Abstract
Previous studies have shown that resistance training with restricted venous blood flow (Kaatsu) results in significant strength gains and muscle hypertrophy. However, few studies have examined the concurrent vascular responses following restrictive venous blood flow training protocols.
Purpose
To examine the effects of 4 weeks of handgrip exercise training, with and without venous restriction, on handgrip strength and brachial artery flow mediated dilation (BAFMD).
Methods
Twelve participants (age=22±1yr; male = 5, female = 7), completed 4 weeks of bilateral handgrip exercise training (Duration: 20 min; Intensity: 60% of the MVC; Cadence: 15 grips*min−1; Frequency: 3 sessions*week−1). During each session venous blood flow was restricted in one arm (Experimental arm = EXP) using a pneumatic cuff placed 4 cm proximal to the antecubital fossa, and inflated to 80 mmHg for the duration of each exercise session. The EXP and control (CON) arm were randomly selected. Handgrip strength was measured using a hydraulic hand dynamometer. Brachial diameters and blood velocity profiles were assessed, using Doppler ultrasonography, before and after 5 min of forearm occlusion (200 mmHg), prior to and at the end of 4 weeks exercise.
Results
Following exercise training, handgrip strength increased 8.32% (p=0.05) in the CON arm and 16.17% (p=0.05) in the EXP arm. BAFMD increased 24.19% (p=0.0001) in the CON arm, and decreased 30.36% (p=0.0001) in the EXP arm.
Conclusion
The data indicate handgrip training combined with venous restriction results in superior strength gains, but reduced BAFMD compared to the non-restricted arm.
Keywords: Blood Flow Restriction, Regional Exercise, Vascular Function, Muscular Strength
Introduction
There have been an increasing number of reports in the literature regarding the effects of exercise training with deliberate restriction of venous blood flow, on skeletal muscle adaptations. This form of training, known as “occlusion-training” or Kaatsu, serves as a powerful stimulant for rapid increases in specific metabolic enzymes, muscle mass and strength (1, 20–22). In fact, the muscle adaptations seen with restrictive venous blood flow training protocols suggests the improvements can be accomplished with much lower intensities of exercise, which may represent an alternative method of training for individuals intolerant to higher intensity training protocols. Interestingly, few studies have examined the concurrent vascular responses following restrictive venous blood flow training protocols.
Findings from our laboratory have consistently reported that regional specific resistance training results in large conduit artery adaptations (2–3, 9). In addition, our data show a direct association between vascular and physical function (e.g. muscular strength) (26). The underlying trigger for such adaptations and associations are believed to be muscular contraction-induced increases in local shear forces which contribute to vascular modifications including endothelial mediated dilators (11). Given the consistent evidence that vascular function is linked to muscular strength (26), we anticipate that the muscular benefits with occlusion training would extend to the vasculature as well.
Thus, the purpose of the present study was to examine the effects of 4 weeks of handgrip exercise training combined with or without restricted venous blood flow on handgrip strength and brachial artery dimensions and vasodilation. We hypothesized that handgrip exercise training with venous restriction would result in superior strength gains and vasoreactivity, compared to the non-restricted arm.
Methods
Twelve participants (Age = 22±1 yr; male = 5, female = 7) were selected from the Kinesiology student body, at Louisiana State University. Before initiation of the study, subjects completed a medical history/health habits questionnaire. In addition, all subjects were familiarized with the equipment and experimental procedures. Exclusion criteria were any diagnoses or evidence of cardiovascular, metabolic, orthopedic, and/or neurological disease; active infection; risk for adverse responses to exercise; and/or taking any medication which may affect cardiovascular function. Each participant signed an informed consent approved by the institutional review board of the Louisiana State University and Agricultural and Mechanical College.
Assessment of Handgrip Strength
Handgrip strength was measured using a hydraulic hand dynamometer (Baseline ®; Irvington, NY). The subject was asked to perform a max voluntary contraction (MVC), standing with the dynamometer at ones side and gripping the dynamometer as hard as they could, for 3 seconds. This was repeated 3 times for each hand. The average of the 3 trials for each hand was considered to be the maximum voluntary handgrip strength. Forearm circumference was examined using a weighted measuring tape positioned 10 cm distal to the midpoint between the lateral epicondyle and olecranon process. All pre-training assessments were performed within the week prior to commencement of training. Handgrip strength trials were performed 5 minutes following ultrasound assessments. These tests were performed before, and following the final week of training. The right arm was assessed first each time and subjects were allowed one minute of rest between handgrip trials.
Assessment of Vascular Function and Blood Velocity Profiles
All brachial artery imaging was conducted by the same ultrasonographer in accordance with the “International Brachial Artery Reactivity Task Force” guidelines (5). Testing was performed between the hours of 7:00 – 11:00 am. Participants were required to refrain from caffeine prior to imaging. Subjects were also instructed to fast and refrain from strenuous activity for 12 hours, and alcohol for 48 hours. In addition, subjects completed a 24-hour history questionnaire recalling past meals, drinks, activities, sleep and medications taken. Baseline ultrasound images were obtained after 20 min of supine rest, in a dark, climate-controlled, quiet room (22–24°C) with the participants arm immobilized and slightly supinated and elevated. An additional 10 minute of rest was given prior to imaging the opposite arm. The right arm was imaged first in each case.
All brachial artery imaging and velocity profiles were obtained using a Hewlett-Packard Sonos 2000 (Bloomfield, CT) Doppler ultrasound, with a 7.5–MHz linear array transducer. Images were obtained in the longitudinal view, approximately 4 cm proximal to the olecranon process, in the anterior/medial plane. Image depth was set at 4 cm, and gain settings were adjusted to provide an optimal view of the anterior and posterior intimal interfaces of the artery and kept constant throughout. Doppler velocity profiles were collected simultaneously using a pulsed Doppler signal at a corrected insonation angle of 60° to the vessel, with the velocity cursor positioned to sample the volume, mid-artery.
Forearm occlusion consisted of inflation of a pneumatic cuff (E-20 rapid cuff inflator; AG-101 air source; D.E. Hokanson, Bellevue, WA) positioned approximately 1 cm distal to the olecranon process, inflated to 200 mmHg for 5 minutes. Images for vessel diameter and velocity profiles were obtained for 30 seconds at rest, and continuously, from the final 30 seconds of occlusion, until 2 minutes following the release of the blood pressure cuff. In addition, heart rate and blood pressure were monitored throughout the imaging process. Heart rate and Blood pressure were recorded using the ECG from the ultrasound and an automated blood pressure device (Datascope-Accutorr 4®, Mindray DS USA; Mahwah, NJ) prior to, during occlusion and following release of the pneumatic cuff. The ultrasound images were recorded digitally and saved on disc for subsequent off-line analysis.
An examination of the blood velocity profile during handgrip exercise was also performed. In one arm, a cuff was placed on the forearm, approximately 4 cm distal to the antecubital fossa. It should be noted that only 6 individuals participated in the experiment to examine blood velocity patterns during exercise, with restricted venous blood flow, after completion of the study. Three females and 3 males were selected for the blood velocity during exercise experiment. The purpose of this additional experiment was to assess the impact of partial forearm vascular occlusion on brachial artery blood velocity patterns. It should be noted that the magnitude of blood flow was not pertinent to this particular investigation, only the pattern of the velocity profiles (e.g. ante- and retrograde profiles). Additionally, when performing the Doppler ultrasound imaging of the brachial artery during handgrip exercise, the blood pressure cuff was placed distal to the position that was used during the actual training intervention. This distal position was chosen to facilitate the placement of the ultrasound probe. The training session followed the same protocol as the study (See Exercise Training, section). A total of five, 10 sec ultrasound images were recorded in each arm; at rest, during 5, 10, 15 and 20 min of exercise.
Data Analysis
Off-line analyses of diameters were analyzed similar to previously published reports (9, 26), using a semi-automated edge-detecting software, Brachial Analyzer (Medical Imaging Applications, LLC; Coralville, IA). The reproducibility of this technique in our laboratory has yielded average mean differences in brachial artery diameter change for days and testers of 1.91% and 1.4%, respectively, with intra-class correlation coefficients of 0.92 and 0.94, respectively (25). Arterial diameters were calculated as the mean distance between the anterior and posterior wall at the blood vessel interface, with the image in diastole, defined as the peak of the r-wave on the electrocardiograph. Resting diameter was defined by the average of 30 seconds of data obtained after 20 minutes of resting conditions. Peak dilation was defined as the largest diameter following release of the occluding cuff. Finally, brachial artery flow mediated dilation (BAFMD) was defined as the percent change in vessel diameter from rest to peak diameter post forearm occlusion.
Blood velocity profiles were analyzed similar to previously published reports (6, 9). Each profile was traced using Image Pro Plus 4.0 software (Media Cybernetics; Bethesda, MD). The antegrade component was defined as the area of tracing above 0 cm/s from the Doppler ultrasound scale and the retrograde component was defined as the area below. The velocity profiles were then divided by the ejection time (s) from that cardiac cycle to subsequently determine the mean velocity (cm/s). The mean velocity (Vmean) during baseline was calculated as the difference between the antegrade and retrograde velocity components. To establish an estimate of oscillatory flow patterns, a ratio was taken between the antegrade and retrograde flow velocities (ante-/retrograde ratio) (6). Shear rate (4 * Vmean (cm/s) / diameter (cm)) was measured at 10 second intervals during reactive hyperemia up to the point of maximum vessel diameter and plotted against time (s). A trapezoidal model was then used to calculate area under the curve (AUC) above baseline (9, 19). During a handgrip training session three blood velocity profiles were traced within 2 minutes of each time point (at rest, during 5, 10, 15 and 20 min of exercise), prior to a muscular contraction and averaged to determine flow velocity patterns during each time point of exercise.
Exercise Training
Exercise training involved gripping a hydraulic hand dynamometer (Baseline®; Irvington, NY) and contracting the forearm at a rate of 15 times*min−1 (1 contraction every 4 sec) at the pace of an electronic metronome, and a resistance of 60% of MVC. The intensity was marked on the gauge of the handgrip dynamometer using a dry-erase marker. The subjects were asked to train for 20 min, 3 days*week−1 for 4 weeks, at Louisiana State University, under the supervision of a lab technician. Throughout the study, the participants were positioned facing two small mirrors to allow for a visual reference of the handgrip dynamometer. Subjects trained both hands, at the same time. However, for one of the limbs the pneumatic blood pressure cuff was placed on the upper arm, 4 cm proximal to the antecubital fossa. The decision which arm would receive the occlusion during training was randomized to avoid a dominant or non-dominant hand bias.
During the training this blood pressure cuff was partially inflated (80 mmHg) to ensure venous occlusion. Although some arterial inflow may have been restricted, the purpose was to induce venous pooling within the forearm vasculature. Previous work utilizing restricted venous blood flow in the legs, have indicated that the application of 100 mmHg was significant enough to restrict venous blood flow and cause venous pooling in the thighs distal to the cuff (14). The current study examined venous restriction in the forearm, and the application of 100 mmHg caused discomfort and exercise intolerance during handgrip training. An occlusion pressure of 80 mmHg was tolerable and sufficient, given that the average resting diastolic blood pressure in the EXP arm for the participants was ~71 mmHg. Subjects were allowed to take 1 min rest periods, after the completion of 5 minutes of training while the cuff remained inflated, but were encouraged to progress through each session.
Statistical Analysis
Statistical analyses were performed using SPSS for Windows (version 17.0). Data are presented as mean ± standard deviation. To determine the effects of the four weeks of handgrip exercise training on handgrip strength and BAFMD, a two (EXP arm vs. CON arm) by two (pre-training vs. post-training) repeated-measures ANCOVA was performed, using the baseline (pre-training) measures as the covariate. To determine gender differences for the magnitude of change in BAFMD and handgrip strength following exercise training, an ANCOVA was performed (Males vs. Females), using the baseline (pre-training) measures, MVC and BAFMD, as the covariate. To examine the change in blood velocity patterns (ante-/retrograde ratio and shear rates) during exercise training, a subsequent two (EXP arm vs. CON arm) by five (Velocity at rest, 5, 10, 15 and 20 minutes of exercise) repeated-measures ANOVA was performed. Differences between means were evaluated using a post-hoc LSD test. An alpha level of p≤0.05 was required for statistical significance.
Results
Twelve participants completed all facets of this study. All individuals were free of symptoms indicative of chronic illness. No one was taking any vascular medication that may influence the results. The baseline characteristics of these individuals are presented in Table 1. Resting systolic and diastolic blood pressure for the participants (pre and post training) averaged 116±9 / 71±8 and 113±8 / 71±7 mmHg, respectively. The average height for males and females in the present study was 185.93±4.89 cm and 168.37±6.8 cm, respectively. The average weight for the males and females was 94.09±13.02 kg and 68.7±10.95 kg, respectively.
Table 1.
Participant Characteristics
| Mean | SD | |
|---|---|---|
| Age (yrs) | 22 | 1 |
| Height (cm) | 175.68 | 10.54 |
| Weight (kg) | 79.38 | 16.88 |
| BMI (kg/m2) | 25 | 3.3 |
| Resting Heart Rate (beats/min) | 66 | 6 |
| Pulse Pressure (mmHg) | 45 | 11 |
Values are mean ± SD for 12 participants
Handgrip Strength and Forearm Circumference
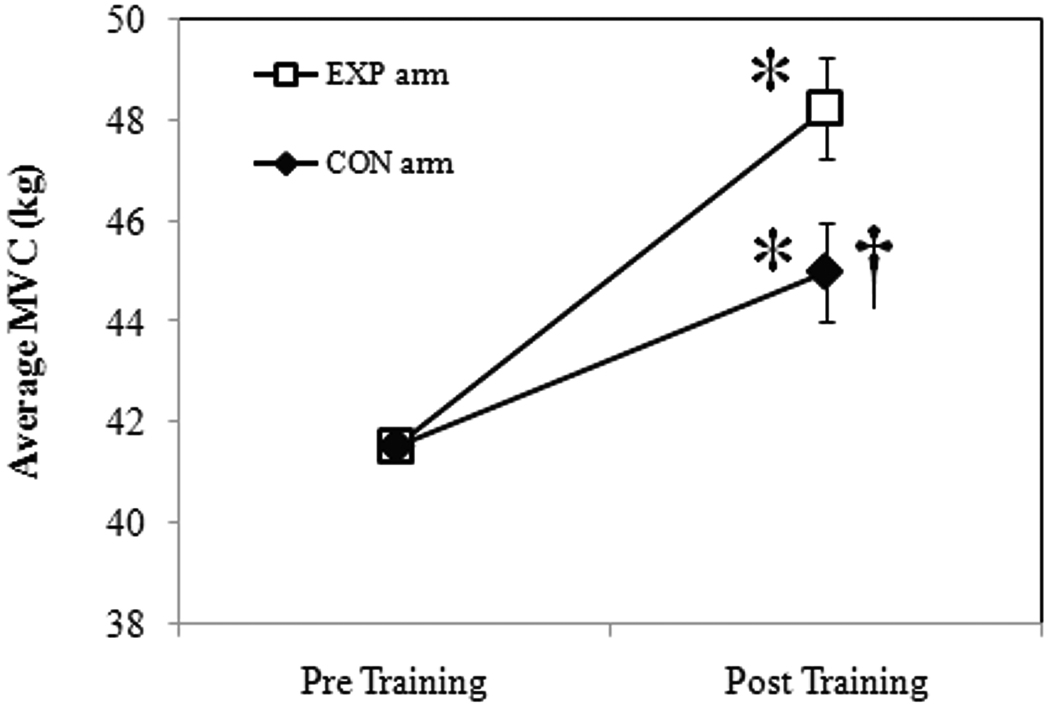
All subjects completed a total of twelve training sessions. There were no significant strength or circumference differences between arms prior to training. The results of the ANCOVA for handgrip strength (covariate=baseline MVC), revealed an increase in strength for the CON arm (8.32%, p=0.05) and the EXP arm (16.17%, p=0.05). These results are illustrated in Figure 1 as the mean change in handgrip strength (kg) between study arms, following 4 weeks of handgrip exercise. It should be noted that there was no significant gender difference between males and females for the magnitude of change in handgrip strength (p=0.36). Following training, there was also a significant increase in forearm circumference for the CON (1.62%: Pre=24.80 vs. Post=25.20 cm, p=0.05) and EXP arms (2.42%: Pre=24.80 vs. Post=25.40 cm, p=0.05).
Figure 1.
Mean change in handgrip strength at baseline (pre-training) and following 4 weeks (post-training) of exercise. Data are presented as mean ± SE. *Significant vs. baseline; †Significant vs. EXP Arm, p≤0.05.
Assessment of Vascular Function and Blood Velocity Profiles
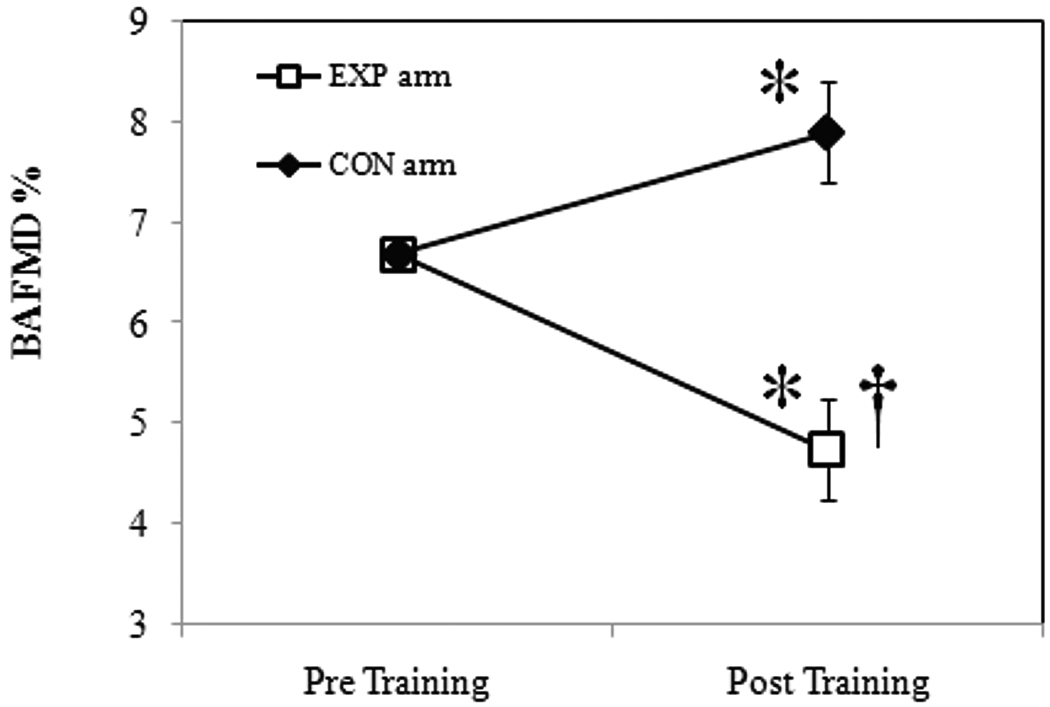
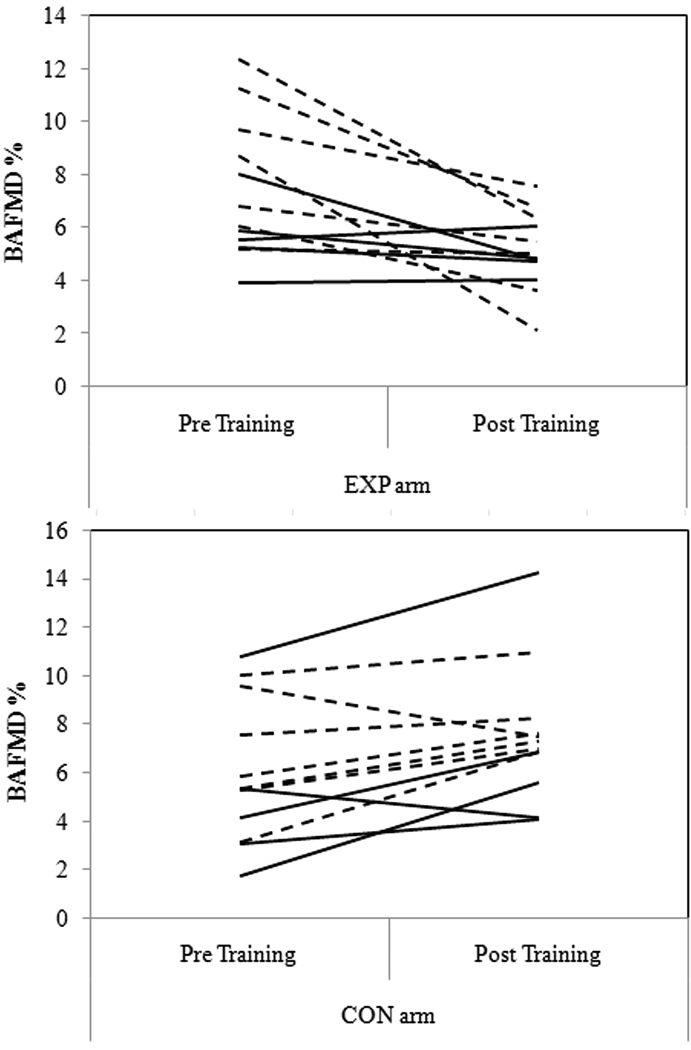
Values for vascular diameters are presented in Table 2. No significant differences in baseline brachial diameter, BAFMD or velocity profiles were noted between arms prior to training. The average baseline brachial diameters for males were; EXP arm = 4.21±0.34 mm and CON arm = 4.29±0.25 mm, and for females; EXP arm = 3.45±0.42 mm, CON arm = 3.54±0.6 mm. Figure 2 presents the results from the ANCOVA for BAFMD, pre- and post-training. BAFMD increased in the CON arm (24.19%, p=0.0001; Absolute change: pre-training=0.22±0.01 mm vs. post-training=0.29±0.11 mm), and decreased in the EXP arm (30.36%, p=0.0001; Absolute change: pre-training=0.27±0.07 mm vs. post-training=0.19±0.06 mm). There were no significant gender differences for the magnitude in change in BAFMD (p=0.11). Figure 3 presents the actual BAFMD values, between study arms, for the individuals following 4 weeks of handgrip exercise. Mean values for blood velocity profiles and shear AUC, pre- and post training, are presented in Table 3.
Table 2.
Vascular diameters at baseline and following 4 weeks of exercise
| Baseline (Pre-training) | Week 4 (post-training) | |
|---|---|---|
| EXP Arm | ||
| Rest Diameter (mm) | 3.76 ± 0.54 | 3.84 ± 0.66 |
| Peak Diameter (mm) | 4.04 ± 0.53 | 4.03 ± 0.68 |
| CON Arm | ||
| Rest Diameter (mm) | 3.85 ± 0.61 | 3.87 ± 0.62 |
| Peak Diameter (mm) | 4.07 ± 0.6 | 4.35 ± 1* |
Values are mean ± SD for 12 participants.
Significant vs. baseline, p ≤ 0.05
Figure 2.
Mean change in BAFMD at baseline (pre-training) and following 4 weeks (post-training) of exercise. Data are presented as mean ± SE. *Significant vs. baseline; †Significant vs. CON Arm, p≤0.05.
Figure 3.
Individual BAFMD values, between study arms, at baseline (pre-training) and following 4 weeks (post training) of handgrip exercise. Solid lines represent male participants, while dashed lines represent female participants.
Table 3.
Blood velocity profiles (resting) and shear stimulus (post cuff release) at baseline and following 4 weeks of exercise
| Baseline (Pre-training) | Week 4 (post-training) | |
|---|---|---|
| EXP Arm | ||
| Antegrade Velocity (cm/s) | 16.5 ± 5.9 | 18.6 ± 6.4 |
| Retrograde Velocity (cm/s) | 3.9 ± 4 | 2.7 ± 3.2 |
| Ante-/Retrograde Ratio (AU) | 15.3 ± 19.9 | 10.34 ± 7 |
| Vmean (cm/s) | 12.6 ± 7.2 | 15.92 ± 7 |
| Shear Stimulus (AUC) | 5472.9 ± 1216.9 | 6776.7 ± 1313.2* |
| CON Arm | ||
| Antegrade Velocity (cm/s) | 19.5 ± 8.7 | 14.7 ± 6.3 |
| Retrograde Velocity (cm/s) | 3.4 ± 3.4 | 3.4 ± 2.3 |
| Ante-/Retrograde Ratio (AU) | 24.9 ± 38.8 | 15.5 ± 27.5 |
| Vmean (cm/s) | 16 ± 10.9 | 11.3 ± 6.7 |
| Shear Stimulus (AUC) | 6579.2 ± 1475.7 | 7664.1 ± 2568.8* |
Values are mean ± SD for 12 participants
Significant vs. baseline, p ≤ 0.05
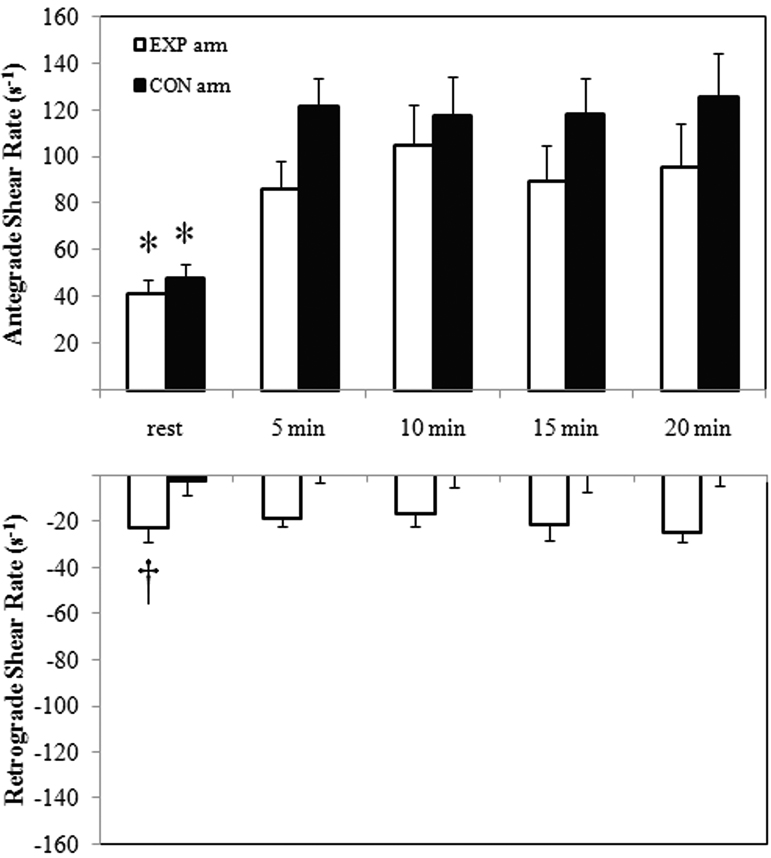
An examination of the blood velocity pattern during exercise revealed upon inflation of the cuff, prior to exercise, the retrograde flow velocity component increased (Pre-inflation=1.27 cm/s vs. Post-inflation=13.49 cm/s, p=0.03), and antegrade velocity decreased (Pre-inflation=17.96 cm/s vs. Post-inflation=14.68 cm/s, p=0.05). Consequently, the ante-/retrograde ratio decreased significantly (Pre-inflation=14.14 cm/s vs. Post-inflation=1.09 cm/s, p=0.05) following cuff inflation in the EXP arm. Furthermore, the retrograde flow velocity component remained for the occluded arm, with no such evidence in the non-occluded arm. Figure 4 illustrates the mean antegrade and retrograde shear rate (s−1) values during the training session.
Figure 4.
Examination of Ante- and Retrograde shear (s−1) in the brachial artery, at rest and during 20 minutes of handgrip exercise, in the EXP and CON arm. *Significant vs. antegrade shear rates during exercise; †Significant vs. CON at rest, p≤0.05.
Discussion
The present study examined the effects of 4 weeks of handgrip exercise training, coupled with and without restricted venous blood flow, on handgrip strength and brachial artery vasodilation. The data indicate that forearm exercise training combined with restricted venous blood flow results in a significantly different change in muscular strength (superior strength gains) and vascular function (reduced vasodilation) compared to the non-restricted arm.
Handgrip Strength and Forearm Circumference
The present study observed significant improvements in handgrip strength and forearm circumference following regional specific handgrip training. These results compare to others who have observed the effects of regional specific exercise on handgrip strength changes (2–3). Alomari and Welsch (2007) reported a 14.5% improvement in grip strength following 4 weeks (5 sessions per week) of handgrip exercise training (3). Interestingly, the present study reports a significantly greater improvement in grip strength in the EXP arm compared to the CON arm.
Previous studies have shown that combining resistance training with restricted venous blood flow results in significant improvements in muscle size and strength (20–22). Consistent with this statement, the present study confirms a 50% greater improvement in handgrip strength in the EXP arm compared to the control. Mechanisms which could have contributed to this increase in strength are not entirely understood, but have been proposed to be the consequence of an upregulation in specific growth factors (e.g. IGF-I) (1) specific metabolic enzymes (e.g. creatine phosphokinase- CPK) (1), the result of hormonal changes (e.g. increase in growth hormone) (1), and/or a preferential recruitment of larger, fast motor units (17, 20).
Vascular Function and Blood Velocity Profiles
Brachial artery resting diameter, prior to training, was similar between arms and to previous reports from our laboratory (2, 8), and significantly associated with BAFMD prior to training (r=0.57, p=0.04). Moreover, the average vasodilatory response following 5 minutes of occlusion, prior to training, were also similar between arms, and in agreement with Dobrosielski et al. (2006), who reported a BAFMD value of 7.7±3.5% in healthy adults (Age=28±8 yrs)(8).
The present data indicate that following 4 weeks of handgrip exercise training BAFMD improved 24.19% in the CON arm. In fact, 10 of 12 arms showed improvements after training, indicating the consistency of the adaptive response. However, the observed improvement is less than previously reported by Allen et al. (2003). In that study, BAFMD improved 62% following 4 weeks of handgrip exercise training using a protocol of 20 min*day−1, 5 days*week−1 (2). The discrepancy between studies could in part be the result of a significantly lower volume of training, in the present study (~50% less).
Typically, improvements in BAFMD with exercise training are believed to be secondary to the changes in shear stress induced by the muscular contractions during the acute bouts of exercise (11). The muscular contraction induced changes in shear stress are thought to alter the endothelial machinery involved in vasodilatory pathways, including increased nitric oxide production, eNOS, PGI2, anti-oxidant defenses, and a reduction in reactive oxygen species, adhesion molecules, and vasoconstriction factors (e.g. endothelin-1) (11, 13, 15). We also acknowledge that the improvement in BAFMD, in the present study, may also be the result of endothelial independent changes in resistance vessel function and microcirculation (11) as the relevant shear stimulus was higher at week 4 in both the CON and EXP arm compared to the pre-training measures. The fact that in the EXP arm BAFMD declined after training despite higher shear AUC is intriguing and suggests a significant alteration in the vasodilatory response to a larger trigger.
Uniquely, the present study observed a significant reduction in BAFMD in the EXP arm. The reduction was apparent in 9 of 12 arms, and appeared to be greatest in those who had the highest pre-training BAFMD, suggesting those individuals “suffered” to a greater extent than those who had lower vascular function prior to training. The fact that the change in vascular function is completely in the opposite direction of the CON arm is quite intriguing. Interestingly, a recent study examined oxidative stress in response to partial vascular occlusion of the upper arm in young men (10). Goldfarb et al. (2008) found an elevation in oxidative stress, as defined by a glutathione ratio and plasma protein carbonyls, in response to moderate intensity resistance training, as well as, with prolonged vascular occlusion (e.g. 20 mmHg less than resting diastolic pressure) (10). The present study utilized a similar occlusion pressure and exercise training intensity, which could have resulted in an elevation in oxidative stress within the EXP arm. This is important considering the inherent effects of oxidative stress on endothelial function (12–13).
An additional explanation for the decline in BAFMD observed in the EXP arm stems from the hypothesis that regular exposure to exercise induced increases in endothelial “shear stress” is the primary signal for a positive expression of endothelial function (11, 13, 15–16, 27). A reduction or alteration of the shear stimulus during exercise may compromise the vascular adaptive response. In fact, in the present study, upon inflation of the cuff and prior to exercise in the EXP arm, the retrograde velocity component increased and the antegrade velocity component decreased. Consequently, the ante-/retrograde ratio decreased significantly following cuff inflation in the EXP arm. The fact that handgrip exercise with restricted venous blood flow has such a large retrograde component, in comparison to antegrade, suggests that there may be a significant change in oscillatory flow patterns and shear stress (6, 16, 23–24). Such a shear stress is thought to promote a proatherogenic phenotype and oxidative stress within the endothelium, resulting in a decline in vascular function (7, 16).
The examination of flow velocity patterns during exercise should be interpreted within the constraints of potential limitations. Our study utilized a non-invasive measure of blood velocity and flow. It is difficult to establish an estimate of oscillatory flow patterns within a vessel with 2 dimensional imaging. However, it is interesting to see that these patterns change when exposed to an increase in resistance (e.g. restricted venous blood flow). The finding that the flow patterns are changed during restrictive flow conditions are in agreement with Tinken et al. (2009), who reported that during handgrip exercise, cuffed arm retrograde flow was higher than in the non-cuffed (24). In fact, BAFMD was not significantly changed in the cuffed arm in response to acute bouts of heating, handgrip, and cycling (24). In another study, Thijssen et al. (2009) reported that acute alterations in flow velocity patterns, at rest, resulted in a reduction in subsequent measures of BAFMD. In that study, it was noted that with increasing occlusion pressures (50–75 mmHg), there was a dose response reduction in reactivity of the brachial artery (23). The apparent reduction in reactivity seen with Thijssen et al. (2009) may also have been, in part, the consequence of a change in pressure exerted upon the arterial wall (18). Interestingly, Padilla et al. (2009) observed a decline in vascular reactivity in the brachial artery when acutely exposed to an increase in hydrostatic pressure (18).
Clinical Relevance and Future Recommendations
Finally, from a clinical perspective, it is critical to understand that alterations in regional blood flow patterns, due to vascular disease (peripheral vascular disease), may affect the exercise adaptation. Perhaps, a change in regional flow patterns in patients with peripheral arterial disease (PAD) may explain why they experience an acute inflammatory response with a subsequent reduction in BAFMD, following an acute bout of exercise (4). Given this evidence, future studies should investigate the effects of moderate intensity exercise training with partial vascular occlusion on markers of oxidative stress (e.g. peroxynitrite, superoxide, and reactive oxygen species) and endothelial function (e.g. flow mediated dilation). In addition, given the milieu of hemodynamic forces exerted upon the arterial wall, future investigations should continue to target the underlying mechanisms involved in vascular responses and adaptation.
Conclusion
These data indicate that forearm exercise training combined with restricted venous blood flow results in a significant increase in muscular strength, coupled with a significant decrease in vascular function (reduced vasodilation). The contrasting change in vascular function following exercise training with venous blood flow restriction in the forearm may, in part, be the consequence of significant alterations in blood flow patterns during handgrip exercise.
Acknowledgements
The authors would like to thank Travis Godawa, Anna Lyons and Vanessa Dueñas for their dedication, commitment, and technical support.
This research was supported by a grant from the National Institute on Aging (1 P01 AG022064) (S.M. Jazwinski).
The results of the present study do not constitute endorsement by ACSM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abe T, Kearns CF, Sato Y. Muscle size and strength are increased following walk training with restricted venous blood flow from the leg muscle, Kaatsu-walk training. J Appl Physiol. 2006;100(5):1460–1466. doi: 10.1152/japplphysiol.01267.2005. [DOI] [PubMed] [Google Scholar]
- 2.Allen JD, Geaghan JP, Greenway F, Welsch MA. Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc. 2003;35(5):847–853. doi: 10.1249/01.MSS.0000064931.62916.8A. [DOI] [PubMed] [Google Scholar]
- 3.Alomari MA, Welsch MA. Regional changes in reactive hyperemic blood flow during exercise training: time-course adaptations. Dyn Med. 2007;6(1) doi: 10.1186/1476-5918-6-1. PMCID: 1779772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreozzi GM, Leone A, Laudani R, Deinite G, Martini R. Acute impairment of the endothelial function by maximal treadmill exercise in patients with intermittent claudication, and its improvement after supervised physical training. Int Angiol. 2007;26(1):12–17. [PubMed] [Google Scholar]
- 5.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 6.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: relationship to physical function. Eur J Appl Physiol. 2009;107(2):219–225. doi: 10.1007/s00421-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a superoxide-producing NADH oxidase. Circ Res. 1998;82(10):1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- 8.Dobrosielski DA, Arce AA, Allen JD, Wood RH, Welsch MA. Biphasic responses of the brachial artery diameter following forearm occlusion: a blunted response in the elderly. Dyn Med. 2006;5:4. doi: 10.1186/1476-5918-5-4. PMCID: 1456951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrosielski DA, Greenway FL, Welsh DA, Jazwinski SM, Welsch MA. Modification of vascular function after handgrip exercise training in 73- to 90-yr-old men. Med Sci Sports Exerc. 2009;41(7):1429–1435. doi: 10.1249/MSS.0b013e318199bef4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldfarb AH, Garten RS, Chee PD, Cho C, Reeves GV, Hollander DB, Thomas C, Aboudehen KS, Francois M, Kraemer RR. Resistance exercise effects on blood glutathione status and plasma protein carbonyls: influence of partial vascular occlusion. Eur J Appl Physiol. 2008;104(5):813–819. doi: 10.1007/s00421-008-0836-1. [DOI] [PubMed] [Google Scholar]
- 11.Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561(Pt 1):1–25. doi: 10.1113/jphysiol.2004.068197. PMCID: 1665322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73(3):411–418. doi: 10.1253/circj.cj-08-1102. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Yoshizumi M. Exercise and endothelial function: role of endothelium-derived nitric oxide and oxidative stress in healthy subjects and hypertensive patients. Pharmacol Ther. 2004;102(1):87–96. doi: 10.1016/j.pharmthera.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Iida H, Kurano M, Takano H, Kubota N, Morita T, Meguro K, Sato Y, Abe T, Yamazaki Y, Uno K, Takenaka K, Hirose K, Nakajima T. Hemodynamic and neurohumoral responses to the restriction of femoral blood flow by KAATSU in healthy subjects. Eur J Appl Physiol. 2007;100(3):275–285. doi: 10.1007/s00421-007-0430-y. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin MH. Endothelium-mediated control of coronary vascular tone after chronic exercise training. Med Sci Sports Exerc. 1995;27(8):1135–1144. [PubMed] [Google Scholar]
- 16.Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104(3):588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myer RA. Does blood flow restriction enhance hypertrophic signaling in skeletal muscle? J Appl Physiol. 2006;100(5):1443–1444. doi: 10.1152/japplphysiol.01636.2005. [DOI] [PubMed] [Google Scholar]
- 18.Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol. 2009;297(3):H1103–H1108. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 19.Pyke KE, Tschakovsky ME. Peak vs. total reactive hyperemia: which determines the magnitude of flow-mediated dilation? J Appl Physiol. 2007;102(4):1510–1519. doi: 10.1152/japplphysiol.01024.2006. [DOI] [PubMed] [Google Scholar]
- 20.Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Takahashi T, Omokawa M, Kinugawa S, Tsutsui H. Intramuscular metabolism during low-intensity resistance exercise with blood flow restriction. J Appl Physiol. 2009;106(4):1119–1124. doi: 10.1152/japplphysiol.90368.2008. [DOI] [PubMed] [Google Scholar]
- 21.Sundberg CJ. Exercise and training during graded leg ischaemia in healthy man with special reference to effects on skeletal muscle. Acta Physiol Scand Suppl. 1994;615:1–50. [PubMed] [Google Scholar]
- 22.Takarada Y, Takazawa H, Sato Y, Takebayashi S, Tanaka Y, Ishii N. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J Appl Physiol. 2000;88(6):2097–2106. doi: 10.1152/jappl.2000.88.6.2097. [DOI] [PubMed] [Google Scholar]
- 23.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53(6):986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 24.Tinken TM, Thijssen DH, Hopkins N, Black MA, Dawson EA, Minson CT, Newcomer SC, Laughlin MH, Cable NT, Green DJ. Impact of shear rate modulation on vascular function in humans. Hypertension. 2009;54(2):278–285. doi: 10.1161/HYPERTENSIONAHA.109.134361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsch MA, Allen JD, Geaghan JP. Stability and reproducibility of brachial artery flow-mediated dilation. Med Sci Sports Exerc. 2002;34(6):960–965. doi: 10.1097/00005768-200206000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Welsch MA, Dobrosielski DA, Arce-Esquivel AA, Wood RH, Ravussin E, Rowley C, Jazwinski SM. The association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc. 2008;40(7):1237–1243. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Z, Zhang Z, Ranjan V, Diamond SL. Shear stress induction of the endothelial nitric oxide synthase gene is calcium-dependent but not calcium-activated. J Cell Physiol. 1997;171(2):205–211. doi: 10.1002/(SICI)1097-4652(199705)171:2<205::AID-JCP11>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]