Abstract
Simian immunodeficiency virus (SIV) and human immunodeficiency virus (HIV) infection results in an early and enduring depletion of intestinal CD4+ T cells. SIV and HIV bind integrin α4β7, thereby facilitating infection of lymphocytes that home to the gut-associated lymphoid tissue (GALT). Using an ex vivo flow cytometry assay, we found that SIVmac239-infected cells expressed significantly lower levels of integrin α4β7 than did uninfected cells. This finding suggested a potential viral effect on integrin α4β7 expression. Using an in vitro model, we confirmed that integrin α4β7 was downregulated on the surfaces of SIVmac239-infected cells. Further, modulation of integrin α4β7 was dependent on de novo synthesis of viral proteins, but neither cell death, the release of a soluble factor, nor a change in activation state was involved. Downregulation of integrin α4β7 may have an unappreciated role in the CD4 depletion of the mucosal-associated lymphoid compartments, susceptibility to superinfection, and/or immune evasion.
Infection of macaques with simian immunodeficiency virus (SIV) and humans with human immunodeficiency virus (HIV), regardless of the route of transmission, results in early establishment of infection in the gut-associated lymphoid tissue (GALT) (3, 23, 25). Consequently, the CD4+ T cells of the GALT are depleted, and intestinal integrity is compromised (4, 21, 37). The mechanism of GALT depletion, as well as the mechanism of viral localization to the GALT, remains poorly understood.
GALT localization is mediated, at least in part, by integrins, a large family of “sticky” cell surface proteins (24, 35, 36). Integrins facilitate conversation between the environment and a cell, thereby influencing cellular adhesion, trafficking, proliferation, and signaling. Consequently, numerous viruses, despite having a small number of proteins, have developed mechanisms to exploit integrins and hence cellular processes, in order to facilitate viral replication and immune evasion (17, 24, 34, 36). Examples of such viruses include human cytomegalovirus (39), rotavirus (14), and SIV/HIV (40). One well-studied integrin, α4β7, mediates migration of lymphocytes to the GALT (31, 33). In 2008, Arthos et al. demonstrated that HIV-1 glycoprotein, gp120, binds integrin α4β7, facilitating infection of CD4+ T cells and increasing viral replication efficiency (1).
Recent in vivo studies have revealed that CD4+ T cells expressing high amounts of integrin α4β7 (integrin α4β7 high) are preferentially infected during acute SIV infection (15, 38). In addition, integrin α4β7 high CD4+ T cells contain greater than one provirus per cell during peak viral infection, suggesting that the cells are unusually susceptible to superinfection. Unexpectedly, superinfection is not observed in integrin α4β7 high CD4+ T cells after peak viral infection (15). Integrin α4β7 high-expressing CD4+ T cells are also depleted from the circulation parallel to the loss of intestinal CD4+ cells, suggesting a fundamental role for integrin α4β7 in SIV pathogenesis (38). The mechanism underlying the depletion of integrin α4β7 high-expressing cells and whether SIV-infected cells are directly or indirectly involved remain unknown. Thus, understanding the single-cell dynamics of integrin α4β7 during SIV infection may improve our understanding of SIV and HIV pathogenesis and clarify the role of integrin α4β7 signaling in mucosal trafficking.
To examine the single-cell dynamics of integrin α4β7 expression during SIV infection, we used a novel, ex vivo, flow cytometry assay (M. Reynolds, unpublished data). We observed that infected, Gag p27+ cells expressed significantly (P = 0.0085) lower levels of integrin α4β7 than uninfected, CD4+ T cells from the same animal, at the same time point. Thus, we hypothesized that SIV decreases integrin α4β7 expression on the surfaces of virus-infected cells. In vitro, integrin α4β7 expression was downregulated on SIVmac239-infected cells as rapidly as 24 h postinfection. Unexpectedly, integrin α4β7 levels were also perturbed on uninfected cells with an increase in number of cells with intermediate integrin α4β7 expression. The modulation of integrin α4β7 was dependent on de novo synthesis of a viral protein(s), but neither cell death, release of a soluble factor, nor a change in activation state were involved. Combined, this finding suggests an as-yet-unidentified viral effect on integrin α4β7 that may influence depletion of the mucosal associated lymphoid compartments, susceptibility to superinfection, and/or immune evasion during SIV infection.
MATERIALS AND METHODS
Ex vivo analysis of Gag p27-positive cells.
Cells from frozen or fresh mesenteric or inguinal lymph node biopsies of SIVmac239-infected Indian rhesus and Mauritian cynomolgus macaques were obtained from multiple time points after infection (Table 1). Sixty million cells were then enriched for CD4 cells by depleting CD8-, CD20-, and CD14-positive cells using selection kits from Miltenyi Biotech (Auburn, CA). After CD4 enrichment, the cells were counted, and five million cells were surface stained with CD3-Alexa Fluor 700 (A700), CD4 peridinin-chlorophyll protein complex (PerCP; BD Biosciences, San Jose, CA), LiveDead fixable violet amine reactive dye (ARD; Invitrogen, Carlsbad, CA), CD95 phycoerythrin-Cy7 (PE-Cy7; eBioscience, San Diego, CA), and integrin α4β7 allophycocyanin (APC; Non-Human Primate Reagent Resource). Phycoerythrin (PE) was used to eliminate remaining CD8- and CD20-positive cells. After surface staining, the cells were washed and fixed with 2% paraformaldehyde (PFA) for 15 min. The cells were then stained for intracellular Gag p27 (NIH AIDS Research and Reference Reagent Program, NIAID, NIH) conjugated to fluorescein isothiocyanate (FITC) combined with permeabilization reagent (Invitrogen, Carlsbad, CA) for 15 min, washed, and fixed with 2% PFA. After staining, the samples were run on an BD-LSRII (BD Biosciences, San Jose, CA), and at least two million events were collected. The samples were then analyzed by FlowJo software version 8.8.6 (TreeStar, Ashland, OR). Replicates were graphed using GraphPad Prism version 5.0a for Macintosh (GraphPad Software, San Diego, CA).
TABLE 1.
Summary of macaques used for ex vivo experimentsa
| Animal | Time postinfection (wks) | Plasma viral load |
|---|---|---|
| cy0151 | 3 | 6.19E + 05 |
| cy0209 | 15 | 1.64E + 05 |
| cy0204 | 24 | 7.66E + 05 |
| cy0166 | 47 | 4.66E + 06 |
| cy0159 | 49 | 1.63E + 05 |
| cy0161 | 72 | 2.08E + 06 |
| cy0166 | 80 | 3.24E + 06 |
| cy0163 | 88 | 2.50E + 06 |
| r00014 | 192 | 8.66E + 06 |
Cells from frozen or fresh mesenteric or inguinal lymph node biopsies of SIVmac239-infected Indian rhesus and Mauritian cynomolgus macaques were obtained from different points of infection and at various viral loads.
Cell preparations and virus.
Blood was drawn from either Indian rhesus macaques or Mauritian cynomolgus macaques into EDTA tubes at the Wisconsin National Primate Research Center (WNPRC) according to protocols approved by the University of Wisconsin Research Animal Resources Center. Peripheral blood mononuclear cells (PBMC) were isolated through density gradient centrifugation. CD8 T cells were depleted by using the nonhuman primate CD8 cell positive selection kit from Miltenyi Biotech. CD8 depleted cells were then incubated at 37°C with 5 μg of concanavalin A/ml at 2 × 106 cells/ml. After 24 h the cells were washed and placed in a similar volume of R15-50 (RPMI 1640 containing 15% fetal calf serum and 50 U of interleukin-2 [IL-2]/ml). After 48 or 72 h, the cells were washed and plated at 2 × 106 cells per well of a 48-well plate for mock or SIVmac239 infection.
Tenofovir was obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAD, NIH. Treatment with tenofovir was initiated 2 h prior to infection at a concentration of 400 μM as indicated (30). After mock infection or infection with SIVmac239 of tenofovir-treated cells, the cells were washed and resuspended in R15-50 containing 400 μM tenofovir throughout the remainder of the experiment.
SIVmac239, kindly generated by T. Friedrich, was grown on CEM*174 cells as previously described (8). J. Lifson generously provided 2,2′-dithiodipyridine (AT-2)-inactivated SIVmac239 generated from SUPT1-CCR Cl 30 cells as previously described (29).
Viral infection.
Viruses were prepared by layering 1 ml of SIVmac239 (9 × 109 50% tissue culture infective doses) or AT-2-inactivated SIVmac239 over 100 μl of 20% sucrose, followed by centrifugation at 14,000 rpm for 60 min at 4°C. After centrifugation, the supernatant was removed, virus resuspended in 100 μl of R-10, and 25 μl of magnetic beads was added. The magnetized virus was then resuspended, and 30 μl was added to each well of the CD8-depleted cells. The plates were then briefly centrifuged and incubated on top of a magnet for 20 min. After infection, the virus was washed off, and the cells were plated in a 48-well plate at 5 × 105 cells/ml in R15-50, and time point samples were collected at 0, 16, 24, 48, 72, and 96 h postinfection (hpi) for flow cytometry. As a control, cells were mock infected with conditioned media and collected at the same time points. All experiments were performed in triplicate and were repeated at least two times.
Transfer of conditioned medium from SIVmac239-inoculated wells.
Cells were either mock infected or infected with SIVmac239 for 48 h as described above. After 48 h, the conditioned medium from the mock- and SIVmac239-inoculated wells was removed and layered over 20% sucrose. Subsequently, the medium was centrifuged at 14,000 rpm for 60 min at 4°C to remove any virions that were present. Next, the supernatant from mock- and SIVmac239-inoculated wells was transferred to CD8-depleted, concanavalin A-stimulated uninfected cells, and time point samples were collected at 24 and 48 hpi for flow cytometry. The experiment was performed in duplicate at least two times.
Antibodies and flow cytometry of in vitro assays.
At each time point, cells were surface stained simultaneously for CD3 A700, CD4 PerCP (BD Biosciences, San Jose, CA), CD95 PE-Cy7, and integrin α4β7 PE (Millennium Pharmaceuticals). Pacific Blue (PB) was used as a dump channel to exclude autofluorescent cells. After surface staining, the cells were washed and fixed with 2% PFA for 15 min. Next, the cells were stained for intracellular Gag p27 as described above. To examine cell death, the cells were surface stained with CD3 A700, CD4 PerCP, α4β7 PE, CD95 PE-Cy7, and a LiveDead Fixable Violet ARD stain (Invitrogen). APC was used as a dump channel. After surface staining, the cells were washed, fixed with 2% PFA, and stained intracellularly with Gag p27 FITC as described above. After staining, samples were run on a BD-LSRII and analyzed by using FlowJo software version 8.8.6. The mean fluorescence intensity (MFI) was determined by using FlowJo software version 8.8.6 after gating integrin α4β7 positive cells in mock, uninfected, and infected cell subsets. Replicates were graphed by using GraphPad Prism.
Statistics.
Replicates were graphed by using GraphPad Prism. A two-tailed t test was used to compare integrin α4β7 expression on infected and uninfected CD4 cells from ex vivo lymph node samples. Bars on graphs represent the standard error of the mean.
RESULTS
Integrin α4β7 expression is decreased on Gag p27-positive cells.
A number of studies suggest that SIV selectively targets and infects integrin α4β7 high expressing cells compared to integrin α4β7 intermediate or low/negative expressing cells (1, 6, 15, 38). Other HIV/SIV receptors, such as CD4 (19, 20, 26, 41), are downregulated after infection, however, the effect of SIV/HIV infection on integrin α4β7 expression has not been previously studied. We isolated lymphocytes from the mesenteric or inguinal lymph nodes of SIVmac239-infected Indian rhesus and Mauritian cynomolgus macaques and enriched CD4+ cells by magnetically depleting CD8+, CD14+, and CD20+ cells. At least two million CD4 enriched cells were monitored for integrin α4β7, CD3, CD4, CD95, violet ARD, and Gag p27 expression. Subsequently, Gag p27+, SIVmac239-infected cells and uninfected, CD4+ cells were gated as illustrated in supplemental Fig. S1 (for supplemental figures, see www.primate.wisc.edu/ehr/files/WNPRC/WNPRC_Laboratories/oconnor/public/publications/%40files/SIV%20mediated%20down-regulation%20of%20integrin%20a4B7%20Supplemental%20Figures.pdf?renderAs=DEFAULT) and assessed independently for integrin α4β7 expression.
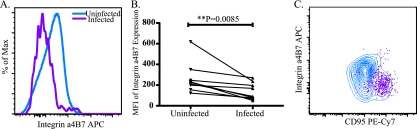
Cells infected with SIVmac239 showed reduced levels of integrin α4β7 compared to uninfected, CD4+ T cells (Fig. 1 A). To quantitate the difference in expression, we analyzed the MFI of integrin α4β7 expression in infected and uninfected cells. Infected cells (n = 9, MFI = 137.2) had significantly (P = 0.0085) lower integrin α4β7 expression than uninfected cells (n = 9, MFI = 229.1) (Fig. 1B).
FIG. 1.
Infected CD4 T cells from the lymph node express significantly less integrin α4β7 than uninfected CD4-positive T cells from the lymph node (n = 9). (A) Representative histogram of integrin α4β7 expression on infected (purple) and uninfected (blue) cells from the lymph node of an SIVmac239-infected Mauritian cynomolgus macaque. (B) MFIs of integrin α4β7 expression on uninfected and infected CD4 T cells. Lines connect samples from the same animal at the same time point. Statistical analysis was performed by using a paired Student t test. (C) Representative contour plot of integrin α4β7 and CD95 expression on uninfected (blue) and infected (purple) cells from the lymph node of a SIVmac239-infected Mauritian cynomolgus macaque.
The reduction in integrin α4β7 expression on infected cells appears to be a general feature of cellular infection with SIV; the same phenomenon was observed in different animals at multiple time points ranging from 3 to 192 weeks postinfection (Table 1). Unexpectedly, a majority of infected cells did not express the CD4 molecule and had low levels of the CD3 molecule (data not shown). This phenomenon most likely occurs because the primary SIV protein responsible for CD4 downregulation, the Nef protein, is produced early during infection, whereas the SIV protein detected by flow cytometry, the Gag protein, is expressed late during infection; however, the fitness of the cells was unknown. To exclude the possibility that the reduction in integrin α4β7 and CD4 was due to death of the infected cells, dead and dying cells were eliminated from analysis by using ARD staining (see supplemental Fig. S1).
Integrin α4β7 low/negative and high subsets are generally comprised of central or effector memory cells, while integrin α4β7 intermediate cells are generally naive (15). The phenotypic heterogeneity of distinct α4β7 subsets could confound interpretation of the ex vivo data demonstrated by Fig. 1C. In addition, our current assay cannot resolve whether the reduction of integrin α4β7 expression on infected cells is associated with a change in activation state. Therefore, we developed an in vitro model to assess the expression of integrin α4β7 on SIVmac239-infected, neighboring SIVmac239-uninfected, and mock-infected cells.
Time-dependent decrease in cell surface expression of integrin α4β7 in vitro on SIVmac239-infected CD4+ T lymphocytes from Indian rhesus macaques.
To examine integrin α4β7 expression on SIVmac239-infected CD4+ T cells in vitro, we isolated lymphocytes from the blood of uninfected Indian rhesus macaques, magnetically depleted the CD8+ T cells, and activated the remaining cells with concanavalin A for 72 h to create a homogeneous population of activated CD4+ targets for SIV infection. After activation, CD8-depleted cells were infected with SIVmac239 or mock infected. Integrin α4β7, CD3, CD4, CD95, and Gag p27 expression was monitored by flow cytometry at 0, 16, 24, 48, 72, and 96 hpi.
The flow cytometry analysis strategy is illustrated in supplemental Fig. S2a. Briefly, lymphocytes were gated based on forward and side scatter and then autofluorescent cells were eliminated to assess the cells that were Gag p27 positive. At all timepoints examined, cells from mock and SIVmac239-infected wells expressed high levels of CD95 confirming that a homogenous, activated subset of cells was present (Fig. S2b). Gag p27+, SIVmac239-infected cells were first detectable at 24 hpi.
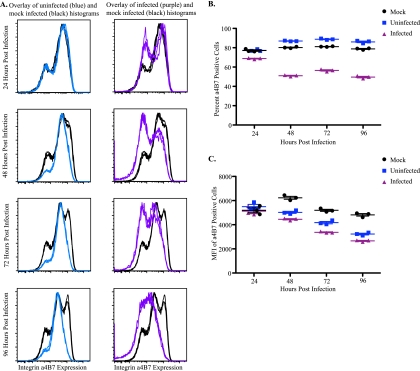
In agreement with the ex vivo data, integrin α4β7 expression decreased on the surfaces of infected cells compared to mock-infected cells beginning at 24 hpi (Fig. 2 A). To quantitate the effect of SIVmac239 infection on integrin α4β7 expression, we analyzed the percentage and MFI of integrin α4β7-positive cells on mock- and SIVmac239-infected subsets (supplemental Fig. S2c). Beginning at 24 hpi, on SIVmac239-infected cells, we observed a decrease in the percentage of integrin α4β7 positive cells (Fig. 2A and B). Similarly, at all subsequent time points examined, the percentage and the MFIs of integrin α4β7-positive cells decreased on infected cells compared to mock-infected cells (Fig. 2A to C). Thus, similar to the ex vivo data, integrin α4β7 expression was reduced on the surfaces of infected cells. Further, integrin α4β7 downregulation on SIVmac239-infected cells occurs in the presence of a homogeneous, CD95-positive population, suggesting that a change in activation state is not the cause (see supplemental Fig. S2b).
FIG. 2.
Integrin α4β7 expression is decreased on SIVmac239-infected cells and elevated on uninfected cells in vitro compared to mock-infected cells from Indian rhesus macaques (n = 9). (A) Representative histograms of integrin α4β7 expression on mock-infected (black), uninfected (blue), and infected (purple) CD4+ T cells in triplicate at multiple time points. (B and C) Representative percentages (B) and MFIs (C) of integrin α4β7-positive cells from mock-infected (black), uninfected (blue), and infected (purple) CD4+ T-cell subsets.
To examine whether uninfected cells within SIVmac239-inoculated cultures also exhibit integrin α4β7 modulation, p27-negative cells from infected wells were selected, and the surface expression of integrin α4β7 was compared to mock-infected cells (Fig. 2A). Integrin α4β7 expression on uninfected and mock-infected subsets was comparable at 24 hpi. Unexpectedly, beginning at 48 hpi, we observed an elevation of integrin α4β7-positive cells in the uninfected subset compared to the mock-infected subset (Fig. 2A and B). Once again, to quantitate the differences we analyzed the percentage and MFIs of integrin α4β7-positive cells. At 48 hpi and after, the percentages of integrin α4β7-positive cells increased, and the MFIs of integrin α4β7-positive cells decreased in uninfected cells (Fig. 2A to C), suggesting that there is a loss of integrin α4β7 high- and low/negative-expressing cells and an increase in integrin α4β7 intermediate-expressing cells. These results demonstrate that SIVmac239 infection differentially alters surface expression of the gut homing receptor, integrin α4β7, on infected and neighboring, uninfected Indian rhesus macaque CD8-depleted T cells.
Alteration of integrin α4β7 expression occurs in multiple macaque species.
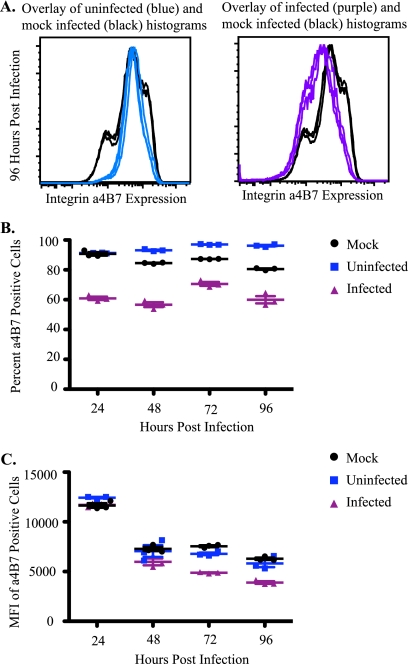
We next examined whether integrin α4β7 modulation was host species specific by repeating the in vitro analysis with lymphocytes from Mauritian cynomolgus macaques. Similar to Indian rhesus macaques, Gag p27 expression was first detected at 24 hpi, although at slightly lower levels (data not shown). Correspondingly, we observed a decrease in the percentage and MFI of integrin α4β7-positive cells from SIVmac239-infected subsets compared to mock-infected subsets (Fig. 3). We also observed an increase in the percentage of integrin α4β7-positive cells beginning at 48 hpi and a slight decrease in the MFIs of integrin α4β7-positive cells beginning at 72 hpi in uninfected cells compared to mock-infected cells (Fig. 3). This supports the hypothesis that the alteration of integrin α4β7 in infected cultures in vitro is characteristic of SIVmac239 infection.
FIG. 3.
Integrin α4β7 is altered on Mauritian cynomolgus macaque cells infected with SIVmac239 compared to mock-infected cells. (A) Representative histogram of integrin α4β7 expression on mock-infected (black), uninfected (blue), and infected (purple) CD4+ T cells in triplicate at 96 h postinfection (n = 6). (B and C) Representative percentages (B) and MFIs (C) of integrin α4β7-positive cells from mock-infected (black), infected (purple), and uninfected (blue) CD4+ T-cell subsets.
Assessment of potential mechanisms for integrin α4β7 modulation.
Finally, we examined several potential mechanisms that could explain the downregulation of integrin α4β7 on infected cells. First, we examined whether viral cytopathy or cell death could indirectly trigger integrin α4β7 modulation. Dead, ARD-positive cells were eliminated as shown in supplemental Fig. S2d prior to identification of infected and uninfected cells. There was a minimal amount of cell death even at 96 hpi, and the elimination of dead and dying cells did not alter the expression of integrin α4β7 on infected, uninfected, or mock-infected cells (data not shown). Indeed, cells in both mock- and SIVmac239-infected wells proliferated throughout the course of infection (data not shown).
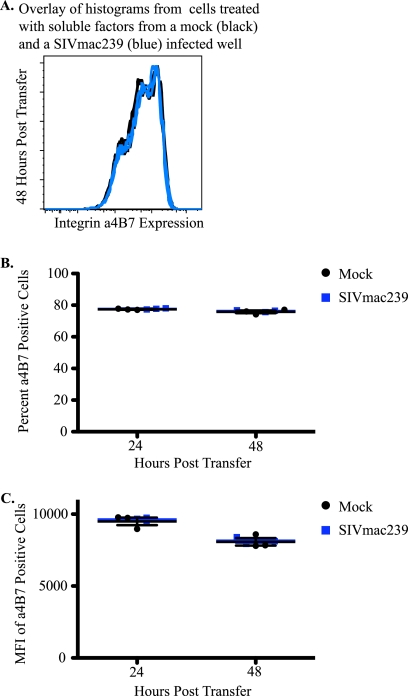
Next, we examined whether SIV infection triggered the release of a soluble factor that modulated integrin α4β7 expression. Briefly, conditioned medium from mock-infected and SIVmac239-infected wells was collected at 48 hpi, and viral particles were removed by centrifugation. The conditioned medium was then transferred to CD8-depleted T cells, and integrin α4β7 expression was examined. SIVmac239 virions in conditioned medium were successfully removed by centrifugation, since transfer did not result in Gag p27 expression (data not shown). Medium transferred from SIVmac239-infected wells did not alter integrin α4β7 expression compared to mock-infected wells (Fig. 4).
FIG. 4.
Integrin α4β7 expression is not altered in the presence of soluble factors from SIV-inoculated wells. (A) Representative histogram of integrin α4β7 expression on cells that received soluble factors from mock-infected (black) or SIVmac239-infected wells (blue) in triplicate 48 h after transfer (n = 2). (B and C) Representative percentages (B) and MFIs (C) of integrin α4β7-positive cells from cells that received soluble factors from mock-infected (black) and SIVmac239-infected (blue) wells.
SIV may also influence modulation of integrin α4β7 on infected and uninfected cells through direct mechanisms such as viral binding, entry, or expression of viral proteins. To examine whether productive viral replication was necessary, we used two independent techniques to block de novo synthesis of viral proteins. In the first experiment, activated, CD8-depleted, Indian rhesus macaque lymphocytes were treated with 400 μM concentrations of the reverse transcriptase inhibitor tenofovir for 2 h prior to infection (12). At all time points examined there was no Gag p27 expression detected, demonstrating that de novo synthesis of viral proteins was blocked (data not shown). There was no detectable difference in integrin α4β7 expression between tenofovir-treated, SIVmac239-infected cells and tenofovir-treated, mock-infected cells (Fig. 5 D and E).
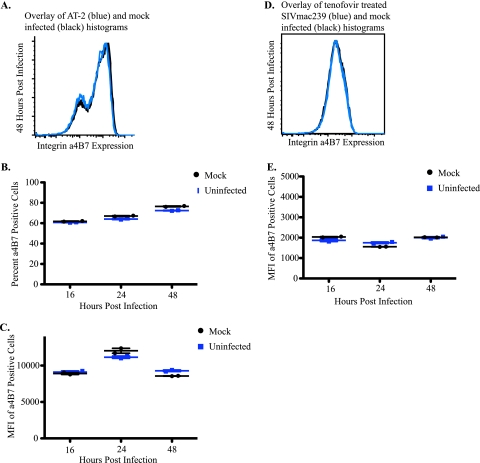
FIG. 5.
Integrin α4β7 expression is not altered in the absence of de novo synthesis of SIVmac239 proteins. (A) Representative histogram of integrin α4β7 expression on mock-infected cells (black) and cells infected with an AT-2-inactivated virus (blue) in duplicate at 48 h postinfection. (B and C) Representative percentages (B) and MFIs (C) of integrin α4β7-positive cells from mock-infected cells (black) and cells infected with an AT-2-inactivated virus (blue). (D) Representative histogram of integrin α4β7 expression on mock-infected (black) and SIVmac239-inoculated cells (blue) after treatment with tenofovir in duplicate at 48 h postinfection. (E) Representative MFIs of integrin α4β7 expression from tenofovir-treated, mock (black)- and SIVmac239 (blue)-inoculated CD4+ T-cell subsets.
In the second experiment, we used AT-2-inactivated SIVmac239 to infect activated, CD8+ T-cell-depleted lymphocytes from Indian rhesus macaques. AT-2-inactivated virions are capable of binding CD4+ cells, fusing, and entering. The viral life cycle is aborted before initiation of reverse transcriptase rendering the virus replication incompetent (2, 28). Similar to the tenofovir-treated cells, there was no Gag p27 expression detected at any of the time points examined (data not shown). Furthermore, the percentages and MFIs of integrin α4β7-positive cells from AT-2-treated and mock-infected wells were indistinguishable (Fig. 5A to C). These results support that de novo synthesis of viral proteins is required for the alteration of integrin α4β7 expression during SIVmac239 infection, but neither cell death nor the release of a soluble factor is involved.
DISCUSSION
SIV and HIV infection results in an early and rapid establishment of infection in the GALT and depletion of the intestinal CD4+ T cells (3, 23, 25). Details surrounding this early stage of pathogenesis remain elusive. Recent work has demonstrated that the HIV envelope protein, gp120, selectively binds to and signals through integrin α4β7, a gut homing integrin (1). Further, it has been demonstrated that cells expressing high levels of integrin α4β7 are preferentially infected and depleted during SIV infection (6, 15, 38). Although integrin α4β7 is not required for viral replication, it is clear that viral engagement of integrin α4β7 provides an advantage to SIV and HIV infection (6). It is within this context that we examined single-cell integrin α4β7 dynamics during SIVmac239 infection.
Using an ex vivo model, we demonstrated that Gag p27+, infected cells from the lymph node express significantly (P = 0.0085) lower quantities of integrin α4β7 compared to uninfected, CD4+ T cells from the lymph node. To our knowledge, these are the first experiments to examine integrin α4β7 on Gag p27+, infected cells by flow cytometry ex vivo. One limitation of this assay is that plasma viral loads need to be >104 copies/ml (Reynolds, unpublished). We are actively working to improve the sensitivity of the ex vivo assay so that we can monitor integrin α4β7 expression in macaques that control SIV infection to determine whether there are differences.
In vitro studies of homogeneous cell populations confirmed that the percentages and MFIs of integrin α4β7-positive cells decreased in infected cells compared to mock-infected cells in a time-dependent manner. Unexpectedly, the percentages of integrin α4β7-positive cells increased and the MFIs decreased in uninfected cells compared to mock-infected cells. Taken together, these data demonstrate that integrin α4β7 downregulation is mediated by SIVmac239 in the presence of a homogeneous, CD95-positive population.
Our initial efforts to identify the mechanism of integrin α4β7 were partially successful, since we demonstrate that de novo synthesis of viral proteins is required. Currently, it is unclear whether de novo synthesis of viral proteins leads to a direct or indirect modulation of integrin α4β7; however, it is unlikely that the presence of a soluble factor, cell death, or a change in activation state is responsible. We are actively exploring the contributions of individual viral proteins to integrin α4β7 modulation.
It is likely that downregulation of integrin α4β7 confers an advantage to the virus. Several viruses, including HIV and SIV, use receptor interference, or the removal of an entry receptor from the surface of the cell, to protect the cell from superinfection and facilitate viral release. Notably, HIV and SIV downregulate CD4 and CCR5 during infection (19, 26, 41). Recently, Cicala et al. demonstrated that integrin α4β7, CD4, and CCR5 colocalize on the cell membrane. Also, due to the extension of integrin α4β7 from the surface of the cell it is likely that integrin α4β7 is the first receptor to engage the virion during infection (6). In addition, integrin α4β7 high cells are preferentially infected. Thus, it is possible that downregulation of integrin α4β7 from the surfaces of SIV-infected cells may prevent superinfection or facilitate viral release. It is also possible that the downregulation of integrin α4β7, due to the close proximity to CD4 and CCR5, is a bystander effect of the downregulation of CD4 and CCR5 from the surface of the cell during SIV infection.
Another advantage of receptor modulation during viral infection is immune evasion. SIV and HIV downregulate CD28, major histocompatibility complex (MHC) I, and MHC II to dampen T-cell activation and immune recognition (19, 41). Integrins are highly potent receptors due to their ability to communicate with the cellular environment and engage multiple receptors, including immune receptors (16, 27). Recruitment of cells expressing integrin α4β7 to the intestines has been associated with graft-versus-host disease and other intestinal inflammatory disorders (5, 18). Therefore, downregulation of integrin α4β7 may decrease the amount of inflammation in the GALT.
In addition to immune evasion or receptor interference, alteration of integrin α4β7 may influence lymphocyte trafficking and subsequently SIV/HIV pathogenesis. SIVsmmPBj is a highly pathogenic strain of SIV that causes acutely lethal disease characterized by profuse watery diarrhea, dehydration, and electrolyte imbalance or metabolic acidosis with gastrointestinal lesions (9-11, 22). In addition, SIVsmmPBj is associated with increased virus replication and immune activation in the GALT (7, 11, 32). Gummuluru et al. noted that lymphocytes from macaques infected with SIVsmmPBj induced expression of integrin αEβ7 and to a lesser extent integrin α4β7 (13, 32). Combined, these findings suggest that recruitment and retention of lymphocytes in the GALT during SIV infection may result in a worsened disease state. Therefore, downregulation of integrin α4β7 may facilitate the circulation of virus-infected cells decreasing the severity of GALT inflammation and tissue damage, lengthening the survival of the host and hence the virus. The downregulation of integrin α4β7 may also have an unappreciated role in the depletion of the mucosal-associated lymphoid compartments.
In conclusion, we successfully examined the single-cell dynamics of integrin α4β7 modulation during SIVmac239 infection. We demonstrated that integrin α4β7 is downregulated on the surfaces of SIVmac239-infected cells both ex vivo and in vitro. Further, on uninfected cells, integrin α4β7 levels were elevated, specifically the integrin α4β7 intermediate subset. This modulation is dependent on de novo synthesis of viral proteins but neither cell death, the release of a soluble factor, or the cellular activation state is involved. Noting that SIV and HIV preferentially infect integrin α4β7 high-expressing cells, it is likely that downregulation of the receptor after viral engagement augments some advantage to the virus. Thus, downregulation of integrin α4β7 on SIV-infected cells may have important implications for pathogenesis, and further investigation could provide novel insights into SIV/HIV-cell interactions and their influence on cellular processes.
Acknowledgments
This research was supported by National Institutes of Health grants R01 AI077376-01 and R21 AI082880-01.
We acknowledge David Watkins and his lab members Shari Piaskowski and Nancy Wilson for their contributions. We also thank the staff at the Wisconsin National Primate Research Center, which is supported by grant P51 RR000167 from the National Center for Research Resources, a component of the National Institutes of Health. Some of the work described here was conducted in a facility constructed with support from Research Facilities Improvement Program grants RR15459-01 and RR020141-01.
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Arthos, J., C. Cicala, E. Martinelli, K. Macleod, D. Van Ryk, D. Wei, Z. Xiao, T. D. Veenstra, T. P. Conrad, R. A. Lempicki, S. McLaughlin, M. Pascuccio, R. Gopaul, J. McNally, C. C. Cruz, N. Censoplano, E. Chung, K. N. Reitano, S. Kottilil, D. J. Goode, and A. S. Fauci. 2008. HIV-1 envelope protein binds to and signals through integrin α4β7, the gut mucosal homing receptor for peripheral T cells. Nat. Immunol. 9:301-309. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, L. O., J. W. J. Bess, E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14(Suppl. 3):S311-S319. [PubMed] [Google Scholar]
- 3.Brenchley, J. M., and D. C. Douek. 2008. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 1:23-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T-cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen, Y. B., H. T. Kim, S. McDonough, R. D. Odze, X. Yao, S. Lazo-Kallanian, T. R. Spitzer, R. Soiffer, J. H. Antin, and J. Ritz. 2009. Upregulation of α4β7 integrin on peripheral T-cell subsets correlates with the development of acute intestinal graft-versus-host disease following allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 15:1066-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cicala, C., E. Martinelli, J. P. McNally, D. J. Goode, R. Gopaul, J. Hiatt, K. Jelicic, S. Kottilil, K. Macleod, A. O'Shea, N. Patel, D. Van Ryk, D. Wei, M. Pascuccio, L. Yi, L. McKinnon, P. Izulla, J. Kimani, R. Kaul, A. S. Fauci, and J. Arthos. 2009. The integrin α4β7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877-20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 8.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10:275-281. [DOI] [PubMed] [Google Scholar]
- 9.Fultz, P. N. 1994. SIVsmmPBj14: an atypical lentivirus. Curr. Top. Microbiol. Immunol. 188:65-76. [DOI] [PubMed] [Google Scholar]
- 10.Fultz, P. N., H. M. McClure, D. C. Anderson, and W. M. Switzer. 1989. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM). AIDS Res. Hum. Retrovir. 5:397-409. [DOI] [PubMed] [Google Scholar]
- 11.Fultz, P. N., and P. M. Zack. 1994. Unique lentivirus-host interactions: SIVsmmPBj14 infection of macaques. Virus Res. 32:205-225. [DOI] [PubMed] [Google Scholar]
- 12.Fung, H. B., E. A. Stone, and F. J. Piacenti. 2002. Tenofovir disoproxil fumarate: a nucleotide reverse transcriptase inhibitor for the treatment of HIV infection. Clin. Ther. 24:1515-1548. [DOI] [PubMed] [Google Scholar]
- 13.Gummuluru, S., F. J. Novembre, B. Seshi, and S. Dewhurst. 1996. SIVsmmPBj14 induces expression of a mucosal integrin on macaque lymphocytes. Virology 215:97-100. [DOI] [PubMed] [Google Scholar]
- 14.Halasz, P., G. Holloway, S. J. Turner, and B. S. Coulson. 2008. Rotavirus replication in intestinal cells differentially regulates integrin expression by a phosphatidylinositol 3-kinase-dependent pathway, resulting in increased cell adhesion and virus yield. J. Virol. 82:148-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kader, M., X. Wang, M. Piatak, J. Lifson, M. Roederer, R. Veazey, and J. J. Mattapallil. 2009. Alpha4(+)beta7(hi)CD4(+) memory T cells harbor most Th-17 cells and are preferentially infected during acute SIV infection. Mucosal Immunol. 2:439-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn, M. L., and G. A. Koretzky. 2006. Integrins and ITAMs: more than just good neighbors. Nat. Immunol. 7:1286-1288. [DOI] [PubMed] [Google Scholar]
- 17.Kalia, M., and S. Jameel. 2009. Virus entry paradigms. Amino Acids. doi: 10.1007/s00726-009-0363-3. [DOI] [PMC free article] [PubMed]
- 18.Kaser, A., and H. Tilg. 2008. Novel therapeutic targets in the treatment of IBD. Expert Opin. Ther. Targets 12:553-563. [DOI] [PubMed] [Google Scholar]
- 19.Keppler, O. T., N. Tibroni, S. Venzke, S. Rauch, and O. T. Fackler. 2006. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc. Biol. 79:616-627. [DOI] [PubMed] [Google Scholar]
- 20.Lama, J. 2003. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr. HIV Res. 1:167-184. [DOI] [PubMed] [Google Scholar]
- 21.Lay, M. D., J. Petravic, S. N. Gordon, J. Engram, G. Silvestri, and M. P. Davenport. 2009. Is the gut the major source of virus in early simian immunodeficiency virus infection? J. Virol. 83:7517-7523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis, M. G., P. M. Zack, W. R. Elkins, and P. B. Jahrling. 1992. Infection of rhesus and cynomolgus macaques with a rapidly fatal SIV (SIVSMM/PBj) isolate from sooty mangabeys. AIDS Res. Hum. Retrovir. 8:1631-1639. [DOI] [PubMed] [Google Scholar]
- 23.Li, Q., L. Duan, J. D. Estes, Z. M. Ma, T. Rourke, Y. Wang, C. Reilly, J. Carlis, C. J. Miller, and A. T. Haase. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148-1152. [DOI] [PubMed] [Google Scholar]
- 24.Luo, B. H., C. V. Carman, and T. A. Springer. 2007. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25:619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mattapallil, J. J., D. C. Douek, B. Hill, Y. Nishimura, M. Martin, and M. Roederer. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093-1097. [DOI] [PubMed] [Google Scholar]
- 26.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell surface CCR5 and CD4. Curr. Biol. 15:714-723. [DOI] [PubMed] [Google Scholar]
- 27.Mocsai, A., M. Zhou, F. Meng, V. L. Tybulewicz, and C. A. Lowell. 2002. Syk is required for integrin signaling in neutrophils. Immunity 16:547-558. [DOI] [PubMed] [Google Scholar]
- 28.Rossio, J. L., M. T. Esser, K. Suryanarayana, D. K. Schneider, J. W. J. Bess, G. M. Vasquez, T. A. Wiltrout, E. Chertova, M. K. Grimes, Q. Sattentau, L. O. Arthur, L. E. Henderson, and J. D. Lifson. 1998. Inactivation of human immunodeficiency virus type 1 infectivity with preservation of conformational and functional integrity of virion surface proteins. J. Virol. 72:7992-8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rutebemberwa, A., J. W. J. Bess, B. Brown, M. Arroyo, M. Eller, B. Slike, V. Polonis, F. McCutchan, J. R. Currier, D. Birx, M. Robb, M. Marovich, J. D. Lifson, and J. H. Cox. 2007. Evaluation of aldrithiol-2-inactivated preparations of HIV type 1 subtypes A, B, and D as reagents to monitor T-cell responses. AIDS Res. Hum. Retrovir. 23:532-542. [DOI] [PubMed] [Google Scholar]
- 30.Sacha, J. B., C. Chung, J. Reed, A. K. Jonas, A. T. Bean, S. P. Spencer, W. Lee, L. Vojnov, R. Rudersdorf, T. C. Friedrich, N. A. Wilson, J. D. Lifson, and D. I. Watkins. 2007. Pol-specific CD8+ T cells recognize simian immunodeficiency virus-infected cells prior to Nef-mediated major histocompatibility complex class I downregulation. J. Virol. 81:11703-11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salmi, M., and S. Jalkanen. 2005. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol. Rev. 206:100-113. [DOI] [PubMed] [Google Scholar]
- 32.Sasseville, V. G., J. B. Rottman, Z. Du, R. Veazey, H. L. Knight, D. Caunt, R. C. Desrosiers, and A. A. Lackner. 1998. Characterization of the cutaneous exanthem in macaques infected with a nef gene variant of SIVmac239. J. Invest. Dermatol. 110:894-901. [DOI] [PubMed] [Google Scholar]
- 33.Shaw, S. K., and M. B. Brenner. 1995. The beta 7 integrins in mucosal homing and retention. Semin. Immunol. 7:335-342. [DOI] [PubMed] [Google Scholar]
- 34.Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of β1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278:20-26. [DOI] [PubMed] [Google Scholar]
- 35.Takada, Y., X. Ye, and S. Simon. 2007. The integrins. Genome Biol. 8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van der Flier, A., and A. Sonnenberg. 2001. Function and interactions of integrins. Cell Tissue Res. 305:285-298. [DOI] [PubMed] [Google Scholar]
- 37.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T-cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 38.Wang, X., H. Xu, A. F. Gill, B. Pahar, D. Kempf, T. Rasmussen, A. A. Lackner, and R. S. Veazey. 2009. Monitoring α4β7 integrin expression on circulating CD4+ T cells as a surrogate marker for tracking intestinal CD4+ T-cell loss in SIV infection. Mucosal Immunol. 2:518-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Warren, A. P., C. N. Owens, L. K. Borysiewicz, and K. Patel. 1994. Down-regulation of integrin alpha 1/beta 1 expression and association with cell rounding in human cytomegalovirus-infected fibroblasts. J. Gen. Virol. 75:3319-3325. [DOI] [PubMed] [Google Scholar]
- 40.Weeks, B. S., M. E. Klotman, S. Dhawan, M. Kibbey, J. Rappaport, H. K. Kleinman, K. M. Yamada, and P. E. Klotman. 1991. HIV-1 infection of human T lymphocytes results in enhanced alpha 5 beta 1 integrin expression. J. Cell Biol. 114:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wildum, S., M. Schindler, J. Munch, and F. Kirchhoff. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J. Virol. 80:8047-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]