Abstract
Purpose
To examine the influence of a unilateral exercise training protocol on brachial artery reactivity (BAR) in 12 men (aged 81 ± 5 yr).
Methods
Brachial artery diameters and blood flow parameters were assessed, in both arms, using high-resolution ultrasonography, before and after 5 min of forearm occlusion, before and at the end of each week of a 4-wk training program. Training consisted of a unilateral handgrip training protocol (nondominant arm) at 60% of maximal voluntary handgrip strength, performed for 4 wk, 4 d·wk −1, 20 min per session, and a cadence of one contraction per 4 s.
Results
After training, handgrip strength increased 6.2% (baseline = 32.4 ± 7.0 kg vs week 4 = 34.4 ± 6.7 kg) in the trained arm only but failed to reach statistical significance (P = 0.10). No statistical changes were observed for blood pressure or resting HR. In contrast, BAR increased 45% (Pre = 2.9% vs Post = 4.1%, P = 0.05) in the trained arm only. Improvements in BAR were observed after the second week of training, without significant changes in the main vasodilatory trigger, defined as the relevant shear stimulus after forearm occlusion (P > 0.05).
Conclusions
These data indicate that a localized short-term exercise program results in significant improvements in vascular function in the trained arm of elderly men compared with the control arm. Furthermore, the findings indicate a statistically significant increase in BAR at the end of the second week of training, despite a similar trigger for dilation versus before training.
Keywords: BRACHIAL ARTERY REACTIVITY, OLDER ADULTS, VASCULAR PLASTICITY, SHEAR RATE
Advancing age is associated with a decline in peak oxygen consumption (peak V̇O2), which is accelerated in the later decades of life (10). The reduction in peak V̇O2 has been attributed to a myriad of factors, including reduced cardiac output reserve and stroke volume (21) and maldistribution of cardiac output (4). Of particular concern are the age-related changes in vasodilatory function of large conduit and resistance vessels, which contribute to the delivery of blood to active skeletal muscle during a time of increasing need (such as during exercise). Previous work by others indicates a strong relation between vasodilatory function and aerobic capacity in older endurance-trained men (26,27) and older women (22). Recent work from our laboratory indicating a link between vascular health and physical function in the elderly (36) further emphasizes the importance of maintaining a healthy vasculature throughout life.
Vasoactivity of large conduits and resistance vessels is partly mediated through the endothelium, which responds to changes in wall shear stress by releasing vasoactive substances (e.g., nitric oxide [16]) to cause smooth muscle relaxation. Measurement of brachial artery reactivity (BAR) in response to 5 min of forearm occlusion is a validated noninvasive tool to assess global vascular health and is associated with vasoreactivity of the coronary arteries (3,30). BAR is reduced in individuals with cardiovascular risk factors (20,29) and with advancing age (6).
Exercise training has emerged as an effective therapy to improve vascular function in the young and middle-aged adults with cardiovascular disease (11). Previous data from our laboratory (1) have shown significant improvements in BAR after 4 wk of unilateral handgrip training in younger individuals. The restorative capacity of exercise training also extends to adults between 50 and 76 yr of age (8,37). However, given the predictions by the National Institute on Aging, that the fastest growing segment of the US population is the age group older than 80 yr, it is important to determine the efficacy of an exercise intervention on vascular health in the “oldest old.”
Interestingly, our previous findings indicate that BAR improved within 4 d of training onset, in younger individuals (1). We are unaware of any exercise training studies in the elderly that have examined the time course of vascular changes. Ultimately, knowledge regarding the time course of vascular changes in the elderly may contribute to the refinement of exercise guidelines designed to preserve functional ability and independence in the elderly.
Accordingly, the purpose of this study was to examine the influence of a unilateral exercise training protocol on BAR, in 73- to 90-yr-old men. It was hypothesized BAR would significantly change in the trained arm only. A second objective of the study was to determine the time course of changes in BAR. It was hypothesized that the changes in BAR would be noticeable after 1 wk of training.
METHODS
Participants
Men older than 70 yr were recruited to participate in this study. Exclusion criteria for the study included the following: 1) active smokers, 2) known alcohol or drug abuse problems, 3) heart attack or stroke in the last 3 months or changes in a resting ECG, 4) American Heart Association Class D (i.e., symptoms of cardiovascular and/ or metabolic disease at rest), 5) poorly controlled high blood pressure or diabetes (i.e., change in medication within the last 6 months), 6) known blood clotting disorders, 7) known blood vessel aneurysm (weakness or enlargements), 8) myasthenia gravis, 9) known acute infection and/or significant emotional distress, 10) adults dementia or other neurological impairment, and 11) inability to see or hear. Each participant signed an informed consent approved by the institutional review board of the Pennington Biomedical Research Center.
Experimental Design
The study was a prospective design consisting of 4 wk of handgrip exercise training of the nondominant arm. The dominant arm of the participant served as the control. The major dependent variable for the study was BAR. A power analysis conducted in our laboratory revealed that, to detect a 30% change in vasoreactivity at 90% power using a prospective design, 10 subjects would be required (35). The resolution of the brachial images in the previous power calculation was limited by a caliper accuracy of 0.028 mm. The analysis system (see “Brachial artery imaging”) used in the current study allows a caliper accuracy of 0.01 mm. HR, blood pressure, blood flow, and estimated shear rate were the major independent variables. Finally, maximal handgrip strength and forearm circumference were also assessed. All variables were examined before training and at the end of each week of the protocol.
Experimental Procedures
Physical fitness
The Physical Activity Scale for the Elderly (PASE) was used to estimate the level of physical activity of each participant before training (33). Participants were asked to avoid changing physical activity behavior during the study. Forearm circumferences of each arm were assessed using a weighted measuring tape approximately 10 cm distal to the midpoint between the lateral epicondyle and the olecranon process. Maximum voluntary handgrip contractions (MVC) of the right and left arms were evaluated using a dynamometer (Baseline®). The participants stood upright while bending forward slightly and were instructed to grip at a maximum effort for 3 s. The average of three consecutive trials was used to determine MVC.
Brachial artery imaging
All brachial artery imaging was conducted by the same ultrasonographer in accordance with the guidelines set forth by the Brachial Artery Reactivity Task Force (7). Brachial artery ultrasound measures were obtained with participants in the supine position, using a 7.5-MHz linear array transducer before, during, and after 5 min of forearm occlusion. Before scanning, the participant was instructed to fast for 12 h. Baseline ultrasound images were obtained after 20 min of supine rest. All images were obtained in the longitudinal view, approximately 4 cm proximal to the olecranon process, in the anterior/medial plane. Image depth was initially set at 4 cm, and gain settings were adjusted to provide an optimal view of the anterior and posterior intimal interfaces of the artery and were kept constant throughout. The arm of the participant was immobilized and slightly supinated. Forearm occlusion consisted of inflation of a blood pressure cuff, positioned approximately 1 cm distal to the olecranon process, to 200 mm Hg for 5 min. Images were obtained at rest and continuously from the final 30 s of occlusion until 5 min after the release of the blood pressure cuff. Doppler velocity profiles were collected simultaneously using a pulsed Doppler signal at an angle of approximately 60° to the vessel. All ultrasound images were recorded digitally, stored on discs, for subsequent offline analysis.
Exercise training
Exercise training involved 20 min of handgrip exercise at an intensity of 60% of maximal handgrip strength for four consecutive days each week. Exercise sessions were not conducted on the same day as BAR assessments. The intervention lasted for 4 wk. For each session, the handgrip dynamometer was squeezed at a rate of one contraction per 4 s. These sessions were monitored by members of the research team. Maximal handgrip strength was assessed on a weekly basis to ensure that the relative training intensity would be maintained during the study.
Brachial Artery Dimensions, Reactivity, and Estimated Shear Analysis
Brachial artery diameters were analyzed using the Brachial Imager software (Medical Imaging Applications, LLC, Coralville, IA). Arterial diameters were calculated as the mean distance between the anterior and the posterior wall at the blood vessel interface, with the image in diastole, defined as the peak of the r-wave. Resting diameter was defined by the average of 30 s of data obtained after 20 min of resting conditions. Peak dilation was defined (by visual inspection of the arterial diameter curve) as the largest diameter after release of the occluding cuff. The value was calculated by the average of 10 images (5 s) surrounding this highest observable peak. Finally, BAR was defined as the absolute (mm) and percent change in vessel diameter from rest to peak. The reproducibility of this technique in our laboratory has yielded intraclass correlation coefficients for days, testers, and readers of 0.92, 0.94 and 0.90, respectively (35).
Blood flow velocity was defined as the average of three mean velocities measured at rest and during the first 10 s of reactive hyperemia. Briefly, three flow velocity integrals (FVI; cm) were randomly selected and traced using Image Pro 4.0 software (Media Cybernetics Inc., Bethesda, MD). The FVI was then divided by the ejection time (s) to subsequently determine the mean velocity (cm·s−1) for each of the selected cardiac cycles. Resting blood flow and hyperemic blood flow (mL·min−1) were then calculated using the after equation: Q̇ = (velocity × HR) × πr2. In accordance with recent recommendations (24), the continued shear stimulus imposed on the vessel wall before peak diameter response (“relevant” shear stimulus) was used to quantify the stimulus for BAR. Shear rate [4 × mean blood velocity (cm·s−1) / diameter (cm)] was measured at 10-s intervals during reactive hyperemia up to the point of maximum vessel diameter and plotted against time (s). A trapezoidal model was then used to calculate area under the curve (AUC) above baseline.
Statistical Analyses
All statistical analyses were performed using SPSS for Windows (version 11.0 [SPSS Inc., Chicago, IL]). To determine the effect of the 4-wk intervention on BAR, a two (trained arm vs nontrained arm) by two (pre vs post) repeated-measures ANOVA was performed. To determine the time course of the changes in BAR across the training protocol, a repeated-measures ANOVA was used. Differences between means were evaluated using a post hoc least significant difference (LSD) test. Relationships among BAR, shear rate, and hyperemic blood flow were examined using Pearson product moment correlations. An α level <0.05 was considered statistically significant.
RESULTS
Participant characteristics
Twelve men (81 ± 5 yr) completed all facets of the study. All individuals were free from symptoms indicative of chronic illness; although two individuals were taking ACE inhibitors, three were on cholesterol lowering medications, and two were taking β-blocking drugs. Dosages were kept constant throughout the study. The average PASE score of this sample indicates that these individuals were moderately active. The baseline characteristics of these individuals are presented in Table 1.
TABLE 1.
Participant characteristics.
| Variable | Baseline |
|---|---|
| Age (yr) | 81 ± 1.4 |
| Height (cm) | 176 ± 1.7 |
| Weight (kg) | 77 ± 3.5 |
| BMI (kg·m−2) | 24.9 ± 1.1 |
| PASE score (AU) | 165 ± 25 |
| Systolic blood pressure (mm Hg) | 133 ± 3 |
| Diastolic blood Pressure (mm Hg) | 76 ± 2 |
| HR (beats·min−1) | 61 ± 3 |
Values are means ± SE.
Forearm circumference, handgrip strength, and hemodynamics
All subjects completed a total of 16 training sessions. Maximal handgrip strength increased 6.2% (baseline = 32.4 ± 7.0 kg vs week 4 = 34.4 ± 6.7 kg) in the trained arm only but failed to reach statistical significance (P = 0.10). No significant change was observed in the forearm circumference of either arm. Also, the results of a paired-samples t-test for pretraining and posttraining measures for cardiovascular hemodynamics did not reveal significant changes in systolic blood pressure (Pre = 133 ± 11 mm Hg vs Post = 131 ± 10; P = 0.08), diastolic blood pressure (Pre = 76 ± 6 mm Hg vs Post = 75 ± 6 mm Hg; P = 0.69), or resting HR (Pre = 61 ± 9 beats·min−1 vs Post = 59 ± 10 beats·min−1; P = 0.32).
Effects of exercise training on BAR
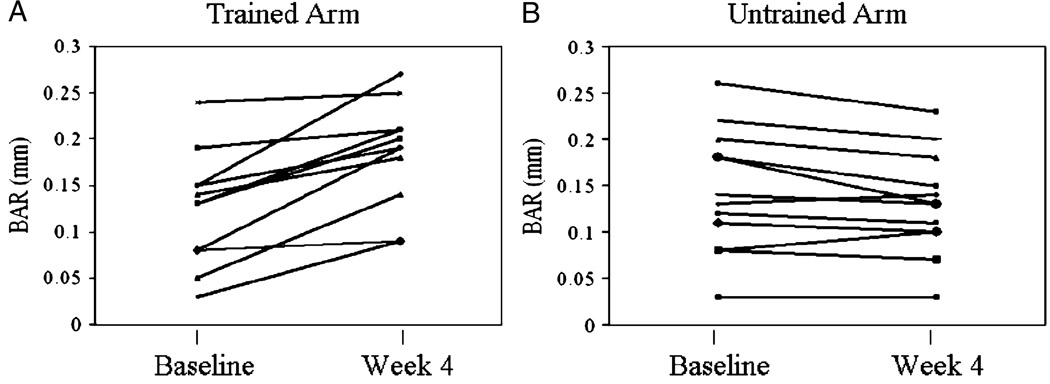
The vascular parameters of the trained and untrained arm during the training program are presented in Table 2. No significant differences were noted between brachial artery diameter at rest and before release of the blood pressure cuff in either arm during the study (data not shown). ANOVA revealed no difference in resting brachial artery diameter between arms before training and during the study. The two (trained arm vs nontrained arm) by two (pre vs post) repeated-measures ANOVA revealed a significant interaction effect, whereby BAR improved 45% in the trained arm only (P = 0.05). No significant differences were observed in pretraining BAR between arms or between pretraining and posttraining BAR in the untrained arm. Individual training responses for both trained and untrained arms are shown in Figure 1. The data clearly show a consistent improvement in BAR, for most trained arms from before to after training. In contrast, no improvements were noted in BAR in any of the untrained arms.
TABLE 2.
Vascular diameters and BAR at baseline and throughout the training program.
| Baseline (Pre) | Week 1 | Week 2 | Week 3 | Week 4 (Post) | |
|---|---|---|---|---|---|
| Trained arm | |||||
| Rest diameter (mm) | 4.47 ± 0.16 | 4.48 ± 0.16 | 4.47 ± 0.16 | 4.47 ± 0.16 | 4.48 ± 0.16 |
| Peak diameter (mm) | 4.60 ± 0.16 | 4.62 ± 0.15 | 4.64 ± 0.15 | 4.64 ± 0.16 | 4.67 ± 0.16 |
| BAR (relative) (%) | 2.9 ± 0.43 | 3.5 ± 0.66 | 3.8* ± 0.49 | 4.0* ± 0.46 | 4.2* ± 0.40 |
| BAR (absolute) (%) | 0.13 ± 0.02 | 0.14 ± 0.02 | 0.17* ± 0.02 | 0.17* ± 0.02 | 0.18* ± 0.02 |
| Time to peak dilation (s) | 47 ± 2 | 44 ± 3 | 48 ± 2 | 47 ± 2 | 51 ± 2 |
| Untrained arm | |||||
| Rest diameter (mm) | 4.64 ± 0.16 | 4.65 ± 0.16 | 4.67 ± 0.16 | 4.66 ± 0.15 | 4.66 ± 0.15 |
| Peak diameter (mm) | 4.78 ± 0.17 | 4.8 ± 0.16 | 4.8 ± 0.16 | 4.8 ± 0.16 | 4.79 ± 0.15 |
| BAR (relative) (%) | 3.1 ± 0.42 | 3.2 ± 0.48 | 2.6 ± 0.41 | 2.8 ± 0.36 | 2.8 ± 0.35 |
| BAR (absolute) (%) | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.12 ± 0.02 | 0.13 ± 0.02 | 0.13 ± 0.02 |
| Time to peak dilation (s) | 48 ± 2 | 41 ± 1 | 46 ± 2 | 44 ± 2 | 45 ± 2 |
Values are means ± SE.
Significant versus baseline, P < 0.05.
FIGURE 1.
Individual changes in BAR for the trained arm (A) and untrained arm (B) before and after the 4-wk intervention.
Time course changes of BAR
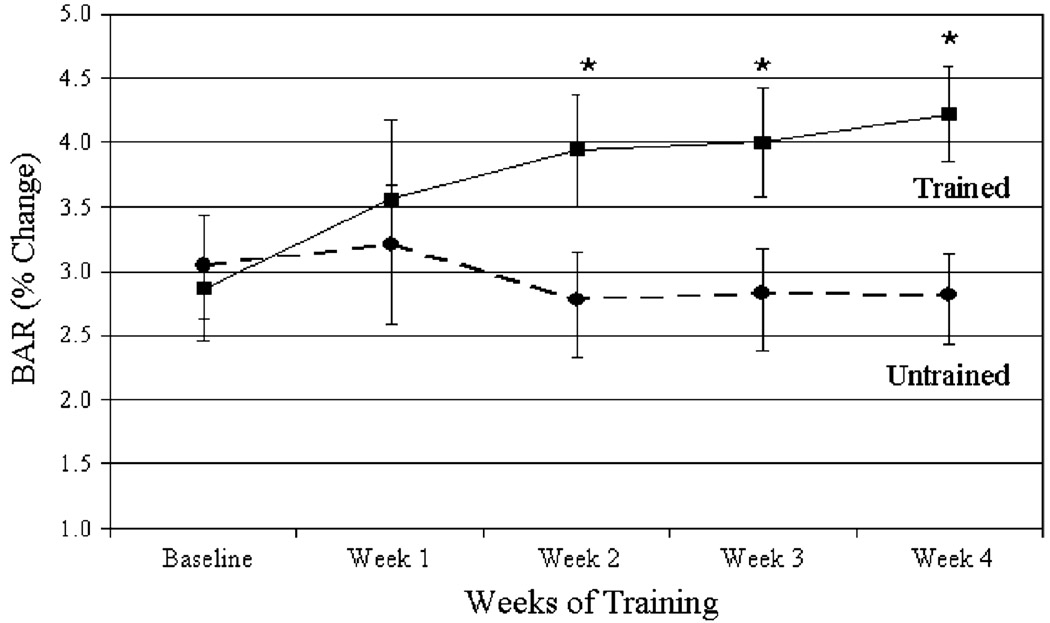
The time course of change in BAR is presented in Figure 2. BAR of the trained arm improved 30% by the second week of training (2.9 ± 1.5 at baseline vs 3.8 ± 1.7 at week 2, P = 0.02) and remained statistically significant compared with pretraining values for the remainder of the intervention (P < 0.05). BAR continued to increase from week 2 to week 4 (maximal improvement 45%), but the changes were not significant from week 2. No significant changes in BAR were noted in the untrained arm during the intervention.
FIGURE 2.
Group mean values ± SE for percent change in BAR across the 4-wk intervention. *P < 0.05 as compared with pretraining.
Effects of exercise training on blood flow and shear rate
Estimates of blood flow at rest and immediately after cuff occlusion for the trained and control arms are presented in Table 3. In addition, the estimated peak wall shear rate immediately after cuff occlusion and the relevant shear stimulus (AUC) are also shown in Table 3. No significant differences in flow and the shear values were observed between arms before training. Estimates of blood flow during reactive hyperemia were significantly higher in the trained arm at weeks 3 and 4 compared with pretraining values. No such changes were noted for the control arm. Peak wall shear rates also increased significantly by week 3 in the trained arm. In contrast, the relevant shear stimulus did not change significantly in the trained arm across the training period but was significantly higher compared with that in the control arm at weeks 2 to 4.
TABLE 3.
Vascular and blood flow parameters at baseline and throughout the training program.
| Baseline (Pre) | Week 1 | Week 2 | Week 3 | Week 4 (Post) | |
|---|---|---|---|---|---|
| Trained arm | |||||
| Resting blood flow (mL·min−1) | 118 ± 43 | 140 ± 40 | 137 ± 34 | 104 ± 19 | 121 ± 33 |
| Hyperemic blood flow (mL·min −1) | 679 ± 132 | 769 ± 147 | 739 ± 80 | 800 ± 112* | 852 ± 95* |
| Peak shear rate (s −1) | 167 ± 21 | 188 ± 23 | 185 ± 18 | 201 ± 21* | 217 ± 22* |
| Shear stimulus (AUC) | 3973 ± 433 | 4188 ± 529 | 4382 ± 482† | 4414 ± 594† | 4895 ± 597† |
| Untrained arm | |||||
| Resting blood flow (mL·min −1) | 99 ± 21 | 120 ± 19 | 111 ± 22 | 123 ± 15 | 123 ± 13 |
| Hyperemic blood flow (mL·min −1) | 829 ± 61 | 811 ± 46 | 762 ± 54 | 764 ± 59 | 775 ± 65 |
| Peak shear rate (s −1) | 183 ± 21 | 179 ± 16 | 162 ± 17 | 165 ± 18 | 173 ± 22 |
| Shear stimulus (AUC) | 4012 ± 423 | 3473 ± 328 | 3578 ± 352 | 3521 ± 415 | 3991 ± 502 |
Values are means ± SE.
Significant versus baseline, P < 0.05.
Significant versus control arm, P < 0.05.
Finally, Pearson product moment correlations revealed significant relationships between the average relevant shear stimulus (AUC) and BAR (r = 0.76, P < 0.001) in either arm across all visits.
DISCUSSION
The purpose of this study was to examine the influence of a unilateral exercise training protocol on BAR in a group of older men. Four weeks of exercise training resulted in a ~45% increase in BAR of the trained arm only. A significant improvement was observed by the end of the second week of training and was maintained throughout the duration of the study. This finding indicates the vascularture’s ability to respond favorably to an exercise stimulus is preserved in individuals in their 9th and 10th decades of life. Moreover, BAR adaptations occur relatively early after the onset of training and are observed before any alterations in the stimulus for dilation.
Unilateral response to exercise training
Before training, brachial artery resting diameters, blood flow, and peak wall shear rates were similar to data from the Louisiana Healthy Aging study (36). The BAR observed in the present study ranged from 2.9% to 4.2%, which is also quite similar to what has been reported in larger investigations of older healthy adults (6,14) including our own (36). Typically, when comparing these findings to younger age groups, BAR is blunted, thus suggesting an age-related decline in the endothelium-dependent function (15,36).
The improvement in BAR in response to exercise training is also in agreement with others (8,37) who studied adults between the age of 50 and 76 yr. Desouza et al. (8) reported a 30% improvement in acetylcholine-induced forearm blood flow in response to 3 months of aerobic exercise, which consisted mostly of walking. More recently, Wray et al. (37) observed a significant improvement in BAR after 6 wk of single-leg knee extensor training. Collectively, the mean age of the participants in the above studies was 63 yr. Thus, the present findings extend our current knowledge of exercise training effects on vascular function to men in their 9th and 10th decades of life. Importantly, the observed changes, in the present study, were quite consistent, in that, all trained arms increased the absolute change in vessel diameter after forearm occlusion. In contrast, there were no improvements in any of the control arms during the same period.
Whereas the present study was not designed to address the underlying mechanisms for change, the findings do provide direct evidence of the plasticity of the vasculature, even in those in their 9th and 10th decades of life. It is hypothesized (17) that the repetitive shear stress associated with muscle contractions signals formation of beneficial endothelial cell phenotype, including increased nitric oxide production, endothelial nitric oxide synthase (eNOS), prostacyclin (PGI2), antioxidant defenses, and a reduction in reactive oxygen species, adhesion molecules, and vasoconstriction factors (e.g., endothelin 1). That said, we acknowledge that the improvement in BAR may, in part, be the result of a change in the trigger for vasodilation. Indeed, both the reactive hyperemic flow response and estimated peak shear rate (23) were significantly higher at week 4 in the trained arm compared with those in the pretraining measures. This increase in postocclusion blood flow after training has been reported previously (2,18,25,37). We speculate that this increase may be secondary to regional adaptations including enhanced endothelial-dependent resistance vessel function (19) and changes in microcirculation (28,34). However, it is important to acknowledge that recent evidence indicates (24) that the continued shear stimulus imposed on the vessel wall before peak diameter response (“relevant” shear stimulus) is a more accurate predictor of BAR than the peak shear stimulus. A lack of change in relevant shear rate (AUC) in the trained arm, in the present study, suggests that the trigger for BAR did not change, yet the reactivity did. This finding would favor a change in the mechanisms underlying BAR.
Time course of change in BAR
Hypothesizing that handgrip training would indeed result in a significant increase in BAR, a second objective was to examine the time course of these vascular adaptations. Evidence for rapid changes in vasoreactivity with training were first reported by Wang et al. (32) who found an increase in vasodilatory responses in the circumflex coronary artery of dogs, owing to a greater release of endothelial-derived NO, after 7 d of exercise training. Studies conducted in our laboratory have confirmed these rapid changes in vasodilatory function in humans (1,2). For example, Allen et al. (1) observed a significant change in BAR after 4 d of handgrip training in young men using a similar training protocol. The findings from the current investigation indicate a statistically significant improvement in the BAR of the trained arm after 8 d of training. Importantly, the change in BAR after only 8 d of training occurred without significant changes in the trigger for dilation compared with pretraining. Thus, the present findings seem to confirm previous studies that indicate that vascular adaptations occur relatively quickly after the onset of training.
It is worth noting that the improvement in BAR was of lesser magnitude and slower in onset than our previous observations in young men (1). The reason for the slower response and lower magnitude in improvement in the current study is not clear but may include both protocol-related differences and/or physiologic differences between young and old. About protocol differences, it is evident that although the relative intensity of the exercise training was the same (60% MVC) between studies, the older men trained at a lower absolute workload (~20 kg in the old vs 26 kg in the young), perhaps suggesting the importance of training volume rather than merely exercise intensity. Thus, further research is needed to determine the threshold of training volume needed to elicit vascular adaptations. However, it is important to appreciate that the strength gains in the trained arm in the present study are nearly identical to those reported by Allen et al. (1) (6.3% increase in handgrip strength).
A second factor that may have influenced the magnitude of the training response in the present study is the size of the brachial artery diameter. Typically, the size of the brachial artery diameter is inversely related to BAR (5,14). Moreover, brachial artery diameter increases with age (14), perhaps due to structural modifications or increased resistance in smaller arterioles downstream. Thus, a lower magnitude of response to training could certainly be a consequence of the pretraining conditions. Specifically, the pretraining average brachial artery diameter in the present study (4.47 mm) is much higher than what Allen et al. (1) reported (3.38 mm), perhaps suggesting that older individuals are closer to their physiologic ceiling. However, the lower magnitude of response in this study could certainly be the consequence of age-related changes within the biological systems that underlie the vascular adaptations to exercise training. This hypothesis has been advanced by others (9) who report that nonfrail octogenarians who undergo strenuous endurance exercise experience attenuated adaptations in V̇O2max and insulin action compared with those between the ages of 60 and 70 yr. Our current findings suggest that this blunted adaptation may apply to the vasculature as well. Clearly, future efforts should focus on understanding the attenuation in the capacity to adapt to training in the elderly, as this may lead to determining the optimal mode and volume (intensity, duration, frequency) of exercise needed to stimulate physiological systems in the elderly.
Practical implications
We have previously discovered that vascular health is associated with measures of physical function in older adults (36). These prior findings fit “the disablement process” (31) and suggest that lower physical function may in part be the consequence of some defect in peripheral vascular function, which contributes to functional limitations and ultimately contribute to loss of dependence and disability in the elderly. The apparent specificity of the observed changes, within the present study, emphasizes the need for a well-rounded exercise program as outlined in the American College of Sports Medicine’s 1998 position statement. Interestingly, findings from studies in heart failure and peripheral arterial disease suggest that training-induced vascular improvements may contribute to improved heart function and aerobic capacity (12,13) and prognosis. Given our current observations, it seems that exercise training is an effective strategy to improve vascular function even in individuals in their 10th decade of life. Future efforts should focus on how/if exercise-induced improvements in vascular function contribute to the preservation of functional ability and independence in the elderly.
CONCLUSIONS
In conclusion, a localized short-term exercise program resulted in significant improvements in BAR of the trained arm of elderly men compared with the control arm. Furthermore, the findings indicate a statistical significant increase in BAR at the end of the second week of training, despite a similar trigger for dilation versus pretraining. The improved BAR was maintained throughout the remainder of the training period but may have, in part, been aided by a larger vasodilatory trigger toward the end of the study. These findings indicate the ability of the vasculature to respond favorably to an exercise stimulus even in individuals in their 9th and 10th decades of life.
Acknowledgments
The authors thank Daniel Credeur, Rachana Vasaiwala, and Kim Landry for their dedication, commitment, and technical support.
This research was supported by a grant from the National Institute on Aging (1 P01 AG022064) (S.M. Jazwinski).
Louisiana Healthy Aging Study: Meghan B. Allen, B.S.; Arturo M. Arce-Esquivel, M.D.; Mark A. Batzer, Ph.D.; Lauri Byerley, Ph.D.; Cathy Champagne, Ph.D.; Katie E. Cherry, Ph.D.; M. Elaine Cress, Ph.D.–Consultant; James P. DeLany, Ph.D.; Jenny Y. Denver, M.S.; Andy Deutsch, Ph.D.; Devon A. Dobrosielski, M.S.; Marla J. Erwin, M.A.; Elizabeth T. Fontham, Ph.D.; Madlyn Frisard, Ph.D.; Paula Geiselman, Ph.D.; Lindsey Goodwin; Tiffany Hall; Scott W. Herke, Ph.D.; Jennifer Hayden, M.S.; Kristi Hebert; Hui-Chen Hsu, Ph.D.; S. Michal Jazwinski, Ph.D.; Sangkyu Kim, Ph.D.; Beth G. Kimball, B.S.; Kim Landry; Daniel LaVie; Matthew Leblanc; Li Li, M.D.; Hui-Yi Lin, Ph.D., M.S.P.H.; Kay Lopez, D.S.N.; John D. Mountz, M.D., Ph.D.; Emily A. Olinde, M.A.; Kim B. Pedersen, Ph.D.; Eric Ravussin, Ph.D.; Paul Remedios; Yolanda Robertson, N.P.; Jennifer Rood, Ph.D.; Henry Rothschild, M.D., Ph.D.; Erin Sandifer; Beth Schmidt, M.S.; Robert Schwartz, M.D.–Consultant; Donald K. Scott, Ph.D.; Jennie L. Silva, M.A.; L. Joseph Su, Ph.D., M.P.H.; Jessica Thomson, Ph.D.; Crystal Traylor, A.P.R.N., M.S.N., W.H.N.P.; Cruz Velasco-Gonzalez, Ph.D.; Jerilyn A. Walker, MS.; David A. Welsh, M.D.; Michael A. Welsch, Ph.D.; Pili Zhang, Ph.D. (Louisiana State University, Baton Rouge; Pennington Biomedical Research Center, Baton Rouge; Louisiana State University Health Sciences Center, New Orleans; University of Alabama, Birmingham).
Footnotes
The results of the present study do not constitute endorsement by ACSM.
REFERENCES
- 1.Allen JD, Geaghan JP, Greenway F, Welsch MA. Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc. 2003;35(5):847–853. doi: 10.1249/01.MSS.0000064931.62916.8A. [DOI] [PubMed] [Google Scholar]
- 2.Alomari MA, Welsch MA. Regional changes in reactive hyperemic blood flow during exercise training: time-course adaptations. Dyn Med. 2007;6(1):1–6. doi: 10.1186/1476-5918-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 4.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100(10):1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 6.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24(2):471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 7.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 8.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 9.Evans EM, Racette SB, Peterson LR, Villareal DT, Greiwe JS, Holloszy JO. Aerobic power and insulin action improve in response to endurance exercise training in healthy 77–87 yr olds. J Appl Physiol. 2005;98(1):40–45. doi: 10.1152/japplphysiol.00928.2004. [DOI] [PubMed] [Google Scholar]
- 10.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112(5):674–682. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 11.Green DJ, Maiorana A, O’Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98(24):2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 13.Hambrecht R, Gielen S, Linke A, et al. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: a randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 14.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8(5):319–328. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 15.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med. 2001;250(1):29–36. doi: 10.1046/j.1365-2796.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- 16.Joannides R, Haefeli WE, Linder L, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–1319. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 17.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maiorana A, O’Driscoll G, Cheetham C, et al. Combined aerobic and resistance exercise training improves functional capacity and strength in CHF. J Appl Physiol. 2000;88(5):1565–1570. doi: 10.1152/jappl.2000.88.5.1565. [DOI] [PubMed] [Google Scholar]
- 19.Maiorana A, O’Driscoll G, Dembo L, Goodman C, Taylor R, Green D. Exercise training, vascular function, and functional capacity in middle-aged subjects. Med Sci Sports Exerc. 2001;33(12):2022–2028. doi: 10.1097/00005768-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Najjar SS, Scuteri A, Lakatta EG. Arterial aging: is it an immutable cardiovascular risk factor? Hypertension. 2005;46(3):454–462. doi: 10.1161/01.HYP.0000177474.06749.98. [DOI] [PubMed] [Google Scholar]
- 21.Ogawa T, Spina RJ, Martin WH, 3rd, et al. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86(2):494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 22.Proctor DN, Koch DW, Newcomer SC, Le KU, Smithmyer SL, Leuenberger UA. Leg blood flow and V̇O2 during peak cycle exercise in younger and older women. Med Sci Sports Exerc. 2004;36(4):623–631. doi: 10.1249/01.mss.0000121951.10417.b5. [DOI] [PubMed] [Google Scholar]
- 23.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102(4):1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 25.Rakobowchuk M, McGowan CL, de Groot PC, Hartman JW, Phillips SM, MacDonald MJ. Endothelial function of young healthy males following whole body resistance training. J Appl Physiol. 2005;98(6):2185–2190. doi: 10.1152/japplphysiol.01290.2004. [DOI] [PubMed] [Google Scholar]
- 26.Rinder MR, Spina RJ, Ehsani AA. Enhanced endothelium-dependent vasodilation in older endurance-trained men. J Appl Physiol. 2000;88(2):761–766. doi: 10.1152/jappl.2000.88.2.761. [DOI] [PubMed] [Google Scholar]
- 27.Rywik TM, Blackman MR, Yataco AR, et al. Enhanced endothelial vasoreactivity in endurance-trained older men. J Appl Physiol. 1999;87(6):2136–2142. doi: 10.1152/jappl.1999.87.6.2136. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki J, Kobayashi T, Uruma T, Koyama T. Time-course changes in arteriolar and venular portions of capillary in young treadmill-trained rats. Acta Physiol Scand. 2001;171(1):77–86. doi: 10.1046/j.1365-201X.2001.00384.x. [DOI] [PubMed] [Google Scholar]
- 29.Taddei S, Virdis A, Mattei P, et al. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91(7):1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 30.Takase B, Uehata A, Akima T, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. Am J Cardiol. 1998;82(12):1535–1539. doi: 10.1016/s0002-9149(98)00702-4. A7–8. [DOI] [PubMed] [Google Scholar]
- 31.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med. 1994;38(1):1–14. doi: 10.1016/0277-9536(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Wolin MS, Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73(5):829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- 33.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 34.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287(5):C1342–C1348. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 35.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Med Sci Sports Exerc. 2002;34(6):960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 36.Welsch MA, Dobrosielski DA, Arce-Esquivel AA, et al. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc. 2008;40(7):1237–1243. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wray DW, Uberoi A, Lawrenson L, Richardson RS. Evidence of preserved endothelial function and vascular plasticity with age. Am J Physiol Heart Circ Physiol. 2006;290(3):H1271–H1277. doi: 10.1152/ajpheart.00883.2005. [DOI] [PubMed] [Google Scholar]