Abstract
Polymorphisms in the cytochrome P450 2C9 (CYP2C9) and vitamin K epoxide reductase complex subunit 1 (VKORC1) genes significantly alter the effective warfarin dose. We determined the frequencies of alleles, single carriers, and double carriers of single nucleotide polymorphisms (SNPs) in the CYP2C9 and VKORC1 genes in a Puerto Rican cohort and gauged the impact of these polymorphisms on warfarin dosage using a published algorithm. A total of 92 DNA samples were genotyped using Luminex® x-MAP technology. The polymorphism frequencies were 6.52%, 5.43% and 28.8% for CYP2C9 *2, *3 and VKORC1-1639 G>A polymorphisms, respectively. The prevalence of combinatorial genotypes was 16% for carriers of both the CYP2C9 and VKORC1 polymorphisms, 9% for carriers of CYP2C9 polymorphisms, 35% for carriers of the VKORC1 polymorphism, and the remaining 40% were non-carriers for either gene. Based on a published warfarin dosing algorithm, single, double and triple carriers of functionally deficient polymorphisms predict reductions of 1.0–1.6, 2.0–2.9, and 2.9–3.7 mg/day, respectively, in warfarin dose. Overall, 60% of the population carried at least a single polymorphism predicting deficient warfarin metabolism or responsiveness and 13% were double carriers with polymorphisms in both genes studied. Combinatorial genotyping of CYP2C9 and VKORC1 can allow for individualized dosing of warfarin among patients with gene polymorphisms, potentially reducing the risk of stroke or bleeding.
Keywords: Warfarin, CYP2C9, VKORC1, Genotyping, Personalized Medicine
INTRODUCTION
Warfarin is an oral anticoagulant considered as the standard-of-care therapy for many thromboembolic disorders. 1–2 More than 24 million prescriptions for warfarin were written in United States in 2007.3–4 Warfarin is frequently associated with unpredictable responses, ranging from occult bleeding to hemorrhage, due in part to a narrow therapeutic index. Therefore, its activity has to be monitored by frequent blood testing for the international normalized ratio and adjustments are often necessary.2
Difference in successful outcomes during warfarin therapy is a multi-factorial issue.5–7 The individual’s unique genetic make-up also plays a cardinal role in the warfarin response. The first gene to be identified as affecting warfarin dose-response was CYP2C9. This gene encodes a particular isoform of CYP450 enzyme, responsible for metabolizing S-warfarin. Approximately 25%–35% of the population have CYP2C9 variants that lead to variably deficient enzyme activity. These variants can cause alterations in initial warfarin dose sensitivities, delays in achieving stable maintenance doses and increased bleeding complications.8–10 Polymorphisms of CYP2C9 include CYP2C9*2 and CYP2C9*3, which are associated with reduced enzyme activity to 70% and 5% of the normal level, respectively. 8,9,11–13 The result is warfarin accumulation and possible hemorrhagic complications.14 The variants have a significant impact on warfarin metabolism in several populations.8–10,15–21 The CYP2C9 status by itself accounts for approximately 15%–20% of the variance in warfarin dose.13,21–22
Warfarin exerts an anticoagulant effect through its inhibition of the VKORC1 gene product.23–25 Patients who are carriers of a common polymorphism in the VKORC1 promoter sequence (− 1639 G>A) require a lower warfarin maintenance dosage.26–27 The − 1639 G>A genotype and related haplotype can independently determine 20%–25% of warfarin dose variance. 21,27 Together, the CYP2C9 and VKORC1 combinatorial genotypes may explain up to 45% of warfarin response variability.10,16,22,27
Current approaches to warfarin induction fail to prevent adverse events. The major flaw of existing warfarin dosing algorithms is that they rely on trial-and-error after an initial warfarin dose of 2 to 10 mg, rather than being tailored to individual genetic and clinical factors. Using pharmacogenetic-based warfarin therapy, clinicians can now estimate a priori the therapeutic dose by genotyping their patients for single-nucleotide polymorphisms (SNPs) that affect warfarin metabolism or sensitivity. Hence, pharmacogenetic-based therapy could reduce medical expenses by preventing inpatients from being kept in the hospital until their therapeutic dose has been determined empirically.
In 2007, the Food and Drug Administration revised the warfarin label to include the impact of the CYP2C9 and VKORC1 polymorphisms in a pharmacogenomic subsection.28 Major and fatal bleeding events occur at rates of 7.2 and 1.3/100 patient-years, respectively, according to a meta-analysis. 29 If genotyping were performed before warfarin prescription, 85,000 serious bleeding events and 17,000 strokes could be avoided annually in the United States alone, saving more than $1 billion in healthcare spending.30 The US National Heart, Lung, and Blood Institute is currently sponsoring a prospective genotype-guided warfarin dosing protocol.31
The primary goal of this study was to determine the frequency of combinations of the CYP2C9 and VKORC1 deficient and null polymorphisms in a Puerto Rican cohort. The results were used for comparison to a cohort of cardiovascular outpatients at Hartford Hospital. The impact on population-wide dose adjustment of the observed prevalence of carriers for combinatorial genotypes is also discussed.
MATERIALS AND METHODS
Study Cohort
One hundred purified human DNA samples present in dried blood spots were used. The dried blood samples were supplied by the Puerto Rico Newborn Screening Program (PRNSP). The analysis was based on a controlled stratified-by-region representative sampling from the target population. Consequently, dried blood samples came from different medical centers in Puerto Rico and were randomly chosen.
Laboratory Analysis
Genomic DNA samples were extracted and purified using Generation DNA Purification kit (QIAGEN Inc., CA, US) following the manufacturer’s protocol. Extracted DNA was stored at −80°C in TRIS-EDTA buffer. Quantification of DNA was performed by fluorescent staining of double-stranded DNA (PicoGreen® dsDNA Quantitation Kit, Molecular Probes, Eugene, OR, US). Fluorescent intensity was measured using a fluorescent micro-titer plate reader (POLARstar OPTIMA, BMG-LABTECH GmbH, Offenburg, Germany).
Genotyping of the CYP2C9 and VKORC1 genes at 12 variable sites, 5 SNPs in CYP2C9 and 7 SNPs in VKORC1, was performed at Genomas (Laboratory of Personalized Health, Hartford, CT, US). The Tag-It™ Mutation Detection assays (Luminex Molecular Diagnostics, Toronto, Canada) were utilized for genotyping. A full explanation of this assay can be found elsewhere.32,33
Statistical Analysis
Allele frequencies (distribution) were determined in the Puerto Rican population for the loci of interest. Test for deviations from Hardy-Weinberg equilibrium (HWE) were used. Departure from HWE were estimated under the null hypothesis of the predictable segregation ratio of specific matching genotypes (P>.05) by use of χ2 goodness-of-fit test with one degree of freedom.
To explore the population-wide impact of polymorphisms on warfarin dose, we classified patients by VKORC1 and CYP2C9 combinatorial genotypes. Warfarin dose was estimated for each combinatorial genotype, using a published equation,16 by assuming a reference idealized person, aged 55 years, male or female with BMI=27. Two values were generated for each combinatorial genotype, incorporating the respective correction factor for sex. The reduction in dose for each combinatorial genotype was calculated as the difference between the dose predicted for that combinatorial genotype and the dose predicted for the wild-type genotype.
RESULTS
The results for the CYP2C9 SNPs are shown in Table 1. The frequency of the alleles *2 and *3 were 6.52% and 5.43%, respectively. With respect to metabolic status, the *3 allele is “highly deficient,” matching a phenotypically defined null metabolizer because this variant only has 5% of the normal metabolic function. Table 2 presents the SNP frequencies for the VKORC1 marker. We tested for 7 different VKORC1 SNPs, but only the − 1639A promoter allele was observed, at 28.8% frequency.
Table 1.
CYP2C9 alleles. Those observed among n=184 from 92 samples are shown in bold
| Allele | DNA Change | Amino-acid Change | Frequency (%) | Allele Count |
|---|---|---|---|---|
| *1 | Reference | Reference | 70.1 | 129 |
| *2 | 430C>T | Arg144Cys | 6.52 | 12 |
| *3 | 1075A>C | Ile359Leu | 5.43 | 10 |
| *4 | 1076T>C | Ile359Tyr | 0 | 0 |
| *5 | 1080C>G | Asp360Glu | 0 | 0 |
| *6 | 818delA | Frameshift | 0.54 | 1 |
Table 2.
VKORC1 alleles. Those observed among n=184 from 92 samples are shown in bold
| Allele | DNA Change | Amino-acid Change | Frequency (%) | Allele Count |
|---|---|---|---|---|
| Reference | Reference | Reference | 71.2 | 131 |
| − 1639 | G>A | Promoter | 28.8 | 53 |
| 85 | G>T | Val29Leu | 0 | 0 |
| 121 | G>T | Ala41Ser | 0 | 0 |
| 134 | T>C | Val45Ala | 0 | 0 |
| 172 | A>G | Arg58Gly | 0 | 0 |
| 1331 | G>A | Val66Met | 0 | 0 |
| 3487 | T>G | Leu128Arg | 0 | 0 |
The carrier prevalence calculated in this study sample, combining polymorphisms in both genes, is shown in Figure 1. The individuals without a polymorphism for either gene (non-carriers) accounted for 40% of the study population. The percentage of subjects with polymorphisms in only a single gene was 9% for CYP2C9 and 35% for VKORC1. Double carrier patients with a single polymorphism in both genes accounted for 13% of the population. Triple carrier patients with a single polymorphism in CYP2C9 and double in VKORC1 accounted for 3.3%. Quadruple carrier patients with double polymorphisms in both genes were not detected in this study.
Fig 1.
Carrier prevalence of CYP2C9/VKORC1 polymorphisms in 92 Puerto Ricans: Eight CYP2C9 (five *1*2; three *1*3) and thirty-two VKORC1 only carriers (twenty-nine GA; three AA); twelve single CYP2C9 and single VKORC1 carriers (five *1*2/GA; six *1*3/GA; one *1*6/GA); three single CYP2C9 and double VKORC1 carriers (two *1*2/AA; one *1*3/AA). Thirty-seven non-carriers were found
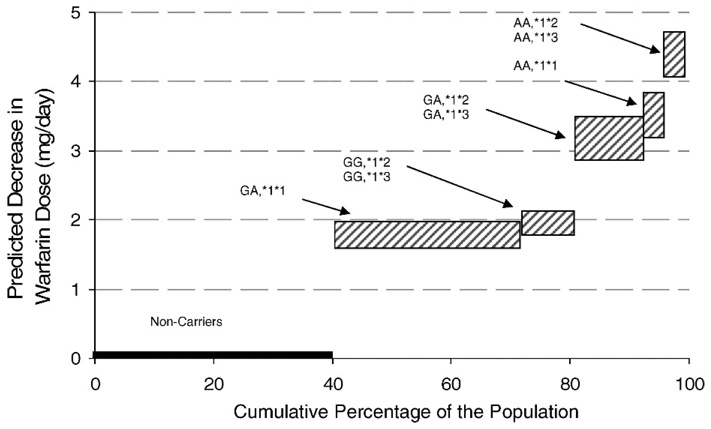
Figure 2 depicts the projected effect of CYP2C9 and VKORC1 combinatorial genotypes on the warfarin dose reduction, based on a published equation. 16 To estimate the effect of these genotypes on clinical dosing practices, we calculated the warfarin dosage for idealized individuals (both sexes) taken from a hypothetical population with the observed genotypic frequencies of our study population. The estimation procedure is based on combinatorial CYP2C9/VKORC1 alleles, age, sex and physical attributes using a published algorithm.16 The model yields stepwise dose reductions dependent on the number of polymorphisms.
Fig 2.
Predicted mean decreases in warfarin dose (mg/day) in hypothetical individuals (55-yo, BMI=27, both genders) taken from a population with the same genotypic frequencies observed in Puerto Ricans. The hatched areas indicate ranges of predicted dose reductions based on a published algorithm16. The upper and lower bars represents the reductions for male and female, respectively
In this study, 40.5% of patients were carriers of a single polymorphism in CYP2C9 (9% *1*2 or *1*3) or VKORC1 (31.5% GA) and, relative to non-carriers (40%), would require a dose decrease in the range of 1.0–1.6 mg/day. Double-carriers, single polymorphism in each gene (13%) or double in VKORC1 (3.2%), accounted for 16.2% of the study population and would require a dose reduction in the range of 2.0–2.95 mg/day. Triple-carriers (CYP2C9 *1*2 or *1*3, VKORC1 AA) accounted for 3.3% of the population and would require a decrease in dose between 2.9–3.7 mg/day.
DISCUSSION
The results from this study demonstrate that 60% of the study population was carrier of one or more polymorphisms resulting in deficient warfarin metabolism (CYP2C9) and/or sensitivity (VKORC1). This portion of the population is at increased risk of warfarin overdosing (bleeding) if given the standard dosage, indicating the need for genotype-guided dosing in the majority of Puerto Ricans. More than one third of the patients, 40%, are carriers of a single polymorphism, and about one fifth, 19.3%, are carriers of 2 or 3 polymorphisms. No subjects were found to be quadruple carriers, with double polymorphisms in both CYP2C9 and VKORC1 genes.
The prevalence of these polymorphisms was slightly lower when compared with an earlier study on unrelated cardiovascular outpatients, ranging 28–88 years old, that were enrolled in a study of dyslipidemias at Hartford Hospital.33 In that population, 44.1% carried a single polymorphism and more than one quarter, 28.3%, carried two or three deficient alleles.33 Although the ethnic composition was predominantly Caucasian, we consider that the observed differences would rather reflect the sampling of the population from cardiovascular (disease load) patients as opposed to newborns in the Puerto Rican random sample.
It is well-known that the prevalence of these allelic variants in people of African ancestry is lower than in Caucasians. Given recent estimates of European, Native American and African admixtures in Puerto Ricans that converge on a ratio of 60:20:20, respectively, 34,35 the observed lower frequencies for these alleles seem to be reasonable.
Notably, one sample from this study population carried the uncommon allele CYP2C9*6 (818delA), which is associated with decreased enzyme activity. CYP2C9*6 is commonly related to African ancestry.36–38 All the CYP2C9 genotypes were wild-type (WT) or heterozygous, and no homozygotes were found. Given the non-WT prevalence of approximately 25%, we would expect 1 or 2 homozygous (including “mixed” homozygotes such as *2*3) according to HW equilibrium, but finding none instead is not a statistically significant deviation.
No statistically significant deviations from HWE were found. HWE is applicable to classical population statistics but may have limited applicability to a population sample of diverse ethno-geographic ancestry. The Puerto Rican population is a three-way admixed population that experienced migration, which might lead us to expect increased heterozygocity. However, such deviations from HWE quickly subside within a single generation under the assumption of random mating. The size and geography of Puerto Rico do not favor isolated subpopulations, and social stratification is likewise not excessive. Considering that the primary admixing events (Spanish settlement for Caucasian influx and the slave trade for African admixture) are many generations past, observing no overt deviations from HWE stands to reason. Larger population samples and genotyping of several other genes are required to elucidate the population genetics of Puerto Rico. Such surveys are the subject of ongoing research by our group.
With regard to dosing algorithms, the combinatorial genotypes yield valuable information for potential DNA-guided adjustments in this population. Individuals with the greatest number of deficient polymorphisms will benefit most from this practice. The predicted warfarin dose reduction (5 mg/day as standard dosage) ranged from 1.6 to 3.7 mg/day for hypothetical patients having the observed combinatorial genotypes. This finding suggests that such a pharmacogenetic approach can then be recommended for reducing adverse events after warfarin administration in Puerto Ricans. Accordingly, risk-associated combinatorial genotype profiles may be assessed in patients with reported adverse events. Currently, our group is focused on conducting the corresponding clinical studies to develop a genomic-based warfarin-dosing algorithm for this population.
Although this report encompasses our initial observations, the multiplexed panel of 5 and 7 alleles for CYP2C9/VKORC1 genes, respectively, and the population sample of 92 subjects address most common and clinically relevant variants for these two genes. Recent studies have demonstrated an increased risk of hemorrhage during long-term therapy in African Americans with CYP2C9 minor variants. 39–41 In Asian populations, where the VKORC1 haplotype predicting low warfarin dose phenotype is more frequent than in Caucasians,42,43 DNA-guided warfarin dosing may also be clinically valuable.44,45 Studies in Caucasian populations show that the CYP2C9 polymorphisms are associated with a 2- to 3-fold increased risk of bleeding during warfarin induction.14,46–49 but not during long-term therapy.50 Evidence from various studies suggests that carriers of combinatorial polymorphisms have a higher risk of severe and life-threatening bleeding episodes due to warfarin-induced over-anticoagulation when compared with patients who carry polymorphisms in only a single gene.39,40 The aggregate annual healthcare cost in the United States is $1.15 billion.30
Whether to genotype an individual’s DNA before or during warfarin treatment, in order to improve clinical outcomes, is an evolving area for regulatory authorities and for the clinical community. Health care will be revolutionized by genotype-guided medicine in clinical practice. The practice of personalized medicine will be increasingly dependent on defining unique, individual drug metabolizing status and target sensitivity by genotyping and tailoring therapy on an individualized basis. Discrepancies in warfarin management from the genotype-guided doses may increase the risk of overdosing and bleeding complications. 46,47 especially during the initiation of warfarin therapy.41,44
Besides typical covariates such as age, sex, body weight, etc., genotyping for combinatorial CYP2C9 and VKORC1 polymorphisms has the potential to become the standard-of-care in future warfarin management. Warfarin dosing represents a current working model for the practice of personalized health care. Genotype-guided pharmacotherapy holds great potential to enhance patient safety based on each individual’s drug metabolism and sensitivity.51
ACKNOWLEDGMENTS
This project has received support from the NCRR Research Centers in Minority Institutions Award Grant G12RR-03051; the PRNSP, Hartford Hospital grant #123260 and Genomas internal research and development funds.
Footnotes
Disclosures
Dr Ruaño is founder and President of Genomas, Inc. Dr Windemuth, Mr Kocherla, Ms Gorowski and Ms Bogaard are full-time employees of Genomas. Dr Seip is a consultant for Genomas.
Author Contributions
Design concept of study: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Acquisition of data: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Data analysis and interpretation: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Manuscript draft: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Statistical expertise: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Administrative, technical, or material assistance: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
Supervision: Duconge, Cadilla, Windemuth, Kocherla, Gorowski, Seip, Bogaard, Renta, Piovanetti, D’Agostino, Santiago-Borrero, Ruaño
REFERENCES
- 1.Lakshminarayan K, Solid CA, Collins AJ, et al. Atrial fibrillation and stroke in the general Medicare population: a 10-year perspective (1992–2002) Stroke. 2006;37:1969–1974. doi: 10.1161/01.STR.0000230607.07928.17. [DOI] [PubMed] [Google Scholar]
- 2.Ansell J, Hirsh J, Poller L, et al. The pharmacology and management of the vitamin K antagonists: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy; Chest; 2004. pp. 204S–2033S. [DOI] [PubMed] [Google Scholar]
- 3.The top 200 generic drugs in 2007 (by units) Drug Topics. 2008;152:156. [Google Scholar]
- 4.The top 200 brand drugs in 2007 (by units) Drug Topics. 2008;152:154. [Google Scholar]
- 5.Couris R, Tataronis G, McCloskey W, et al. Dietary vitamin K variability affects International Normalized Ratio coagulation indices. Int J Vitam Nutr Res. 2006;76:65–74. doi: 10.1024/0300-9831.76.2.65. [DOI] [PubMed] [Google Scholar]
- 6.Hylek EM. Oral anticoagulants: Pharmacologic issues for use in the elderly. Clin Geriatr Med. 2001;17:1–13. doi: 10.1016/s0749-0690(05)70102-6. [DOI] [PubMed] [Google Scholar]
- 7.Absher RK, Moore ME, Parker MH. Patient-specific factors predictive of warfarin dosage requirements. Ann Pharmacother. 2002;36:1512–1517. doi: 10.1345/aph.1C025. [DOI] [PubMed] [Google Scholar]
- 8.Linder MW, Looney S, Adams JE, et al. Warfarin dose adjustments based on CYP2C9 genetic polymorphisms. J Thromb Thrombolysis. 2002;14:227–232. doi: 10.1023/a:1025052827305. [DOI] [PubMed] [Google Scholar]
- 9.Scordo MG, Pengo V, Spina E, et al. Influence of CYP2C9 and CYP2C19 genetic polymorphisms on warfarin maintenance dose and metabolic clearance. Clin Pharmacol Ther. 2002;72:702–710. doi: 10.1067/mcp.2002.129321. [DOI] [PubMed] [Google Scholar]
- 10.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106:2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 11.Kamali F, Pirmohamed M. The future prospects of pharmacogenetics in oral anticoagulation therapy. Br J Clin Pharmacol. 2006;61:746–751. doi: 10.1111/j.1365-2125.2006.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rettie AE, Wienkers LC, Gonzalez FJ, et al. Impaired (S)-warfarin metabolism catalysed by R144C allelic variant of CYP2C9. Pharmacogenetics. 1994;4:39–42. doi: 10.1097/00008571-199402000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Voora D, Eby C, Linder MW, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thromb Haemost. 2005;93:700–705. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 14.Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. doi: 10.1001/jama.287.13.1690. [DOI] [PubMed] [Google Scholar]
- 15.Reynolds KK, Valdes R, Jr, Hartung BR, Linder MW. Individualizing warfarin therapy. Personalized Medicine. 2007;4:11–31. doi: 10.2217/17410541.4.1.11. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Shennan M, Reynolds KK, et al. Estimation of warfarin maintenance dose based on VKORC1 (− 1639 G>A) and CYP2C9 genotypes. Clin Chem. 2007;53:1199–1205. doi: 10.1373/clinchem.2006.078139. [DOI] [PubMed] [Google Scholar]
- 17.Wu AH. Use of genetic and non-genetic factors in warfarin dosing algorithms. Pharmacogenomics. 2007;8:851–861. doi: 10.2217/14622416.8.7.851. [DOI] [PubMed] [Google Scholar]
- 18.Mushiroda T, Ohnishi Y, Saito S, et al. Association of VKORC1 and CYP2C9 polymorphisms with warfarin dose requirements in Japanese patients. J Hum Genet. 2006;51:249–253. doi: 10.1007/s10038-005-0354-5. [DOI] [PubMed] [Google Scholar]
- 19.Wadelius M, Sorlin K, Wallerman O, et al. Warfarin sensitivity related to CYP2C9, CYP3A5, ABCB1 (MDR1) and other factors. Pharmacogenomics J. 2004;4:40–48. doi: 10.1038/sj.tpj.6500220. [DOI] [PubMed] [Google Scholar]
- 20.Wadelius M, Chen LY, Downes K, et al. Common VKORC1 and GGCX polymorphisms associated with warfarin dose. Pharmacogenomics J. 2005;5:262–270. doi: 10.1038/sj.tpj.6500313. [DOI] [PubMed] [Google Scholar]
- 21.Wadelius M, Chen LY, Eriksson N, et al. Association of warfarin dose with genes involved in its action and metabolism. Hum Genet. 2007;121:23–34. doi: 10.1007/s00439-006-0260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman MA, Wilke RA, Caldwell MD, et al. Relative impact of covariates in prescribing warfarin according to CYP2C9 genotype. Pharmacogenetics. 2004;14:539–547. doi: 10.1097/01.fpc.0000114760.08559.dc. [DOI] [PubMed] [Google Scholar]
- 23.Rost S, Fregin A, Ivaskevicius V, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 24.Li T, Chang CY, Jin DY, Stafford DW, et al. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–544. doi: 10.1038/nature02254. [DOI] [PubMed] [Google Scholar]
- 25.Suttie JW. The biochemical basis of warfarin therapy. Adv Exp Med Biol. 1987;214:3–16. doi: 10.1007/978-1-4757-5985-3_2. [DOI] [PubMed] [Google Scholar]
- 26.Yuan HY, Chen JJ, Lee MT, et al. A novel functional VKORC1 promoter polymorphism is associated with inter-individual and interethnic differences in warfarin sensitivity. Hum Mol Genet. 2005;14:1745–1751. doi: 10.1093/hmg/ddi180. [DOI] [PubMed] [Google Scholar]
- 27.Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med. 2005;352:2285–2293. doi: 10.1056/NEJMoa044503. [DOI] [PubMed] [Google Scholar]
- 28. [Last accessed: August, 2007];Princeton, NJ: Bristol-Myers Squibb Pharma Company; Coumadin Label. Available at http://www.fda.gov/cder/foi/label/2007/009218s105lblv2.pdf.
- 29.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [DOI] [PubMed] [Google Scholar]
- 30.McWilliam A, Lutter R, Nardinelli C. Health care savings from personalized medicine using genetic testing: the case of warfarin. [Last accessed: August 11, 2009];Working Paper. AE1-Brookings Joint Center for Regulatory Studies. 2006 Available at http://www.reg-markets.org/publications/index.php?tab=author&authorid=5.
- 31.Rosenberg Y. Slide presentation. NHLB/NIH workshop; 2006. Rationale and outline of an NHLB/NIH Initiative for a large, multicenter randomized trial of genotype-guided dosage of warfarin therapy. [Google Scholar]
- 32.Gordon J, Merante F, Weiss S, Zastawny R. Pharmacogenetic P-450 screening using the Tag-It universal bead-based array platform. In: Wong SH, Linder MW, Valdes R, editors. Pharmacogenomics and Proteomics: Enabling the Practice of Personalized Medicine. Washington, DC: 2006. AAC Press. [Google Scholar]
- 33.Ruano G, Thompson PD, Bower B, et al. High carrier prevalence of combinatorial CYP2C9 and VKORC1 genotypes affecting warfarin dosing. Personalized Medicine. 2008;5:225–232. doi: 10.2217/17410541.5.3.225. [DOI] [PubMed] [Google Scholar]
- 34.Salari K, Choudhry S, Tang H, et al. Genetic admixture and asthma-related phenotypes in Mexican-American and Puerto Rican asthmatics. Genet Epidemiol. 2005;29:76–86. doi: 10.1002/gepi.20079. [DOI] [PubMed] [Google Scholar]
- 35.Choudhry S, Coyle NE, Tang H, et al. Population stratification confounds genetic association studies among Latinos. Hum Genet. 2006;118:652–664. doi: 10.1007/s00439-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 36.Kidd RS, Curry TB, Gallagher S, et al. Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics. 2001;11:803–808. doi: 10.1097/00008571-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Allabi AC, Gala JL, Desager JP, et al. Genetic polymorphisms of CYP2C9 and CYP2C19 in the Beninese and Belgian populations. Br J Clin Pharmacol. 2003;56:653–657. doi: 10.1046/j.1365-2125.2003.01937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanira MO, Al-Mukhaini MK, Al Hinai AT, et al. Frequency of CYP2C9 genotypes among Omani patients receiving warfarin and its correlation with warfarin dose. Community Genet. 2007;10:32–37. doi: 10.1159/000096279. [DOI] [PubMed] [Google Scholar]
- 39.Schalekamp T, Brasse BP, Roijers JF, et al. VKORC1 and CYP2C9 genotypes and acenocoumarol anticoagulation status: interaction between both genotypes affects overanticoagulation. Clin Pharmacol Ther. 2006;80:13–22. doi: 10.1016/j.clpt.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 40.Schwarz UI, Stein CM. Genetic determinants of dose and clinical outcomes in patients receiving oral anticoagulants. Clin Pharmacol Ther. 2006;80:7–12. doi: 10.1016/j.clpt.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther. 2007;83:312–320. doi: 10.1038/sj.clpt.6100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Veenstra DL, You JH, Rieder MJ, et al. Association of vitamin K epoxide reductase complex 1 (VKORC1) variants with warfarin dose in a Hong Kong Chinese patient population. Pharmacogenet Genomics. 2005;15:687–691. doi: 10.1097/01.fpc.0000174789.77614.68. [DOI] [PubMed] [Google Scholar]
- 43.Cho HJ, Sohn KH, Park HM, et al. Factors affecting the interindividual variability of warfarin dose requirement in adult Korean patients. Pharmacogenomics. 2007;8:329–337. doi: 10.2217/14622416.8.4.329. [DOI] [PubMed] [Google Scholar]
- 44.Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2007;25:45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- 45.Womack C. As Part of Retrofitting, FDA Panel Votes to Re-label Warfarin for PGx; Is Dx Far Behind? Pharmacogenomics Reporter. 2005 12-1-0005. [Google Scholar]
- 46.Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med. 2005;7:97–104. doi: 10.1097/01.gim.0000153664.65759.cf. [DOI] [PubMed] [Google Scholar]
- 47.Lindh JD, Lundgren S, Holm L, et al. Several-fold increase in risk of overanticoagulation by CYP2C9 mutations. Clin Pharmacol Ther. 2005;78:540–550. doi: 10.1016/j.clpt.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Visser LE, van-Vliet M, van-Schaik RH, et al. The risk of overanticoagulation in patients with CYP2C9*2 or CYP2C9*3 alleles on acenocoumarol or phenprocoumon. Pharmacogenetics. 2004;4:27–33. doi: 10.1097/00008571-200401000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Voora D, Eby C, Linder MW, et al. Prospective dosing of warfarin based on cytochrome P-450 2C9 genotype. Thromb Haemost. 2005;93:700–705. doi: 10.1160/TH04-08-0542. [DOI] [PubMed] [Google Scholar]
- 50.Taube J, Halsall D, Baglin T. Influence of cytochrome P-450 CYP2C9 polymorphisms on warfarin sensitivity and risk of over-anticoagulation in patients on long-term treatment. Blood. 2000;96:1816–1819. [PubMed] [Google Scholar]
- 51.Ruano G. Quo Vadis Personalized medicine? Personalized Medicine. 2004;1:1. doi: 10.1517/17410541.1.1.1. [DOI] [PubMed] [Google Scholar]