Abstract
The purpose of this study was to examine the flow velocity pattern of the brachial artery and to determine its relationship to measures of physical function. Subjects from the Louisiana Healthy Aging Study (n = 95; age = 84 ± 10 years) were evaluated. Brachial artery flow velocities and dimensions were measured using high-resolution ultrasonography. The continuous scale of physical function and performance test (CS-PFP10) was used to assess physical function. This test is based on the performance of 11 activities of daily living. Total CS-PFP10 score was 39.51 ± 21.21 U. Mean antegrade and retrograde velocities at rest were 14.2 ± 4.7 and 3.6 ± 2.2 cm/s, respectively. Ante-/retrograde ratio was 5.5 ± 4.6. Brachial artery diameter was 4.3 ± 0.7 mm. Pulse pressure and vascular conductance were 66 ± 18 mmHg, and 0.9 ± 0.5 ml/min/mmHg, respectively. Vascular conductance (r = −0.34), ante-/retrograde ratio (r = −0.42) and CS-PFP10 (r = −0.65) were inversely and retrograde velocity (r = 0.40) and pulse pressure (r = 0.36), were directly associated with age. Retrograde velocity was inversely related to vascular conductance (r = −0.27) and CS-PFP10 total score (r = −0.45). A MANOVA revealed that those with the higher CS-PFP10 scores had a lower retrograde velocity (P = 0.0001), but this association was, in part, age-dependent. Among nonagenarians (n = 52), those in the lower tertiles of the CS-PFP10 scores had significantly higher retrograde velocities compared to those in the higher tertiles (P = 0.035). These data indicate an increase in brachial retrograde velocity with age. These hemodynamic changes are related to a decline in physical function.
Keywords: Blood flow, Flow velocity, Aging, Functional ability
Introduction
Aging is associated with alterations in structural and functional properties of large arteries (Lakatta and Levy 2003a, b; Lakatta 2003; Miyachi et al. 2005; Yashin et al. 2006; Yin 1980), including an increase in intima-media thickness (Vaitkevicius et al. 1993) and arterial stiffness (Proctor et al. 1998) and altered vascular tone (Lakatta and Levy 2003a, b; Lakatta 2003). The consequences of these changes may include alterations in flow velocity patterns. Eventually, such changes may contribute to alterations in blood flow distribution, reduced tissue perfusion (Proctor et al. 1998), and attenuate physical function (Welsch et al. 2008).
The flow velocity pattern is of importance, considering hemodynamic forces on endothelial cell expression. In vivo shear stress appears to be a signal for the expression of antiatherogenic, but oscillatory flow patterns produces proatherogenic gene expression (Laughlin et al. 2008). Green et al. (2002) noted that with increasing intensities of exercise, the retrograde component of the brachial artery flow velocity integral increased. Green et al. (2002) speculated this increase to be the result of an increase in down-stream pressure, attributed to an increase in sympathetic vasoconstriction of inactive vascular beds (Rowell 1993). It is presently unclear to what extent age-related alterations of the vasculature influences arterial flow velocity patterns.
The purpose of this study was to examine brachial artery antegrade and retrograde flow velocities in the elderly, and to determine their relationship to physical function. It was hypothesized that the antegrade and retrograde flow velocity would decrease and increase with age, respectively. Moreover, it was hypothesized these velocity patterns would be related to physical function.
Methods
Participants in this study represent a subset from the Louisiana Healthy Aging Study. Sampling was performed via random selection based on voter registration lists and the medicare beneficiary enrollment data file from the Center of Medicare and Medicaid Services. Methods of recruitment included mail, follow-up phone calls, and in the case of the nonagenarians, a home visit to explain the study in detail. Exclusion criteria for the Louisiana Healthy Aging Study included individuals scoring below 25 on the minimental status exam (Folstein et al. 1975) and individuals in American Heart Association Class D (i.e., symptoms of cardiovascular and/or metabolic disease at rest). In addition, individuals with significant dysrhythmias were excluded for the purpose of these analyses. Each participant signed an informed consent approved by the institutional review boards of the host institutions.
Brachial artery imaging and analysis
All brachial artery imaging were performed using a Toshiba Powervision SSA-380A, in accordance with guidelines (Corretti et al. 2002). Prior to imaging, subjects were instructed to fast and refrain from exercise for 12 h, and alcohol for 48 h. Subjects were asked to rest in a supine position for 20 min before imaging. All images were obtained in longitudinal view, approximately 4 cm proximal to the olecranon process, in the anterior/medial plane. Flow velocity measurements were obtained using a pulsed Doppler signal at an angle of 60° to the vessel. All data were analyzed using the Brachial Imager software (Medical Imaging Applications, LLC). Arterial diameters were calculated as the mean distance between the anterior and posterior wall of the blood vessel interface, with the image in diastole, defined as the peak of the r-wave from the electrocardiograph and systole defined as peak of the antegrade velocity signal. Brachial diameter was defined by the average of 30 s of recorded data.
Flow velocity signals, blood flow and vascular conductance
Flow velocity signals were calculated in a similar manner to Dobrosielski et al. (2008). Each flow velocity signal was manually traced using Image Pro Plus 4.0 software. The antegrade signal was defined as the area of tracing above 0 cm/s and the retrograde signal was defined as the area below (Fig. 1). The antegrade and retrograde ratio (ante-/retrograde ratio) was the quotient of the velocity values. The flow velocity signals were then divided by the ejection time (s) from that cardiac cycle to subsequently determine the mean velocity (cm/s). The mean flow velocity integral (FVI) was calculated as the difference between the antegrade and retrograde flow velocity signals. Mean blood flow (ml/min) was estimated from the equation: blood flow = (mean FVI × heart rate) × πr2 (r = blood vessel radius). Finally, vascular conductance was calculated as mean blood flow/mean arterial pressure (Wray et al. 2006).
Fig. 1.
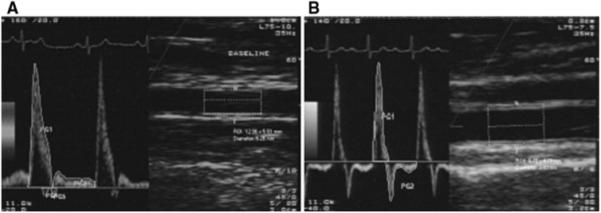
Examples of flow velocity tracings. a An image from a 67-year old, with an average antegrade and retrograde velocity of 24.77 and 1.24 cm/s, respectively, and b an image from a 97-year old, with an average antegrade and retrograde velocity of 15.55 and 5.10 cm/s, respectively
Physical function test
The CS-PFP10 test was used to assess functional ability (Cress et al. 1996). This scale is based on the performance of 11 activities of daily living. Participant instructions and protocols were standardized. Five separate physical domain scores are averaged to yield a total score of between 0 and 100. The physical domains assessed include, upper-body strength, lower-body strength, upper-body flexibility, balance and coordination, and endurance.
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 16.0). Data are presented as mean ± standard deviation. To examine the influence of age on brachial ± artery dimensions, flow velocity integrals, and physical function scores, a Pearson product moment correlation was used. To compare the flow velocities, vascular conductance and physical function, for specific functional categories (CS-PFP10 total score <25; 25 to <57; and >57 U), a multivariate analysis of variance (MANOVA) was used. In order to control for the age related association between variables, a second MANOVA, for the nonagenarians (individuals ≥90 years), was used to compare flow velocities, vascular conductance and physical function, among functional tertiles. Differences between categories were evaluated using post hoc comparisons. An alpha level of (P < 0.05) was required for statistical significance.
Results
The characteristics for 95 individuals (84 ± 10 years) are presented in Table 1. Of the participants, 35% were between 60 and 80 years, 8% ranged 81–89 years and 57% made up the nonagenarian group. Among the participants, 75% had some form of diagnosed cardiovascular disease (e.g. hypertension 64%, congestive heart failure 14%, and peripheral vascular disease 1%). Other pathologies included arthritis 66%, diabetes 14%, and cancer 11%. The average number of prescribed medications was 5 (range 0–17) (Table 2). Mean antegrade and retrograde velocities were 14.2 ± 4.7 and 3.6 ± 2.2 cm/s, respectively. The ante/retrograde ratio was 5.54 ± 4.57. Mean blood flow and vascular conductance were 88.6 ± 45.4 ml/min and 0.9 ± 0.5 ml/min/mmHg. The average total CS-PFP10 score was 39.5. ± 21.2, ranging 3.6–81.4 U.
Table 1.
Participant characteristics
| Variable | Mean | SD |
|---|---|---|
| Age (years) | 84 | 10 |
| Pulse pressure (mmHg) | 66 | 18 |
| Antegrade velocity (cm/s) | 14.2 | 4.7 |
| Retrograde velocity (cm/s) | 3.6 | 2.2 |
| Ante-/retrograde index (cm/s) | 5.5 | 4.6 |
| Resting flow (mL/min) | 88 | 45 |
| Brachial diameter (mm) | 4.3 | 0.7 |
| Conductance (ml/min/mmHg) | 0.9 | 0.5 |
| CS-PFP10 score (U) | 39.5 | 21.2 |
Values are mean ± SD for 95 participants
Table 2.
Prescribed medications
| Medication | Number of participants |
Percent (%) |
|---|---|---|
| Cardiovascular | ||
| Nitrates | 9 | 10 |
| Angiotensin II blockers | 14 | 15 |
| Alpha adrenergic blockers | 17 | 18 |
| Anti coagulants | 18 | 19 |
| Calcium channel blockers | 21 | 22 |
| ACE inhibitors | 22 | 23 |
| β-Adrenergic blockers | 26 | 27 |
| Diuretics | 33 | 35 |
| Others | ||
| Insulin | 14 | 15 |
| Anti inflammatory | 18 | 19 |
| Anti depressants | 19 | 20 |
| Statins | 32 | 34 |
Values are for 95 participants
Age, blood pressure, flow velocities and blood flow, brachial artery
Diameter and physical function
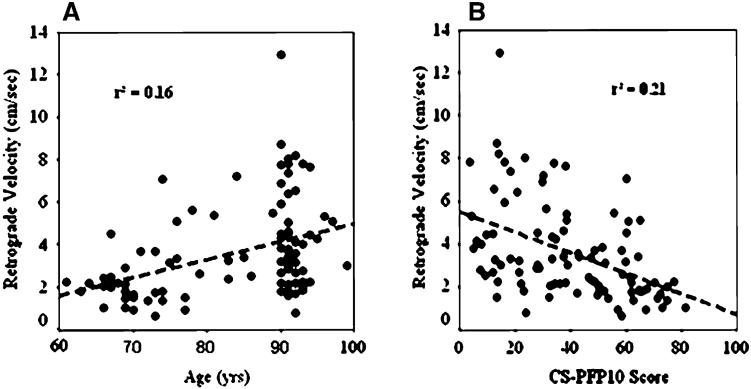
Age was inversely related to blood flow (r = −0.31), vascular conductance (r = −0.34), ante-/retrograde ratio (r = −0.42) and CS-PFP10 (r = −0.65), and directly related to pulse pressure (r = 0.36), and retrograde flow (r = 0.40) (Table 3). The regression equation for retrograde velocity and age [retrograde velocity = −3.49 + 0.08 (age); P = 0.0001] shows an approximate 60% increase per decade after age 60. Antegrade flow was associated with vascular conductance (r = 0.51), whereas, retrograde flow was inversely related to vascular conductance (r = −0.27), and the CS-PFP10 scores (r = −0.45). The ante-/retrograde ratio was related to CS-PFP10 scores (r = 0.35). The associations between retrograde velocity and age, and retrograde velocity and the CS-PFP10 scores are presented in Figs. 2a, b.
Table 3.
Associations between age, pulse pressure, flow velocities, resting flow, brachial diameter, vascular conductance and physical function
| Age | Pulse pressure |
Antegrade velocity |
Retrograde velocity |
Ante-/retrograde Index |
Resting flow |
Brachial diameter |
Conductance | |
|---|---|---|---|---|---|---|---|---|
| Age (years) |
r = 1 P = 0 |
|||||||
| Pulse pressure (mmHg) |
r = 0.36** P = 0.0001 |
r = 1 P = 0 |
||||||
| Antegrade velocity (cm/s) |
r = −0.12 P = 0.26 |
r = 0.78 P = 0.45 |
r = 1 P = 0 |
|||||
| Retrograde velocity (cm/s) |
r = 0.40** P = 0.001 |
r = 0.15 P = 0.14 |
r = 0.24* P = 0.02 |
r = 1 P = 0 |
||||
| Ante-/retrograde index (cm/s) |
r = −0.42** P = 0.001 |
r = 0.14 P = 0.19 |
r = 0.33** P = 0.001 |
r = −0.57** P = 0.0001 |
r = 1 P = 0 |
|||
| Resting Xow (ml/min) |
r = −0.31** P = 0.003 |
r = −0.09 P = 0.39 |
r = 0.52** P = 0.0001 |
r = −0.26* P = 0.01 |
r = 0.34** P = 0.001 |
r = 1 P = 0 |
||
| Brachial diameter (mm) |
r = 0.037 P = 0.72 |
r = −0.08 P = 0.44 |
r = −0.36** P = 0.0001 |
r = −0.19 P = 0.06 |
r = −0.18 P = 0.08 |
r = 0.43** P = 0.0001 |
r = 1 P = 0 |
|
| Conductance (ml/min/mmHg) |
r = −0.34** P = 0.001 |
r = −0.17 P = 0.11 |
r = 0.51** P = 0.0001 |
r = −0.27* P = 0.01 |
r = 0.37** P = 0.0001 |
r = 0.97** P = 0.0001 |
r = 0.38** P = 0.0001 |
r = 1 P = 0 |
| CS-PFP10 (U) |
r = −0.65** P = 0.0001 |
r = −0.20 P = 0.06 |
r = −0.45** P = 0.0001 |
r = −0.45** P = 0.0001 |
r = 0.35** P = 0.0001 |
r = 0.23* P = 0.02 |
r = 0.20 P = 0.05 |
r = 0.26* P = 0.01 |
(P ≤ 0.01)
(P ≤ 0.05)
Fig. 2.
a, b The relation between retrograde velocity (cm/s) and age (years); and retrograde velocity (cm/s) and physical function
Blood flow velocity and physical function categories
The results of the MANOVA, comparing flow velocities among CS-PFP10 functional categories, revealed a main effect (P = 0.0001). The analysis revealed no difference for antegrade velocity (P = 0.51). However, individuals in the highest functional class had lower retrograde flow velocities (P = 0.02), then those in the middle functional class, whom had significantly lower retrograde velocities compared to those in the lowest functional classes (P = 0.03). In addition, individuals in the highest functional class had significantly higher resting blood flows and vascular conductance, and lower pulse pressures than those in the lower functional classes. However, when age was entered into the model as a covariate, the main effect for retrograde velocity increased (P = 0.12), indicating a significant age contribution.
Blood flow velocity and physical function categories in nonagenarians
The results of the second MANOVA, comparing flow velocities and CS-PFP10 functional tertiles, in the nonagenarians, also revealed a main effect (P = 0.04). As indicated in Fig. 3, those individuals in the higher functional tertiles had a significantly lower retrograde velocity compared to those in the lower functional tertiles (P = 0.01). Interestingly, there was also a significant difference in antegrade velocity between those in the higher functional tertiles compared to those in lower. Importantly, there were no significant differences between pulse pressure, medications and incidence of diseases among the functional tertiles for the nonagenarians.
Fig. 3.
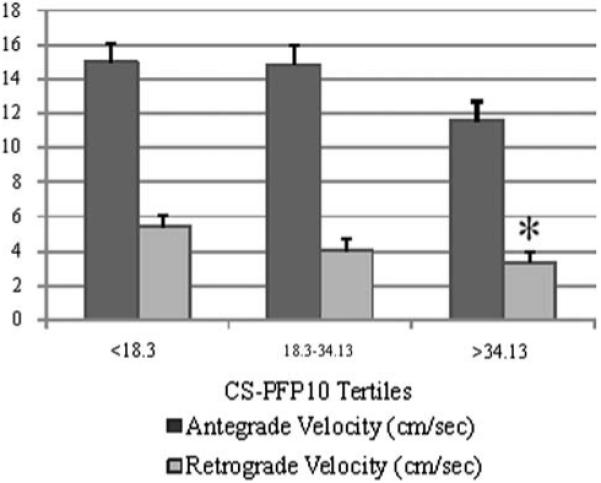
Antegrade and retrograde velocities (cm/s) per CS-PFP10 score tertiles for nonagenarians. *P < 0.05 versus lowest tertile
Discussion
The aim of this study was to examine flow velocity patterns of the brachial artery, at rest, in a subset of participants from the Louisiana Health Aging Study. With advancing age, there was a decrease in the average blood flow velocity with a subsequent reduction in basal blood flow. With advancing age, antegrade velocity did not change, while retrograde velocity increased. Furthermore, individuals with greater physical function scores exhibited a lower retrograde velocity, although this was in part influenced by age. Importantly, among nonagenarians, those in the lower tertiles of physical function had higher retrograde velocity, compared to those in the higher tertiles. These data indicate an age-dependent change in flow velocity patterns, which may, in part, contribute to lower physical functionality.
Age, blood pressure, flow velocities, brachial artery diameter and physical function
The present study confirms an age-dependent increase in pulse pressure [PP = 11.68 + 0.65 (age); P = 0.0001). The age-trajectory in the present study is similar to reports from the Framingham Heart Study (Yashin et al. 2006). The increase in pulse pressure with aging is believed to be the consequence of a decrease in large artery compliance (Van Bortel and Spek 1998). The age-dependent decline in brachial blood flow and velocity has been reported (Seals et al. 2008). The age associated reductions in blood flow are mediated by corresponding decreases in vascular conductance (Dinenno et al. 1999, 2001; Miyachi et al. 2005), and are closely related to decreases in estimated leg oxygen consumption.
The antegrade velocity did not change, while retrograde velocity increased, with age. This finding suggests those factors that define the retrograde signal change perhaps to a greater extent than those factors associated with the antegrade velocity (Ogawa et al. 1992). Evidence for this stems from a study which reported an increase in local peripheral pressure using strategically placed pressure cuVs resulted in an augmentation in the retrograde, but not the antegrade velocity profile in young individuals (Baccelli et al. 1985). The age-dependent increase in pulse pressure, in the current study suggests an imbalance between arterial flow and arterial impedance (Izzo and Mitchell 2007), and a decrease in vascular conductance. The association between the retrograde flow velocity and vascular conductance (r = −0.27) is in line with Baccelli et al. (1985). The current study did not find an association between age and brachial diameter at rest, which has been reported (Koch et al. 2003). The average diameter tended to be bigger in participants who were taller (r = 0.46), larger (r = 0.43), or males (r = 0.63). This study also observed that average brachial arteries were bigger than studies reporting values for individuals under the age of 65 years (Allen et al. 2000; Koch et al. 2003). The lack of an age-dependent enlargement of the brachial artery in the present study suggest that any such changes may have occurred before age 60.
The present study shows a similar decline in physical function scores as reported from another cohort from the Louisiana Healthy Aging Study (Welsch et al. 2008). The regression of CS-PFP10 scores against age in the present study [CS-PFP10 = 150.67–1.33 (age); P = 0.0001] suggests an approximate 1.3% per year decrease in function among adults over 60 years of age.
Blood flow velocity and physical function
There was an apparent relation between retrograde velocity and physical function scores. The evidence for this relationship stems from two analyses, which indicate that individuals in the highest category of physical function exhibited a lower retrograde velocity. In the first analyses, participants were classified as low, moderate and highly functional according to the CSPFP-10 scores. The rationale for this classification is based on the probability of independence graph developed by Cress et al. (1996). In that study, individuals who scored above the threshold score of 57 were classified as independent, those who scored below 26 rated themselves as limited in physical function, leaving the middle group as a possible “at risk” population (Cress et al. 1996). The fact this association was, in part, driven by age, required a second analysis, on the nonagenarians only. Among this group, there was no significant difference between the number or type of medications, or the incidence of disease compared to the entire study population. The second analysis performed on this group revealed those with the highest functional scores had a lower retrograde velocity.
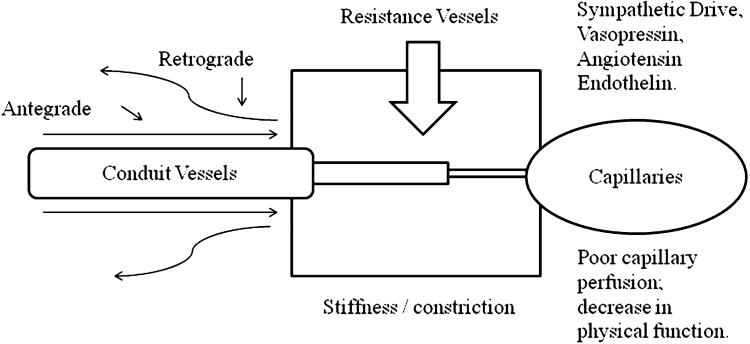
Explanation for the apparent associations may reflect age-dependent alterations in peripheral impedance (Rowell 1993). The mechanisms by which peripheral impedance increases with age are summarized in Fig. 4, and include a change in autonomic balance toward an increased hyper-adrenergic state, other hormonal influences, or a decrease in the production or effects of nitricoxide (NO) (Lakatta 2003). This increase in peripheral impedance appears to attenuate vascular conductance (Dinenno et al. 2005) and increase peripheral resistance, which may limit exercise tolerance (Dinenno et al. 2005). The fact that, the nonagenarians, in the highest tertile of physical function, exhibited lower retrograde velocities may indicate a different age-trajectory in peripheral impedance (Green et al. 2002).
Fig. 4.
Proposed mechanisms by which retrograde velocity integrals may increase with age
Finally, future work is needed to determine the extent of the change of the retrograde flow velocity component on the actual changes in hemodynamic forces on the arterial wall. It could be hypothesized that the above-stated changes in retrograde velocity with advancing age could contribute to greater oscillatory flow. To that extent, it is perhaps interesting to consider the ante-/retrograde ratio, in the present study. This ratio decreased with age, and was also directly related to CS-PFP10 scores (r = 0.35). This may point toward a change in the beat-to-beat hemodynamic forces on the arterial wall. If hemodynamic forces are thought to have both beneficial and negative effects on endothelial cell expression (Laughlin et al. 2008), it could be argued that individuals with higher oscillatory flow patterns produce pro-atherogenic factors, such as reactive oxygen species (De Keulenaer et al. 1998).
Conclusion
The present study indicates a decrease in average blood flow velocity and a subsequent reduction in basal blood flow, with advancing age. With advancing age, retrograde velocity increased significantly, while antegrade velocity did not. Furthermore, individuals with greater physical function scores exhibited a lower retrograde velocity, although this was in part influenced by age. Among nonagenarians, those in the lower tertiles of physical function had significantly higher retrograde velocity, compared to those in the higher tertiles. These data indicate an agedependent change in flow velocity patterns, which may, in part, be a consequence of arterial senescence, and contribute to a decline in physical function.
Acknowledgments
This research was supported by a grant from the Louisiana Board of Regents through the Millennium Trust Health Excellence Fund [HEF(2001-06)-02] (S.M. Jazwinski), and by a grant from the National Institute on Aging (1 P01 AG022064) (S.M. Jazwinski).
Contributor Information
Daniel P. Credeur, Department of Kinesiology, Louisiana State University, 112 Long Field House, Baton Rouge, LA 70803, USA, dcrede1@tigers.lsu.edu
Devon A. Dobrosielski, Wake Forest University, Winston-Salem, NC, USA
Arturo A. Arce-Esquivel, University of Missouri, Columbia, MO, USA
Michael A. Welsch, Department of Kinesiology, Louisiana State University, 112 Long Field House, Baton Rouge, LA 70803, USA
References
- Allen JD, Wilson JB, Tulley RT, Lefevre M, Welsch MA. Influence of age and normal plasma fibrinogen levels on flow-mediated dilation in healthy adults. Am J Cardiol. 2000;86:703–705. doi: 10.1016/s0002-9149(00)01060-2. [DOI] [PubMed] [Google Scholar]
- Baccelli G, Pignoli P, Corbellinni E, Pizzolati PL, Bassini M, Longo T, Zanchetti A. Hemodynamic factors changing blood flow velocity waveform and profile in normal human brachial artery. Angiology. 1985;36:1–8. doi: 10.1177/000331978503600101. [DOI] [PubMed] [Google Scholar]
- Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallace P, Vita J, Vogel R, International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- Cress ME, Buchner DM, Questad KA, Esselman PC, de Lateur BJ, Scwartz RS. Continuous-scale physical functional performance in healthy older adults: a validation study. Arch Phys Med Rehabil. 1996;77:1243–1250. doi: 10.1016/s0003-9993(96)90187-2. [DOI] [PubMed] [Google Scholar]
- De Keulenaer GW, Chappell DC, Ishizaka N, Nerem RM, Alexander RW, Griendling KK. Oscillatory and steady laminar shear stress differentially affect human endothelial redox state: role of a super-oxide-producing NADH oxidase. Circ Res. 1998;82:1094–1101. doi: 10.1161/01.res.82.10.1094. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Stauffer BL, Seals DR. Reductions in basal limb blood flow and vascular conductance with human ageing: role for augmented alpha-adrenergic vasoconstriction. J Physiol. 2001;536:977–983. doi: 10.1111/j.1469-7793.2001.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic {alpha}-.adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol (Lond) 2005;567:311–321. doi: 10.1113/jphysiol.2005.087668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrosielski DA, Greenway F, Welsch MA. Modification of vasoreactivity in elderly men following handgrip exercise training. Med Sci Sports Exer. 2008 in press. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Green D, Cheetham C, Reed C, Dembo L, O’Driscoll G. Assessment of brachial artery blood flow across the cardiac cycle: retro-grade flows during cycle ergometry. J Appl Physiol. 2002;93:361–368. doi: 10.1152/japplphysiol.00051.2002. [DOI] [PubMed] [Google Scholar]
- Izzo JL, Jr, Mitchell GF. Aging and arterial structure-function relations. Adv Cardiol. 2007;44:19–34. doi: 10.1159/000096701. [DOI] [PubMed] [Google Scholar]
- Koch DW, Leuenberger UA, Proctor DN. Augmented leg vaso-constriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part I: aging arteries: a “set up” for vascular disease. Circulation. 2003a;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part II: the aging heart in health: links to heart disease. Circulation. 2003b;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Newcomer SC, Bender SB. Importance of hemodynamic forces as signals for exercise-induced changes in endothelial cell phenotype. J Appl Physiol. 2008;104:588–600. doi: 10.1152/japplphysiol.01096.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi M, Tanaka H, Kawano H, Okajima M, Tabata I. Lack of age-related decreases in basal whole leg blood flow in resistance-trained men. J Appl Physiol. 2005;99:1384–1390. doi: 10.1152/japplphysiol.00061.2005. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Spina RJ, Martin WH, 3rd, Kohrt WM, Schechtman KB, Holloszy JO, Ehsani AA. Effects of aging, sex, and physical training on cardiovascular responses to exercise. Circulation. 1992;86:494–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffer DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol. 1998;85:68–75. doi: 10.1152/jappl.1998.85.1.68. [DOI] [PubMed] [Google Scholar]
- Rowell L. Human Cardiovascular Control. Oxford University Press; New York: 1993. pp. 212–213. [Google Scholar]
- Seals DR, DeSouza CA, Donato AJ, Tanaka H. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–1332. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaitkevicius PV, Fleg JL, Engel JH, O’Connor FC, Wright JG, Lakatta LE, Yin FC, Lakatta EG. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88:1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, Spek JJ. Influence of aging on arterial compliance. J Hum Hypertens. 1998;12:583–586. doi: 10.1038/sj.jhh.1000669. [DOI] [PubMed] [Google Scholar]
- Welsch MA, Dobrosielski DA, Arce-Esquivel A, Wood RH, Ravussin E, Jazwinski SM, Rowley C. the association between flow-mediated dilation and physical function in older men. Med Sci Sports Exerc. 2008;40:1237–1243. doi: 10.1249/MSS.0b013e31816c5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray DW, Donato AJ, Nishiyama SK, Richardson RS. Acute sympathetic vasoconstriction at rest and during dynamic exercise in cyclists and sedentary humans. J Appl Physiol. 2006;102:704–712. doi: 10.1152/japplphysiol.00984.2006. [DOI] [PubMed] [Google Scholar]
- Yashin AI, Akushevich IV, Arbeev KG, Akushevich L, Ukraintseva SV, Kulminski A. Insights on aging and exceptional longevity from longitudinal data: novel findings from the Framingham Heart Study. Age (Dordr) 2006;28:363–374. doi: 10.1007/s11357-006-9023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin FCP. The aging heart: the aging vasculature and its effects on the heart. Vol. 12. Raven Press; New York: 1980. pp. 139–146. [Google Scholar]