBDMSCl
tert-butyldimethylsilyl chloride
- THF
tetrahydrofuran
References
- Abe I, Seki T, Umehara K, Miyase T, Noguchi H, Sakakibara J, Ono T. Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 2000;268:767–771. doi: 10.1006/bbrc.2000.2217. [DOI] [PubMed] [Google Scholar]
- Adhami VM, Ahmad N, Mukhtar H. Molecular targets for green tea in prostate cancer prevention. J. Nutr. 2003;133(Suppl.):2417S–2424S. doi: 10.1093/jn/133.7.2417S. [DOI] [PubMed] [Google Scholar]
- Ahn W-S, Huh SW, Bae S-M, Lee I-P, Lee J-M, Namkoong S-E, Kim C-K, Sin J-I. A major constituent of green tea, EGCG, inhibits the growth of a human cervical cancer cell line, CaSki cells, through apoptosis, G(1) arrest, and regulation of gene expression. DNA Cell Biol. 2003a;22:217–224. doi: 10.1089/104454903321655846. [DOI] [PubMed] [Google Scholar]
- Ahn W-S, Yoo J, Huh SW, Kim C-K, Lee J-M, Namkoong S-E, Bae S-M, Lee IP. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003b;12:383–390. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–1240. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- Boik J. Flavonoids. In: Boik J, editor. Natural Compounds in Cancer Therapy-Promising Nontoxic Antitumor Agents from Plants & Other Sources. Oregon Medicinal Press LLC; Princeton, MN: 2001. pp. 251–273. [Google Scholar]
- Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31:151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- Cardellina JH, II, Munro MH, Fuller RW, Manfredi KP, McKee TC, Tischler M, Bokesch HR, Gustafson KR, Beutler JA, Boyd MR. A chemical screening strategy for the dereplication and prioritization of HIV-inhibitory aqueous natural products extracts. J. Nat. Prod. 1993;56:1123–1129. doi: 10.1021/np50097a016. [DOI] [PubMed] [Google Scholar]
- Chiu AE, Chan JL, Kern DG, Kohler S, Rehmus WE, Kimball AB. Double-blinded, placebo-controlled trial of green tea extracts in the clinical and histologic appearance of photoaging skin. Dermatol. Surg. 2005;31:855–860. doi: 10.1111/j.1524-4725.2005.31731. [DOI] [PubMed] [Google Scholar]
- Chow H-HS, Cai Y, Hakim IA, Crowell JA, Shahi F, Brooks CA, Dorr RT, Hara Y, Alberts DS. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003;9:3312–3319. [PubMed] [Google Scholar]
- Conney AH. Enzyme induction and dietary chemicals as approaches to cancer chemoprevention: the Seventh DeWitt S. Goodman Lecture. Cancer Res. 2003;63:7005–7031. [PubMed] [Google Scholar]
- Demeule M, Michaud-Levesque J, Annabi B, Gingras D, Boivin D, Jodoin J, Lamy S, Bertrand Y, Beliveau R. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr. Med. Chem. Anti-Cancer Agents. 2002;2:441–463. doi: 10.2174/1568011023353930. [DOI] [PubMed] [Google Scholar]
- Doss MX, Potta SP, Hescheler J, Sachinidis A. Trapping of growth factors by catechins: a possible therapeutical target for prevention of proliferative diseases. J. Nutr. Biochem. 2005;16:259–266. doi: 10.1016/j.jnutbio.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Elmets CA, Singh D, Tubesing K, Matsui M, Katiyar S, Mukhtar H. Cutaneous photoprotection from ultraviolet injury by green tea polyphenols. J. Am. Acad. Dermatol. 2001;44:425–432. doi: 10.1067/mjd.2001.112919. [DOI] [PubMed] [Google Scholar]
- Haslam E. Natural polyphenols (vegetable tannins) as drugs: possible modes of action. J. Nat. Prod. 1996;59:205–215. doi: 10.1021/np960040+. [DOI] [PubMed] [Google Scholar]
- Henning SM, Niu Y, Lee NH, Thames GD, Minutti RR, Wang H, Go VL, Heber D. Bioavailability and antioxidant activity of tea flavanols after consumption of green tea, black tea, or a green tea extract supplement. Am. J. Clin. Nutr. 2004;80:1558–1564. doi: 10.1093/ajcn/80.6.1558. [DOI] [PubMed] [Google Scholar]
- Katiyar SK, Elmets CA, Agarwal R, Mukhtar H. Protection against ultraviolet-B radiation-induced local and systemic suppression of contact hypersensitivity and edema responses in C3H/HeN mice by green tea polyphenols. Photochem Photobiol. 1995;62:855–861. doi: 10.1111/j.1751-1097.1995.tb09147.x. [DOI] [PubMed] [Google Scholar]
- Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (-)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- Kohri T, Nanjo F, Suzuki M, Seto R, Matsomoto N, Yamakawa M, Hojo H, Hara Y, Desai D, Amin S, Conaway CC, Chung F-L. Synthesis of (-)-[4-3H]epigallocatechin gallate and its metabolic fate in rats after intravenous administration. J. Agric. Food. Chem. 2001;49:1042–1048. doi: 10.1021/jf0011236. [DOI] [PubMed] [Google Scholar]
- Kolb HC, Van Nieuwenhze MS, Sharpless KB. Catalytic asymmetric dihydroxylation. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- Lamy S, Gingras D, Beliveau R. Green tea catechins inhibit vascular endothelial growth factor receptor phosphorylation. Cancer Res. 2002;62:381–385. [PubMed] [Google Scholar]
- Lee WJ, Shim J-Y, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol. Pharmacol. 2005;68:1018–1030. doi: 10.1124/mol.104.008367. [DOI] [PubMed] [Google Scholar]
- Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–8121. [PubMed] [Google Scholar]
- Li L, Chan TH. Enantioselective synthesis of epigallocatechin-3-gallate (EGCG), the active polyphenol component from green tea. Org. Lett. 2001;3:739–741. doi: 10.1021/ol000394z. [DOI] [PubMed] [Google Scholar]
- Nel RJJ, Van Rensberg H, Van Heerden PS, Ferreira D. Stereoselective synthesis of flavonoids, Part 8. Free phenolic flavan-3-ols. J. Chem. Res. 1999;10:606–607. 2610–2625. (S) (M) [Google Scholar]
- Pisters KM, Newman RA, Coldman B, Shin DM, Khuri FR, Hong WK, Glisson BS, Lee JSJ. Phase I trial of oral green tea extract in adult patients with solid tumors. Clin. Oncol. 2001;19:1830–1838. doi: 10.1200/JCO.2001.19.6.1830. [DOI] [PubMed] [Google Scholar]
- SciFinder/Medline database search 2006 May; www.cas.org/SCIFINDER/SCHOLAR/index.html.
- Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (-)-epigallocatechin gallate with (-)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res. 1999;59:44–47. [PubMed] [Google Scholar]
- Ullmann U, Haller J, Decourt JP, Girault N, Girault J, Richard-Caudron AS, Pineau B, Weber P. A single ascending dose study of epigallocatechin gallate in healthy volunteers. J. Int. Med. Res. 2003;31:88–101. doi: 10.1177/147323000303100205. [DOI] [PubMed] [Google Scholar]
- Van Rensburg H, Van Heerden PS, Bezuidenhoudt BCB, Ferreira D. Enantioselective synthesis of the four catechin diastereomer derivatives. Tetrahedron Lett. 1997a;38:3089–3092. [Google Scholar]
- Van Rensburg H, Van Heerden PS, Ferreira D. Enantioselective synthesis of flavonoids, Part 3. Trans- and cis- flavan-3-ol methyl ether acetates. J. Chem. Soc., Perkin Trans. 1. 1997b:3415–3421. [Google Scholar]
- Zhou Y-D, Kim Y-P, Li X-C, Baerson SR, Agarwal AK, Hodges TW, Ferreira D, Nagle DG. Hypoxia-inducible factor-1 activation by (-)-epicatechin gallate: Potential adverse effects of cancer chemoprevention with high-dose green tea extracts. J. Nat. Prod. 2004;67:2063–2069. doi: 10.1021/np040140c. [DOI] [PMC free article] [PubMed] [Google Scholar]