Abstract
Complement, part of the innate immune system, acts to remove pathogens and unwanted host material. Complement is known to function in all tissues, including the central nervous system (CNS). In this study, we demonstrated the importance of the complement system within the CNS in the development of behavioral seizures following Theiler's murine encephalomyelitis virus (TMEV) infection. C57BL/6 mice, deficient in complement component C3, developed significantly fewer behavioral seizures following TMEV infection, whereas mice depleted of complement component C3 in the periphery through treatment with cobra venom factor had a seizure rate comparable to that of control mice. These studies indicate that C3 participates in the induction of acute seizures during viral encephalitis.
The complement system, a component of the innate immune system, functions to recognize and eliminate pathogens and unwanted host material (1). Activation of complement can occur by the classical, alternative, lectin, and terminal pathways (1). The classical pathway is activated by antigen-antibody complexes, some viruses, Gram-negative bacteria, or C-reactive protein complexes (4). The alternative pathway is activated by lipopolysaccharides and polysaccharides on the surfaces of viruses, bacteria, fungi, and parasites. The lectin pathway is activated by mannose or N-acetylglucosamine on the surfaces of bacteria and other pathogens (4). The complement system, consisting of >40 proteins, is highly regulated by the expression of complement inhibitors and complement receptors, as the complement system can have deleterious effects when unregulated (1). Two important steps in the complement cascade are the cleavage of the multifunctional complement proteins C3 and C5 into C3a and C3b proteins and C5a and C5b proteins, respectively. The anaphylatoxins C3a and C5a function to recruit leukocytes and induce inflammation. C3b functions in opsonization, the process of coating pathogens or particulate material with opsonin and thus making it more susceptible to phagocytosis. C5b functions to initiate the assembly of the C5b-C9 complex, the membrane attack complex (MAC), leading to pathogen lysis. Proteins of the complement system are found throughout all tissues and bodily fluids. Although primarily produced by hepatocytes in the liver, other cell types constitutively express low levels of complement proteins (1).
Complement proteins are constitutively produced by neurons, microglia, astrocytes, and oligodendrocytes in the central nervous system (CNS) (14, 16, 44; reviewed in references 1 and 13). Astrocytes, the predominant glial cell type in the brain, are comparable to hepatocytes in terms of the number of complement components they produce (4). The levels of various complement mRNAs and proteins are markedly increased in the CNS following viral infection; for example, C1q and C3 proteins and mRNAs are increased in the rat brain following infection with Borna disease virus (11), and C1q protein and mRNA are increased in the rhesus macaque brain following infection with simian immunodeficiency virus (10). In both cases, the increase in the production of C1q in the brain was localized to microglia/macrophages (10, 11). Complement activation has also been shown to be involved in the control of other viral infections, such as the spread of West Nile virus to the CNS (24).
We have been studying the role of the innate immune system in the development of acute behavioral seizures following CNS infection of C57BL/6 mice with a neurotropic virus (19, 23). Infection with Theiler's murine encephalomyelitis virus (TMEV) results in acute seizures developing in more than 50% of C57BL/6 mice (both male and female) generally between days 3 and 10 postinfection (p.i.) (23). Two proinflammatory cytokines, tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), and concomitant inflammatory changes in the brain (perivascular cuffing comprised of infiltrating mononuclear cells, infiltration of macrophages, and/or activation of microglial cells and gliosis) were implicated as contributors to the development of acute seizures (19). In contrast, the proinflammatory cytokine IL-1, TMEV-specific CD8+ T cells, and viral persistence were discounted as playing a role in seizures (19). It was found that both the pattern of days on which the mice were observed to have seizures and the seizure score (Racine scale) for any given day varied from mouse to mouse (23). Typically, day 3 p.i. was the first day on which a few mice were observed to have seizures, day 6 p.i. was the peak of behavioral seizure activity, and the majority of seizures had a seizure score of 3 (forelimb clonus) and above (score of 4, rearing; score of 5, rearing and falling). The seizures were afebrile and appeared limbic in nature (23). The seizure frequency was observed to be one per mouse per 2-h observation period, and the duration of the seizures was typically 1 to 2 min (23, 31). Mice experiencing seizures were impaired in both coordination and motor function (23).
The role of complement in the development of seizures has been studied in humans with Rasmussen's encephalitis (RE) (41). Although several different viruses have been detected in the tissues of humans with RE by PCR and in situ hybridization, these results are still controversial. Instead it is thought that an autoimmune process underlies RE. Notwithstanding the unknown etiology of RE, the activation of the complement cascade is thought to be a critical component of disease pathogenesis. Several activated components of the complement system (C4, C8, and MAC) were shown to be present in discrete patches of neurons in the cortex of three out of five patients with active RE by immunohistochemistry (41). As a means of demonstrating in vivo that MAC deposition on neurons could trigger seizures, the individual components of the MAC (C5b6, C7, C8, and C9) were sequentially infused into the rat hippocampus, and assembly of the MAC triggered both behavioral and electrographic seizures as well as cytotoxicity (42).
In our current study, we examined the role that complement may play in the development of behavioral seizures in the TMEV-induced seizure model. Through the use of mice deficient in complement component C3 and through depletion of complement component C3 in the periphery, we were able to demonstrate the importance of the complement system within the CNS in the development of seizures in the TMEV-induced seizure model.
MATERIALS AND METHODS
Animals and infection.
Males of the C57BL/6 and DBA/2 strains of mice were purchased from the Jackson Laboratory (Bar Harbor, ME). C57BL/6 × DBA/2 (C57 × DBA) F1 mice were bred on-site. Male and female mice in which complement component C3 has been disrupted (B6.129S4-C3tm1Crr/J) on a C57BL/6 background were kindly provided by John Weis, University of Utah.
On day 0, 5- to 6-week-old mice were anesthetized with isoflurane by inhalation and infected intracerebrally with 2 × 104 or 2 × 105 PFU of the Daniels (DA) strain of TMEV or mock injected with 20 μl of phosphate-buffered saline (PBS). The DA strain of TMEV was propagated as previously described (47).
The care and use of the mice in this study were in accordance with the guidelines prepared by the Committee on Care and Use of Laboratory Animals, Institute of Laboratory Animals Resources, National Research Council (26a).
Observations.
To monitor for seizure activity, mice were observed continuously for a 2-h period, randomly chosen between the hours of 9:00 a.m. and 5:00 p.m. each day p.i. Seizure activity was graded using the Racine scale as follows: stage 1, mouth and facial movements; stage 2, head nodding; stage 3, forelimb clonus; stage 4, rearing; and stage 5, rearing and falling (5, 28).
Histology.
Additional mice were euthanized with isoflurane on days 3, 7, 10, 14, and 21 p.i. in order to observe the neurological manifestations of the seizures. The animals were perfused first with PBS and then with a buffered 4% paraformaldehyde solution. The brains were harvested and fixed in 4% paraformaldehyde, divided into five coronal slabs per brain and embedded in paraffin. Multiple 4-μm-thick tissue sections, containing sections from all five coronal slabs per brain, were cut, mounted on slides, and stained via various methods as described below. The tissue section of only one of the five coronal slabs represented per slide contained the hippocampal/dentate gyral regions of the brain. Luxol fast blue-stained slides were used to evaluate neuronal cell loss in the hippocampus in a blinded fashion using one slide per brain (n = 3 to 18 brains per experimental group). Neuronal cell loss in the pyramidal cell layer of the hippocampus, from CA1 to CA3, was scored as follows: score 0, no damage; score 1, 10 to 29% cell loss (10 to 29% of the pyramidal cell layer from CA1 to CA3 is missing); score 2, 30 to 59% cell loss; and score 3, >60% cell loss (19). An undamaged control brain would receive a score of 0 with no cell loss, and thus, 100% of the cells are present. A score was given for each of the two hippocampi present in a brain, and then the scores were summed so the highest possible score for cell loss per brain could be 6 (the highest score, 3, for two regions of the brain). Cytoarchitecture of the hippocampus was referenced in Fig. 43 to 49 in The Mouse Brain in Stereotaxic Coordinates (12).
Immunohistochemistry.
DA viral-antigen-positive cells and astrocytes were detected on paraffin sections using hyperimmune rabbit serum against TMEV and glial fibrillary acidic protein (GFAP) antibody (Dako Corp., Carpinteria, CA), respectively, as previously described (37, 39). The slides were labeled using the avidin-biotin peroxidase complex technique with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich, St. Louis, MO) in 0.01% hydrogen peroxide (Sigma) in PBS. The specificity of antibody binding was confirmed by parallel staining minus the hyperimmune serum or the GFAP antibody.
Enumeration of DA viral-antigen-positive cells was performed in a blinded fashion with a light microscope using one slide per brain and evaluating tissue sections from all five coronal slabs represented per slide (n = 3 to 11 brains per experimental group). DA viral-antigen-positive cells were enumerated and summed in the following brain regions in C3-deficient mice and C57BL/6 mice: frontal lobe, septum, caudoputamen, hippocampus, midbrain, and cortex. DA viral-antigen-positive cells were enumerated and summed in the following brain regions in cobra venom factor (CVF)-treated mice: frontal lobe, hippocampus, and cortex. No DA viral antigen was detected in the additional three brain regions of CVF-treated mice.
The extent of gliosis was semiquantified by scoring GFAP-positive (GFAP+) activated astrocytes in the hippocampus and dentate gyrus in a blinded fashion using one slide per brain (n = 3 to 16 brains per experimental group). Activated astrocytes have larger cell bodies and fatter processes and stain more intensely than quiescent astrocytes. Gliosis was scored as follows: score 0, no damage (<50 activated astrocytes present); score 1, mild (50 to 350 activated astrocytes present); score 2, moderate (351 to 700 activated astrocytes present); and score 3, severe (>700 activated astrocytes present) (19). A score was given for each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain, and then the scores were summed so the highest possible score for gliosis per brain could be 12 (the highest score, 3, for four regions of the brain). For a control (labeled PBS) for GFAP staining, untreated mice (n = 2) and PBS-treated mice (n = 3; one each sacrificed on days 3, 5, and 7 p.i.) were evaluated as described above, and the scores were averaged.
RCA-I lectin histochemistry.
Activated microglia and macrophages were identified by biotinylated Ricinus communis agglutinin I (RCA-I) (Vector Laboratories Inc., Burlingame, CA) as previously described (32, 35, 36, 38). One slide per brain for 3 to 14 brains per experimental group was examined in a blinded fashion. RCA-I-positive (RCA-I+) cells in each of the two hippocampi present in a brain and each of the two dentate gyri present in a brain were enumerated and summed. For a control (labeled PBS) for RCA-I staining, PBS-treated mice (n = 3; one each sacrificed on days 3, 5, and 7 p.i.) were evaluated as described above, and the values were averaged.
PCR arrays.
Five- to 6-week-old C57BL/6 mice (three to four per group) infected with 2 × 104 PFU DA virus or injected with PBS were euthanized with isoflurane on days 2 and 6 p.i., and their brains were harvested and frozen. The brains from naïve mice were used as a normal control. RNA was isolated by homogenizing the brains in Trizol reagent (Invitrogen, San Diego, CA), performing chloroform extraction, and then further purifying the RNA by means of the RNeasy maxikit (Qiagen, Chatsworth, CA). From the RNA, cDNA was made using Moloney murine leukemia virus (MMLV) reverse transcriptase (Invitrogen) according to the manufacturer's recommendations and using random primers. cDNAs from three or four brains were pooled from the different groups of mice: PBS-injected mice on day 2 p.i., infected mice on day 2 p.i., PBS-injected mice on day 6 p.i, infected mice with no seizures on day 6 p.i, and infected mice with seizures on day 6 p.i. cDNA was assayed on a LC480 Light Cycler (Roche, Indianapolis, IN) 96-well block, via a PCR array specific for mouse inflammatory cytokines and receptors (SABiosciences, Frederick, MD) per the manufacturer's instructions.
QRT-PCR.
The template for quantitative real-time (QRT)-PCR for the detection of C3 was the individual cDNAs as generated for the PCR arrays but without pooling the cDNAs (three per group) from the different groups of mice (mice injected with PBS and observed on day 6 p.i., infected mice with no seizures observed on day 6 p.i., and infected mice with seizures observed on day 6 p.i.). QRT-PCR was performed using a LC480 Light Cycler and the SYBR green method of amplicon detection. β-Actin was used to normalize the data. The level (L) of gene expression was determined using the formula: L = (2−Ct)(108), where Ct is cycle threshold. A relative normalized expression level for each sample was determined by dividing the level of the gene of interest by the level of β-actin.
QRT-PCR for the detection of DA viral RNA was performed as described above with the following modifications. Five- to 6-week-old mice infected with 2 × 105 PFU DA virus were euthanized with isoflurane on days 14 and 19 p.i. for C3-deficient mice (7 mice without seizures) and C57BL/6 mice (4 mice with seizures and 3 mice without seizures), and their brains were harvested and frozen. RNA was isolated, and cDNA was generated as described above for the PCR arrays and QRT-PCR for C3. β-Actin was used to normalize the data. Primers were designed so as to amplify within the 2C region of the TMEV genome.
CVF treatment.
C57BL/6 mice were injected intraperitoneally (i.p.) with 30 μg of CVF (Quidel, San Diego, CA) in 100 μl of PBS approximately 22 h prior to infection with 2 × 105 PFU of the DA strain of TMEV and on days 1 and 3 p.i. (34). CVF is a structural and functional analog of C3 that replaces C3b in the formation of a functional C3 convertase that is not readily inactivated by regulatory proteins and that depletes plasma C3 due to C3 cleavage by the convertase that is >250-fold more stable (30). Multiple, small doses of CVF from the cobra Naja naja were well tolerated and depleted plasma complement, in particular C3, in vivo in all species tested (7). This depletion (hypocomplementemia) was maintained for 5 to 6 days. The effect of CVF treatment on other plasma complement components (C5 and C6) varied by species (7). Enzyme-linked immunosorbent assay analysis previously demonstrated that treatment of male C57BL/6 mice with a single dose of CVF (30 μg i.p.) resulted in a reduction in the level of mouse serum antigenic C3 to less than 3% of the initial C3 level within 4 h of treatment, and this reduced level of C3 persisted for at least 48 h (33). The level of C3 in serum remained below 10% of the initial C3 level up to day 4 (33).
Statistical analysis.
The StatView program (SAS Institute Inc., Cary, NC) was used for all statistical analyses performed. Analysis of variance (ANOVA), followed when necessary by Fisher's protected least significant difference (PLSD) posthoc test, was performed to determine group differences for continuous data (C3 mRNA expression, DA viral RNA, DA viral-antigen-positive cells, and activated microglia/macrophages). The chi-square test was utilized for nominal data (whether the mice had seizures [yes or no]). Finally, the unpaired two-group Mann-Whitney U test was performed for all nonparametric analyses (neuronal cell loss and gliosis).
RESULTS
C3 is important for seizures.
The levels of expression of mRNAs for inflammatory cytokines and receptors were assayed via PCR arrays as described in Materials and Methods. mRNA expression was compared in mice injected with PBS and mice infected with the Daniels (DA) strain of Theiler's murine encephalomyelitis virus (TMEV) on day 2 p.i., prior to the development of seizures, and on day 6 p.i., when seizures were clearly evident. mRNA expression in the brains of PBS-injected mice on days 2 and 6 p.i. was not different from mRNA expression in the brains of control mice. We found that the expression of C3 mRNA was elevated as early as day 2 p.i. and continued to rise through day 6 p.i. On day 2 p.i., the expression of C3 mRNA was 5-fold greater in the DA virus-infected mice than in the PBS-injected mice. On day 6 p.i., the levels of expression of C3 mRNA were 13.5-fold and 47.7-fold greater in infected mice without seizures and infected mice with seizures, respectively, than in the PBS-injected mice. Therefore, on day 6 p.i., the expression of C3 mRNA was 3.5-fold greater in the infected mice with seizures than in the infected mice without seizures.
In order to confirm the involvement of C3 in the development of seizures in our TMEV-induced seizure model, C3 mRNA expression levels were assayed via quantitative real-time (QRT)-PCR as described in Materials and Methods. mRNA expression was compared in PBS-injected and DA virus-infected mice on day 6 p.i. On day 6 p.i., mRNA expression of C3 was significantly increased in infected mice both with seizures and mice without seizures compared to PBS-injected control mice (P < 0.05) (Fig. 1). Both the PCR arrays and the QRT-PCR analyses demonstrated that mRNA expression of the C3 component of complement was increased following infection with TMEV. However, the PCR arrays demonstrated a difference between mice with seizures and mice without seizures, whereas the QRT-PCR analyses did not confirm this difference. Therefore, additional methods must be employed to determine the contribution of C3 to the development of seizures in this model.
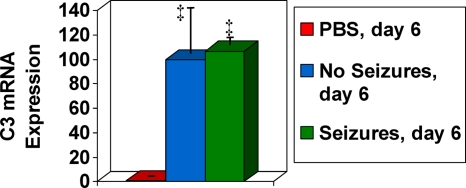
FIG. 1.
mRNA expression levels for C3. mRNA expression was compared, using QRT-PCR specific for C3, between DA virus-infected mice on day 6 p.i. (mice with no seizures and mice with seizures on day 6) and PBS-injected control mice. C3 mRNA expression is shown on the y axis as a relative normalized expression level. C3 mRNA expression significantly increased in mice with seizures and mice without seizures on day 6 compared to PBS-injected control. Values that are significantly different (P < 0.05) from the value for the PBS-injected control mice are indicated (‡). Results are depicted as means plus standard errors of the means (SEMs) (error bars) for groups with three mice per group.
We used C3-deficient mice to test how C3 could influence whether DA virus-infected mice developed seizures. C3-deficient mice or wild-type mice were infected with DA virus and observed for the development of seizures through day 21 p.i. Seventeen percent of mice deficient in C3 developed seizures (Racine scale stages 3 to 5) compared to 52.4% of infected wild-type mice (P = 0.0001) (Fig. 2).
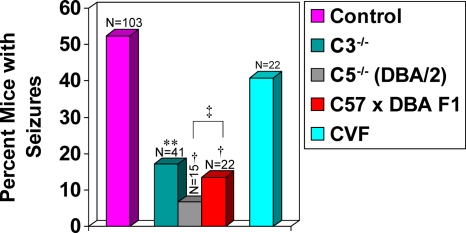
FIG. 2.
Frequency of seizures (Racine scale stage 3 to 5) in C3- and C5-deficient and treated mice. Significantly fewer C3-deficient (C3−/−) mice (17%) developed seizures compared to wild-type control mice. **, P = 0.0001. In contrast, no significant difference in seizure frequency was seen in mice undergoing cobra venom factor (CVF) treatment compared to wild-type control mice. Significantly fewer DBA/2 mice (C5−/−; 6.7%) and C57 × DBA F1 mice (13.6%) developed seizures compared to wild-type control mice. †, P < 0.001. Also, significantly fewer DBA/2 mice developed seizures compared to C57 × DBA F1 mice. ‡, P < 0.05. The total number of mice infected (N) is shown over the individual bars of the graph. Percent mice with seizures (y axis) is calculated as follows: (number of mice with seizures/total number of mice infected) × 100.
We examined neuronal cell loss, viral persistence, and inflammation in C3-deficient mice infected with DA virus (Fig. 3 to 5 ). Data for days 3 and 7 p.i. for mice from three of the groups (C57BL/6 mice without seizures, C3-deficient mice with seizures, and C3-deficient mice without seizures) were combined, as there were no differences between C57BL/6 mice without seizures on days 3 and 7 p.i. and the numbers of C3-deficient mice in the groups with seizures and without seizures were low. In this study, there were no C57BL/6 mice with seizures on day 3 p.i.
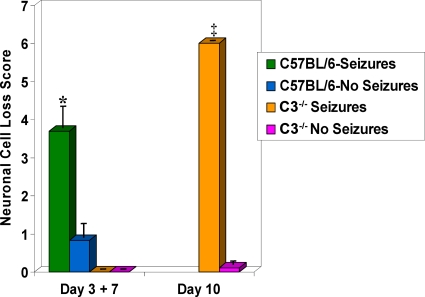
FIG. 3.
Neuronal cell loss within the hippocampus in C3-deficient mice infected with DA virus. Neuronal cell loss was scored as described in Materials and Methods for mice sacrificed on days 3, 7, and 10 p.i. Neuronal cell loss was significantly greater in the C57BL/6 mice with seizures on day 7 p.i. than in C57BL/6 mice without seizures on days 3 and 7 p.i. The C3-deficient mice (C3−/−) with seizures had significantly greater neuronal cell loss than the C3-deficient mice without seizures on day 10 p.i. ‡, P < 0.05; *, P < 0.01. Results are depicted as means plus SEMs (error bars) for groups with 3 to 18 mice per group.
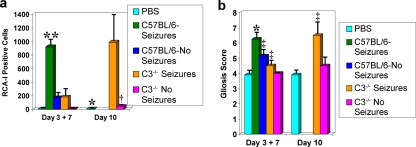
FIG. 5.
Inflammation within the hippocampus and dentate gyrus of C3-deficient mice infected with DA virus. Activated microglia/macrophages (a), detected through RCA-I lectin histochemistry, and activated astrocytes (gliosis) (b), detected through GFAP immunohistochemistry, are measures of inflammation. (a) The numbers of RCA-I+ cells were significantly greater in C57BL/6 mice with seizures on day 7 p.i. than in PBS-injected control mice, C57BL/6 mice without seizures on days 3 and 7 p.i., and C3-deficient mice with or without seizures on days 3 and 7 p.i. On day 10 p.i., C3-deficient mice with seizures had a significantly greater number of RCA-I+ cells than both the PBS-injected control mice and the C3-deficient mice without seizures. *, P < 0.01; †, P < 0.001; **, P < 0.0001. Results are depicted as means plus SEMs (error bars) for groups with 3 to 14 mice per group. (b) Gliosis was scored as described in Materials and Methods. Gliosis was significantly greater in C57BL/6 mice with seizures on day 7 p.i. than in PBS-injected control mice, C57BL/6 mice without seizures on days 3 and 7 p.i., and C3-deficient mice with or without seizures on days 3 and 7 p.i. Gliosis was also significantly greater in C57BL/6 mice without seizures on days 3 and 7 p.i. than in PBS-injected control mice and C3-deficient mice without seizures on days 3 and 7 p.i. Gliosis was significantly greater in C3-deficient mice with seizures than in PBS-injected control mice by day 10 p.i. ‡, P < 0.05; *, P < 0.01. Results are depicted as means plus SEMs (error bars) for groups with 3 to 16 mice per group.
Neuronal cell loss was apparent in C57BL/6 mice without seizures on days 3 and 7 p.i. and in C57BL/6 mice with seizures on day 7 p.i. (Fig. 3). C57BL/6 mice with seizures on day 7 p.i. had significantly greater neuronal cell loss than C57BL/6 mice without seizures on days 3 and 7 p.i. (P < 0.01). No neuronal cell loss was apparent in C3-deficient mice with or without seizures on days 3 and 7 p.i. However, neuronal cell loss was apparent in C3-deficient mice with or without seizures on day 10 p.i. (Fig. 3). Thus, the C3-deficient mice with seizures had significantly greater neuronal cell loss than the C3-deficient mice without seizures on day 10 p.i. (P < 0.05).
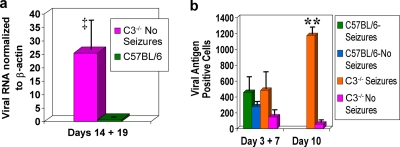
Interestingly, viral genome and viral protein were found to persist in C3-deficient mice infected with DA virus (Fig. 4). Data for days 14 and 19 p.i. for mice from two groups (C57BL/6 mice with seizures and C57BL/6 mice without seizures) were combined to study the persistence of the viral genome. Viral genome was found, in a significantly greater quantity, in C3-deficient mice without seizures on days 14 and 19 p.i. than in C57BL/6 mice with and without seizures at the same time points (P < 0.05) (Fig. 4a). The levels of viral protein in C3-deficient mice with and without seizures on days 3 and 7 p.i. were found to be comparable to the levels in C57BL/6 mice with seizures on day 7 p.i. and C57BL/6 mice without seizures on days 3 and 7 p.i. (difference not significant) (Fig. 4b). However, by day 10 p.i., the number of viral-antigen-positive cells was significantly greater in the C3-deficient mice with seizures than in the C3-deficient mice without seizures (P < 0.0001).
FIG. 4.
DA viral persistence in the brains of C3-deficient mice. DA viral RNA as determined by QRT-PCR (a) and DA viral-antigen-positive cells as determined by immunohistochemistry (b) demonstrate the presence of DA viral RNA and protein in the brains of C3-deficient mice, both with and without seizures. (a) The level of viral RNA was normalized against β-actin mRNA levels. DA viral RNA was significantly greater in the C3-deficient mice without seizures on days 14 and 19 p.i. than in C57BL/6 mice with seizures (4 mice) and without seizures (3 mice) on days 14 and 19 p.i. ‡, P < 0.05. Results are depicted as means plus SEMs (error bars) for groups with seven mice per group. (b) There were no significant differences in the numbers of DA viral-antigen-positive cells between C57BL/6 mice with seizures on day 7 p.i. or C57BL/6 mice without seizures on days 3 and 7 p.i. compared to C3-deficient mice with or without seizures on days 3 and 7 p.i. DA viral antigen was significantly greater in the C3-deficient mice with seizures than in C3-deficient mice without seizures on day 10 p.i. **, P < 0.0001. Results are depicted as means plus SEMs (error bars) for groups with 3 to 11 mice per group.
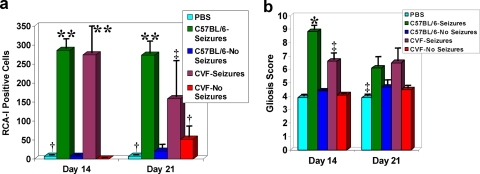
Inflammation in C3-deficient mice infected with DA virus was measured via Ricinus communis agglutinin I (RCA-I) lectin histochemical detection of activated microglia/macrophages and glial fibrillary acidic protein (GFAP) immunohistochemical detection of activated astrocytes (gliosis) (Fig. 5). The numbers of activated microglia/macrophages in C3-deficient mice with and without seizures on days 3 and 7 p.i. were found to be significantly less than those in C57BL/6 mice with seizures on day 7 p.i. (P < 0.0001) but comparable to those in C57BL/6 mice without seizures on days 3 and 7 p.i. and PBS-injected control mice (difference not significant) (Fig. 5a). However, by day 10 p.i., the numbers of activated microglia/macrophages in C3-deficient mice with seizures were found to be significantly greater than the numbers in both the PBS-injected control mice (P < 0.01) and the C3-deficient mice without seizures (P < 0.001) (Fig. 5a). The gliosis score for C57BL/6 mice with seizures on day 7 p.i. was significantly greater than the scores for PBS-injected control mice (P < 0.01), C57BL/6 mice without seizures on days 3 and 7 p.i. (P < 0.05), C3-deficient mice with seizures on days 3 and 7 p.i. (P < 0.05), and C3-deficient mice without seizures on days 3 and 7 p.i. (P < 0.01) (Fig. 5b). The gliosis score was also significantly greater in C57BL/6 mice without seizures on days 3 and 7 p.i. than in PBS-injected control mice (P < 0.05) and C3-deficient mice without seizures on days 3 and 7 p.i. (P < 0.05) (Fig. 5b). However, by day 10 p.i., the gliosis score for C3-deficient mice with seizures was found to be significantly greater than the score in PBS-injected control mice (P < 0.05) (Fig. 5b).
In summary, we examined neuronal cell loss, viral persistence, and inflammation in C3-deficient mice infected with DA virus (Fig. 3 to 5). We found that although neuronal cell loss was undetectable in C3-deficient mice early after infection (days 3 and 7 p.i.), neuronal cell loss was significant in C3-deficient mice with seizures by the time that acute behavioral seizures resolved (day 10 p.i.). Virus was found to persist in C3-deficient mice through the time that acute behavioral seizures resolved and beyond (days 14 and 19 p.i.). As for inflammation, we examined the numbers of activated microglia/macrophages and gliosis. Although the numbers of activated microglia/macrophages were low in C3-deficient mice early after infection, the numbers were significant in C3-deficient mice with seizures by the time that acute behavioral seizures resolved. The gliosis score for C3-deficient mice with seizures was comparable to that for PBS-injected control mice early after infection; however, the gliosis score for C3-deficient mice with seizures was significantly higher than the score for PBS-injected control mice by the time that acute behavioral seizures resolved. Therefore, the extent of neuronal cell loss and inflammation seen in C57BL/6 mice with seizures for days 3 and 7 p.i. was seen for C3-deficient mice with seizures on day 10 p.i., and virus persisted in C3-deficient mice.
In order to determine the importance of C3 in the CNS versus C3 in the periphery, mice were treated with cobra venom factor (CVF) before and after infection. This treatment has been shown to deplete C3 in the periphery (outside the CNS) (33, 34). Our initial CVF injection occurred 22 h prior to infection, and booster injections were given on days 1 and 3 p.i. C3 was depleted at the time of infection, and the reduced level of C3 would have been maintained due to the booster injections at least through day 7 p.i. As acute seizures develop between days 3 and 10 p.i. and the peak of seizure activity occurs on day 6 p.i., this treatment regiment should have resulted in depletion of C3 through the peak of seizure activity in these mice. CVF-treated mice and wild-type mice were observed for the development of seizures through day 21 p.i. Forty-one percent of CVF-treated mice developed seizures (Racine scale stage 3 to 5) compared to infected wild-type mice (difference not significant) (Fig. 2). These data indicate that C3 within the CNS, but not C3 in the periphery, contributes to the development of seizures.
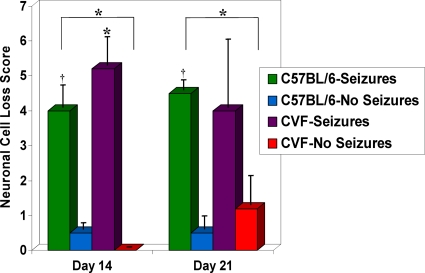
We examined neuronal cell loss, viral persistence, and inflammation in CVF-treated mice infected with DA virus (Fig. 6 to 8 ). Neuronal cell loss was very similar in CVF-treated and wild-type mice on days 14 and 21 p.i. (Fig. 6). C57BL/6 mice with seizures on day 14 p.i. had significantly greater neuronal cell loss than C57BL/6 mice without seizures (P < 0.001) and CVF-treated mice without seizures (P < 0.01). CVF-treated mice with seizures on day 14 p.i. also had significantly greater neuronal cell loss than C57BL/6 mice without seizures and CVF-treated mice without seizures (P < 0.01). On day 21 p.i., only C57BL/6 mice with seizures had significantly greater neuronal cell loss than C57BL/6 mice without seizures (P < 0.001) and CVF-treated mice without seizures (P < 0.01).
FIG. 6.
Neuronal cell loss within the hippocampus of CVF-treated and untreated mice infected with DA virus. Neuronal cell loss was scored as described in Materials and Methods for mice sacrificed on days 14 and 21 p.i. Neuronal cell loss was significantly greater in the untreated mice with seizures than in CVF-treated mice and untreated mice without seizures on days 14 and 21 p.i. The CVF-treated mice with seizures had significantly greater neuronal cell loss than the CVF-treated mice and untreated mice without seizures on day 14 p.i. *, P < 0.01; †, P < 0.001. Results are depicted as means plus SEMs (error bars) for groups with 3 to 12 mice per group.
FIG. 8.
Inflammation within the hippocampus and dentate gyrus of CVF-treated and untreated mice infected with DA virus. Activated microglia/macrophages (a), detected through RCA-I lectin histochemistry and activated astrocytes (gliosis) (b), detected through GFAP immunohistochemistry, are measures of inflammation. (a) The numbers of RCA-I+ cells were significantly greater in C57BL/6 mice and CVF-treated mice with seizures than in PBS-injected control mice and C57BL/6 mice and CVF-treated mice without seizures on day 14 p.i. The numbers of RCA-I+ cells were significantly greater in C57BL/6 mice with seizures than in PBS-injected control and C57BL/6 mice and CVF-treated mice without seizures on day 21 p.i. The numbers of RCA-I+ cells were significantly greater in CVF-treated mice with seizures than in PBS-injected control and C57BL/6 mice without seizures on day 21 p.i. ‡, P < 0.05; †, P < 0.001; **, P < 0.0001. Results are depicted as means plus SEMs (error bars) for groups with three to seven mice per group. (b) Gliosis was scored as described in Materials and Methods. Gliosis was significantly greater in C57BL/6 mice with seizures than in PBS-injected control mice, CVF-treated mice with seizures, and C57BL/6 mice and CVF-treated mice without seizures on day 14 p.i. Also, on day 14 p.i., gliosis was significantly greater in CVF-treated mice with seizures than in PBS-injected control mice and C57BL/6 mice and CVF-treated mice without seizures. There was significantly less gliosis in PBS-injected control mice than in C57BL/6 mice and CVF-treated mice with seizures on day 21 p.i. ‡, P < 0.05; *, P < 0.01. Results are depicted as means plus SEMs (error bars) for groups with three to seven mice per group.
Viral protein was found to persist in CVF-treated mice with seizures through day 14 p.i. (Fig. 7). The number of antigen-positive cells was significantly greater in the CVF-treated mice with seizures than in the CVF-treated mice without seizures (P < 0.05), the C57BL/6 mice with seizures (P < 0.05), and the C57BL/6 mice without seizures (P < 0.01 [data not shown]) on day 14 p.i. By day 21 p.i., viral-antigen-positive cells were not detected in CVF-treated and wild-type mice, both with and without seizures (data not shown).
FIG. 7.
DA viral persistence in the brains of CVF-treated mice with seizures. DA viral-antigen-positive cells, as determined by immunohistochemistry, demonstrate the presence of DA viral protein in the brains of CVF-treated mice with seizures on day 14 p.i. C57BL/6 mice with seizures were assayed on day 14 p.i. for comparison. DA viral antigen was significantly greater in the CVF-treated mice with seizures than in both the CVF-treated mice without seizures and the C57BL/6 mice with seizures. ‡, P < 0.05. Results are depicted as means plus SEMs (error bars) for groups with five or six mice per group.
Inflammation in CVF-treated mice infected with DA virus was measured via RCA-I lectin histochemical detection of activated microglia/macrophages and GFAP immunohistochemical detection of activated astrocytes (gliosis) (Fig. 8). The numbers of activated microglia/macrophages in C57BL/6 mice and CVF-treated mice with seizures were found to be significantly greater than those in PBS-injected control mice (P < 0.001), C57BL/6 mice without seizures (P < 0.0001), and CVF-treated mice without seizures (P < 0.0001) on day 14 p.i. (Fig. 8a). On day 21 p.i., the numbers of activated microglia/macrophages in C57BL/6 mice with seizures were found to be significantly greater than those in PBS-injected control mice (P < 0.001), C57BL/6 mice without seizures (P < 0.0001), and CVF-treated mice without seizures (P < 0.001). On day 21 p.i., the numbers of activated microglia/macrophages in CVF-treated mice with seizures were found to be significantly greater than those in PBS-injected control mice (P < 0.05) and C57BL/6 mice without seizures (P < 0.05) (Fig. 8a). The gliosis score for C57BL/6 mice with seizures was significantly greater than the scores for PBS-injected control mice (P < 0.01), C57BL/6 mice without seizures (P < 0.01), CVF-treated mice with seizures (P < 0.01), and CVF-treated mice without seizures on day 14 p.i. (P < 0.01) (Fig. 8b). Also, on day 14 p.i., the gliosis score for CVF-treated mice with seizures was significantly greater than the scores for PBS-injected control mice (P < 0.05), C57BL/6 mice without seizures (P < 0.05), and CVF-treated mice without seizures (P < 0.05) (Fig. 8b). As expected, on day 21 p.i., the PBS-injected control mice had a significantly lower gliosis score than the C57BL/6 mice with seizures (P < 0.05) and the CVF-treated mice with seizures (P < 0.05) did (Fig. 8b).
In summary, we examined neuronal cell loss, viral persistence, and inflammation in CVF-treated mice infected with DA virus (Fig. 6 to 8). We found that the extents of neuronal cell loss and inflammation were comparable in CVF-treated mice with seizures and C57BL/6 mice with seizures and in CVF-treated mice without seizures and C57BL/6 mice without seizures for days 14 and 21 p.i. Virus was found to persist in CVF-treated mice with seizures through day 14 p.i., although virus was cleared by day 21 p.i.
C5 may also be important for seizures.
The DBA/2 strain of mouse is deficient in the complement component C5. We tested the DBA/2 strain to determine the role of C5 in seizure development in DA virus-infected mice. DBA/2 and C57 × DBA F1 mice were infected with DA virus (2 × 104 PFU) and monitored for the development of seizures through day 21 p.i. Interestingly, 6.7% of DBA/2 mice (male) and 13.6% of C57 × DBA F1 mice (12 males and 10 females) also developed seizures (Racine scale stage 3 to 5) following infection with DA virus compared to infected C57BL/6 mice (P < 0.001) (Fig. 2). In addition, significantly fewer DBA/2 mice developed seizures compared to the C57 × DBA F1 mice (P < 0.05) (Fig. 2).
DISCUSSION
In our previous study of the TMEV-induced seizure model, we implicated the innate immune response, specifically cytokine production and inflammation, as contributing to the development of acute behavioral seizures (19). Complement is part of the innate immune response and can contribute to the release of cytokines and the induction of inflammation (1). More specifically, activation of C3 has been shown to result in the release of TNF-α and IL-6 (46). It has been reported that the complement activation fragments (C3a and C5a) could synergistically upregulate lipopolysaccharide-induced production of the TNF-α and IL-6 cytokines (46). In our previous exploration of the importance of both TNF-α and IL-6 in the TMEV-induced seizure model, it was found that mice that were deficient in either TNF receptor I or IL-6 were significantly less susceptible to the development of seizures (19). Also, TNF-α and IL-6 mRNA expression levels were increased in TMEV-infected mice experiencing seizures (19). Thus, the contribution that C3 makes to the development of seizures, as demonstrated in the present study through the use of C3-deficient mice, may be through the TNF-α and IL-6 signaling pathways. We are developing plans to investigate both the interactions between C3 and the cytokines, TNF-α and IL-6, which may lead to the development of acute seizures, and the importance of TNF-α, IL-6, and C3 in the development of epilepsy using this TMEV-induced seizure model.
In the CNS, inflammation can consist of gliosis and the infiltration of macrophages and/or the activation of microglial cells. C3a and C5a have been shown to induce activation of microglia (25), and C5a is chemotactic for microglia and astrocytes in vitro (2, 43). The presence of macrophages/activated microglia and activated astrocytes (gliosis), detected via RCA-I lectin histochemistry and GFAP immunohistochemistry, respectively, has been previously demonstrated for the TMEV-induced seizure model (19). Both the number of RCA-I+ cells and the extent of gliosis were significantly greater in TMEV-infected mice experiencing seizures than in TMEV-infected mice without seizures for particular time points p.i. (19). The presence of activated microglia and activated astrocytes suggests a role for complement-induced inflammation in the development of seizures in the TMEV-induced seizure model. This is supported by the current study in which inflammation was examined in C3-deficient mice and CVF-treated mice. Early after infection (days 3 and 7 p.i.), mice deficient in C3 (with or without seizures), and therefore lacking in C3 activation, demonstrated significantly reduced migroglial/macrophage activation and gliosis (Fig. 5). C3-deficient mice with seizures did develop a significant amount of inflammation by day 10 p.i., which probably occurred as a result of the seizures (Fig. 5). Mice treated with CVF, and therefore lacking in complement activation in the periphery but not the CNS, demonstrated inflammation comparable to that in untreated mice (Fig. 8).
CVF has long been known to deplete C3 in the periphery (serum or plasma and peritoneal lavage fluid) from the time that it was first purified (7) through the development of a recombinant form (20) to the present (22, 45). As for the CNS, complement proteins are constitutively expressed at low levels by cells locally in the CNS, and thus, complement activation within the CNS is not exclusively dependent on complement from the systemic circulation. Examination of complement, and more specifically C3, in the CNS following CVF treatment has been performed previously (8). It was found that CVF treatment did not eliminate complement activation and deposition in the brain following a complement-activating insult (hypoxic-ischemic brain injury) to the CNS. The potential local sources of C3 within the injured brain were determined to be activated microglia and cells of the endothelium, ependyma, and choroid plexus cell types (8). The presence of complement activation within the CNS explains the results of Kim and Perlman (18) in which antibody-induced demyelination was reduced by 85 to 90% in mouse hepatitis virus strain JHM-infected FcRγ−/− RAG1−/− mice (FcRγ, Fc receptor gamma), but demyelination was reduced by only 76% in JHM-infected RAG1−/− mice treated with CVF to deplete complement. As complement activation occurs within the CNS following viral infection and we see an increase in C3 mRNA expression within the brain following TMEV infection, we would not expect CVF treatment to eliminate TMEV-induced complement activation within the CNS.
With respect to complement and neurons, prominent constitutive synthesis of many complement proteins and complement receptors (C3a receptor [C3aR] and C5aR) has been localized to the hippocampal neurons, as well as the pyramidal cortical neurons and cerebellar Purkinje neurons, in healthy murine brains (9, 26, 27, 44). Complement components C3, C5, and C6, both mRNA and protein, were found to be expressed in the granular neurons of the dentate gyrus and the pyramidal neurons of the CA1 to CA3 regions of the hippocampus (44). The expression of complement proteins in the pyramidal neurons of the CA1 to CA3 regions of the hippocampus coincides with the region of marked neuron loss previously described for the TMEV-induced seizure model (19, 23). Quantification of neuron loss demonstrated that TMEV-infected mice experiencing seizures had a significantly greater amount of neuron loss than TMEV-infected mice without seizures (19). Activation of complement within these neurons may play a role in the resultant neuron loss. The current study supports this in that early after infection (days 3 and 7 p.i.), mice deficient in C3 (with or without seizures), again lacking in complement activation, do not experience neuronal cell loss (Fig. 3). Also in support of activation of complement within neurons playing a role in neuron loss are the following findings. (i) Cell death of hippocampal pyramidal neurons in TMEV-infected C57BL/6 mice early after infection is not caused by direct viral infection of the neurons (6). (ii) Mice treated with CVF, again lacking in complement activation in the periphery but not the CNS, experience neuronal cell loss comparable to that in untreated mice (Fig. 6). Of all the CNS cell types, neurons are the most susceptible to complement-mediated cell lysis, as they express only very low levels of most complement inhibitors (reviewed in reference 13). If the majority of neurons that are lost are inhibitory neurons, then excitotoxicity by the remaining excitatory neurons would be expected to be the cause of the resultant seizures. If, however, seizures occur in the absence of complement activation within neurons, then neuronal cell loss can still occur, as seen for C3-deficient mice with seizures on day 10 p.i. (Fig. 3), probably as a result of the seizures.
In our previous study of the TMEV-induced seizure model, we discounted a role for viral persistence in the development of acute behavioral seizures, as C57BL/6 mice (viral clearance) experienced seizures and OT-I mice (viral persistence) experienced comparable seizures, while SJL/J mice (viral persistence) did not experience seizures (19). In the current study, we again discount a role for viral persistence, as viral genome (Fig. 4a) and viral protein (Fig. 4b) persist in C3-deficient mice, which have a much reduced frequency of seizures (Fig. 2), while viral protein (Fig. 7) persists in CVF-treated mice, which have a seizure frequency comparable to that of control mice (Fig. 2). Conversely, viral persistence in both the C3-deficient and CVF-treated mice suggests a role for complement in the clearance of virus. Previously, in support of this concept, studies treating TMEV-infected SJL/J mice with CVF suggested that complement played a role in limiting the spread of TMEV within the CNS (29), and the titer of TMEV was shown to be increased, on days 6, 7 and 8 p.i., in the spinal cords of ddY mice treated with an antibody against complement type 3 receptor (21).
In addition to the C3 gene, genes within and outside the major histocompatibility complex (MHC) may contribute to the development of seizures in the TMEV-induced seizure model. DBA/2 mice are C5 deficient and significantly less susceptible to seizure development than C57BL/6 mice are (6.7% compared to 50%, respectively). However, the difference in seizure susceptibility between C57BL/6 mice (C3 and C5 sufficient) and DBA/2 mice (C5 deficient) may arise due to differences in the genetic background as opposed to differences in the presence (C57BL/6) or absence (DBA/2) of C5. C57BL/6 mice and DBA/2 mice differ at the MHC locus in that C57BL/6 mice have the H-2b allele and DBA/2 mice have the H-2d allele. We previously demonstrated that another inbred strain of mice that has the H-2d allele at the MHC locus, BALB/c mice (C3 and C5 sufficient), do not experience seizures following TMEV infection (0%; 0/17) (23). In addition, we previously showed that another inbred strain of mice that are C5 deficient, FVB/N mice (H-2q), do not experience seizures following TMEV infection (0%; 0/20) (23). Therefore, it is difficult to correlate the occurrence of seizures with only the presence or absence of C5. Interestingly, 13.6% of C57 × DBA F1 mice also developed seizures, and the intermediate phenotype of the C57 × DBA offspring demonstrates heritability of the seizure susceptibility. The data suggest that C5 may contribute to the development of seizures in conjunction with other genes, within and/or outside the MHC locus, in the TMEV-induced seizure model.
Previously, we suggested that the TMEV-induced seizure model could be a potential model for human mesial temporal lobe epilepsy (TLE) with hippocampal sclerosis based on the loss of neurons and the presence of gliosis in both conditions (19). We extend this comparison in the present study by demonstrating the importance of complement in the TMEV-induced seizure model. Human mesial TLE is a seizure disorder in which complement is suspected to play a role in pathogenesis (17). Human mesial TLE is most likely a multifactorial disease involving multiple susceptibility genes and various environmental factors, including CNS infections (40). A large-scale gene expression profiling (microarray) study of human mesial TLE has implicated complement component C3 (upregulated at the transcriptional level) in the pathophysiology of this disease (17). By immunohistochemistry, altered expression of C3 was demonstrated at the protein level (perivascular infiltration of C3-positive leukocytes) and/or MAC was detected on neurons within the entorhinal cortex (EC) of nine out of 11 mesial TLE patients. These are indications of complement activation within the EC, which provides the main excitatory input to the hippocampus (17). A large-scale gene expression study of the post-status epilepticus (post-SE) rodent model, an animal model which mimics the mesial TLE syndrome, also implicated the complement pathway in epileptogenesis (15). Several components of the complement pathway (C1qa, C1qc, C3, and C4a) were upregulated in the CA3 region of the hippocampus and the EC region, but not in the cerebellum, at the latent period time point (1 week after electrically induced SE; the epileptogenesis phase), and this upregulation remained through the chronic period time point (3 to 4 months post-SE; the epilepsy phase) (15). This study has been extended to examine expression of complement components at the protein level in the animal model and to compare the experimental model findings to humans with TLE (3). Protein expression of the complement components C1q and C3d mainly occurred in microglial cells, perivascular astrocytes, and some hippocampal neurons in the animal model. Examination of surgical specimens from TLE patients with hippocampal sclerosis demonstrated that expression of the C1q and C3 genes was significantly upregulated in patient material and that protein expression of C1q, C3c, C3d, and MAC was increased in the CA1 to CA4 and hilus regions of the hippocampus. C1q, C3c, and C3d were expressed in reactive astrocytes, microglial cells, and neurons, whereas MAC was predominantly expressed in activated microglial cells and macrophages. In general, in the TLE specimens, complement activation in astrocytes correlated with areas of gliosis and complement activation in microglial cells correlated with areas of neuronal loss (3). As a potential model for human mesial TLE with hippocampal sclerosis, the TMEV-induced seizure model will be used to study the pathogenesis underlying the progression from acute seizures, through the latent period (epileptogenesis), to the development of spontaneous seizures (epilepsy).
In this study, we have demonstrated the importance of complement activation within the CNS in the development of acute behavioral seizures following viral infection. Complement appears to be activated as part of the innate immune response to viral infection. This in turn leads to the production/release of IL-6 and the induction of inflammation. Neurons are lost, and ultimately, seizures result. Further work is required to elucidate other genes within and outside the MHC that may contribute to seizure susceptibility.
Acknowledgments
We thank Michael K. Burrow, Daniel J. Doty, and Jordan T. Sim for excellent technical assistance. We thank Kathleen Borick for outstanding preparation of the manuscript.
This work was supported by funding from NIH 1R01NS065714-01A1, the Margolis Foundation, CURE, and the Emma Mary Deland Foundation.
Footnotes
Published ahead of print on 28 April 2010.
REFERENCES
- 1.Alexander, J. J., A. J. Anderson, S. R. Barnum, B. Stevens, and A. J. Tenner. 2008. The complement cascade: yin-yang in neuroinflammation-neuro-protection and -degeneration. J. Neurochem. 107:1169-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, R. C., L. Harvath, and M. E. Dubois-Dalcq. 1990. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J. Neurosci. Res. 27:400-407. [DOI] [PubMed] [Google Scholar]
- 3.Aronica, E., K. Boer, E. A. van Vliet, S. Redeker, J. C. Baayen, W. G. M. Spliet, P. C. van Rijen, D. Troost, F. H. L. da Silva, W. J. Wadman, and J. A. Gorter. 2007. Complement activation in experimental and human temporal lobe epilepsy. Neurobiol. Dis. 26:497-511. [DOI] [PubMed] [Google Scholar]
- 4.Barnum, S. R. 1995. Complement biosynthesis in the central nervous system. Crit. Rev. Oral Biol. Med. 6:132-146. [DOI] [PubMed] [Google Scholar]
- 5.Benkovic, S. A., J. P. O'Callaghan, and D. B. Miller. 2004. Sensitive indicators of injury reveal hippocampal damage in C57BL/6J mice treated with kainic acid in the absence of tonic-clonic seizures. Brain Res. 1024:59-76. [DOI] [PubMed] [Google Scholar]
- 6.Buenz, E. J., B. M. Sauer, R. G. Lafrance-Corey, C. Deb, A. Denic, C. L. German, and C. L. Howe. 2009. Apoptosis of hippocampal pyramidal neurons is virus independent in a mouse model of acute neurovirulent picornavirus infection. Am. J. Pathol. 175:668-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cochrane, C. G., H. J. Müller-Eberhard, and B. S. Aikin. 1970. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J. Immunol. 105:55-69. [PubMed] [Google Scholar]
- 8.Cowell, R. M., J. M. Plane, and F. S. Silverstein. 2003. Complement activation contributes to hypoxic-ischemic brain injury in neonatal rats. J. Neurosci. 23:9459-9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davoust, N., J. Jones, P. F. Stahel, R. S. Ames, and S. R. Barnum. 1999. Receptor for the C3a anaphylatoxin is expressed by neurons and glial cells. Glia 26:201-211. [DOI] [PubMed] [Google Scholar]
- 10.Depboylu, C., M. K. H. Schäfer, W. J. Schwaeble, T. A. Reinhart, H. Maeda, H. Mitsuya, R. Damadzic, D. M. Rausch, L. E. Eiden, and E. Weihe. 2005. Increase of C1q biosynthesis in brain microglia and macrophages during lentivirus infection in the rhesus macaque is sensitive to antiretroviral treatment with 6-chloro-2′,3′-dideoxyguanosine. Neurobiol. Dis. 20:12-26. [DOI] [PubMed] [Google Scholar]
- 11.Dietzschold, B., W. Schwaeble, M. K. H. Schäfer, D. C. Hooper, Y. M. Zehng, F. Petry, H. Sheng, T. Fink, M. Loos, H. Koprowski, and E. Weihe. 1995. Expression of C1q, a subcomponent of the rat complement system, is dramatically enhanced in brains of rats with either Borna disease or experimental allergic encephalomyelitis. J. Neurol. Sci. 130:11-16. [DOI] [PubMed] [Google Scholar]
- 12.Franklin, K. B. J., and G. Paxinos. 1997. The mouse brain in stereotaxic coordinates. Academic Press, San Diego, CA.
- 13.Gasque, P., Y. D. Dean, E. P. McGreal, J. VanBeek, and B. P. Morgan. 2000. Complement components of the innate immune system in health and disease in the CNS. Immunopharmacology 49:171-186. [DOI] [PubMed] [Google Scholar]
- 14.Gasque, P., M. Fontaine, and B. P. Morgan. 1995. Complement expression in human brain. Biosynthesis of terminal pathway components and regulators in human glial cells and cell lines. J. Immunol. 154:4726-4733. [PubMed] [Google Scholar]
- 15.Gorter, J. A., E. A. van Vliet, E. Aronica, T. Breit, H. Rauwerda, F. H. Lopes da Silva, and W. J. Wadman. 2006. Potential new antiepileptogenic targets indicated by microarray analysis in a rat model for temporal lobe epilepsy. J. Neurosci. 26:11083-11110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hosokawa, M., A. Klegeris, J. Maguire, and P. L. McGeer. 2003. Expression of complement messenger RNAs and proteins by human oligodendroglial cells. Glia 42:417-423. [DOI] [PubMed] [Google Scholar]
- 17.Jamali, S., F. Bartolomei, A. Robaglia-Schlupp, A. Massacrier, J.-C. Peragut, J. Régis, H. Dufour, R. Ravid, P. Roll, S. Pereira, B. Royer, N. Roeckel-Trevisiol, M. Fontaine, M. Guye, J. Boucraut, P. Chauvel, P. Cau, and P. Szepetowski. 2006. Large-scale expression study of human mesial temporal lobe epilepsy: evidence for dysregulation of the neurotransmission and complement systems in the entorhinal cortex. Brain 129:625-641. [DOI] [PubMed] [Google Scholar]
- 18.Kim, T. S., and S. Perlman. 2005. Virus-specific antibody, in the absence of T cells, mediates demyelination in mice infected with a neurotropic coronavirus. Am. J. Pathol. 166:801-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirkman, N. J., J. E. Libbey, K. S. Wilcox, H. S. White, and R. S. Fujinami. 2010. Innate but not adaptive immune responses contribute to behavioral seizures following viral infection. Epilepsia 51:454-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kock, M. A., B. E. Hew, H. Bammert, D. C. Fritzinger, and C.-W. Vogel. 2004. Structure and function of recombinant cobra venom factor. J. Biol. Chem. 279:30836-30843. [DOI] [PubMed] [Google Scholar]
- 21.Kohanawa, M., M. Asano, T. Minagawa, and A. Nakane. 1997. Macrophage antigen-1 positive cells are essential in the defense against Theiler's virus strain GD VII infection in the spinal cord. Microb. Pathog. 23:33-38. [DOI] [PubMed] [Google Scholar]
- 22.Leendertse, M., R. J. L. Willems, R. Flierman, A. F. De Vos, M. J. M. Bonten, and T. van der Poll. 2010. The complement system facilitates clearance of Enterococcus faecium during murine peritonitis. J. Infect. Dis. 201:544-552. [DOI] [PubMed] [Google Scholar]
- 23.Libbey, J. E., N. J. Kirkman, M. C. P. Smith, T. Tanaka, K. S. Wilcox, H. S. White, and R. S. Fujinami. 2008. Seizures following picornavirus infection. Epilepsia 49:1066-1074. [DOI] [PubMed] [Google Scholar]
- 24.Mehlhop, E., K. Whitby, T. Oliphant, A. Marri, M. Engle, and M. S. Diamond. 2005. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J. Virol. 79:7466-7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Möller, T., C. Nolte, R. Burger, A. Verkhratsky, and H. Kettenmann. 1997. Mechanisms of C5a and C3a complement fragment-induced [Ca2+]i signaling in mouse microglia. J. Neurosci. 17:615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataf, S., P. F. Stahel, N. Davoust, and S. R. Barnum. 1999. Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci. 22:397-402. [DOI] [PubMed] [Google Scholar]
- 26a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 27.O'Barr, S. A., J. Caguioa, D. Gruol, G. Perkins, J. A. Ember, T. Hugli, and N. R. Cooper. 2001. Neuronal expression of a functional receptor for the C5a complement activation fragment. J. Immunol. 166:4154-4162. [DOI] [PubMed] [Google Scholar]
- 28.Racine, R. J. 1972. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 32:281-294. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, M., C. F. Lucchinetti, R. J. Clark, T. L. Yakash, H. Markowitz, and V. A. Lennon. 1988. Immunoglobulins and complement in demyelination induced in mice by Theiler's virus. J. Immunol. 140:800-806. [PubMed] [Google Scholar]
- 30.Singer, L., H. R. Colten, and R. A. Wetsel. 1994. Complement C3 deficiency: human, animal, and experimental models. Pathobiology 62:14-28. [DOI] [PubMed] [Google Scholar]
- 31.Stewart, K.-A. A., K. S. Wilcox, R. S. Fujinami, and H. S. White. 1 December 2009, posting date. Theiler's virus infection chronically alters seizure susceptibility. Epilepsia [Epub ahead of print] doi: 10.1111/j.1528-1167.2009.02405.x. [DOI] [PubMed]
- 32.Suzuki, H., H. Franz, T. Yamamoto, Y. Iwasaki, and H. Konno. 1988. Identification of the normal microglial population in human and rodent nervous tissue using lectin-histochemistry. Neuropathol. Appl. Neurobiol. 14:221-227. [DOI] [PubMed] [Google Scholar]
- 33.Szalai, A. J., D. E. Briles, and J. E. Volanakis. 1996. Role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect. Immun. 64:4850-4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szalai, A. J., S. B. Digerness, A. Agrawal, J. F. Kearney, R. P. Bucy, S. Niwas, J. M. Kilpatrick, Y. S. Babu, and J. E. Volanakis. 2000. The Arthus reaction in rodents: species-specific requirement of complement. J. Immunol. 164:463-468. [DOI] [PubMed] [Google Scholar]
- 35.Tsunoda, I., Y. Iwasaki, H. Terunuma, K. Sako, and Y. Ohara. 1996. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol. (Berl.) 91:595-602. [DOI] [PubMed] [Google Scholar]
- 36.Tsunoda, I., L.-Q. Kuang, J. E. Libbey, and R. S. Fujinami. 2003. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 162:1259-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsunoda, I., I. J. McCright, L.-Q. Kuang, A. Zurbriggen, and R. S. Fujinami. 1997. Hydrocephalus in mice infected with a Theiler's murine encephalomyelitis virus variant. J. Neuropathol. Exp. Neurol. 56:1302-1313. [DOI] [PubMed] [Google Scholar]
- 38.Tsunoda, I., T. Tanaka, Y. Saijoh, and R. S. Fujinami. 2007. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am. J. Pathol. 171:1563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsunoda, I., Y. Wada, J. E. Libbey, T. S. Cannon, F. G. Whitby, and R. S. Fujinami. 2001. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J. Virol. 75:7494-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Gassen, K. L., M. de Wit, M. J. Koerkamp, M. G. Rensen, P. C. van Rijen, F. C. Holstege, D. Lindhout, and P. N. de Graan. 2008. Possible role of the innate immunity in temporal lobe epilepsy. Epilepsia 49:1055-1065. [DOI] [PubMed] [Google Scholar]
- 41.Whitney, K. D., P. I. Andrews, and J. O. McNamara. 1999. Immunoglobulin G and complement immunoreactivity in the cerebral cortex of patients with Rasmussen's encephalitis. Neurology 53:699-708. [DOI] [PubMed] [Google Scholar]
- 42.Xiong, Z.-Q., W. Qian, K. Suzuki, and J. O. McNamara. 2003. Formation of complement membrane attack complex in mammalian cerebral cortex evokes seizures and neurodegeneration. J. Neurosci. 23:955-960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yao, J., L. Harvath, D. L. Gilbert, and C. A. Colton. 1990. Chemotaxis by a CNS macrophage, the microglia. J. Neurosci. Res. 27:36-42. [DOI] [PubMed] [Google Scholar]
- 44.Yu, J. X., B. M. Bradt, and N. R. Cooper. 2002. Constitutive expression of proinflammatory complement components by subsets of neurons in the central nervous system. J. Neuroimmunol. 123:91-101. [DOI] [PubMed] [Google Scholar]
- 45.Zaidi, T. S., T. Zaidi, and G. B. Pier. 2010. Role of neutrophils, MyD88-mediated neutrophil recruitment, and complement in antibody-mediated defense against Pseudomonas aeruginosa keratitis. Invest. Ophthalmol. Vis. Sci. 51:2085-2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang, X., Y. Kimura, C. Fang, L. Zhou, G. Sfyroera, J. D. Lambris, R. A. Wetsel, T. Miwa, and W.-C. Song. 2007. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood 110:228-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zurbriggen, A., and R. S. Fujinami. 1989. A neutralization-resistant Theiler's virus variant produces an altered disease pattern in the mouse central nervous system. J. Virol. 63:1505-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]