Abstract
While few children and young adults have cross-protective antibodies to the pandemic H1N1 2009 (pdmH1N1) virus, the illness remains mild. The biological reasons for these epidemiological observations are unclear. In this study, we demonstrate that the bulk memory cytotoxic T lymphocytes (CTLs) established by seasonal influenza viruses from healthy individuals who have not been exposed to pdmH1N1 can directly lyse pdmH1N1-infected target cells and produce gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α). Using influenza A virus matrix protein 1 (M158-66) epitope-specific CTLs isolated from healthy HLA-A2+ individuals, we further found that M158-66 epitope-specific CTLs efficiently killed both M158-66 peptide-pulsed and pdmH1N1-infected target cells ex vivo. These M158-66-specific CTLs showed an effector memory phenotype and expressed CXCR3 and CCR5 chemokine receptors. Of 94 influenza A virus CD8 T-cell epitopes obtained from the Immune Epitope Database (IEDB), 17 epitopes are conserved in pdmH1N1, and more than half of these conserved epitopes are derived from M1 protein. In addition, 65% (11/17) of these epitopes were 100% conserved in seasonal influenza vaccine H1N1 strains during the last 20 years. Importantly, seasonal influenza vaccination could expand the functional M158-66 epitope-specific CTLs in 20% (4/20) of HLA-A2+ individuals. Our results indicated that memory CTLs established by seasonal influenza A viruses or vaccines had cross-reactivity against pdmH1N1. These might explain, at least in part, the unexpected mild pdmH1N1 illness in the community and also might provide some valuable insights for the future design of broadly protective vaccines to prevent influenza, especially pandemic influenza.
Since its first identification in North America in April 2009, the novel pandemic H1N1 2009 (pdmH1N1) virus has been spreading in humans worldwide, giving rise to the first pandemic in the 21st century (13, 18). The pdmH1N1 virus contains a unique gene constellation, with its NA and M gene segments being derived from the Eurasian swine lineage while the other gene segments originated from the swine triple-reassortant H1N1 lineage. The triple-reassortant swine viruses have in turn derived the HA, NP, and NS gene segments from the classical swine lineage (20). The 1918 pandemic virus gave rise to both the seasonal influenza H1N1 and the classical swine H1N1 virus lineages (41). Evolution in different hosts during the subsequent 90 years has led to increasing antigenic differences between recent seasonal H1N1 viruses and swine H1 viruses (42). Thus, younger individuals have no antibodies that cross neutralize pdmH1N1, while those over 65 years of age are increasingly likely to have cross-neutralizing antibodies to pdmH1N1 (10, 25).
Currently available seasonal influenza vaccines do not induce cross-reactive antibodies against this novel virus in any age group (10, 25). In animal models, it has been shown that pdmH1N1 replicated more efficiently and caused more severe pathological lesions than the current seasonal influenza virus (28). However, most patients with pdmH1N1 virus infection show a mild illness comparable to seasonal influenza (9, 42). The incidence of severe cases caused by pdmH1N1 was not significantly higher than that caused by human seasonal influenza viruses (43). These findings imply that seasonal influenza A virus-specific memory T cells preexisting in previously infected individuals may have cross-protection to this novel pdmH1N1.
Cross-reactivity of influenza A virus-specific T-cell immunity against heterosubtypic strains which are serologically distinct has been demonstrated (5, 29, 33, 47). Humans who have not been exposed to avian influenza A (H5N1) virus do have cross-reactive memory CD4 and CD8 T cells to a wide range of H5N1 peptides (33, 47). More recently, one study also showed that some seasonal influenza A virus-specific memory T cells in individuals without exposure to prior pdmH1N1 infection can recognize pdmH1N1 (24). However, the results in most of these studies were determined by the gamma interferon (IFN-γ) responses to influenza virus peptides. Although the recalled IFN-γ response is commonly used to detect memory CD4 and CD8 T cells, the activated T cells that bind major histocompatibility complex (MHC)-presented peptide are not necessarily capable of lysing the target cells (6). In addition, the peptides, but not the whole virus, may not be able to fully represent the human cross-response against the virus as a whole. Therefore, in addition to cytokine production, the demonstration of direct antigen-specific cytotoxicity of cytotoxic T lymphocytes (CTLs) against both peptide-pulsed and virus-infected target cells is needed for better understanding of human CTL responses against pdmH1N1 virus.
In this study, using bulk memory CTLs and epitope-specific CTLs established by seasonal influenza A viruses and epitope-specific peptide from healthy individuals, respectively, we evaluated their cross-cytotoxicity and cytokine responses to pdmH1N1. We also examined the expression of chemokine receptors CXCR3 and CCR5, which could help CTLs to migrate to the site of infection. In addition, to understand whether the seasonal influenza vaccines have benefit for people who have not been exposed to pdmH1N1, we further examined the ability of seasonal influenza vaccines to induce the conserved M158-66 epitope-specific CTLs in HLA-A2-seropositive healthy individuals.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood obtained from 12 healthy adult volunteers recruited between May and July 2009. A brief questionnaire was conducted when the blood sample was collected to confirm that there was no clinical history of pdmH1N1 infection in these donors. A microneutralization assay was used to confirm that there was no detectable antibody against pdmH1N1 in these healthy adult volunteers. Twenty HLA-A2-positive adult donors were recruited before the 2008 influenza season and vaccinated with one dose of 2008-2009 trivalent seasonal influenza vaccine, comprised of A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, and B/Florida/4/2006-like strains, manufactured by GlaxoSmithKline Biologicals. The blood was collected before and 1 month after vaccination. Monocyte-derived macrophages (MDMs) were generated from mononuclear cells as we described previously (60). PBMCs and MDMs were cultured in RPMI 1640 (Invitrogen) medium supplemented with 10% autologous serum. B cells from PBMCs were stimulated and expanded via CD40 using NIH 3T3 cells transfected with the human CD40 ligand as we described before (52, 58, 59). The research protocol was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster. The T2 (deficient in human transporter associated with antigen processing) and Madin-Darby canine kidney (MDCK) cell lines (both from ATCC) were routinely maintained in Iscove's modified Dulbecco's medium and Dulbecco's modified Eagle's medium (Invitrogen), respectively.
Influenza virus and peptide.
As described in our previous study (36), seasonal influenza A/Brisbane/59/2007 (H1N1)-like and A/Brisbane/10/2007 (H3N2)-like viruses were cultured in MDCK cells. A/California/04/2009 pandemic pdmH1N1 was propagated in embryonated chicken eggs. These viruses were purified by adsorption to and elution from turkey red blood cells. The virus titer was determined by titration in MDCK cells with daily observation of cytopathogenic effect and was confirmed by hemagglutination assay. Handling of the pandemic virus was performed in a biosafety level 3 facility. The HLA-A2-restricted influenza virus M1 peptide (GILGFVFTL) (Chinese Peptide, China) and human papillomavirus (HPV) peptide (FQQLFLNTL) (AnaSpec, CA) were dissolved in dimethyl sulfoxide (DMSO) at 1 mg/ml and stored at −70°C until use.
Ex vivo expansion of influenza A virus-specific bulk CTLs.
PBMCs were infected with seasonal influenza H1N1 and H3N2 viruses at a multiplicity of infection (MOI) of 3 in the absence of human serum. After 1 h of viral adsorption, unadsorbed viruses were washed away with excess phosphate-buffered saline. The cells were then cultured in RPMI 1640 medium plus 10% human AB serum and 1 mg/ml phytohemagglutinin (PHA) (Sigma). On day 7, CD8+ T cells were isolated by using a CD8 T-cell isolation kit (Miltenyi). Autologous PBMCs that were infected by influenza virus were irradiated and cocultured with the purified CD8+ T cells at 1:1 in RPMI 1640 medium supplemented with 10% human AB serum, 1 mg/ml PHA, and 50 IU/ml recombinant human interleukin-2 (IL-2) for 5 more days. On day 12, IFN-γ+ CD8+ T cells were sorted as the effector cells with an IFN-γ secretion assay kit (Miltenyi Biotec). Briefly, CD8+ T cells were attached to IFN-γ catch reagent and isolated by positive selection with IFN-γ detection antibody-phycoerythrin (PE) and anti-PE microbeads. The purity of IFN-γ-positive CD8+ T cells was routinely more than 95% as determined by flow cytometric analysis.
Ex vivo expansion of influenza M158-66-specific CTLs.
PBMCs from healthy HLA-A2-positive individuals who had detectable CD3+ CD8+ M158-66 tetramer-positive (M158-66-tetramer+) cells before influenza vaccination were stimulated with 1 μg/ml M1 peptide in RPMI 1640 medium plus 10% human AB serum and recombinant IL-2 (Gibco). On day 10, CD3+ CD8+ M1-tetramer+ cells were sorted with a FACSAira and incubated with irradiated allogeneic feeder cells in the presence of PHA to expand M158-66-specific CTLs. At the indicated time points, the cells were examined for HLA-A*0201-restricted M158-66 tetramer staining of CD3+ CD8+ cells.
Flow cytometry and tetramer analysis.
The following anti-human monoclonal antibodies (MAbs) were used in this study: anti-HLA-A2 (BB7.2), anti-CD45RO (UCHL1), anti-CCR7 (3D12), anti-CD62L (DREG-56), anti-IFN-γ (25723.11), anti-TNF-α (6401.1111), antiperforin (G9), anti-granzyme B (GB11), and anti-CD107a (H4A3) (all from BD Biosciences) and anti-CD3 (HIT3a), anti-CD8 (HIT8a), anti-CD19 (HIB19), anti-CCR5 (HEK/1/85a), and anti-CXCR3 (TG1/CXCR3) (all from BioLegend). For intracellular staining, cells were fixed, permeabilized, and then labeled with the indicated antibody as we described before (36, 44, 59). In order to examine tetramer staining, cells were incubated with anti-CD3, anti-CD8, and HLA-A2/M158-66 tetramer (Beckman Coulter, CA) for 30 min and then analyzed by flow cytometry. All data were acquired on a BD FACSAria with FACS Diva (BD Biosciences) and analyzed using FlowJo software (TreeStar).
Cytotoxicity assay and granule exocytosis examination.
The cytotoxicity assay with T2 cells as target cells was performed with the Live/Dead cell-mediated cytotoxicity kit (Invitrogen) as described in our previous studies (36, 52). Briefly, DiOC18-labeled T2 cells were pulsed with M158-66 or HPV peptide in the presence of β2-microglobulin for 2 h and then cocultured with expanded M158-66-specific CTLs at the indicated effector cell/target cell (E/T) ratio in the presence of propidium iodide (PI) for 4 h. After incubation, the cytotoxicity was analyzed by flow cytometry and calculated as the percentage of DiO+ PI+ cells out of total DiO+ cells.
To determine the cytotoxicity of influenza virus-specific CTLs against influenza virus-infected target cells, target cells (autologous MDMs or B cells) were infected with H1N1, H3N2, or pdmH1N1 virus. After 1 h, the cells were washed and then cocultured with influenza virus bulk CTLs or M158-66-specific CTLs at the indicated E/T ratio for 4 to 6 h. After incubation, total cells were stained with anti-CD3 and EthD-2 as we described before (44). Dead target cells were identified as EthD-2-positive cells out of CD3-negative cells, and cell death was analyzed by flow cytometry.
To examine the granule exocytosis of CTLs, B and T2 cells were loaded with M158-66 or negative control HPV peptide and then cocultured with influenza virus M158-66-specific CTLs in the presence of fluorescein isothiocyanate (FITC)-anti-human CD107a MAb for 4 h. The expression of CD107a on the CTL surface was determined by flow cytometry.
MHC I-restricted CD8 T-cell epitope analysis.
Total human MHC I-restricted CD8 T-cell epitopes of all influenza A viruses were retrieved from the IEDB database. The epitopes that induced a positive T-cell response were included in this study for analysis. For overlapping epitopes, each one was included. When one or more epitopes were contained in another one, only the longest epitope was included for analysis, except for the M158-66 epitope (GILGFVFTL) used in this study. To determine the conservation of CD8 T-cell epitopes in pdmH1N1, all protein sequences of pdmH1N1, with A/California/04/2009(H1N1) (CA04) and A/California/07/2009(H1N1) (CA07) as representative strains, were searched against CD8 T-cell epitopes. These epitopes conserved in pdmH1N1 were further examined in the sequences of seasonal influenza vaccine H1N1 strains from 1989 to 2009 to calculate the frequency of epitopes invariant in the vaccine strains compared with pdmH1N1. Only the epitopes with 100% conservation were included for analysis.
Statistical analysis.
Data were expressed as means ± standard errors of the means (SEM). Statistical analysis was performed with Student's paired t test or one-way analysis of variance (ANOVA) with a multiple-comparison test using Prism 5 (GraphPad Software). A P value of <0.05 was considered significant.
RESULTS
Cross-response of bulk CTLs against pdmH1N1 in healthy individuals.
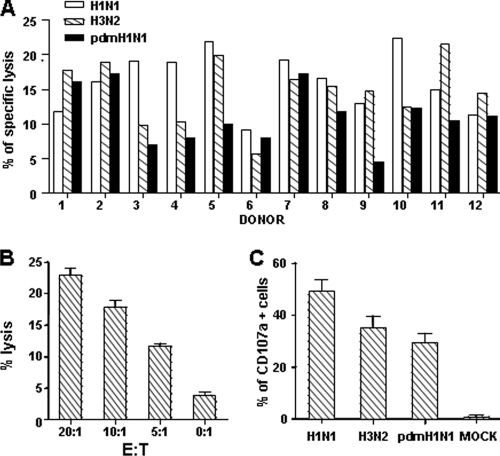
The cross-responses of the whole CTL repertoire against pdmH1N1 virus were first determined in healthy individuals without exposure to prior pdmH1N1 infection as confirmed by pdmH1N1 antibody testing. By stimulation of PBMCs with both H1N1 and H3N2 viruses from 2008-2009 seasonal influenza vaccine strains, seasonal influenza A virus-specific bulk CTLs were expanded and IFN-γ-secreting CD8 T cells were sorted as effector cells (E). In order to define the CTL cross-reactivity against pdmH1N1 virus, the direct cytotoxicity against influenza virus-infected target cells was examined. As shown in Fig. 1A, CTLs expanded by seasonal influenza viruses not only directly lysed seasonal H1N1- and H3N2-infected autologous MDMs but also efficiently killed the pdmH1N1-infected cells (target [T]), indicating that bulk CTLs induced by seasonal influenza A virus (H1N1 and H3N2) have cross-responses to pdmH1N1. Among 12 healthy donors, 5 had similar levels of CTL responses to seasonal influenza H1N1, H3N2, and pdmH1N1 viruses, whereas the others exhibited lower but substantial cross-CTL responses to pdmH1N1 compared to those against either seasonal H1N1 or H3N2 virus-infected cells (Fig. 1A). In addition, the cross-responses of bulk CTLs against pdmH1N1 virus were increased with the increase of the E/T ratio (Fig. 1B).
FIG. 1.
Bulk memory CTL cross-response against pdmH1N1 in healthy individuals. (A) PBMCs from healthy individuals were stimulated with both H1N1 and H3N2 viruses from 2008-2009 seasonal influenza vaccine strains to expand virus-specific bulk CTLs, of which IFN-γ-secreting CD8 T cells (effector [E]) were purified and then cocultured with autologous MDMs (target [T]) infected or not with H1N1, H3N2, and pdmH1N1 viruses at an E/T ratio of 10:1. Data for 12 different individuals are shown. (B) Human influenza virus-specific bulk CTLs were cocultured with pdmH1N1 virus-infected autologous MDMs at the indicated E/T ratios. After 4 h, cell death of MDMs was analyzed by flow cytometry. Data for 12 different individuals are shown. (C) Effector cells were cocultured with MDMs at an E/T ratio of 10:1 in the presence of anti-human CD107a antibody. After 4 h, CD107a expression on CD8 T cells was examined by flow cytometry. Data for 12 different individuals are shown.
Granule exocytosis is the main pathway for CTLs to lyse virus-infected target cells (34). Upon engagement with target cells, the secretory lysosomes that contain cytolytic granules fuse with the cell membrane, allowing the release of perforin and enzymes (granule exocytosis) to exert killing (8, 14). During this process, CD107a, a lysosomal membrane protein, redistributes to the cell surface. Thus, surface expression of CD107a is used to examine the exocytosis of killer cells (1, 7, 8). We next determined the granule exocytosis of bulk CTLs by detecting CD107a expression. As shown in Fig. 1C, similar to the case for seasonal H1N1 and H3N2 viruses, pdmH1N1 virus-infected MDMs also induced substantial granule exocytosis of CTLs expanded by seasonal influenza viruses (P > 0.05).
Cross-cytokine responses of bulk CTLs against pdmH1N1 in healthy individuals.
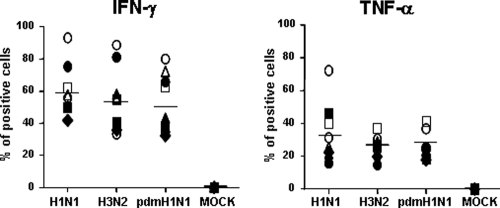
In addition to the direct lysis of infected cells, virus-specific CTLs also secrete effector cytokines to exert antiviral effects (37, 50, 55), among which IFN-γ and TNF-α are predominantly produced in influenza virus-specific CTLs in the lung (31). Therefore, the IFN-γ and TNF-α responses of bulk CTLs against the whole influenza virus were examined, despite the fact that their production is only an indirect surrogate marker for T-cell function. After a 4-hour coculture of bulk CTLs induced by seasonal influenza viruses (H1N1 and H3N2) and virus-infected MDMs, intracellular cytokine expression was analyzed by flow cytometry. As shown in Fig. 2, comparable frequencies of IFN-γ- and TNF-α-expressing cells were detected in the bulk CTLs stimulated by seasonal vaccine strain H1N1-, seasonal vaccine strain H3N2-, and pdmH1N1-infected MDMs.
FIG. 2.
Cytokine expressions of bulk memory CTLs against pdmH1N1. PBMCs from healthy individuals were stimulated with both H1N1 and H3N2 viruses from 2008-2009 seasonal influenza vaccine strains to expand virus-specific bulk CTLs, of which IFN-γ-secreting CD8 T cells (effector [E]) were purified and then cocultured with autologous MDMs (target [T]) infected with or without H1N1, H3N2, and pdmH1N1 viruses at an E/T ratio of 10:1 for 4 h in the presence of brefeldin A. The cells were then fixed, permeabilized, and examined for IFN-γ and TNF-α expression in CD8 T cells. Data for eight different individuals are shown.
Ex vivo expansion of M158-66 epitope-specific memory CTLs.
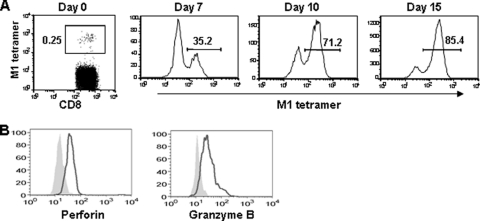
The M158-66 epitopes from seasonal vaccine strains (H1N1 and H3N2) are conserved in pdmH1N1, and the frequency of CTLs against a certain epitope in the individual PBMCs was very low. In order to investigate the function of epitope-specific memory CTLs, we expanded M158-66 epitope-specific memory CTLs with M158-66 peptide, which is a dominant HLA-A*0201-restricted epitope. PBMCs were isolated from healthy HLA-A2-positive individuals who had detectable CD3+ CD8+ M158-66-tetramer+ cells before influenza vaccination and were incubated with M158-66 peptide and recombinant IL-2 for expansion of epitope-specific CTLs. On day 10, CD3+ CD8+ M1-tetramer+ cells were isolated and further stimulated with irradiated allogeneic feeder cells in the presence of PHA to expand M158-66-specific CTLs. As shown in Fig. 3A, the epitope-specific memory CTLs were largely expanded by ex vivo peptide over 15 days of stimulation.
FIG. 3.
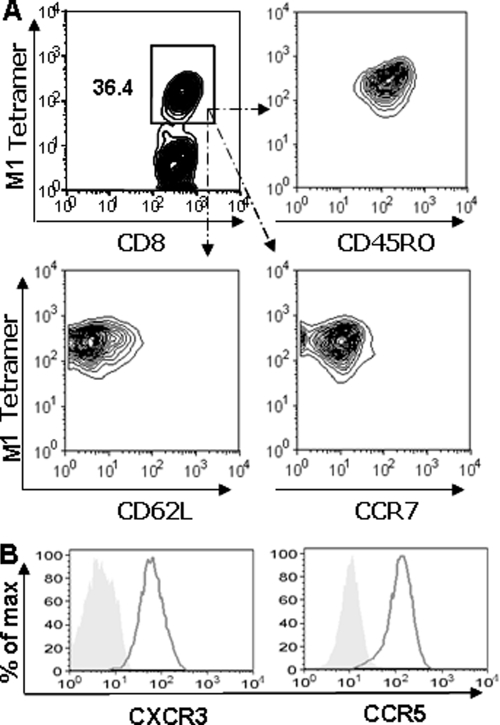
Ex vivo expansion of M158-66 epitope-specific memory CTLs. (A) PBMCs from healthy HLA-A2-seropositive individuals were stimulated with influenza M158-66 peptide to expand the epitope-specific CTLs. HLA-A2/M158-66 peptide tetramer+ CD8 T cells were analyzed by flow cytometry based on the gating of CD3+ CD8+ T cells. (B) Expression of perforin and granzyme B in HLA-A2/M158-66 peptide tetramer+ CD3+ CD8+ T cells. The results shown are representative of four different individuals.
The cytotoxicity of killer cells is usually mediated by cytolytic granules. The granule-mediated apoptosis is induced by the complementary action of granzymes, where granzyme B plays an essential role in inducing apoptosis, and perforin, a crucial effector molecule facilitating the entry of granzymes into the target cells (27, 35). We next examined the expression of granules in the epitope-specific memory CTLs expanded by M158-66 peptide. As shown in Fig. 3B, intracellular staining analysis demonstrated that a large fraction of these expanded memory CTLs expressed perforin and granzyme B.
Cross-responses of epitope-specific memory CTLs against pdmH1N1.
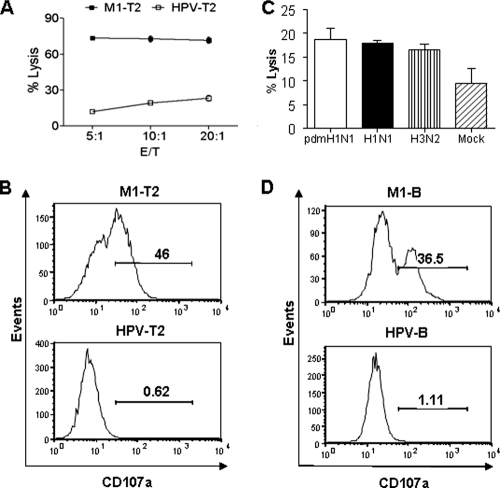
To confirm the cross-responses of CTLs against pdmH1N1, we further investigated the function of epitope-specific CTLs after expansion in four HLA-A2+ individuals. As shown in Fig. 4A, the expanded M158-66 epitope-specific memory CTLs had strong cytotoxicity specifically against influenza virus M158-66 peptide-pulsed target cells compared to that against control HPV peptide-pulsed cells. In parallel with the cytotoxicity, significant granule exocytosis, as evidenced by the high level of surface expression of CD107a, was detected on the epitope-specific memory CTLs stimulated by M158-66 peptide-pulsed target cells but not on those stimulated by HPV peptide-pulsed cells (Fig. 4B).
FIG. 4.
Cross-responses of M158-66 epitope-specific memory CTLs against pdmH1N1. (A) PBMCs from healthy HLA-A2-seropositive individuals were stimulated with influenza M158-66 peptide to expand the epitope-specific CTLs. T2 cells were loaded with influenza virus M1 (M1-T2) or negative control HPV peptide (HPV-T2) and then cocultured with M158-66 epitope-specific CTLs at the indicated E/T ratios. After 4 h, specific lysis was determined by flow cytometry. (B) M158-66 epitope-specific CTLs were cocultured with peptide-pulsed T2 cells in the presence of anti-CD107a antibody for 4 h. The surface expression of CD107a on CD8 T cells was examined. (C) Autologous B cells infected with or without H1N1, H3N2, or pdmH1N1 virus were cocultured with M158-66 epitope-specific CTLs for 6 h. B-cell death was analyzed by flow cytometry. (D) B cells were loaded with M158-66 (M1-B) or negative control HPV peptide (HPV-B) and then cocultured with M158-66 epitope-specific CTLs in the presence of anti-CD107a antibody. After 4 h, the surface expression of CD107a on CD8 T cells was examined. The results shown are representative of four independent experiments.
To define more precisely the CTL cross-reactivity against pdmH1N1, the cytotoxicity against virus-infected target cells by M158-66 epitope-specific CTLs was further studied. Autologous B cells were infected with seasonal H1N1 or H3N2 or pdmH1N1 virus and used as the target cells. As shown in Fig. 4C, the epitope-specific memory CTLs expanded by M158-66 peptide showed comparable cytotoxicity against pdmH1N1 and seasonal influenza H1N1 and H3N2 virus-infected target cells. In addition, a strong granule exocytosis was demonstrated in M158-66 peptide-specific memory CTLs upon engagement with B cells pulsed with this peptide but not those pulsed with the HPV (Fig. 4D). Taken together, these data indicated that the epitope-specific memory CTLs could efficiently recognize and kill the cells infected by the heterologous strains as long as the virus conserved this epitope.
Effector memory phenotype and trafficking receptor expression in M158-66 epitope-specific memory CTLs.
Memory T cells can be divided into two subgroups, central memory cells (CD62Lhigh CCR7+) and effector memory cells (CD62Llow CCR7−), based on the expression of homing receptors. These two groups have distinct traffic pathways and responses upon restimulation. Upon pathogen challenge, the effector memory CTLs can traffic to the peripheral tissue sites, immediately lyse target cells, and produce cytokines to contain the invading pathogens (6, 48). We therefore examined the phenotype of the CTLs. As demonstrated in Fig. 5A, all the M158-66 epitope-specific CTLs were CD45RO+ CD62Llow CCR7−, indicating that these CTLs were effector memory cells.
FIG. 5.
Effector memory phenotype and trafficking receptor expression in M158-66 epitope-specific memory CTLs. M158-66 epitope-specific CTLs were examined for the expression of memory phenotype CD45RO, CD62L, and CCR7 and of the trafficking receptor CXCR3 and CCR5 by flow cytometry. The results shown are representative of four independent experiments.
In order to clear the invading virus, the virus-specific CD8 T cells must migrate to sites of infection (56), which is mediated by the interaction of chemokine receptors and chemokines. It is known that CCR5 expressed on memory CTLs is needed for their recruitment to the airways during the early stages of the recall response (30). CXCR3 also plays a primary role in the early recruitment of memory CD8 T cells from the circulation to the lung airways during respiratory virus infection (30). Therefore, we further examined the expression of these chemokine receptors on the epitope-specific CTLs. As shown in Fig. 5B, almost all the M158-66 peptide-specific memory CTLs expressed CCR5 and CXCR3. Taken together, these findings indicated that these memory CTLs had the potential to quickly traffic to the site of infection and immediately clear influenza virus upon viral challenge.
In vivo expansion of M158-66 epitope-specific memory CTLs by seasonal influenza vaccine.
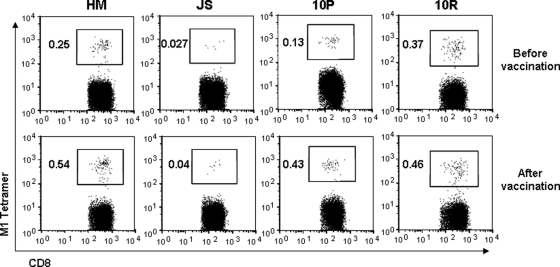
As the M158-66 epitope-specific memory CTLs could efficiently recognize and kill pdmH1N1-infected cells, we further determined whether the currently available seasonal influenza vaccine (GlaxoSmithKline Biologicals) could expand these CTLs. HLA-A2/M158-66-tetramer+ CD8+ T cells in frozen PBMCs isolated from 20 HLA-A2-positive individuals who received seasonal 2008-2009 influenza vaccine before influenza season were analyzed. Among them, four individuals had detectable HLA-A2/M158-66 tetramer-positive CTLs before vaccination. Importantly, their HLA-A2/M158-66 tetramer-positive CTLs were increased 1 month after seasonal influenza vaccination (Fig. 6). This expansion by seasonal influenza vaccine varied among the donors in terms of the frequency of HLA-A2/M158-66-tetramer+ CTLs, with the highest increase being about 3-fold and the lowest 40% in these individuals.
FIG. 6.
In vivo expansion of M158-66 epitope-specific CTLs by seasonal influenza vaccine. PBMCs from healthy HLA-A2-seropositive individuals were isolated before and 1 month after seasonal 2008-2009 influenza vaccination. HLA-A2/M158-66 tetramer staining was performed and analyzed by flow cytometry. The numbers shown here indicate the frequency of HLA-A2/M158-66-tetramer+ cells within the population of CD3+ CD8+ T cells. Among 20 HLA-A2-positive individuals, only 4 individuals had an increase of HLA-A2/M158-66 tetramer-positive CTLs 1 month after vaccination. Results from those four donors are shown.
Conservation of CD8 T-cell epitopes in pdmH1N1 and seasonal vaccine strains.
We next determined how many CD8 T-cell epitopes derived from previous influenza A viruses are conserved in pdmH1N1. CD8 T-cell epitopes of influenza A viruses of different subtypes, including H1N1, H3N2, and H5N1 were searched, and a total of 94 experimentally defined CD8 T-cell epitopes were obtained from the IEDB database. Of these, 17 epitopes are conserved in pdmH1N1, with CA04 and CA07 as representative strains (Table 1). As shown in Table 1, more than half of these conserved CD8 T-cell epitopes are derived from M1 protein, but no conserved epitope was found in HA protein.
TABLE 1.
MHC class I-restricted CD8 T-cell epitopes conserved in pdmH1N1 CA04 and CA07
| Epitope no. | Protein | Sequence | IEDB ID |
|---|---|---|---|
| 1 | M1 | GILGFVFTL | 20354 |
| 2 | M1 | ILSPLTKGIL | 27350 |
| 3 | M1 | IRHENRMVL | 28309 |
| 4 | M1 | KTRPILSPLTK | 33844 |
| 5 | M1 | LTKGILGFVFTLTVPSERG | 39989 |
| 6 | M1 | RGLQRRRFVQNALNGNG | 53918 |
| 7 | M1 | RMVLASTTAK | 54953 |
| 8 | M1 | SIIPSGPLK | 58567 |
| 9 | M1 | ALASCMGLIY | 97192 |
| 10 | NA | CVNGSCFTV | 7291 |
| 11 | NA | SWPDGAELPF | 62486 |
| 12 | NP | ELRSRYWAIRTRSG | 13264 |
| 13 | NP | QLVWMACHSAA | 97583 |
| 14 | NS1 | GEISPLPSL | 19312 |
| 15 | PA | CELTDSSWI | 6183 |
| 16 | PA | FMYSDFHFI | 17119 |
| 17 | PA | SLENFRAYV | 59069 |
We then determined how many of these 17 epitopes conserved in pdmH1N1 were invariant in the seasonal influenza vaccine H1N1 strains from 1989 to 2009. Six H1N1 viruses, A/Singapore/6/1986(H1N1), A/Bayern/7/1995(H1N1), A/Beijing/262/1995(H1N1), A/New Caledonia/20/1999(H1N1), A/Solomon Islands/3/2006(H1N1), and A/Brisbane/59/2007(H1N1), have been used as vaccine strains during this period. All full-length protein sequences were retrieved from the NCBI, and the conservation of these epitopes was ascertained. As shown in Table 2, about 65% (11/17) of these epitopes were completely conserved in the vaccine H1N1 strains. Similar to the epitopes conserved in pdmH1N1, most of the invariant T-cell epitopes in the vaccine strains are derived from the internal M1 protein.
TABLE 2.
MHC class I-restricted CD8 T-cell epitopes conserved in all seasonal vaccine H1N1 strains from 1989 to 2009 and in pdmH1N1 CA04 and CA07
| Epitope no. | Protein | Sequence | IEDB ID |
|---|---|---|---|
| 1 | M1 | GILGFVFTL | 20354 |
| 2 | M1 | ILSPLTKGIL | 27350 |
| 3 | M1 | IRHENRMVL | 28309 |
| 4 | M1 | KTRPILSPLTK | 33844 |
| 5 | M1 | LTKGILGFVFTLTVPSERG | 39989 |
| 6 | M1 | RGLQRRRFVQNALNGNG | 53918 |
| 7 | M1 | RMVLASTTAK | 54953 |
| 8 | M1 | ALASCMGLIY | 97192 |
| 9 | NA | SWPDGAELPF | 62486 |
| 10 | NS1 | GEISPLPSL | 19312 |
| 11 | PA | FMYSDFHFI | 17119 |
DISCUSSION
T-cell-mediated immune responses play critical roles in host defenses against influenza A virus infection. The cross-reactive memory T cells, especially CTLs, are believed to improve virus clearance and reduce the severity of illness even in the absence of virus-specific antibodies, although they cannot prevent influenza infection (40). The cross-reactivity of influenza A virus-specific T cells to heterosubtypic strains has been reported (5, 15, 23, 29, 33, 47, 51). However, little is known about the cross-responses of seasonal influenza virus-specific CTLs against pdmH1N1. In this study, we further demonstrated that both bulk memory CTLs and epitope-specific CTLs established by seasonal human influenza A viruses or vaccine in healthy individuals had cross-responses against pdmH1N1 virus, as shown by the direct lysis of pdmH1N1-infected cells and expression of IFN-γ and TNF-α upon pdmH1N1 challenge. Thus, we clearly demonstrated the cross-cytotoxicity of the preexisting seasonal influenza A virus-specific CTLs against pdmH1N1.
It is known that CTLs may not provide sterilizing immunity but may promote viral clearance and alleviate illness severity, which have been extensively studied in animals (3, 38, 53, 54). There are more accumulating data showing that T-cell responses can protect against influenza. High levels of CD8 T-cell response are correlated with reduced viral shedding in persons without specific antibody (40). Most importantly, the cross-protection of such CTLs has been demonstrated in humans in vivo by the Cleveland family study, in which persons who had experienced symptomatic H1N1 influenza were found to be partially protected from the following pandemic H2N2 virus infection in 1957 (17, 46). Indeed, even for adults over 60 years old, the cross-reactive T-cell responses play some role despite the fact that they have some level of preexisting antibodies, as the cellular responses, but not antibodies, are correlated with protection against influenza illness among individuals older than 60 years (39).
It is interesting that the disease severity seems not to be related to the preexisting cross-reactive memory T cells during infection with the highly pathogenic avian influenza virus, although cross-reactive memory T cells have been detected in healthy populations by determining the IFN-γ responses (29, 32, 33, 47). The fact that lethal H5N1 influenza viruses are resistant to the antiviral effects of IFN-α, IFN-γ, or TNF-α may partially explain this (49).
pdmH1N1 has been shown to replicate more efficiently and cause more severe pathological lesions than the current seasonal influenza virus in animal models (28). However, the illness caused by this novel pandemic pdmH1N1 in most patients is mild (9, 42). Recently, some degree of cross-reactive T-cell IFN-γ responses against pdmH1N1 virus was detected in a healthy population (21, 24). Here we provide further evidence of the preexistence of cross-reactively functional memory CTLs to pdmH1N1 in healthy individuals. In addition, these cross-reactive CTLs expressed CCR5 and CXCR3, which could help cross-reactive CTLs migrate to the sites of infection, as pdmH1N1-infected cells express the ligands of CCR5 (CCL3, CCL4, and CCL5) and CXCR3 (CXCL10) (57). Our data suggest that the preexisting cross-reactive CTLs might contribute, at least in part, to the mild illness in this pandemic.
Since there is a very low frequency of influenza virus-specific T cells in human peripheral blood, most studies have used IFN-γ responses to investigate the cross-reactive T-cell responses or have used influenza virus peptide-pulsed cell lines or cell lines in which HA, NA, and M proteins are expressed as target cells to determine the cross-reactive CTLs of in vitro-expanded CD8 T-cell lines (5, 23, 29, 51). However, these assays have some limitations. First, IFN-γ-secreting T cells are not necessarily capable of lysing the target cells (6). Second, influenza virus peptides or HA, NA, and M proteins cannot fully represent the virus as a whole. To overcome these limitations, here we used the whole virus-infected and peptide-pulsed autologous primary cells as the target cells to examine the killing capacities of virus-specific CTLs. Although the assays we used here have obvious advantages, they still have limitations, as using in vitro-amplified and purified virus-specific CD8 T cells to examine their cytotoxic activities may not fully represent the in vivo situation. A prospective population-based case-control study may make it possible to further confirm our current findings.
Here, we have shown that 17 of 94 experimentally defined CD8 T-cell epitopes derived from the previous influenza A viruses are conserved in pdmH1N1, and more than half of these epitopes are derived from the M1 proteins. No conserved epitope was found in HA protein. In addition, we further demonstrated that about 65% (11/17) of the MHC class I-restricted CD8 T-cell epitopes in pdmH1N1 were completely conserved in seasonal influenza vaccine H1N1 strains during the last 20 years, and most of these invariant epitopes in vaccine strains are derived from M1 protein. Consistent with our findings, previous studies also demonstrated that the HLA-A2-restricted M158-66 epitope is the most dominant and frequently recognized CD8 T-cell epitope in humans, and memory responses to influenza virus are usually stronger in individuals with the HLA-A2 genotype than in those with other genotypes (2, 4). Importantly, we also found that seasonal influenza vaccination could expand the cross-reactively functional M158-66 epitope-specific memory CTLs in 20% (4 out of 20) HLA-A2-positive individuals. These findings suggest that vaccination with seasonal influenza vaccines might still be of benefit in this pandemic, at least in some cases, in terms of the induction of cross-reactive CTLs. Indeed, recent epidemiological studies also showed that seasonal trivalent inactivated influenza vaccine (TIV) could provide some protection (16, 19), although the seasonal influenza vaccine does not stimulate protective antibody responses to pdmH1N1.
The ability of commercially available influenza vaccines to induce human T-cell responses may vary depending on the internal protein contents of vaccines from different manufacturers (12). Here we showed that TIV (GlaxoSmithKline) induced HLA-A2/M158-66-tetramer+ CD8+ T cells in 20% of HLA-2-positive individuals, as this vaccine contains a relatively high level of M protein (11). Consistent with our finding, recent studies also showed that TIV (Sanofi Pasteur) could induce HLA-A2/M1-tetramer+ CD8+ T cells in 12 to 25% of HLA-2-positive individuals (45, 51). Live attenuated influenza vaccine (LAIV) is expected to induce T-cell responses more efficiently than TIV, as it contains all the viral internal proteins. He et al. reported that the frequency of influenza A virus-specific IFN-γ-producing CD4 and CD8 T cells significantly increased after LAIV but not TIV immunization in children of ages 5 to 9 years (26). A larger proportion of elderly volunteers who received both LAIV and TIV than of those who received TIV alone experienced a postvaccination rise in anti-influenza A virus CTL activity (22). These studies suggest that LAIV might have more benefits than TIV in providing cross-protection against variant influenza viruses, and this needs to be further confirmed by a well-designed case-control study in the future.
In conclusion, we clearly demonstrated that memory CTLs established by seasonal human influenza A viruses or vaccines could cross-react against pdmH1N1 virus. Our data suggest that individuals who were infected with seasonal human influenza A viruses previously or who received seasonal human influenza vaccines may derive benefit, at least in part, from the preexisting cross-reactive memory CTLs to reduce the severity of pdmH1N1 infection even without protective antibodies. These data may also provide some valuable insights for the future design of broadly protective vaccines to improve protection against influenza, especially pandemic influenza.
Acknowledgments
This work was supported in part by the Area of Excellence program on influenza supported by the University Grants Committee of the Hong Kong Special Administrative Region, China (project no. AoE/M-12/06) (J.S.M.P., Y.-L.L., and W.T.); the General Research Fund, Research Grants Council of Hong Kong (HKU 777108 M, HKU777407, and HKU768108) (W.T. and Y.-L.L.); the Research Fund for the Control of Infectious Diseases, Hong Kong SAR government (07060482) (W.T.); Seed Funding for Basic Research, University Research Committee, the University of Hong Kong (200611159224) (W.T.); postgraduate studentships, University of Hong Kong (H.M., G.Q., and J. Z.); and the Edward Sai-Kim Hotung Pediatric Education and Research Fund (Y.-L.L.).
Footnotes
Published ahead of print on 21 April 2010.
REFERENCES
- 1.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 2.Assarsson, E., H. H. Bui, J. Sidney, Q. Zhang, J. Glenn, C. Oseroff, I. N. Mbawuike, J. Alexander, M. J. Newman, H. Grey, and A. Sette. 2008. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J. Virol. 82:12241-12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender, B. S., T. Croghan, L. Zhang, and P. A. Small, Jr. 1992. Transgenic mice lacking class I major histocompatibility complex-restricted T cells have delayed viral clearance and increased mortality after influenza virus challenge. J. Exp. Med. 175:1143-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boon, A. C., G. de Mutsert, Y. M. Graus, R. A. Fouchier, K. Sintnicolaas, A. D. Osterhaus, and G. F. Rimmelzwaan. 2002. The magnitude and specificity of influenza A virus-specific cytotoxic T-lymphocyte responses in humans is related to HLA-A and -B phenotype. J. Virol. 76:582-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boon, A. C., G. de Mutsert, D. van Baarle, D. J. Smith, A. S. Lapedes, R. A. Fouchier, K. Sintnicolaas, A. D. Osterhaus, and G. F. Rimmelzwaan. 2004. Recognition of homo- and heterosubtypic variants of influenza A viruses by human CD8+ T lymphocytes. J. Immunol. 172:2453-2460. [DOI] [PubMed] [Google Scholar]
- 6.Brown, L. E., and A. Kelso. 2009. Prospects for an influenza vaccine that induces cross-protective cytotoxic T lymphocytes. Immunol. Cell Biol. 87:300-308. [DOI] [PubMed] [Google Scholar]
- 7.Bryceson, Y. T., M. E. March, D. F. Barber, H. G. Ljunggren, and E. O. Long. 2005. Cytolytic granule polarization and degranulation controlled by different receptors in resting NK cells. J. Exp. Med. 202:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryceson, Y. T., M. E. March, H. G. Ljunggren, and E. O. Long. 2006. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214:73-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao, B., X. W. Li, Y. Mao, J. Wang, H. Z. Lu, Y. S. Chen, Z. A. Liang, L. Liang, S. J. Zhang, B. Zhang, L. Gu, L. H. Lu, D. Y. Wang, and C. Wang. 2009. Clinical features of the initial cases of 2009 pandemic influenza A (H1N1) virus infection in China. N. Engl. J. Med. 361:2507-2517. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2009. Serum cross-reactive antibody response to a novel influenza A (H1N1) virus after vaccination with seasonal influenza vaccine. MMWR Morb. Mortal. Wkly. Rep. 58:521-524. [PubMed] [Google Scholar]
- 11.Chaloupka, I., A. Schuler, M. Marschall, and H. Meier-Ewert. 1996. Comparative analysis of six European influenza vaccines. Eur. J. Clin. Microbiol. Infect. Dis. 15:121-127. [DOI] [PubMed] [Google Scholar]
- 12.Co, M. D., L. Orphin, J. Cruz, P. Pazoles, K. M. Green, J. Potts, A. M. Leporati, J. A. Babon, J. E. Evans, F. A. Ennis, and M. Terajima. 2009. In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses. Vaccine 27:319-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawood, F. S., S. Jain, L. Finelli, M. W. Shaw, S. Lindstrom, R. J. Garten, L. V. Gubareva, X. Xu, C. B. Bridges, and T. M. Uyeki. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 14.Djeu, J. Y., K. Jiang, and S. Wei. 2002. A view to a kill: signals triggering cytotoxicity. Clin. Cancer Res. 8:636-640. [PubMed] [Google Scholar]
- 15.Doherty, P. C., and A. Kelso. 2008. Toward a broadly protective influenza vaccine. J. Clin. Invest. 118:3273-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Echevarria-Zuno, S., J. M. Mejia-Arangure, C. Grajales-Muniz, C. Gonzalez-Bonilla, and V. H. Borja-Aburto. 2010. Seasonal vaccine effectiveness against pandemic A/H1N1 reply. Lancet 375:802-803. [Google Scholar]
- 17.Epstein, S. L. 2006. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J. Infect. Dis. 193:49-53. [DOI] [PubMed] [Google Scholar]
- 18.Fraser, C., C. A. Donnelly, S. Cauchemez, W. P. Hanage, M. D. Van Kerkhove, T. D. Hollingsworth, J. Griffin, R. F. Baggaley, H. E. Jenkins, E. J. Lyons, T. Jombart, W. R. Hinsley, N. C. Grassly, F. Balloux, A. C. Ghani, N. M. Ferguson, A. Rambaut, O. G. Pybus, H. Lopez-Gatell, C. M. Alpuche-Aranda, I. B. Chapela, E. P. Zavala, D. M. Guevara, F. Checchi, E. Garcia, S. Hugonnet, and C. Roth. 2009. Pandemic potential of a strain of influenza A (H1N1): early findings. Science 324:1557-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia-Garcia, L., J. L. Valdespino-Gomez, E. Lazcano-Ponce, A. Jimenez-Corona, A. Higuera-Iglesias, P. Cruz-Hervert, B. Cano-Arellano, A. Garcia-Anaya, E. Ferreira-Guerrero, R. Baez-Saldana, L. Ferreyra-Reyes, S. Ponce-de-Leon-Rosales, C. Alpuche-Aranda, M. H. Rodriguez-Lopez, R. Perez-Padilla, and M. Hernandez-Avila. 2009. Partial protection of seasonal trivalent inactivated vaccine against novel pandemic influenza A/H1N1 2009: case-control study in Mexico City. BMJ 339:b3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garten, R. J., C. T. Davis, C. A. Russell, B. Shu, S. Lindstrom, A. Balish, W. M. Sessions, X. Xu, E. Skepner, V. Deyde, M. Okomo-Adhiambo, L. Gubareva, J. Barnes, C. B. Smith, S. L. Emery, M. J. Hillman, P. Rivailler, J. Smagala, M. de Graaf, D. F. Burke, R. A. Fouchier, C. Pappas, C. M. Alpuche-Aranda, H. Lopez-Gatell, H. Olivera, I. Lopez, C. A. Myers, D. Faix, P. J. Blair, C. Yu, K. M. Keene, P. D. Dotson, Jr., D. Boxrud, A. R. Sambol, S. H. Abid, K. St George, T. Bannerman, A. L. Moore, D. J. Stringer, P. Blevins, G. J. Demmler-Harrison, M. Ginsberg, P. Kriner, S. Waterman, S. Smole, H. F. Guevara, E. A. Belongia, P. A. Clark, S. T. Beatrice, R. Donis, J. Katz, L. Finelli, C. B. Bridges, M. Shaw, D. B. Jernigan, T. M. Uyeki, D. J. Smith, A. I. Klimov, and N. J. Cox. 2009. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science 325:197-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge, X., V. Tan, P. L. Bollyky, N. E. Standifer, E. A. James, and W. W. Kwok. 2010. Assessment of seasonal influenza A virus-specific CD4 T-cell responses to 2009 pandemic H1N1 swine-origin influenza A virus. J. Virol. 84:3312-3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorse, G. J., M. J. Campbell, E. E. Otto, D. C. Powers, G. W. Chambers, and F. K. Newman. 1995. Increased anti-influenza A virus cytotoxic T cell activity following vaccination of the chronically ill elderly with live attenuated or inactivated influenza virus vaccine. J. Infect. Dis. 172:1-10. [DOI] [PubMed] [Google Scholar]
- 23.Gotch, F., A. McMichael, G. Smith, and B. Moss. 1987. Identification of viral molecules recognized by influenza-specific human cytotoxic T lymphocytes. J. Exp. Med. 165:408-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenbaum, J. A., M. F. Kotturi, Y. Kim, C. Oseroff, K. Vaughan, N. Salimi, R. Vita, J. Ponomarenko, R. H. Scheuermann, A. Sette, and B. Peters. 2009. Pre-existing immunity against swine-origin H1N1 influenza viruses in the general human population. Proc. Natl. Acad. Sci. U. S. A. 106:20365-20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hancock, K., V. Veguilla, X. Lu, W. Zhong, E. N. Butler, H. Sun, F. Liu, L. Dong, J. R. DeVos, P. M. Gargiullo, T. L. Brammer, N. J. Cox, T. M. Tumpey, and J. M. Katz. 2009. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N. Engl. J. Med. 361:1945-1952. [DOI] [PubMed] [Google Scholar]
- 26.He, X. S., T. H. Holmes, C. Zhang, K. Mahmood, G. W. Kemble, D. B. Lewis, C. L. Dekker, H. B. Greenberg, and A. M. Arvin. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756-11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heusel, J. W., R. L. Wesselschmidt, S. Shresta, J. H. Russell, and T. J. Ley. 1994. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76:977-987. [DOI] [PubMed] [Google Scholar]
- 28.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jameson, J., J. Cruz, M. Terajima, and F. A. Ennis. 1999. Human CD8+ and CD4+ T lymphocyte memory to influenza A viruses of swine and avian species. J. Immunol. 162:7578-7583. [PubMed] [Google Scholar]
- 30.Kohlmeier, J. E., S. C. Miller, J. Smith, B. Lu, C. Gerard, T. Cookenham, A. D. Roberts, and D. L. Woodland. 2008. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity 29:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohlmeier, J. E., and D. L. Woodland. 2009. Immunity to respiratory viruses. Annu. Rev. Immunol. 27:61-82. [DOI] [PubMed] [Google Scholar]
- 32.Kreijtz, J. H., G. de Mutsert, C. A. van Baalen, R. A. Fouchier, A. D. Osterhaus, and G. F. Rimmelzwaan. 2008. Cross-recognition of avian H5N1 influenza virus by human cytotoxic T-lymphocyte populations directed to human influenza A virus. J. Virol. 82:5161-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, L. Y., L. A. Ha do, C. Simmons, M. D. de Jong, N. V. Chau, R. Schumacher, Y. C. Peng, A. J. McMichael, J. J. Farrar, G. L. Smith, A. R. Townsend, B. A. Askonas, S. Rowland-Jones, and T. Dong. 2008. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 118:3478-3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lieberman, J. 2003. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nat. Rev. Immunol. 3:361-370. [DOI] [PubMed] [Google Scholar]
- 35.Lowin, B., M. C. Peitsch, and J. Tschopp. 1995. Perforin and granzymes: crucial effector molecules in cytolytic T lymphocyte and natural killer cell-mediated cytotoxicity. Curr. Top. Microbiol. Immunol. 198:1-24. [DOI] [PubMed] [Google Scholar]
- 36.Mao, H., W. Tu, G. Qin, H. K. Law, S. F. Sia, P. L. Chan, Y. Liu, K. T. Lam, J. Zheng, M. Peiris, and Y. L. Lau. 2009. Influenza virus directly infects human natural killer cells and induces cell apoptosis. J. Virol. 83:9215-9222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matikainen, S., J. Siren, J. Tissari, V. Veckman, J. Pirhonen, M. Severa, Q. Sun, R. Lin, S. Meri, G. Uze, J. Hiscott, and I. Julkunen. 2006. Tumor necrosis factor alpha enhances influenza A virus-induced expression of antiviral cytokines by activating RIG-I gene expression. J. Virol. 80:3515-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mbawuike, I. N., Y. Zhang, and R. B. Couch. 2007. Control of mucosal virus infection by influenza nucleoprotein-specific CD8+ cytotoxic T lymphocytes. Respir. Res. 8:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McElhaney, J. E., D. Xie, W. D. Hager, M. B. Barry, Y. Wang, A. Kleppinger, C. Ewen, K. P. Kane, and R. C. Bleackley. 2006. T cell responses are better correlates of vaccine protection in the elderly. J. Immunol. 176:6333-6339. [DOI] [PubMed] [Google Scholar]
- 40.McMichael, A. J., F. M. Gotch, G. R. Noble, and P. A. Beare. 1983. Cytotoxic T-cell immunity to influenza. N. Engl. J. Med. 309:13-17. [DOI] [PubMed] [Google Scholar]
- 41.Peiris, J. S., L. L. Poon, and Y. Guan. 2009. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J. Clin. Virol. 45:169-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peiris, J. S., W. W. Tu, and H. L. Yen. 2009. A novel H1N1 virus causes the first pandemic of the 21(st) century. Eur. J. Immunol. 39:2946-2954. [DOI] [PubMed] [Google Scholar]
- 43.Presanis, A. M., D. De Angelis, A. Hagy, C. Reed, S. Riley, B. S. Cooper, L. Finelli, P. Biedrzycki, and M. Lipsitch. 2009. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 6:e1000207.19997612 [Google Scholar]
- 44.Qin, G., H. Mao, J. Zheng, S. F. Sia, Y. Liu, P. L. Chan, K. T. Lam, J. S. Peiris, Y. L. Lau, and W. Tu. 2009. Phosphoantigen-expanded human gammadelta T cells display potent cytotoxicity against monocyte-derived macrophages infected with human and avian influenza viruses. J. Infect. Dis. 200:858-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rastogi, D., C. Wang, X. Mao, C. Lendor, P. B. Rothman, and R. L. Miller. 2007. Antigen-specific immune responses to influenza vaccine in utero. J. Clin. Invest. 117:1637-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rimmelzwaan, G. F., R. A. Fouchier, and A. D. Osterhaus. 2007. Influenza virus-specific cytotoxic T lymphocytes: a correlate of protection and a basis for vaccine development. Curr. Opin. Biotechnol. 18:529-536. [DOI] [PubMed] [Google Scholar]
- 47.Roti, M., J. Yang, D. Berger, L. Huston, E. A. James, and W. W. Kwok. 2008. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J. Immunol. 180:1758-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 49.Seo, S. H., E. Hoffmann, and R. G. Webster. 2002. Lethal H5N1 influenza viruses escape host anti-viral cytokine responses. Nat. Med. 8:950-954. [DOI] [PubMed] [Google Scholar]
- 50.Seo, S. H., and R. G. Webster. 2002. Tumor necrosis factor alpha exerts powerful anti-influenza virus effects in lung epithelial cells. J. Virol. 76:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Terajima, M., J. Cruz, A. M. Leporati, L. Orphin, J. A. Babon, M. D. Co, P. Pazoles, J. Jameson, and F. A. Ennis. 2008. Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients. J. Virol. 82:9283-9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu, W., Y. L. Lau, J. Zheng, Y. Liu, P. L. Chan, H. Mao, K. Dionis, P. Schneider, and D. B. Lewis. 2008. Efficient generation of human alloantigen-specific CD4+ regulatory T cells from naive precursors by CD40-activated B cells. Blood 112:2554-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ulmer, J. B., T. M. Fu, R. R. Deck, A. Friedman, L. Guan, C. DeWitt, X. Liu, S. Wang, M. A. Liu, J. J. Donnelly, and M. J. Caulfield. 1998. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J. Virol. 72:5648-5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Webby, R. J., S. Andreansky, J. Stambas, J. E. Rehg, R. G. Webster, P. C. Doherty, and S. J. Turner. 2003. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc. Natl. Acad. Sci. U. S. A. 100:7235-7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong, G. H., and D. V. Goeddel. 1986. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. Nature 323:819-822. [DOI] [PubMed] [Google Scholar]
- 56.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 21:29-70. [DOI] [PubMed] [Google Scholar]
- 57.Woo, P. C., E. T. Tung, K. H. Chan, C. C. Lau, S. K. Lau, and K. Y. Yuen. 2010. Cytokine profiles induced by the novel swine-origin influenza A/H1N1 virus: implications for treatment strategies. J. Infect. Dis. 201:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng, J., Y. Liu, Y. L. Lau, and W. Tu. 2010. CD40-activated B cells are more potent than immature dendritic cells to induce and expand CD4(+) regulatory T cells. Cell Mol. Immunol. 7:44-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zheng, J., Y. Liu, G. Qin, P. L. Chan, H. Mao, K. T. Lam, D. B. Lewis, Y. L. Lau, and W. Tu. 2009. Efficient induction and expansion of human alloantigen-specific CD8 regulatory T cells from naive precursors by CD40-activated B cells. J. Immunol. 183:3742-3750. [DOI] [PubMed] [Google Scholar]
- 60.Zhou, J., H. K. Law, C. Y. Cheung, I. H. Ng, J. S. Peiris, and Y. L. Lau. 2006. Differential expression of chemokines and their receptors in adult and neonatal macrophages infected with human or avian influenza viruses. J. Infect. Dis. 194:61-70. [DOI] [PMC free article] [PubMed] [Google Scholar]