Abstract
Due to the inherent immune evasion properties of the HIV envelope, broadly neutralizing HIV-specific antibodies capable of suppressing HIV infection are rarely produced by infected individuals. We examined the feasibility of utilizing genetic engineering to circumvent the restricted capacity of individuals to endogenously produce broadly neutralizing HIV-specific antibodies. We constructed a single lentiviral vector that encoded the heavy and light chains of 2G12, a broadly neutralizing anti-HIV human antibody, and that efficiently transduced and directed primary human B cells to secrete 2G12. To evaluate the capacity of this approach to provide protection from in vivo HIV infection, we used the humanized NOD/SCID/γcnull mouse model, which becomes populated with human B cells, T cells, and macrophages after transplantation with human hematopoietic stem cells (hu-HSC) and develops in vivo infection after inoculation with HIV. The plasma of the irradiated NOD/SCID/γcnull mice transplanted with hu-HSC transduced with the 2G12-encoding lentivirus contained 2G12 antibody, likely secreted by progeny human lymphoid and/or myeloid cells. After intraperitoneal inoculation with high-titer HIV-1JR-CSF, mice engrafted with 2G12-transduced hu-HSC displayed marked inhibition of in vivo HIV infection as manifested by a profound 70-fold reduction in plasma HIV RNA levels and an almost 200-fold reduction in HIV-infected human cell numbers in mouse spleens, compared to control hu-HSC-transplanted NOD/SCID/γcnull mice inoculated with equivalent high-titer HIV-1JR-CSF. These results support the potential efficacy of this new gene therapy approach of using lentiviral vectors encoding a mixture of broadly neutralizing HIV antibodies for the treatment of HIV infection, particularly infection with multiple-drug-resistant isolates.
While broadly neutralizing human immunodeficiency virus (HIV)-specific antibodies have the capacity to prevent or suppress HIV infection, they are rarely produced by infected individuals, thereby markedly compromising the ability of the humoral response to control HIV infection (reviewed in reference 28). The high degree of sequence variability in the gp120 structure limits the number of highly conserved epitopes available for targeting by neutralizing antibodies (40). In addition, HIV utilizes several mechanisms to shield the limited number of conserved neutralizing epitopes from the potentially potent antiviral effects of HIV envelope-specific antibodies (14). First, the envelope protein is heavily glycosylated, and the linkage of the most immunoreactive envelope peptide structures to poorly immunogenic glycans shields them from antibody binding (37). Second, exposure of neutralizing epitopes not protected from antibody binding by glycosylation is greatly reduced by trimerization of the gp120-gp41 structure (5). Third, susceptibility of other neutralizing epitopes to antibodies is greatly reduced by limiting their accessibility to antibody binding to the brief transient phase of conformational changes that occur only during binding of the envelope protein to its cellular receptors, CD4 and CCR5 or CXCR4 (41). These intrinsic structural features of gp120 greatly reduce the capacity of natural HIV infection or vaccination to generate broadly neutralizing antibodies able to prevent or control infection. Despite these constraints, rare human antibodies with broad anti-HIV neutralizing activity, i.e., 2G12, b12, 2F5, and 4E10, have been isolated (2).
The capacity of passive immunization with neutralizing antibodies to prevent infection was suggested by challenge studies demonstrating that transferred neutralizing antibodies protected monkeys from infection by simian immunodeficiency virus (SIV) and simian-human immunodeficiency virus (SHIV) (15). These studies were extended to humans, including several studies that examined the effect of passive immunotherapy using 2G12, 2F5, and 4E10 on inhibition of HIV replication in infected individuals (20). Passive immunotherapy with a triple combination of 2G12, 2F5, and 4E10 delayed viral rebound after the cessation of highly active antiretroviral therapy (HAART), and activity of 2G12 was critical for inhibitory activity by this antibody combination (18). The key role of 2G12 in suppressing HIV replication was supported by the development of viral rebound in parallel with the emergence of HIV isolates resistant to neutralization by 2G12 (19).
While HIV infection may be controlled by the lifelong treatment of HIV-infected individuals with periodic infusions of neutralizing-antibody cocktails every few weeks, this is not a practical or cost-effective therapeutic approach. Eliciting these antibodies by vaccination has not been successful. Therefore, we investigated whether we could circumvent the mechanisms that limit the endogenous production of broadly neutralizing HIV-specific antibodies using a molecular genetic approach to generate B cells that secrete these protective antibodies. In a proof-of-concept study, we examined the capacity of a single lentiviral vector to express the heavy and light chains of the 2G12 antibody, a well-studied anti-HIV human antibody that has broad neutralizing activity both against T cell line-adapted and primary HIV isolates (31). The 2G12 antibody was generated by applying murine/human xenohybridoma technology to establish human hybridoma cell lines from B cells isolated from HIV-infected individuals (16), and it targets the high-mannose and/or hybrid glycans of residues 295, 332, and 392 and peripheral glycans from residues 386 and 448 on gp120. In the current study we demonstrated that a lentiviral vector encoding the heavy and light chains of the 2G12 antibody reprogrammed B cells in vitro to secrete 2G12 with functional neutralizing activity. Furthermore, we demonstrated that the 2G12 lentiviral vector genetically modified human hematopoietic stem cells (hu-HSC), enabling them to differentiate in vivo into progeny cells that secreted 2G12 antibody that inhibited the development of in vivo HIV infection in humanized mice.
MATERIALS AND METHODS
Cells and cell culture.
The 293T cell line, used for lentiviral production, was maintained, before being used for transfection, for fewer than 15 passages in Iscove's modified Dulbecco's medium (IMDM) (Cellgro, Manassas, VA) supplemented with heat-inactivated fetal calf serum (FCS) (10%, vol/vol; Atlanta Biological, Lawrenceville, GA), penicillin (100 U/ml; Life Technologies, Carlsbad, California), streptomycin (10 μg/ml; Life Technologies), HEPES buffer (10 mM; MP Biomedicals, Solon, OH), and l-glutamine (2 mM; Life Technologies). The NSO cell line, which is capable of producing recombinant antibodies (26), and the TZM-bl cell line (36) (obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with heat-inactivated FCS (10%, vol/vol), penicillin (100 U/ml), streptomycin (10 μg/ml), and 1× MEM nonessential amino acids (Sigma, St. Louis, MO). For NSO cells, the DMEM was also supplemented with NTCC 109 medium (10%, vol/vol; Sigma).
Highly purified B cells (>90% purity) were isolated from donor leukopacks from HIV-naïve donors using MACS CD19 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. Following isolation, the highly purified B cells were activated for 48 h with 10 μg/ml of lipopolysaccharide (LPS) (Sigma) to increase their susceptibility to lentiviral vector transduction as described previously (34). Highly purified (>95% purity) human hematopoietic stem cells (hu-HSC) were isolated from umbilical cord blood from HIV-naïve donors using MACS CD34 microbeads (Miltenyi Biotec) according to the manufacturer's instructions and were aliquoted and frozen until use. The hu-HSC were rapidly thawed, washed, and cultured prior to transduction for 48 h as described previously (6) in IMDM supplemented with FCS (1.5% vol/vol), BIT 9500 (20%, vol/vol; Stem Cell Technologies, Vancouver, BC, Canada), penicillin (100 U/ml), streptomycin (10 μg/ml), and cytokines (Flt-3 ligand [100 ng/ml], stem cell factor [SCF] [100 ng/ml], granulocyte colony-stimulating factor [GCSF] [10 ng/ml], and interleukin-6 [IL-6] [10 ng/ml] [all obtained from Peprotech, Rocky Hill, NJ]).
Cloning of the 2G12 heavy and light chains into the lentiviral vector.
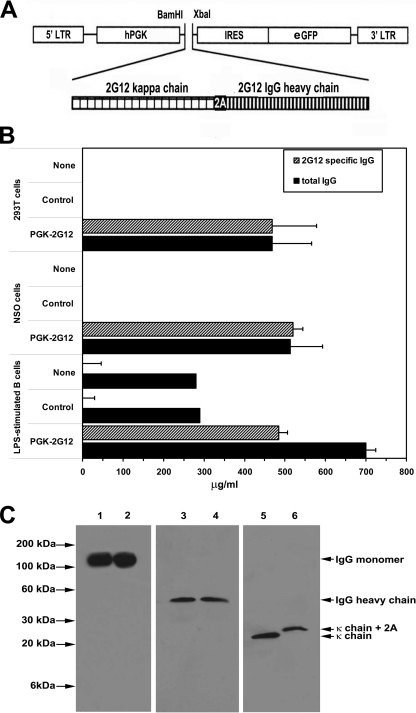
The mRNA sequences encoding the light and heavy chain genes that combine into the secreted 2G12 antibody (1, 16) were combined into a single transcript linked by a “self-cleaving” 2A peptide (30) (light chain-2A-heavy chain) using a PCR strategy (12) as shown in Fig. 1 A. Briefly, the first PCR amplification used a forward primer specific for the 5′ leader sequence of the light chain with an added BamHI restriction site (primer 1) and a return primer which contains the sequence encoding half of the 2A sequence followed by the terminal light chain region (primer 3). The second PCR amplification used a forward primer containing the full 2A sequence followed by the heavy chain leader sequence (primer 4) and a return primer specific for the terminal sequence of the heavy chain and including an XbaI restriction site (primer 2). The two PCR products were mixed, and the combined light chain-2A-heavy chain sequence was generated by PCR amplification with primer 1 and primer 2. The product was cloned into the BamHI/XbaI restriction site of a lentiviral transfer vector regulated by the human phosphoglycerate kinase (hPGK) promoter (hPGK.ires.emcvwt.eGFP.Wpre) (7) upstream of an internal ribosome entry site (IRES)-regulated enhanced green fluorescent protein (eGFP) reporter gene.
FIG. 1.
Cells transfected with the 2G12-lentivector or transduced with 2G12-expressing lentivirus produce 2G12 antibody. (A) PCR cloning of the vector expressing the 2G12 light chain-2A-2G12 heavy chain construct into the hPGK.ires.emcvwt.eGFP.Wpre construct downstream of the 5′ long terminal repeat (LTR) and the human phosphoglycerate kinase (hPGK) promoter and upstream of an internal ribosome entry site (IRES)-regulated enhanced green fluorescent protein (eGFP) reporter gene was performed as described in Materials and Methods to generate the 2G12-lentivector construct. (B) 293T cells were untransfected or transfected with either a control eGFP-expressing lentivector or the 2G12-lentivector. NSO cells or LPS-stimulated primary B cells were untransduced or transduced either with a control eGFP-expressing lentivirus or the 2G12-expressing lentivirus. One week later, the level of 2G12 antibody was quantified by ELISA. The data represent the averages ± standard errors of the means (SEM) from three separate experiments performed in triplicate and are presented as μg/ml normalized to cultures of 1 × 106 cells/ml. (C) Comparison of the structures of lentivector-produced 2G12 antibody and monoclonal 2G12 antibody. Monoclonal 2G12 antibody (lanes 1, 3, and 5) or supernatant collected from 2G12-lentivector-transduced 293T cells (lanes 2, 4, and 6) were run on SDS-polyacrylamide gels under nonreducing conditions (lanes 1 and 2) or reducing conditions (lanes 3 to 6), and the gels were subjected to Western blotting with the indicated antibody.
Transfection and lentiviral vector production.
The pseudotyped HIV-based third-generation lentivirus carrying the light chain-2A-heavy chain construct of the 2G12 antibody was generated by calcium phosphate-mediated cotransfection of 293T cells with four plasmids: a cytomegalovirus (CMV) promoter-driven packaging construct expressing the gag and pol genes, a Rous sarcoma virus (RSV) promoter-driven construct expressing the rev gene, a CMV promoter-driven construct expressing the vesicular stomatitis virus (VSV) G envelope gene, and a self-inactivating transfer construct driven by the hPGK promoter containing HIV cis-acting sequences and an expression cassette for the 2G12 light chain-2A-heavy chain coding sequence inserted upstream of an IRES-regulated eGFP (2G12-lentivector) or an empty expression plasmid as described previously (8). The culture medium was replaced about 16 h after transfection and then harvested 24 h and 48 h later, filtered, and ultracentrifuged. The lentivirus-containing pellet was resuspended in sterile phosphate-buffered saline (PBS), frozen in aliquots, and stored at −80°C until use. The concentration of the lentiviral vector in the aliquots was determined by measuring the p24 concentration in the supernatant by enzyme-linked immunosorbent assay (ELISA) (see below).
Lentiviral transduction of target cells.
LPS-activated primary B cells, cytokine-stimulated primary hu-HSC, or NSO cells were harvested, washed, resuspended in 0.5 ml of growth medium with added Polybrene (4 μg/ml), and plated into 24-well plates (1 ×105 cells/well). The indicated lentivirus (100 ng of p24 antigen, ∼108 transducing units/ml) was added to each well, and after centrifugation, (2,500 rpm for 30 min at room temperature) the plates were incubated overnight at 37°C. The next day, growth medium was added to each well and the plates were cultured for an additional 2 to 7 days. The transduction efficiency of defined cell populations was determined by measuring cellular expression of the eGFP reporter gene after cell staining with antibodies to cell-specific surface markers by flow cytometry as described previously (29). The indicated antibodies (anti-human CD19-phycoerythrin [PE], anti-human CD19-allophycocyanin [APC]-Cy7, anti-human CD8-PE-Cy5, and anti-human CD4-PE [Biolegend, San Diego CA] and anti-human CD34-PE and anti-human CD45-APC [BD Biosciences, Franklin Lakes, NJ]) were added to cells suspended in fluorescence-activated cell sorter (FACS) buffer, incubated for 30 min at 4°C in the dark, washed, and resuspended in 0.25 ml of FACS buffer. Fluorescent cells were enumerated using an LSRII (BD Biosciences), and the acquired data were analyzed using FlowJo software (Treestar, Ashland, OR).
Measurement of total IgG and 2G12 IgG.
Total IgG was measured by incubating culture supernatant or serum directly in triplicate wells (100 μl/well) in Maxisorp ELISA plates (Nunc, Rochester, NY) for 1 h at 37°C. The plates were blocked with Tris-buffered saline (TBS) blocking buffer with added 5% casein, washed, and incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG antibody for 1 h at 37°C. After the wells were washed, substrate (Sigmafast OPD; Sigma) was added and incubated for 30 min. The reaction was stopped with 4 N sulfuric acid, and specific absorbance was measured using a microplate reader. The amount of 2G12 IgG produced was quantified using a modified ELISA (39). Briefly, Maxisorp ELISA plates were coated with M1G1 antibody, an anti-idiotype antibody specific for 2G12 (39), overnight at 4°C in NaHCO3 (pH 8.0) coating buffer. The plates were blocked with TBS blocking buffer with added 5% casein for 1 h at 37°C. Samples were diluted in PBS and incubated in triplicate wells for 1 h at 37°C, the wells were washed, and HRP-conjugated anti-human IgG antibody (1:1,000 dilution; Southern Biotech, Birmingham, AL) was added to the wells and incubated for 1 h at 37°C. After the wells were washed, Sigmafast OPD substrate was added and specific absorbance was determined as described above.
Western immunoblot analysis of 2G12 antibody structure.
Proper assembly of the 2G12 antibody heavy and light chains was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western immunoblotting as described previously (21, 23). Supernatant collected from 293T cells transduced with the 2G12 lentivector and monoclonal 2G12 antibody obtained from the NIH AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH) were boiled in either a nonreducing or a reducing (containing 25 mM mercaptoethanol) SDS sample buffer, electrophoresed on a 4 to 20% precast linear gradient Tris-HCl Ready Gel (Bio-Rad, Hercules, CA), and transferred to an Immunoblot polyvinylidene difluoride membrane (Bio-Rad, Hercules, CA). The membrane was blocked for 1 h with PBS containing 5% milk powder, incubated with either goat anti-human IgG antibody conjugated to HRP (Southern Biotech) or goat anti-human kappa chain antibody conjugated to HRP (Southern Biotech) for 1 h, and then washed four times with PBS containing 0.1% Tween. Bound antibody was detected with the Western Lightning chemiluminescence system (GE Healthcare, Boston, MA) according to the manufacturer's instructions.
HIV neutralization assay.
HIV-neutralizing antibody activity was quantified using the TZM-bl luciferase reporter gene assay as described previously (25). TZM-bl (previously designated clone JC53-bl [clone 13]) is a HeLa cell line engineered to express CD4, CCR5 and CXCR4, and a HIV long terminal repeat-regulated luciferase reporter gene upon infection of the cells with diverse isolates of HIV. Neutralizing activity was measured by adding 1 μl of high-titer HIVJR-CSF (> 3,000 tissue culture infective doses [TCID]) to 150 μl of culture supernatants (at a 1:1 dilution). After incubation for 1 h at 37°C in a 96-well flat-bottom culture plate, freshly trypsinized TZM-bl cells (103 cells in 50 μl) were added to each well. After 2 days of culture, the cells were harvested and lysed with 200 μl of lysis buffer (Promega, Madison, WI), and luciferase activity was quantified using a DC Berthold TubeMaster (Berthold Technologies, Oak Ridge, TN) and expressed as relative light units.
Generation of hu-NOD/SCID/γcnull mice.
The pathogen-free, non-obese-diabetic/severe combined immunodeficient mouse line harboring a complete null mutation of the common cytokine receptor γ chain (NOD/SCID/γcnull mice; 8 to 12 weeks old) used in this study were a kind gift of Leonard Shultz (Jackson Laboratory, Bar Harbor, ME) (10) and were housed and maintained as described previously (32). All animal studies were approved by the Einstein Institutional Animal Care and Use Committee and were consistent with the guidelines for the care and use of laboratory animals. Sublethally irradiated (400 rads) NOD/SCID/γcnull mice were intravenously injected with highly purified human cord blood CD34 cells (1 × 105 cells). The CD34 cells were mock transduced, transduced with empty lentivector, or transduced with 2G12-lentivector. After transplantation, the mice were maintained on a regimen of sulfamethoxazole and trimethoprim in their drinking water, and their engraftment was monitored by flow cytometric analysis of peripheral blood for the presence of human CD45+ cells.
Infection of hu-NOD/SCID/γcnull mice with HIV.
After documentation that the peripheral blood of the transplanted hu-NOD/SCID/γcnull mice was populated with human T cells, mice were injected intraperitoneally with 1 ml of HIVJR-CSF virus (>10,000 TCID) as described previously (24). One week later, HIV infection of the mice was quantified by measurement of plasma HIV RNA levels using the Versant HIV bDNA 3.0 assay (Siemens Healthcare Diagnostics, Deerfield, IL) according to the manufacturer's instructions. In addition, we quantified the number of HIV-infected cells in the mouse spleens by limiting-dilution coculture as described previously (4). Briefly, 5-fold dilutions of splenocytes were added to HIV-naïve, phytohemagglutinin (PHA)/IL-2-activated human peripheral blood mononuclear cells (PBMCs) (1 × 106 cells/well). After 7 days, HIV p24 antigen in the supernatant was quantified by an ELISA (12).
RESULTS
Construction of the lentiviral vector expressing the 2G12 heavy and light chain antibody sequences.
As shown in Fig. 1A, a lentiviral expression construct encoding the heavy chain and light chain of the human HIV-neutralizing antibody 2G12 (28) as a single transcript linked with a 2A-self-cleaving peptide regulated by the hPGK (12) upstream of an IRES-EGFP reporter sequence was generated using the PCR-based cloning strategy (12). The membrane region of the IgG heavy chain gene expressed by the 2G12-lentivector was deleted to maximize secretion of the vector-encoded 2G12 antibody. After translation of the light chain-2A-heavy chain transcript, the 2A peptide undergoes spontaneous cleavage generating near-complete stoichiometric separation of the linked IgG light and heavy chains. A third-generation, four-plasmid lentiviral vector system was used to generate high-titer lentivirus expressing this construct by transient transfection of 293T packaging cells as described previously (12). We chose the lentiviral vector system because lentiviral vectors can transduce quiescent hu-HSC much more efficiently than murine gamma retroviral vectors (3, 13) and mediate subsequent in vivo expression by mature progeny cells after transplantation of transduced hu-HSC into NOD/SCID/γcnull mice (7).
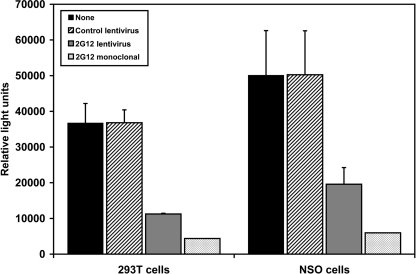
The capacity of the 2G12-lentivector to encode functional 2G12 antibody was examined by transfecting 293T cells with either a control lentivector or the 2G12-lentivector or by transducing the murine myeloma cell line NSO (26) or LPS-activated primary human B cells with either a control lentivirus or the 2G12-expressing lentivirus. Seven days later, the fractions of 293T cells, NSO cells, and primary B cells expressing the eGFP reporter gene were 86%, 59%, and 32%, respectively. 2G12 antibody was detected in the culture supernatant, constituting 100% of the total IgG secreted by the 293T and NSO cells and ∼70% of the total IgG secreted by the LPS-activated B cells (Fig. 1B). To determine if the lentivector-encoded 2G12 heavy and light chains assembled properly, we used SDS-PAGE analyzed under nonreducing and reducing conditions to compare the 2G12 antibody produced by 2G12-lentivector-transfected 293T cells to control monoclonal 2G12 antibody, followed by Western immunoblotting. When the SDS-polyacrylamide gel was run under nonreducing conditions, bands with a molecular mass of about 150 kDa, which corresponds to the molecular mass of the natural form of IgG composed of two pairs of heavy and light chains, were detected after Western blotting with anti-human IgG antibody (Fig. 1C, lanes 1 and 2). When the 2G12 antibodies electrophoresed on an SDS-polyacrylamide gel were run under reducing conditions, Western blot analysis with anti-human IgG antibody or with anti-human kappa chain antibody detected either an ∼60-kDa band, which corresponds to the molecular mass of IgG heavy chain (Fig. 1C, lanes 3 and 4), or an ∼30-kDa band, corresponding to the kappa chain (Fig. 1C, lanes 5 and 6), respectively. After 2A-mediated self-cleavage of the translated light chain-2A-heavy chain transcript into the heavy and light chains, the 22-amino-acid 2A peptide sequence remains linked to the light chain. This increases the molecular mass of the 2G12-lentivector-encoded kappa chain by about 2.2 kDa (Fig. 1C, lane 6). Taken together, these results indicated that the 2G12 heavy and light chains produced by 2G12-lentivector-transfected 293T cells assembled appropriately. Functional activity of the 2G12 antibody secreted by the 293T cells transfected with the 2G12 lentivector and the NSO cells transduced with 2G12-expressing lentivirus was indicated by its capacity to neutralize HIV infection (Fig. 2).
FIG. 2.
2G12 antibody secreted by transduced cells inhibits HIV infection. 293T cells and NSO cells were either untransduced or transduced with a control eGFP-expressing lentivirus or the 2G12-expressing lentivirus. One week later, supernatant was harvested, incubated for 1 h with HIVJR-CSF, and then added to culture wells containing adherent TZM-bl cells. Two days later, luciferase activity in the cell lysate was measured and reported as relative light units. Monoclonal 2G12 antibody was added as a positive control. The data represent the averages ± SEM from three separate experiments performed in duplicate.
In vivo differentiation of 2G12-transduced human HSC into mature human B cells after transplantation into NOD/SCID/γcnull mice is associated with the in vivo production of 2G12 antibody.
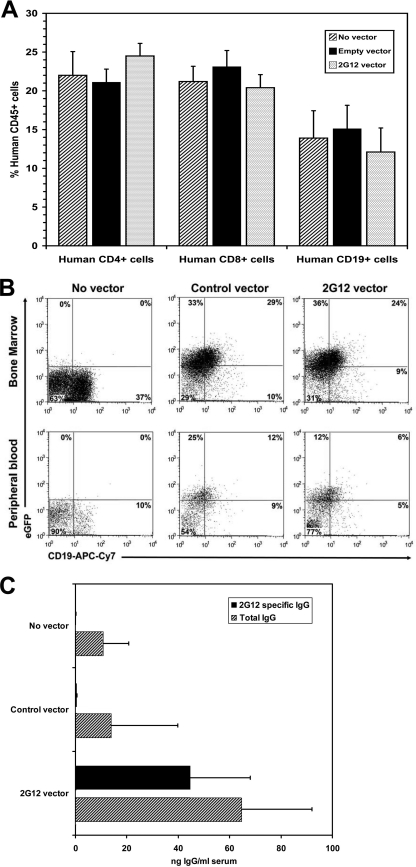
NOD/SCID/γcnull mice efficiently support the differentiation of hu-HSC into mature human myeloid and lymphoid cells (10). Purified hu-HSC transplanted intravenously into these irradiated NOD/SCID/γcnull mice differentiate and populate up to 70% of the peripheral leukocytes in the peripheral blood of these mice with human CD45 cells, including human T cells, B cells, and monocytic cells (10). We used this humanized mouse model to evaluate the capacity of hu-HSC-mediated gene therapy to generate progeny human cells that secrete 2G12 and thereby protect the human CD4 T cells from HIV infection. After transduction with the 2G12-expressing lentivirus, greater than 45% of the hu-HSC expressed the eGFP marker gene. The transduced hu-HSC cells were then transplanted by intravenous injection into sublethally irradiated NOD/SCID/γcnull mice. By 12 weeks after transplantation, in vivo differentiation of the transplanted hu-HSC was indicated by the detection of human CD45-positive cells in the peripheral blood, spleens, and bone marrow of about 30% of the transplanted mice. Analysis of the engrafted mice demonstrated that transduction of the hu-HSC with the 2G12-expressing lentivirus did not affect the degree of peripheral engraftment of the mice with human CD45+, CD4+, CD8+, and CD19+ cells (Fig. 3A). Differentiation of lentivirus-transduced hu-HSC was indicated by the detection in the bone marrow and peripheral blood of the transplanted mice of human CD19-positive cells, a large fraction of which expressed GFP (Fig. 3B). Human IgG was detected in the sera of all of the transplanted mice, and 2G12 antibody (∼40 ng/ml) constituted greater than 60% of the total human IgG in mice transplanted with 2G12-lentivirus-transduced CD34 cells (Fig. 3C). Thus, lentivirus can transduce hu-HSC and program progeny B cells to differentiate in vivo into 2G12 antibody-producing cells.
FIG. 3.
NOD/SCID/γcnull mice transplanted with hu-HSC transduced with 2G12-expressing lentivirus become populated with human B cells, and their serum contains 2G12 antibody. Irradiated NOD/SCID/γcnull mice were transplanted with hu-HSC that were either untransduced (n = 9 mice) or transduced with a control eGFP-expressing lentivirus (n = 9 mice) or the 2G12-expressing lentivirus (n = 8 mice). About 3 months after transplantation, the mice were analyzed. (A) The peripheral blood of the transplanted mice was examined for the presence of human CD4+, CD8+, and CD19+ cells after gating on human CD45+ expression. The data represent the averages values ± SEM for the indicated cell population for each group of mice. (B) The peripheral blood and bone marrow of the NOD/SCID/γcnull mice were analyzed by flow cytometry to quantify the fraction of human B cells expressing the lentivirus-encoded eGFP. Representative dot plots of peripheral blood and bone marrow cells analyzed for human CD19 and eGFP expression with the percentage positive for each quadrant are shown. (C) The levels of human IgG and 2G12 IgG in plasma samples from the engrafted mice were quantified by ELISA in duplicate. The data represent the average values ± SEM of human IgG and 2G12 IgG measured in the plasma for each group of mice.
Inhibition of the onset of HIV infection in mice systemically expressing the 2G12 antibody.
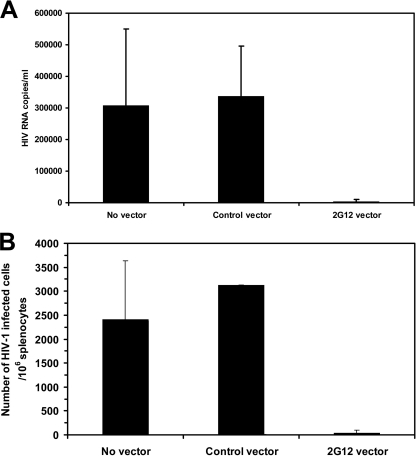
Population of the lymphoid tissue of the NOD/SCID/γcnull murine model with human T cells supports the development of productive HIV infection after in vivo challenge of these mice with HIV (35). This permitted us to examine whether the 2G12 antibody produced by lentivector-transduced human cells inhibited in vivo HIV infection. NOD/SCID/γcnull mice transplanted with hu-HSC transduced with the 2G12-expressing lentivirus and populated with human T cells were inoculated intraperitoneally with high-titer HIVJR-CSF (∼8,000 TCID). Seven days later, the mice were sacrificed and the plasma and spleens were obtained. The level of plasma HIV RNA was reduced by over 70-fold in the NOD/SCID/γcnull mice transplanted with hu-HSC transduced with the 2G12-expressing lentivirus compared to NOD/SCID/γcnull mice transplanted with hu-HSC transduced with the control lentivirus (Fig. 4A). Furthermore, there was an almost 200-fold reduction in the number of HIV-infected cells in the mouse spleens (Fig. 4B). Thus, endogenously produced 2G12 encoded by the 2G12-expressing lentivirus markedly inhibited the in vivo replication of HIV.
FIG. 4.
NOD/SCID/γcnull mice transplanted with hu-HSC transduced with the 2G12-expressing lentivirus are resistant to in vivo HIV infection. NOD/SCID/γcnull mice transplanted with hu-HSC transduced with either a control lentivirus or the 2G12-expressing lentivirus that became populated with human T cells were challenged by intraperitoneal injection of HIV-1JR-CSF. Seven days later, the mice were bled and the spleens were harvested. (A) Plasma from each group of mice was collected and pooled, and HIV RNA levels were determined by the bDNA assay. Values are shown as average number of HIV RNA copies/ml ± SEM. (B) HIV infection was quantified by limiting-dilution coculture of mouse splenocytes with activated HIV-seronegative human PBMCs. One week later, the HIV p24 antigen levels in the culture supernatants were measured and the 50% TCID was calculated for splenocytes from engrafted mice that were either untransduced (n = 4 mice) or transduced with either a control eGFP-expressing lentivirus (n = 4 mice) or the 2G12-expressing lentivirus (n = 4 mice). Values are presented as averages ± SEM of TCID50/106 splenocytes.
DISCUSSION
HIV-infected individuals display very limited production of broadly neutralizing HIV-specific antibodies due to several mechanisms, including the poor immunogenicity of neutralizing epitopes (2) and the cross-reactivity of neutralizing epitopes with self-antigens (9), which markedly compromises the ability of the humoral response to control HIV infection. In the current study we examined whether we could use gene transfer technology to bypass the inherent limitations in the adaptive immune system that prevent the generation of broadly neutralizing antibodies by HIV infection or vaccination. Previous studies reported that fibroblasts transduced ex vivo with retroviral vectors encoding HIV-specific antibodies transplanted into mice displayed sustained in vivo HIV-specific antibody production that reduced viral burden levels after transplantation into HIV-infected humanized mice (27). These studies were extended to macaques, where intravenously injected adeno-associated virus (AAV) vectors encoding neutralizing immunoadhesions transduced postmitotic organs and produced SIV-specific neutralizing antibodies that protected the majority of AAV-injected macaques from infection after intravenous SIV challenge (11).
In the current study we have extended those findings by demonstrating that transduction with the 2G12 lentiviral vectors directed hu-HSC to differentiate into human progeny cells that secreted the broadly neutralizing anti-HIV antibody 2G12 into the serum, which was associated with marked inhibition of the subsequent development of infection in HIV-inoculated humanized mice. The 2G12 lentiviral vector construct used in the current study had the membrane region of the IgG heavy chain gene deleted to maximize secretion of the vector-encoded 2G12 antibody. Consequently, the transduced B cells did not express surface 2G12 IgG and would not proliferate in response to antigenic stimulation. We plan to generate a 2G12 construct containing the membrane region of the IgG heavy chain gene for future studies to determine whether transduced B cells display in vivo expansion after HIV infection or immunization with gp120. Since 2G12 antibody production by the lentiviral vector was driven by the constitutively active PGK promoter, it is possible that cells of any hu-HSC-derived lineage could have secreted 2G12, including CD4 T cells. An intriguing possibility is that human CD4 T cells and macrophages derived from the 2G12 lentiviral vector-transduced hu-HSC may have been protected from HIV infection by endogenously produced 2G12. Future studies will employ lentiviral vectors containing a B cell-specific promoter to compare this approach to the physiological approach of limiting antibody production to B cells. A major advantage of this approach compared to passive immunization is that it obviates the need for lifelong intravenous injections of anti-HIV antibodies at weekly to monthly intervals. Furthermore, this approach would readily permit the delivery of antibodies that have been molecularly engineered to display increased functional activity (38) and of newly identified broadly reactive antibodies as they become described. In addition, the transduced B cells would likely provide the constant high levels of serum anti-HIV antibodies that are required for optimal neutralizing activity (20). Transducing the human HSC with a mixture of lentivectors encoding other broadly neutralizing antibodies in addition to 2G12, such as 2F5 and 4E10, would provide patients with serum levels of anti-HIV antibodies recognizing different epitopes which would likely retard the in vivo emergence of resistant isolates. While HIV isolates resistant to a single monoclonal antibody (MAb) are readily generated, HIV isolates that are resistant to 2G12, 2F5, and 4E10 have been generated only after prolonged in vitro culture, and these multiresistant isolates display markedly impaired replication fitness (22). While this approach would be subject to concerns associated with retroviral HSC-targeted gene therapy, extensive sequence analysis of lentiviral vector integration sites from three individuals treated with infusion of lentiviral vector gene-modified CD4+ cells demonstrated no enrichment for integration sites near proto-oncogene 5′ ends or within tumor suppressor genes (17, 33). Thus, gene therapy using lentiviral vectors encoding a mixture of broadly neutralizing HIV antibodies may represent a new therapeutic approach for the treatment of HIV infection, particularly for patients infected with HIV isolates resistant to multiple antiretroviral drugs.
Acknowledgments
This work was supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases grant AI67136 and Einstein/MMC Center for AIDS Research grant AI51519).
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Buchacher, A., R. Predl, K. Strutzenberger, W. Steinfellner, A. Trkola, M. Purtscher, G. Gruber, C. Tauer, F. Steindl, A. Jungbauer, et al. 1994. Generation of human monoclonal antibodies against HIV-1 proteins; electrofusion and Epstein-Barr virus transformation for peripheral blood lymphocyte immortalization. AIDS Res. Hum. Retroviruses 10:359-369. [DOI] [PubMed] [Google Scholar]
- 2.Burton, D. R., R. C. Desrosiers, R. W. Doms, W. C. Koff, P. D. Kwong, J. P. Moore, G. J. Nabel, J. Sodroski, I. A. Wilson, and R. T. Wyatt. 2004. HIV vaccine design and the neutralizing antibody problem. Nat. Immunol. 5:233-236. [DOI] [PubMed] [Google Scholar]
- 3.Cockrell, A. S., and T. Kafri. 2007. Gene delivery by lentivirus vectors. Mol. Biotechnol. 36:184-204. [DOI] [PubMed] [Google Scholar]
- 4.Dadachova, E., M. C. Patel, S. Toussi, C. Apostolidis, A. Morgenstern, M. W. Brechbiel, M. K. Gorny, S. Zolla-Pazner, A. Casadevall, and H. Goldstein. 2006. Targeted killing of virally infected cells by radiolabeled antibodies to viral proteins. PLoS Med. 3:e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, K. L., E. S. Gray, P. L. Moore, J. M. Decker, A. Salomon, D. C. Montefiori, B. S. Graham, M. C. Keefer, A. Pinter, L. Morris, B. H. Hahn, and G. M. Shaw. 2009. High titer HIV-1 V3-specific antibodies with broad reactivity but low neutralizing potency in acute infection and following vaccination. Virology 387:414-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorrell, C., O. I. Gan, D. S. Pereira, R. G. Hawley, and J. E. Dick. 2000. Expansion of human cord blood CD34(+)CD38(−) cells in ex vivo culture during retroviral transduction without a corresponding increase in SCID repopulating cell (SRC) frequency: dissociation of SRC phenotype and function. Blood 95:102-110. [PubMed] [Google Scholar]
- 7.Follenzi, A., L. E. Ailles, S. Bakovic, M. Geuna, and L. Naldini. 2000. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 25:217-222. [DOI] [PubMed] [Google Scholar]
- 8.Follenzi, A., and L. Naldini. 2002. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 346:454-465. [DOI] [PubMed] [Google Scholar]
- 9.Haynes, B. F., J. Fleming, E. W. St Clair, H. Katinger, G. Stiegler, R. Kunert, J. Robinson, R. M. Scearce, K. Plonk, H. F. Staats, T. L. Ortel, H. X. Liao, and S. M. Alam. 2005. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science 308:1906-1908. [DOI] [PubMed] [Google Scholar]
- 10.Hiramatsu, H., R. Nishikomori, T. Heike, M. Ito, K. Kobayashi, K. Katamura, and T. Nakahata. 2003. Complete reconstitution of human lymphocytes from cord blood CD34+ cells using the NOD/SCID/gammacnull mice model. Blood 102:873-880. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, P. R., B. C. Schnepp, J. Zhang, M. J. Connell, S. M. Greene, E. Yuste, R. C. Desrosiers, and K. Reed Clark. 2009. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat. Med. 15:901-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joseph, A., J. H. Zheng, A. Follenzi, T. Dilorenzo, K. Sango, J. Hyman, K. Chen, A. Piechocka-Trocha, C. Brander, E. Hooijberg, D. A. Vignali, B. D. Walker, and H. Goldstein. 2008. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J. Virol. 82:3078-3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kafri, T. 2004. Gene delivery by lentivirus vectors an overview. Methods Mol. Biol. 246:367-390. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson Hedestam, G. B., R. A. Fouchier, S. Phogat, D. R. Burton, J. Sodroski, and R. T. Wyatt. 2008. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat. Rev. Microbiol. 6:143-155. [DOI] [PubMed] [Google Scholar]
- 15.Kramer, V. G., N. B. Siddappa, and R. M. Ruprecht. 2007. Passive immunization as tool to identify protective HIV-1 Env epitopes. Curr. HIV Res. 5:642-655. [DOI] [PubMed] [Google Scholar]
- 16.Kunert, R., F. Ruker, and H. Katinger. 1998. Molecular characterization of five neutralizing anti-HIV type 1 antibodies: identification of nonconventional D segments in the human monoclonal antibodies 2G12 and 2F5. AIDS Res. Hum. Retroviruses 14:1115-1128. [DOI] [PubMed] [Google Scholar]
- 17.Levine, B. L., L. M. Humeau, J. Boyer, R. R. MacGregor, T. Rebello, X. Lu, G. K. Binder, V. Slepushkin, F. Lemiale, J. R. Mascola, F. D. Bushman, B. Dropulic, and C. H. June. 2006. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc. Natl. Acad. Sci. U. S. A. 103:17372-17377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manrique, A., P. Rusert, B. Joos, M. Fischer, H. Kuster, C. Leemann, B. Niederost, R. Weber, G. Stiegler, H. Katinger, H. F. Gunthard, and A. Trkola. 2007. In vivo and in vitro escape from neutralizing antibodies 2G12, 2F5, and 4E10. J. Virol. 81:8793-8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehandru, S., B. Vcelar, T. Wrin, G. Stiegler, B. Joos, H. Mohri, D. Boden, J. Galovich, K. Tenner-Racz, P. Racz, M. Carrington, C. Petropoulos, H. Katinger, and M. Markowitz. 2007. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J. Virol. 81:11016-11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montefiori, D. C. 2005. Neutralizing antibodies take a swipe at HIV in vivo. Nat. Med. 11:593-594. [DOI] [PubMed] [Google Scholar]
- 21.Nagahira, K., Y. Fukuda, T. Nasu, H. Kawashima, C. Noguchi, T. Kurihara, S. Oikawa, and T. Nakanishi. 1998. Construction and expression of a mouse-human chimeric antibody against human tumor necrosis factor-alpha. Immunol. Lett. 64:139-144. [DOI] [PubMed] [Google Scholar]
- 22.Nakowitsch, S., H. Quendler, H. Fekete, R. Kunert, H. Katinger, and G. Stiegler. 2005. HIV-1 mutants escaping neutralization by the human antibodies 2F5, 2G12, and 4E10: in vitro experiments versus clinical studies. AIDS 19:1957-1966. [DOI] [PubMed] [Google Scholar]
- 23.Osiecki, K., L. Xie, J. H. Zheng, R. Squires, M. Pettoello-Mantovani, and H. Goldstein. 2005. Identification of granulocyte-macrophage colony-stimulating factor and lipopolysaccharide-induced signal transduction pathways that synergize to stimulate HIV type 1 production by monocytes from HIV type 1 transgenic mice. AIDS Res. Hum. Retroviruses 21:125-139. [DOI] [PubMed] [Google Scholar]
- 24.Pettoello-Mantovani, M., T. R. Kollmann, N. F. Katopodis, C. Raker, A. Kim, S. Yurasov, H. Wiltshire, and H. Goldstein. 1998. thy/liv-SCID-hu mice: a system for investigating the in vivo effects of multidrug therapy on plasma viremia and human immunodeficiency virus replication in lymphoid tissues. J. Infect. Dis. 177:337-346. [DOI] [PubMed] [Google Scholar]
- 25.Polonis, V. R., B. K. Brown, A. R. Borges, S. Zolla-Pazner, D. S. Dimitrov, M. Y. Zhang, S. W. Barnett, R. M. Ruprecht, G. Scarlatti, E. M. Fenyo, D. C. Montefiori, F. E. McCutchan, and N. L. Michael. 2008. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 375:315-320. [DOI] [PubMed] [Google Scholar]
- 26.Rossmann, C., N. Sharp, G. Allen, and D. Gewert. 1996. Expression and purification of recombinant, glycosylated human interferon alpha 2b in murine myeloma NSo cells. Protein Expr. Purif. 7:335-342. [DOI] [PubMed] [Google Scholar]
- 27.Sanhadji, K., L. Grave, J. L. Touraine, P. Leissner, C. Rouzioux, R. Firouzi, L. Kehrli, J. C. Tardy, and M. Mehtali. 2000. Gene transfer of anti-gp41 antibody and CD4 immunoadhesin strongly reduces the HIV-1 load in humanized severe combined immunodeficient mice. AIDS 14:2813-2822. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava, I. K., J. B. Ulmer, and S. W. Barnett. 2005. Role of neutralizing antibodies in protective immunity against HIV. Hum. Vaccin. 1:45-60. [DOI] [PubMed] [Google Scholar]
- 29.Sun, J., T. Soos, V. N. Kewalramani, K. Osiecki, J. H. Zheng, L. Falkin, L. Santambrogio, D. R. Littman, and H. Goldstein. 2006. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J. Virol. 80:1850-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szymczak, A. L., C. J. Workman, Y. Wang, K. M. Vignali, S. Dilioglou, E. F. Vanin, and D. A. Vignali. 2004. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 22:589-594. [DOI] [PubMed] [Google Scholar]
- 31.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang, E. J., M. Pettoello-Mantovani, C. M. Anderson, K. Osiecki, D. Moskowitz, and H. Goldstein. 2002. Development of a novel transgenic mouse/SCID-hu mouse system to characterize the in vivo behavior of reservoirs of human immunodeficiency virus type 1-infected cells. J. Infect. Dis. 186:1412-1421. [DOI] [PubMed] [Google Scholar]
- 33.Wang, G. P., B. L. Levine, G. K. Binder, C. C. Berry, N. Malani, G. McGarrity, P. Tebas, C. H. June, and F. D. Bushman. 2009. Analysis of lentiviral vector integration in HIV+ study subjects receiving autologous infusions of gene modified CD4+ T cells. Mol. Ther. 17:844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warncke, M., B. Vogt, J. Ulrich, M. D. von Laer, W. Beyer, H. Klump, B. Micheel, and A. Sheriff. 2004. Efficient in vitro transduction of naive murine B cells with lentiviral vectors. Biochem. Biophys. Res. Commun. 318:673-679. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe, S., K. Terashima, S. Ohta, S. Horibata, M. Yajima, Y. Shiozawa, M. Z. Dewan, Z. Yu, M. Ito, T. Morio, N. Shimizu, M. Honda, and N. Yamamoto. 2007. Hematopoietic stem cell-engrafted NOD/SCID/IL2Rgamma null mice develop human lymphoid systems and induce long-lasting HIV-1 infection with specific humoral immune responses. Blood 109:212-218. [DOI] [PubMed] [Google Scholar]
- 36.Wei, X., J. M. Decker, H. Liu, Z. Zhang, R. B. Arani, J. M. Kilby, M. S. Saag, X. Wu, G. M. Shaw, and J. C. Kappes. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob. Agents Chemother. 46:1896-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 38.West, A. P., Jr., R. P. Galimidi, C. P. Foglesong, P. N. Gnanapragasam, K. E. Huey-Tubman, J. S. Klein, M. D. Suzuki, N. E. Tiangco, J. Vielmetter, and P. J. Bjorkman. 2009. Design and expression of a dimeric form of human immunodeficiency virus type 1 antibody 2G12 with increased neutralization potency. J. Virol. 83:98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolbank, S., R. Kunert, G. Stiegler, and H. Katinger. 2003. Characterization of human class-switched polymeric (immunoglobulin M [IgM] and IgA) anti-human immunodeficiency virus type 1 antibodies 2F5 and 2G12. J. Virol. 77:4095-4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wyatt, R., and J. Sodroski. 1998. The HIV-1 envelope glycoproteins: fusogens, antigens, and immunogens. Science 280:1884-1888. [DOI] [PubMed] [Google Scholar]
- 41.Zwick, M. B., R. Kelleher, R. Jensen, A. F. Labrijn, M. Wang, G. V. Quinnan, Jr., P. W. Parren, and D. R. Burton. 2003. A novel human antibody against human immunodeficiency virus type 1 gp120 is V1, V2, and V3 loop dependent and helps delimit the epitope of the broadly neutralizing antibody immunoglobulin G1 b12. J. Virol. 77:6965-6978. [DOI] [PMC free article] [PubMed] [Google Scholar]